Ceramide and Related Molecules in Viral Infections
Abstract
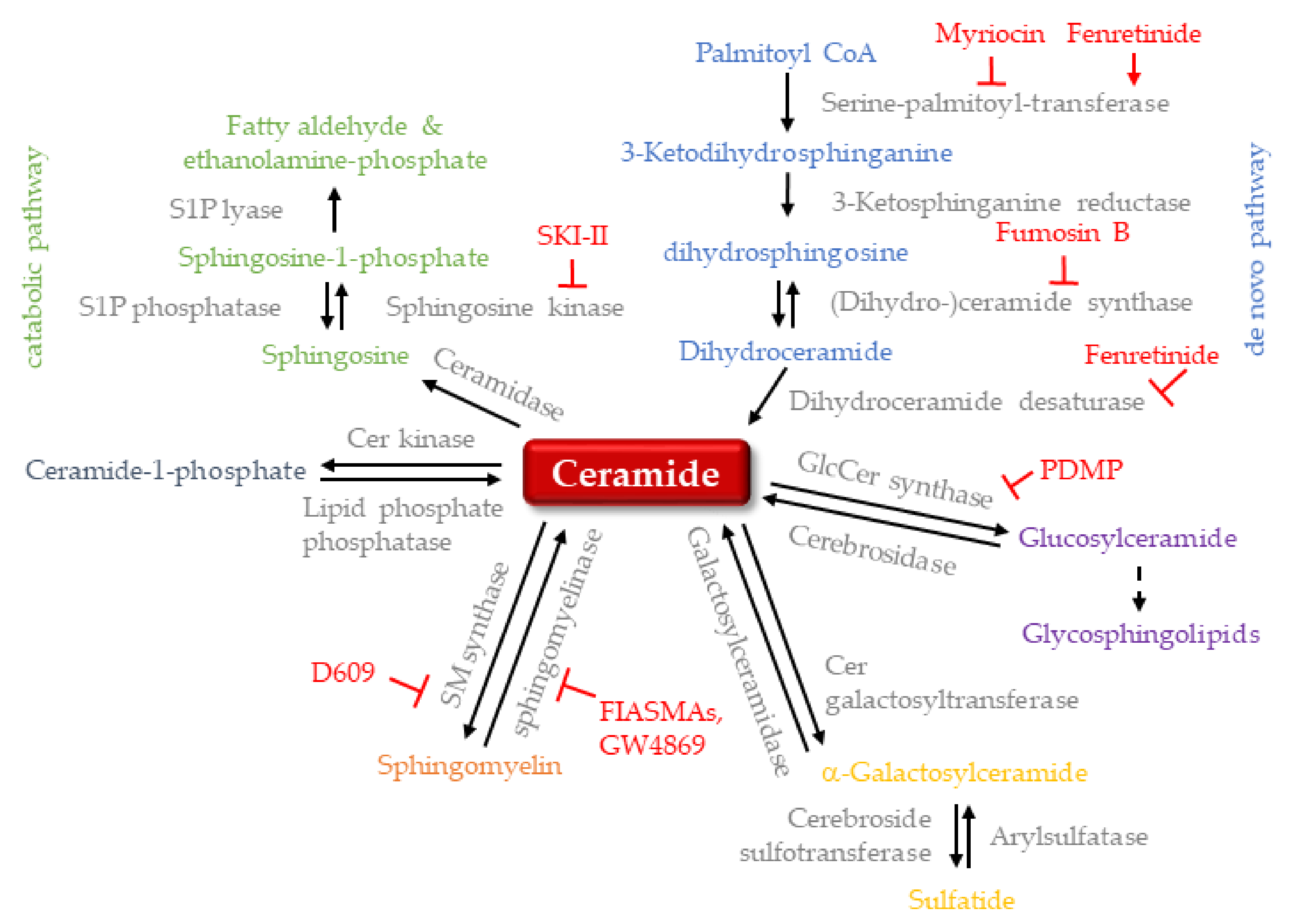
1. Introduction
2. Viral Entry
2.1. Viral Attachment
2.2. Viral Penetration and Uncoating
2.3. Targeting Sphingolipids to Prevent Viral Entry
3. Viral Gene Expression and Replication
3.1. Viral Replication and Assembly
3.2. Anti-Viral Properties of Ceramide-Metabolism Inhibitors
4. Virion Release
5. Viral-Induced Apoptosis and Morbidity
6. Viral Immune Evasion and Immune Modulation
6.1. Viral Immune Evasion
6.2. Invariant Natural Killer T Cells in Viral Infections
6.3. Potential Clinical Applications of α-GalCer
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Viruses | |
| B19V | human parvovirus B19 |
| EBOV | ebola virus |
| EMCV-D | encephalomyocarditis virus |
| HBV | hepatitis B virus |
| HCMV | human cytomegalovirus |
| HCV | hepatitis C virus |
| HIV-1 | human immunodeficiency virus type 1 |
| HPV | human papillomavirus |
| HSV-1 | herpes simplex virus 1 |
| HSV-2 | herpes simplex virus 2 |
| IAV | influenza A virus |
| IBV | infectious bronchitis virus |
| JEV | Japanese encephalitis virus |
| LCMV | lymphocytic choriomeningitis virus |
| MV | measles virus |
| SARS-CoV | severe acute respiratory syndrome corona virus |
| SFV | Semliki Forest virus |
| SV40 | simian virus 40 |
| VSV | vesicular stomatitis virus |
| WNV | West Nile virus |
| ZIKV | Zika virus |
| Sphingolipids and sphingolipid-metabolizing enzymes | |
| α-CalCer | α-galactosylceramide |
| ASM | acid sphingomyelinase |
| CERT | ceramide transport protein |
| dhS1P | dihydrosphingosine 1-phosphate |
| FIASMA | functional inhibitor of acid sphingomyelinase |
| GalCer | galactosylceramide |
| Gb3 | globotriaosylceramide |
| Gb4Cer | globoside/globotetraosylceramide |
| GlcCerS | glucosylceramide synthase |
| GM3-NeuAc | N-acetylneuraminyllactosylceramide |
| GSL | glycosphingolipids |
| NSM | neutral sphingomyelinase |
| SM | sphingomyelin |
| SMS2 | sphingomyelin synthase 2 |
| SphK1 | sphingosine kinase 1 |
| SPT | serine palmitoyltransferase |
| Other | |
| ACE2 | angiotensin converting enzyme 2 |
| COVID-19 | corona virus disease 2019 |
| DC-SIGN | dendritic cell-specific intracellular adhesion molecule-3-grabbing non-integrin |
| HA | hemagluttinin |
| iNKT | invariant natural killer T cell |
| mTOR | mammalian target of rapamycin |
| OSBP | oxysterol-binding protein |
| PKD | protein kinase D |
| RanBP3 | Ran-binding protein 3 |
| TGN | trans golgi network |
References
- Narimatsu, S.; Soeda, S.; Tanaka, T.; Kishimoto, Y. Solubilization and partial characterization of fatty acyl-CoA:sphingosine acyltransferase (ceramide synthetase) from rat liver and brain. Biochim. Biophys. Acta 1986, 877, 334–341. [Google Scholar]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef]
