Supramolecular Chromatographic Separation of C60 and C70 Fullerenes: Flash Column Chromatography vs. High Pressure Liquid Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Silica-Bound C-Butylpyrogallol[4]arene Stationary Phase 3a and 3b
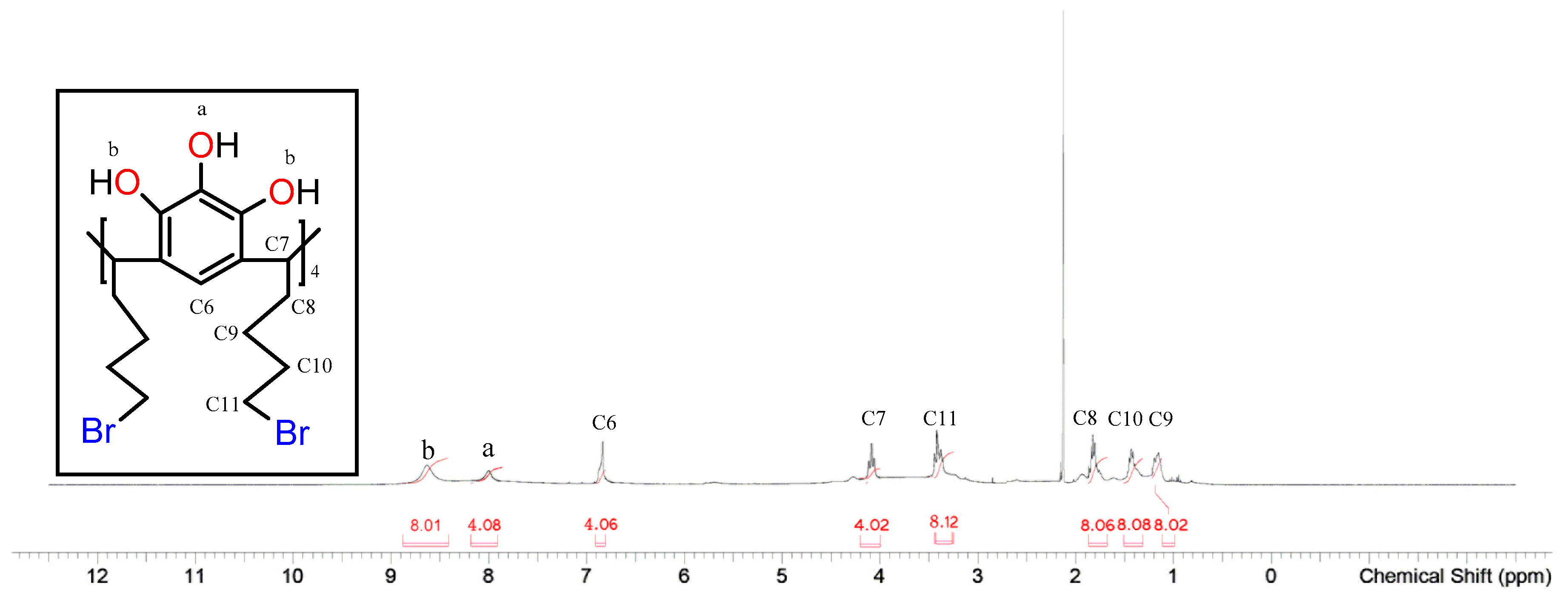
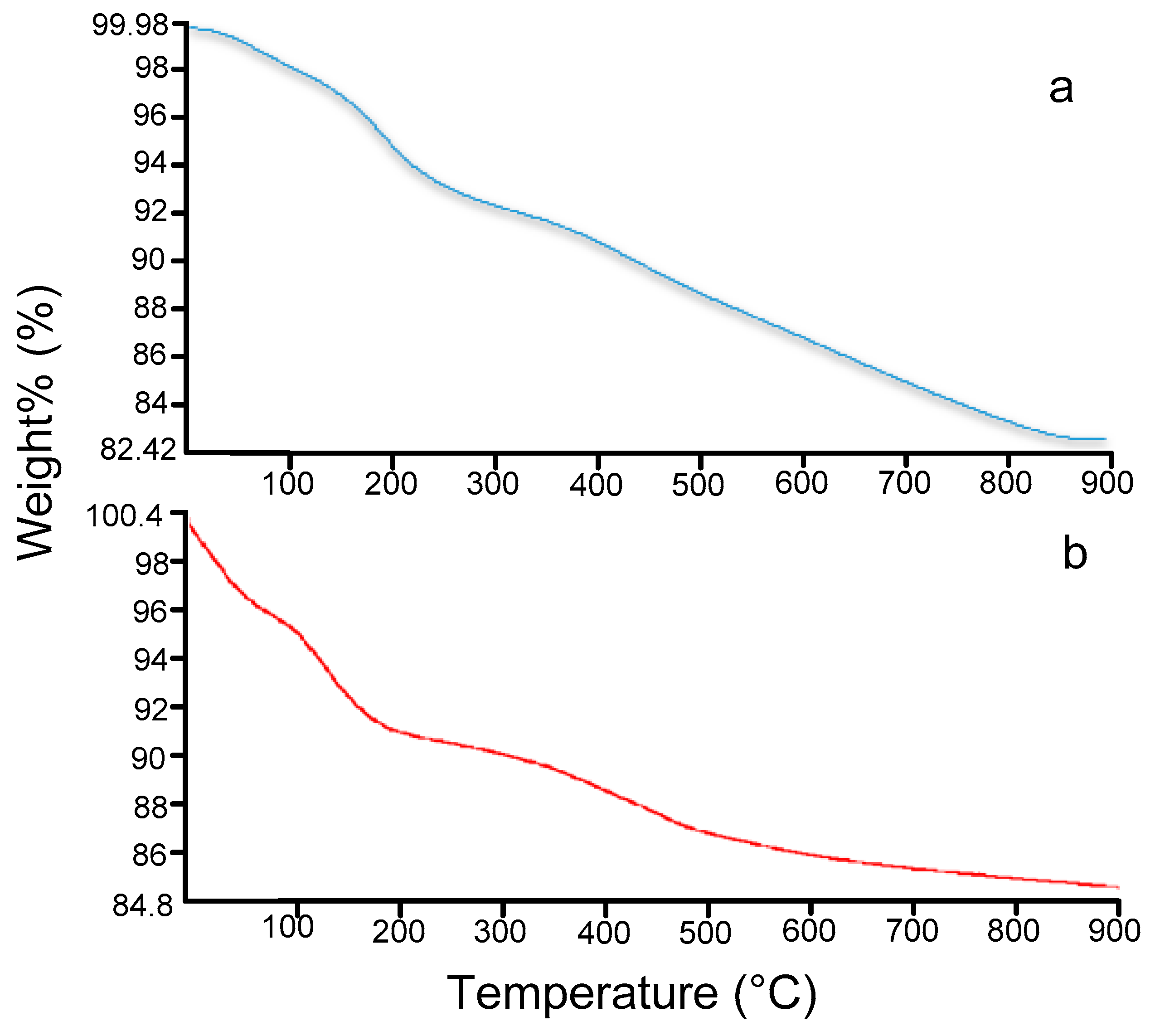
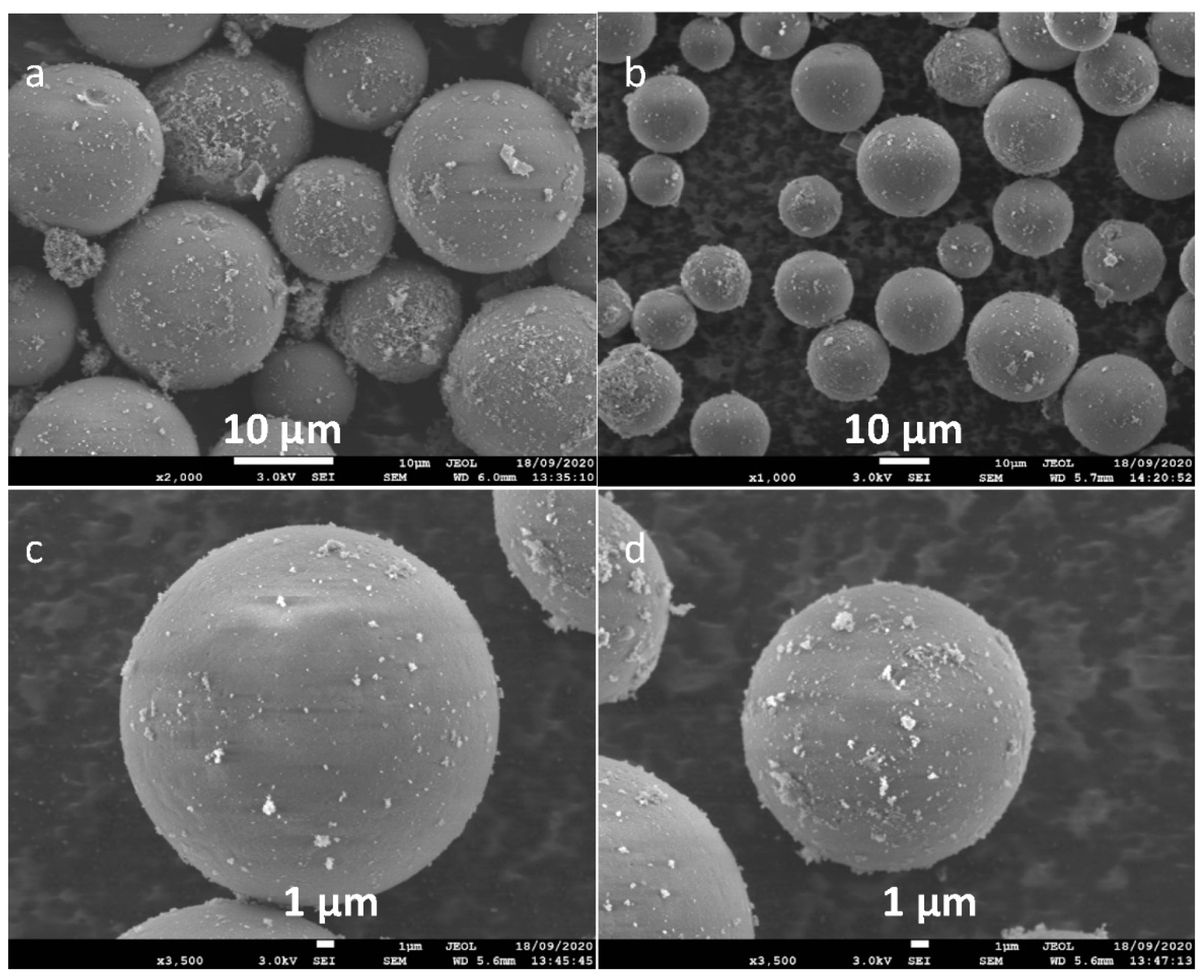
2.2. Characterisation of the Silica-Bound C-Butylpyrogallol[4]arene Stationary Phases 3a and 3b
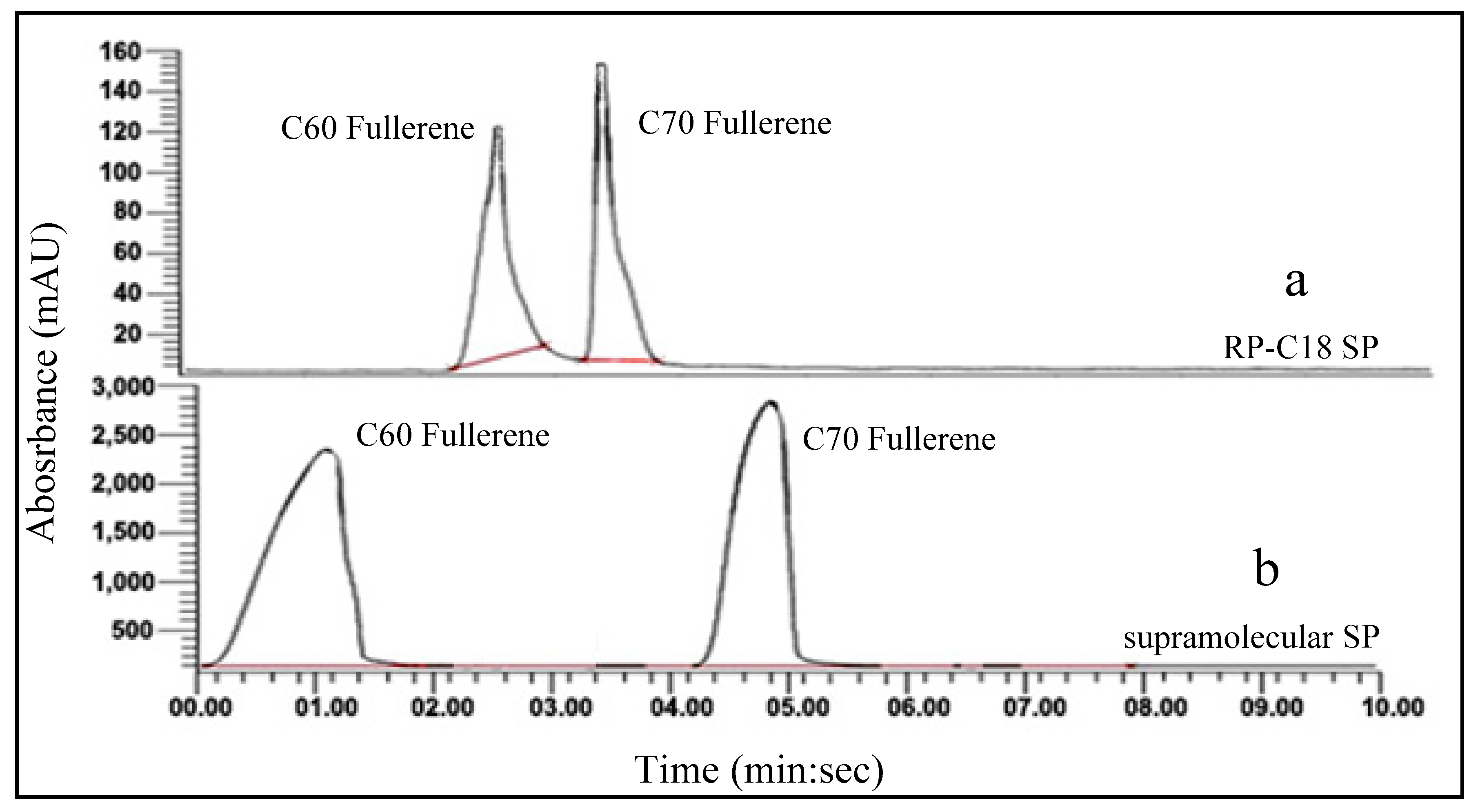
2.3. Supramolecular Chromatographic Separation of C60 and C70 Fullerenes
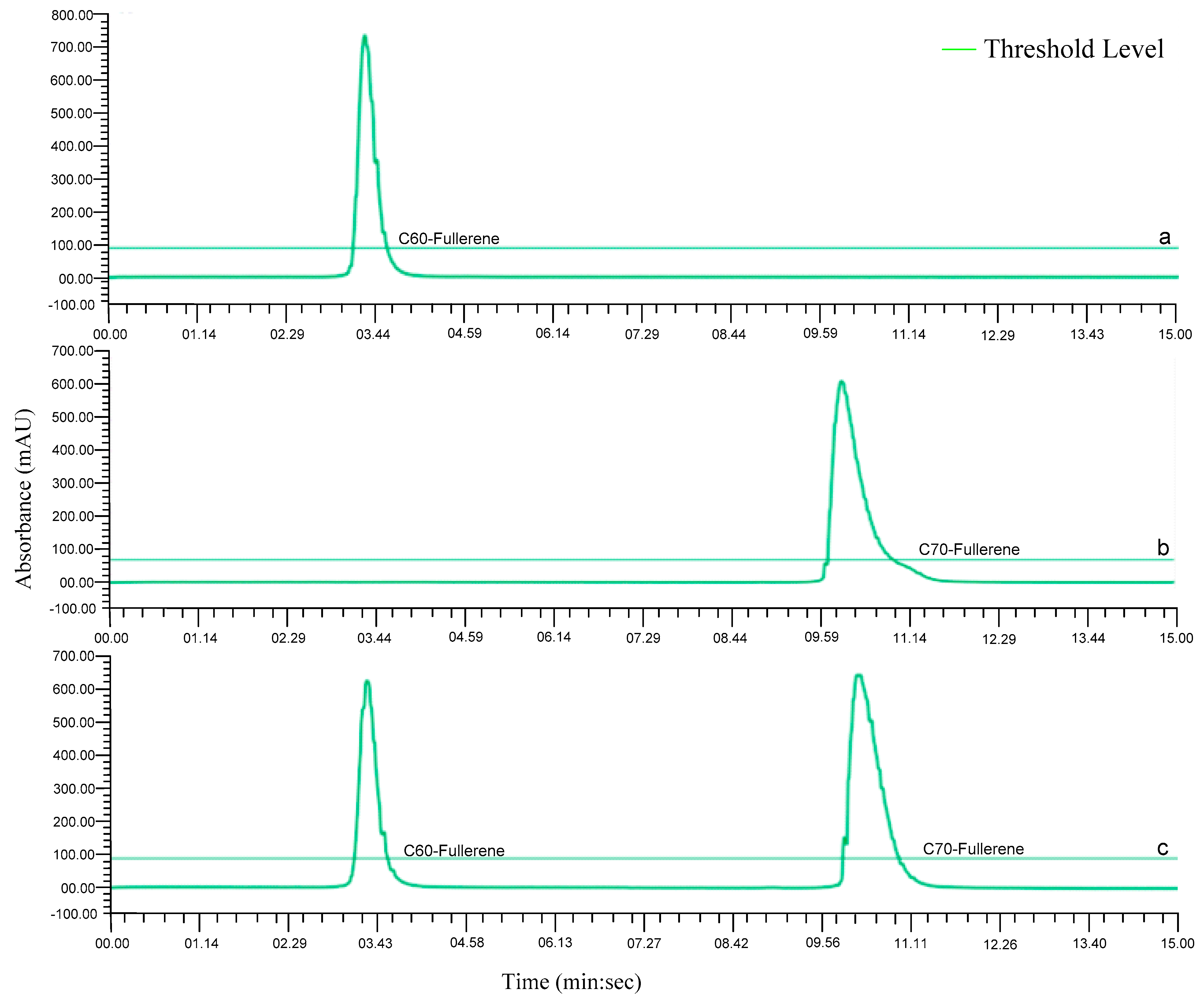
2.3.1. HPLC Separation of C60 and C70-Fullerenes
2.3.2. Flash Column Separation of C60 and C70-Fullerenes
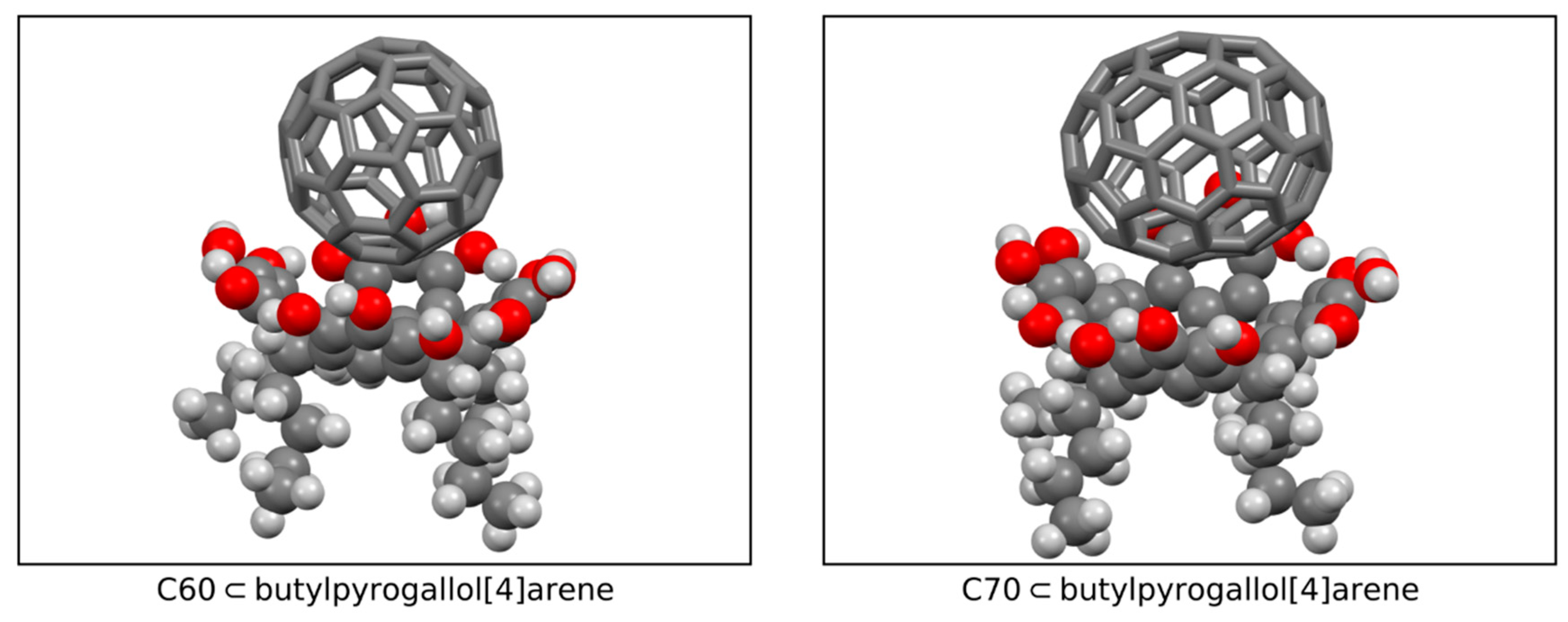
2.4. Quantum Chemistry Calculations
3. Materials and Methods
3.1. Reagents and Instruments
3.2. Synthesis of C-Hydroxybutyl-Pyrogallol[4]arene 1
3.3. Synthesis of C-Bromobutylcalix[4]pyrogallolarene 2
3.4. Preparation of C-Butylpyrogallol[4]arene Bound Silica Gel HPLC Stationary Phase 3a
3.5. Preparation of C-Butylpyrogallol[4]arene Bound Silica Gel Flash Column Stationary Phase 3b
3.6. Column Packing
3.7. HPLC Separation Procedure
3.8. Flash Column Chromatographic Procedure
3.9. In Silico Study for the Host–Guest Interaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Balch, A.L.; Olmstead, M.M. Reactions of Transition Metal Complexes with Fullerenes (C60, C70, etc.) and Related Materials. Chem. Rev. 1998, 98, 2123–2166. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, W.; Zuckermann, R.N.; Li, Z.; Zhao, X.; Liu, Y. The Organic Flatland—Recent Advances in Synthetic 2D Organic Layers. Adv. Mater. 