- Schneider, P.B.; Kennedy, E.P. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J. Lipid. Res. 1967, 8, 202–209. [Google Scholar] [CrossRef]
- Beckmann, N.; Gulbins, E.; Becker, K.A.; Carpinteiro, A. Sphingomyelinase, Acidic. In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: New York, NY, USA, 2017; pp. 1–8. [Google Scholar]
- Grassme, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, N.; Sharma, D.; Gulbins, E.; Becker, K.A.; Edelmann, B. Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons. Front. Physiol. 2014, 5, 331. [Google Scholar] [CrossRef] [PubMed]
- Draeger, A.; Babiychuk, E.B. Ceramide in plasma membrane repair. Handb. Exp. Pharmacol. 2013, 341–353. [Google Scholar] [CrossRef]
- Ryu, W.-S. Chapter 3—Virus Life Cycle. In Molecular Virology of Human Pathogenic Viruses; Ryu, W.-S., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 31–45. [Google Scholar]
- Chen, C.S.; Rosenwald, A.G.; Pagano, R.E. Ceramide as a modulator of endocytosis. J. Biol. Chem. 1995, 270, 13291–13297. [Google Scholar] [CrossRef]
- Volpert, G.; Ben-Dor, S.; Tarcic, O.; Duan, J.; Saada, A.; Merrill, A.H., Jr.; Pewzner-Jung, Y.; Futerman, A.H. Oxidative stress elicited by modifying the ceramide acyl chain length reduces the rate of clathrin-mediated endocytosis. J. Cell Sci. 2017, 130, 1486–1493. [Google Scholar] [CrossRef]
- Srnka, C.A.; Tiemeyer, M.; Gilbert, J.H.; Moreland, M.; Schweingruber, H.; de Lappe, B.W.; James, P.G.; Gant, T.; Willoughby, R.E.; Yolken, R.H.; et al. Cell surface ligands for rotavirus: Mouse intestinal glycolipids and synthetic carbohydrate analogs. Virology 1992, 190, 794–805. [Google Scholar] [CrossRef]
- Martínez, M.A.; López, S.; Arias, C.F.; Isa, P. Gangliosides Have a Functional Role during Rotavirus Cell Entry. J. Virol. 2013, 87, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Hammache, D.; Piéroni, G.; Yahi, N. Role of glycosphingolipid microdomains in CD4-dependent HIV-1 fusion. Glycoconj. J. 2000, 17, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. Molecular Mechanism of HIV-1 Entry. Trends Microbiol. 2019, 27, 878–891. [Google Scholar] [CrossRef]
- Dennison, S.M.; Anasti, K.M.; Jaeger, F.H.; Stewart, S.M.; Pollara, J.; Liu, P.; Kunz, E.L.; Zhang, R.; Vandergrift, N.; Permar, S.; et al. Vaccine-Induced HIV-1 Envelope gp120 Constant Region 1-Specific Antibodies Expose a CD4-Inducible Epitope and Block the Interaction of HIV-1 gp140 with Galactosylceramide. J. Virol. 2014, 88, 9406–9417. [Google Scholar] [CrossRef] [PubMed]
- Magérus-Chatinet, A.; Yu, H.; Garcia, S.; Ducloux, E.; Terris, B.; Bomsel, M. Galactosyl ceramide expressed on dendritic cells can mediate HIV-1 transfer from monocyte derived dendritic cells to autologous T cells. Virology 2007, 362, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Alfsen, A.; Bomsel, M. HIV-1 gp41 Envelope Residues 650–685 Exposed on Native Virus Act as a Lectin to Bind Epithelial Cell Galactosyl Ceramide. J. Biol. Chem. 2002, 277, 25649–25659. [Google Scholar] [CrossRef]