2015, 27, 5762–5770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, J.; Zhang, Y.; Chen, J.; Zhao, Y.; Niu, Y.; Yu, C. Cerium Dioxide-Doped Carboxyl Fullerene as Novel Nanoprobe and Catalyst in Electrochemical Biosensor for Amperometric Detection of the CYP2C19* 2 Allele in Human Serum. Biosens. Bioelectron. 2018, 102, 94–100. [Google Scholar] [CrossRef]
- Krishna, V.; Anand, S.; Maytin, E.; Grobmyer, S. Polyhydroxy-Fullerene Sunscreen Active Agents and Compositions. U.S. Patent US20190053991A1, 21 February 2019. [Google Scholar]
- Martins, M.; Azoia, N.G.; Melle-Franco, M.; Ribeiro, A.; Cavaco-Paulo, A. Permeation of Skin with (C60) Fullerene Dispersions. Eng. Life Sci. 2017, 17, 732–738. [Google Scholar] [CrossRef]
- Castro, E.; Cerón, M.R.; Garcia, A.H.; Kim, Q.; Etcheverry-Berríos, A.; Morel, M.J.; Díaz-Torres, R.; Qian, W.; Martinez, Z.; Mendez, L. A New Family of Fullerene Derivatives: Fullerene-Curcumin Conjugates for Biological and Photovoltaic Applications. RSC Adv. 2018, 8, 41692–41698. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Kasuya, Y.; Ji, Q.; Sathish, M.; Shrestha, L.K.; Ishihara, S.; Minami, K.; Morita, H.; Yamazaki, T.; Hanagata, N. Vortex-Aligned Fullerene Nanowhiskers as a Scaffold for Orienting Cell Growth. ACS Appl. Mater. Interfaces 2015, 7, 15667–15673. [Google Scholar] [CrossRef] [PubMed]
- Neretin, I.S.; Lyssenko, K.A.; Antipin, M.Y.; Slovokhotov, Y.L.; Boltalina, O.V.; Troshin, P.A.; Lukonin, A.Y.; Sidorov, L.N.; Taylor, R. C60F18, a Flattened Fullerene: Alias a Hexa-Substituted Benzene. Angewandte Chemie 2000, 112, 3411–3414. [Google Scholar] [CrossRef]
- Amsharov, K.Y.; Holzwarth, J.; Roshchyna, K.; Sharapa, D.I.; Hampel, F.; Hirsch, A. Synthesis, Structural Characterization, and Crystal Packing of the Elusive Pentachlorinated Azafullerene C59NCl5. Chem. Eur. J. 2017, 23, 9014–9017. [Google Scholar] [CrossRef]