- Cook, D.G.; Fantini, J.; Spitalnik, S.L.; Gonzalez-Scarano, F. Binding of Human Immunodeficiency Virus Type I (HIV-1) Gp120 to Galactosylceramide (GalCer): Relationship to the V3 Loop. Virology 1994, 201, 206–214. [Google Scholar] [CrossRef]
- Yahi, N.; Baghdiguian, S.; Moreau, H.; Fantini, J. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J. Virol. 1992, 66, 4848–4854. [Google Scholar] [CrossRef]
- Harouse, J.; Bhat, S.; Spitalnik, S.; Laughlin, M.; Stefano, K.; Silberberg, D.; Gonzalez-Scarano, F. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science 1991, 253, 320–323. [Google Scholar] [CrossRef]
- Puri, A.; Hug, P.; Jernigan, K.; Barchi, J.; Kim, H.-Y.; Hamilton, J.; Wiels, J.; Murray, G.J.; Brady, R.O.; Blumenthal, R. The neutral glycosphingolipid globotriaosylceramide promotes fusion mediated by a CD4-dependent CXCR4-utilizing HIV type 1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA 1998, 95, 14435–14440. [Google Scholar] [CrossRef]
- Puri, A.; Hug, P.; Jernigan, K.; Rose, P.; Blumenthal, R. Role of Glycosphingolipids in HIV-1 Entry: Requirement of Globotriosylceramide (Gb3) in CD4/CXCR4-dependent Fusion. Biosci. Rep. 1999, 19, 317–325. [Google Scholar] [CrossRef]
- Hammache, D.; Yahi, N.; Maresca, M.; Piéroni, G.; Fantini, J. Human Erythrocyte Glycosphingolipids as Alternative Cofactors for Human Immunodeficiency Virus Type 1 (HIV-1) Entry: Evidence for CD4-Induced Interactions between HIV-1 gp120 and Reconstituted Membrane Microdomains of Glycosphingolipids (Gb3 and GM3). J. Virol. 1999, 73, 5244–5248. [Google Scholar] [CrossRef]
- Popik, W.; Alce, T.M.; Au, W.C. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J. Virol. 2002, 76, 4709–4722. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, H.; Yoshii, H.; Tanaka, Y.; Sato, H.; Yamamoto, N.; Kubo, Y. Raft localization of CXCR4 is primarily required for X4-tropic human immunodeficiency virus type 1 infection. Virology 2009, 386, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sometani, A.; Yamazaki, Y.; Horiike, G.; Mizutani, Y.; Masuda, H.; Yamada, M.; Tahara, H.; Xu, G.; Miyamoto, D.; et al. Sulphatide binds to human and animal influenza A viruses, and inhibits the viral infection. Biochem. J. 1996, 318 Pt 2, 389–393. [Google Scholar] [CrossRef]
- Suzuki, Y.; Matsunaga, M.; Matsumoto, M. N-Acetylneuraminyllactosylceramide, GM3-NeuAc, a new influenza A virus receptor which mediates the adsorption-fusion process of viral infection. Binding specificity of influenza virus A/Aichi/2/68 (H3N2) to membrane-associated GM3 with different molecular species of sialic acid. J. Biol. Chem. 1985, 260, 1362–1365. [Google Scholar]
- Bally, M.; Rydell, G.E.; Zahn, R.; Nasir, W.; Eggeling, C.; Breimer, M.E.; Svensson, L.; Höök, F.; Larson, G. Norovirus GII.4 Virus-like Particles Recognize Galactosylceramides in Domains of Planar Supported Lipid Bilayers. Angew. Chem. Int. Ed. 2012, 51, 12020–12024. [Google Scholar] [CrossRef]
- Otsuki, N.; Sakata, M.; Saito, K.; Okamoto, K.; Mori, Y.; Hanada, K.; Takeda, M. Both Sphingomyelin and Cholesterol in the Host Cell Membrane Are Essential for Rubella Virus Entry. J. Virol. 2018, 92, e01130-17. [Google Scholar] [CrossRef] [PubMed]
- Grassmé, H.; Riehle, A.; Wilker, B.; Gulbins, E. Rhinoviruses Infect Human Epithelial Cells via Ceramide-enriched Membrane Platforms. J. Biol. Chem. 2005, 280, 26256–26262. [Google Scholar] [CrossRef]
- Dreschers, S.; Franz, P.; Dumitru, C.A.; Wilker, B.; Jahnke, K.; Gulbins, E. Infections with Human Rhinovirus Induce the Formation of Distinct Functional Membrane Domains. Cell. Physiol. Biochem. 2007, 20, 241–254. [Google Scholar] [CrossRef]
- Bentley, J.K.; Newcomb, D.C.; Goldsmith, A.M.; Jia, Y.; Sajjan, U.S.; Hershenson, M.B. Rhinovirus Activates Interleukin-8 Expression via a Src/p110β Phosphatidylinositol 3-Kinase/Akt Pathway in Human Airway Epithelial Cells. J. Virol. 2007, 81, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, D.X.; Tam, J.P. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008, 369, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Orchard, R.C.; Wilen, C.B.; Doench, J.G.; Baldridge, M.T.; McCune, B.T.; Lee, Y.C.; Lee, S.; Pruett-Miller, S.M.; Nelson, C.A.; Fremont, D.H.; et al. Discovery of a proteinaceous cellular receptor for a norovirus. Science 2016, 353, 933–936. [Google Scholar] [CrossRef]
- Orchard, R.C.; Wilen, C.B.; Virgin, H.W. Sphingolipid biosynthesis induces a conformational change in the murine norovirus receptor and facilitates viral infection. Nat. Microbiol. 2018, 3, 1109–1114. [Google Scholar] [CrossRef]
- Quattrocchi, S.; Ruprecht, N.; Bönsch, C.; Bieli, S.; Zürcher, C.; Boller, K.; Kempf, C.; Ros, C. Characterization of the Early Steps of Human Parvovirus B19 Infection. J. Virol. 2012, 86, 9274–9284. [Google Scholar] [CrossRef]
- Bönsch, C.; Zuercher, C.; Lieby, P.; Kempf, C.; Ros, C. The Globoside Receptor Triggers Structural Changes in the B19 Virus Capsid That Facilitate Virus Internalization. J. Virol. 2010, 84, 11737–11746. [Google Scholar] [CrossRef]
- Miller, M.E.; Adhikary, S.; Kolokoltsov, A.A.; Davey, R.A. Ebolavirus Requires Acid Sphingomyelinase Activity and Plasma Membrane Sphingomyelin for Infection. J. Virol. 2012, 86, 7473–7483. [Google Scholar] [CrossRef] [PubMed]
- Aizaki, H.; Morikawa, K.; Fukasawa, M.; Hara, H.; Inoue, Y.; Tani, H.; Saito, K.; Nishijima, M.; Hanada, K.; Matsuura, Y.; et al. Critical Role of Virion-Associated Cholesterol and Sphingolipid in Hepatitis C Virus Infection. J. Virol. 2008, 82, 5715–5724. [Google Scholar] [CrossRef] [PubMed]