- Coustel, N.; Bernier, P.; Aznar, R.; Zahab, A.; Lambert, J.; Lyard, P. Purification of C60 by a Simple Crystallization Procedure. J. Chem. Soc. Chem. Commun. 1992, 1402–1403. [Google Scholar] [CrossRef]
- Nagata, K.; Dejima, E.; Kikuchi, Y.; Hashiguchi, M. Efficient and Scalable Method for [60]Fullerene Separation from a Fullerene Mixture: Selective Complexation of Fullerenes with DBU in the Presence of Water. Org. Process Res. Dev. 2005, 9, 660–662. [Google Scholar] [CrossRef]
- Manolova, N.; Rashkov, I.; Legras, D.; Beguin, F. Separation of C60/C70 Mixture on Activated Carbon and Activated Carbon Fibres. Carbon 1995, 33, 209–213. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Wang, H.; Yan, X. High-Performance Separation of Fullerenes on Metal–Organic Framework MIL-101 (Cr). Chem. Eur. J. 2011, 17, 11734–11737. [Google Scholar] [CrossRef]
- Guillaume, Y.C.; Peyrin, E. Geometrical Model for the Retention of Fullerenes in High-Performance Liquid Chromatography. Anal. Chem. 1999, 71, 1326–1331. [Google Scholar] [CrossRef]
- Casadei, N.; Thomassin, M.; Guillaume, Y.; André, C. A Humic Acid Stationary Phase for the High Performance Liquid Chromatography Separation of Buckminsterfullerenes: Theoretical and Practical Aspects. Anal. Chim. Acta 2007, 588, 268–273. [Google Scholar] [CrossRef]
- Xiao, J.; Meyerhoff, M.E. High-Performance Liquid Chromatography of C60, C70, and Higher Fullerenes on Tetraphenylporphyrin-Silica Stationary Phases using Strong Mobile Phase Solvents. J. Chromatogr. A. 1995, 715, 19–29. [Google Scholar] [CrossRef]
- . Yi, H.; Zeng, G.; Lai, C.; Huang, D.; Tang, L.; Gong, J.; Chen, M.; Xu, P.; Wang, H.; Cheng, M. Environment-Friendly Fullerene Separation Methods. Chem. Eng. J. 2017, 330, 134–145. [Google Scholar] [CrossRef]