- Hug, P.; Lin, H.M.; Korte, T.; Xiao, X.; Dimitrov, D.S.; Wang, J.M.; Puri, A.; Blumenthal, R. Glycosphingolipids promote entry of a broad range of human immunodeficiency virus type 1 isolates into cell lines expressing CD4, CXCR4, and/or CCR5. J. Virol 2000, 74, 6377–6385. [Google Scholar] [CrossRef]
- Puri, A.; Rawat, S.S.; Lin, H.M.; Finnegan, C.M.; Mikovits, J.; Ruscetti, F.W.; Blumenthal, R. An inhibitor of glycosphingolipid metabolism blocks HIV-1 infection of primary T-cells. Aids 2004, 18, 849–858. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nemoto-Sasaki, Y.; Tanikawa, T.; Oka, S.; Tsuchiya, K.; Zama, K.; Mitsutake, S.; Sugiura, T.; Yamashita, A. Sphingomyelin Synthase 2, but Not Sphingomyelin Synthase 1, Is Involved in HIV-1 Envelope-mediated Membrane Fusion. J. Biol. Chem. 2014, 289, 30842–30856. [Google Scholar] [CrossRef] [PubMed]
- Avota, E.; Gulbins, E.; Schneider-Schaulies, S. DC-SIGN Mediated Sphingomyelinase-Activation and Ceramide Generation Is Essential for Enhancement of Viral Uptake in Dendritic Cells. PLoS Pathog. 2011, 7, e1001290. [Google Scholar] [CrossRef]
- Avota, E.; Koethe, S.; Schneider-Schaulies, S. Membrane dynamics and interactions in measles virus dendritic cell infections. Cell. Microbiol. 2013, 15, 161–169. [Google Scholar] [CrossRef]
- Tsai, B.; Gilbert, J.M.; Stehle, T.; Lencer, W.; Benjamin, T.L.; Rapoport, T.A. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003, 22, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Campanero-Rhodes, M.A.; Smith, A.; Chai, W.; Sonnino, S.; Mauri, L.; Childs, R.A.; Zhang, Y.; Ewers, H.; Helenius, A.; Imberty, A.; et al. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. J. Virol. 2007, 81, 12846–12858. [Google Scholar] [CrossRef] [PubMed]
- Greber, U.F.; Singh, I.; Helenius, A. Mechanisms of virus uncoating. Trends Microbiol. 1994, 2, 52–56. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Kanou, K.; Kawase, M.; Matsuyama, S. Clinical Isolates of Human Coronavirus 229E Bypass the Endosome for Cell Entry. J. Virol. 2017, 91, e01387-16. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; He, S.; Martínez-Romero, C.; Kouznetsova, J.; Tawa, G.; Xu, M.; Shinn, P.; Fisher, E.G.; Long, Y.; Motabar, O.; et al. Synergistic drug combination effectively blocks Ebola virus infection. Antivir. Res. 2017, 137, 165–172. [Google Scholar] [CrossRef]