- Boyd, P.D.; Reed, C.A. Fullerene− Porphyrin Constructs. Acc. Chem. Res. 2005, 38, 235–242. [Google Scholar] [CrossRef]
- Süß, S.; Michaud, V.; Amsharov, K.; Akhmetov, V.; Kaspereit, M.; Damm, C.; Peukert, W. Quantitative Evaluation of Fullerene Separation by Liquid Chromatography. J. Phys. Chem. C 2019, 123, 16747–16756. [Google Scholar] [CrossRef]
- Mekapothula, S.; Addicoat, M.A.; Boocock, D.J.; Wallis, J.D.; Cragg, P.J.; Cave, G.W. Silica Bound Co-Pillar[4+1]Arene as a Novel Supramolecular Stationary Phase. Chem. Commun. 2020, 56, 1792–1794. [Google Scholar] [CrossRef]
- Mekapothula, S.; Wonanke, A.D.; Addicoat, M.A.; Wallis, J.D.; Boocock, D.J.; Cave, G.W. A Supramolecular Cavitand for Selective Chromatographic Separation of Peptides using LC-MS/MS: A Combined in Silico and Experimental Approach. New J. Chem. 2021, 45, 141–146. [Google Scholar] [CrossRef]
- Gutsche, C.D. Calixarenes: An Introduction; Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Kimata, K.; Hosoya, K.; Araki, T.; Tanaka, N. [2-(1-Pyrenyl) Ethyl] Silyl Silica Packing Material for Liquid Chromatographic Separation of Fullerenes. J. Org. Chem. 1993, 58, 282–283. [Google Scholar] [CrossRef]
- Li, L.; Liu, M.; Da, S.; Feng, Y. High Performance Liquid Chromatography of Aromatic Carboxylic Acids on p-Tert-Butyl-Calix[8]Arene-Bonded Silica Gel Stationary Phase. Talanta 2004, 62, 643–648. [Google Scholar] [CrossRef]
- Śliwka-Kaszyńska, M.; Jaszczołt, K.; Kołodziejczyk, A.; Rachoń, J. 1, 3-Alternate-25,27-Dibenzoiloxy-26, 28-Bis-[3-Propyloxy]-Calix[4]Arene-Bonded Silica Gel as a New Type of HPLC Stationary Phase. Talanta 2006, 68, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.; Dzung, N.T.K. Calixarene-Based Molecules for Cation Recognition. Sensors 2002, 2, 397–416. [Google Scholar] [CrossRef]
- Cabrera, K.; Wieland, G.; Schäfer, M. High-Performance Liquid Chromatographic Separation of Fullerenes (C60 and C70) using Chemically Bonded γ-Cyclodextrin as Stationary Phase. J. Chromatogr. A 1993, 644, 396–399. [Google Scholar] [CrossRef]
- Andersson, T.; Nilsson, K.; Sundahl, M.; Westman, G.; Wennerström, O. C60 Embedded in γ-Cyclodextrin: A Water-Soluble Fullerene. J. Chem. Soc. Chem. Commun. 1992, 8, 604–606. [Google Scholar] [CrossRef]
- Atwood, J.L.; Koutsantonis, G.A.; Raston, C.L. Purification of C60 and C70 by Selective Complexation with Calixarenes. Nature 1994, 368, 229–231. [Google Scholar] [CrossRef]
- Song, J.; Aratani, N.; Shinokubo, H.; Osuka, A. A Porphyrin Nanobarrel that Encapsulates C60. J. Am. Chem. Soc. 2010, 132, 16356–16357. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N. Preferential Precipitation of C70 Over C60 with p-Halohomooxacalix[3]Arenes. Org. Biomol. Chem. 2003, 1, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Funck, M.; Guest, D.P.; Cave, G.W. Microwave-Assisted Synthesis of Resorcin[4]Arene and Pyrogallol[4]Arene Macrocycles. Tetrahedron Lett. 2010, 51, 6399–6402. [Google Scholar] [CrossRef]
- Cave, G.W.; Antesberger, J.; Barbour, L.J.; McKinlay, R.M.; Atwood, J.L. Inner Core Structure Responds to Communication between Nanocapsule Walls. Angew. Chem. Int. Ed. 2004, 43, 5263–5266. [Google Scholar] [CrossRef] [PubMed]
- Heaven, M.W.; Cave, G.W.; McKinlay, R.M.; Antesberger, J.; Dalgarno, S.J.; Thallapally, P.K.; Atwood, J.L. Hydrogen-Bonded Hexamers Self-Assemble as Spherical and Tubular Superstructures on the Sub-Micron Scale. Angew. Chem. Int. Ed. 2006, 45, 6221–6224. [Google Scholar] [CrossRef] [PubMed]
- Cave, G.W.; Dalgarno, S.J.; Antesberger, J.; Ferrarelli, M.C.; McKinlay, R.M.; Atwood, J.L. Investigations into Chain Length Control Over Solid-State Pyrogallol[4]Arene Nanocapsule Packing. Supramol. Chem. 2008, 20, 157–159. [Google Scholar] [CrossRef]
- Bassil, D.B.; Dalgarno, S.J.; Cave, G.W.; Atwood, J.L.; Tucker, S.A. Spectroscopic Investigations of Adma Encapsulated in Pyrogallol[4]Arene Nanocapsules. J. Phys. Chem. B. 2007, 111, 9088–9092. [Google Scholar] [CrossRef]
- DeStefano, J.; Langlois, T.; Kirkland, J. Characteristics of Superficially-Porous Silica Particles for Fast HPLC: Some Performance Comparisons with Sub-2-µm Particles. J. Chromatogr. Sci. 2008, 46, 254–260. [Google Scholar] [CrossRef]
- Hara, T.; Kobayashi, H.; Ikegami, T.; Nakanishi, K.; Tanaka, N. Performance of Monolithic Silica Capillary Columns with Increased Phase Ratios and Small-Sized Domains. Anal. Chem. 2006, 78, 7632–7642. [Google Scholar] [CrossRef] [PubMed]
- Majors, R.E. High-Performance Liquid Chromatography on Small Particle Silica Gel. Anal. Chem. 1972, 44, 1722–1726. [Google Scholar] [CrossRef]
- Kirkland, J. Superficially Porous Silica Microspheres for the Fast High-Performance Liquid Chromatography of Macromolecules. Anal. Chem. 1992, 64, 1239–1245. [Google Scholar] [CrossRef]
- Flídrová, K.; Liška, A.; Ludvík, J.; Eigner, V.; Lhoták, P. Fullerene Recognition by 5-Nitro-11,17,23,29-Tetramethylcalix[5]Arene. Tetrahedron Lett. 2015, 56, 1535–1538. [Google Scholar] [CrossRef]
- González-Delgado, A.M.; Giner-Casares, J.J.; Brezesinski, G.; Regnouf-de-Vains, J.; Camacho, L. Langmuir Monolayers of an Inclusion Complex Formed by a New Calixarene Derivative and Fullerene. Langmuir 2012, 28, 12114–12121. [Google Scholar] [CrossRef]
- Yu, Q.; Feng, Y.; Shi, Z.; Yang, J. HPLC Separation of Fullerenes on the Stationary Phases of Two Lewis Bases Modified Magnesia–Zirconia. Anal. Chim. Acta 2005, 536, 39–48. [Google Scholar] [CrossRef]
- Ohta, H.; Saito, Y.; Nagae, N.; Pesek, J.J.; Matyska, M.T.; Jinno, K. Fullerenes Separation with Monomeric Type C30 Stationary Phase in High-Performance Liquid Chromatography. J. Chromatogr. A 2000, 883, 55–66. [Google Scholar] [CrossRef]
- Yu, Q.; Shi, Z.; Lin, B.; Wu, Y.; Feng, Y. HPLC Separation of Fullerenes on Two Charge-Transfer Stationary Phases. J. Sep. Sci. 2006, 29, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Carboni, A.; Helmus, R.; Parsons, J.R.; Kalbitz, K.; de Voogt, P. A Method for the Determination of Fullerenes in Soil and Sediment Matrices using Ultra-High Performance Liquid Chromatography Coupled with Heated Electrospray Quadrupole Time of Flight Mass Spectrometry. J. Chromatogr. A 2016, 1433, 123–130. [Google Scholar] [CrossRef]
- Coutant, D.E.; Clarke, S.A.; Francis, A.H.; Meyerhoff, M.E. Selective Separation of Fullerenes on Hydroxyphenyl-triphenylporphyrin–silica Stationary Phases. J. Chromatogr. A 1998, 824, 147–157. [Google Scholar] [CrossRef]
- Yu, Q.; Lin, B.; He, H.; Shi, Z.; Feng, Y. Preparation of Pyrenebutyric Acid Bonded Silica Stationary Phases for the Application to the Separation of Fullerenes. J. Chromatogr. A 2005, 1083, 23–31. [Google Scholar] [CrossRef]
- Kimata, K.; Hirose, T.; Moriuchi, K.; Hosoya, K.; Araki, T.; Tanaka, N. High-Capacity Stationary Phases Containing Heavy Atoms for HPLC Separation of Fullerenes. Anal. Chem. 1995, 67, 2556–2561. [Google Scholar] [CrossRef]
- NACALAI TESQUE, INC. [Cosmosil]. 2020. Available online: https://www.nacalai.co.jp/global/cosmosil/index/Buckyprep-D.html (accessed on 27 January 2021).
- Jinno, K.; Uemura, T.; Ohta, H.; Nagashima, H.; Itoh, K. Separation and Identification of Higher Molecular Weight Fullerenes by High-Performance Liquid Chromatography with Monomeric and Polymeric Octadecylsilica Bonded Phases. Anal. Chem. 1993, 65, 2650–2654. [Google Scholar] [CrossRef]
- Kasprowiak, A.; Cazier-Dennin, F.; Danjou, P. Flash Chromatography System: A Practical Tool for Demonstrating the Influence of Column Characteristics on Chromatographic Resolution. J. Chem. Educ. 2020, 97, 1145–1150. [Google Scholar] [CrossRef]
- Selmani, S.; Shen, M.Y.; Schipper, D.J. Iptycene-Functionalized Silica Gel for the Purification of Fullerenes using Flash Chromatography. RSC Adv. 2017, 7, 19026–19029. [Google Scholar] [CrossRef]
- Addicoat, M.A.; Metha, G.F. Kick: Constraining a Stochastic Search Procedure with Molecular Fragments. J. Comput. Chem. 2009, 30, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 61; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-Consistent-Charge Density-Functional Tight-Binding Method for Simulations of Complex Materials Properties. Phys. Rev. B 1998, 58, 7260. [Google Scholar] [CrossRef]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.; Goddard, W.A., III; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Te Velde, G.T.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Onufriev, A.V.; Case, D.A. Generalized Born Implicit Solvent Models for Biomolecules. Ann. Rev. Biophys. 2019, 48, 275–296. [Google Scholar] [CrossRef] [PubMed]

| Chromatographic Technique | Shape of Silica Gel | Particle Size (µm) | Surface Area (m2/g) | Pore Volume (cc/g) | Pore Size |
|---|---|---|---|---|---|
| Flash column | spherical | 50 | 500 | 0.70 | 500 |
| HPLC | spherical | 5 | 120 | 0.95 | 70 |
| Guests | ∆GBE (kcal mol−1) | Experimental Chromatography | ||
|---|---|---|---|---|
| Gas Phase | Solvent Phase (Toluene) | HPLC (min:sec) | Flash Column (min:sec) | |
| C60-fullerene | −133.385 | −123.862 | 1:08 | 03:26 |
| C70-fullerene | −135.569 | −125.355 | 4:85 | 10:38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekapothula, S.; Wonanke, A.D.D.; Addicoat, M.A.; Boocock, D.J.; Wallis, J.D.; Cave, G.W.V. Supramolecular Chromatographic Separation of C60 and C70 Fullerenes: Flash Column Chromatography vs. High Pressure Liquid Chromatography. Int. J. Mol. Sci. 2021, 22, 5726. https://doi.org/10.3390/ijms22115726
Mekapothula S, Wonanke ADD, Addicoat MA, Boocock DJ, Wallis JD, Cave GWV. Supramolecular Chromatographic Separation of C60 and C70 Fullerenes: Flash Column Chromatography vs. High Pressure Liquid Chromatography. International Journal of Molecular Sciences. 2021; 22(11):5726. https://doi.org/10.3390/ijms22115726
Chicago/Turabian StyleMekapothula, Subbareddy, A. D. Dinga Wonanke, Matthew A. Addicoat, David J. Boocock, John D. Wallis, and Gareth W. V. Cave. 2021. "Supramolecular Chromatographic Separation of C60 and C70 Fullerenes: Flash Column Chromatography vs. High Pressure Liquid Chromatography" International Journal of Molecular Sciences 22, no. 11: 5726. https://doi.org/10.3390/ijms22115726
APA StyleMekapothula, S., Wonanke, A. D. D., Addicoat, M. A., Boocock, D. J., Wallis, J. D., & Cave, G. W. V. (2021). Supramolecular Chromatographic Separation of C60 and C70 Fullerenes: Flash Column Chromatography vs. High Pressure Liquid Chromatography. International Journal of Molecular Sciences, 22(11), 5726. https://doi.org/10.3390/ijms22115726