- Simmons, G.; Reeves, J.D.; Grogan, C.C.; Vandenberghe, L.H.; Baribaud, F.; Whitbeck, J.C.; Burke, E.; Buchmeier, M.J.; Soilleux, E.J.; Riley, J.L.; et al. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology 2003, 305, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Voisset, C.; Lavie, M.; Helle, F.; De Beeck, A.O.; Bilheu, A.; Bertrand-Michel, J.; Tercé, F.; Cocquerel, L.; Wychowski, C.; Vu-Dac, N.; et al. Ceramide enrichment of the plasma membrane induces CD81 internalization and inhibits hepatitis C virus entry. Cell. Microbiol. 2008, 10, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, C.M.; Rawat, S.S.; Puri, A.; Wang, J.M.; Ruscetti, F.W.; Blumenthal, R. Ceramide, a target for antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2004, 101, 15452–15457. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, C.M.; Blumenthal, R. Fenretinide inhibits HIV infection by promoting viral endocytosis. Antivir. Res. 2006, 69, 116–123. [Google Scholar] [CrossRef]
- Gobeil, L.-A.; Lodge, R.; Tremblay, M.J. Differential HIV-1 Endocytosis and Susceptibility to Virus Infection in Human Macrophages Correlate with Cell Activation Status. J. Virol. 2012, 86, 10399–10407. [Google Scholar] [CrossRef]
- Luisoni, S.; Suomalainen, M.; Boucke, K.; Tanner, L.B.; Wenk, M.R.; Guan, X.L.; Grzybek, M.; Coskun, Ü.; Greber, U.F. Co-option of Membrane Wounding Enables Virus Penetration into Cells. Cell Host Microbe 2015, 18, 75–85. [Google Scholar] [CrossRef]
- Murakami, K.; Tenge, V.R.; Karandikar, U.C.; Lin, S.-C.; Ramani, S.; Ettayebi, K.; Crawford, S.E.; Zeng, X.-L.; Neill, F.H.; Ayyar, B.V.; et al. Bile acids and ceramide overcome the entry restriction for GII.3 human norovirus replication in human intestinal enteroids. Proc. Natl. Acad. Sci. USA 2020, 117, 1700–1710. [Google Scholar] [CrossRef]
- Peters, S.; Schlegel, J.; Becam, J.; Avota, E.; Sauer, M.; Schubert-Unkmeir, A. Neisseria meningitidis Type IV Pili Trigger Ca(2+)-Dependent Lysosomal Trafficking of the Acid Sphingomyelinase To Enhance Surface Ceramide Levels. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Simonis, A.; Hebling, S.; Gulbins, E.; Schneider-Schaulies, S.; Schubert-Unkmeir, A. Differential Activation of Acid Sphingomyelinase and Ceramide Release Determines Invasiveness of Neisseria meningitidis into Brain Endothelial Cells. PLoS Pathog. 2014, 10, e1004160. [Google Scholar] [CrossRef]
- Gaspar, E.B.; Mortara, R.A.; Andrade, L.O.; da Silva, C.V. Lysosomal exocytosis: An important event during invasion of lamp deficient cells by extracellular amastigotes of Trypanosoma cruzi. Biochem. Biophys. Res. Commun. 2009, 384, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Luo, M. Influenza virus entry. Adv. Exp. Med. Biol. 2012, 726, 201–221. [Google Scholar] [CrossRef]
- Razinkov, V.I.; Cohen, F.S. Sterols and Sphingolipids Strongly Affect the Growth of Fusion Pores Induced by the Hemagglutinin of Influenza Virus. Biochemistry 2000, 39, 13462–13468. [Google Scholar] [CrossRef] [PubMed]
- Audi, A.; Soudani, N.; Dbaibo, G.; Zaraket, H. Depletion of Host and Viral Sphingomyelin Impairs Influenza Virus Infection. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Erdreich-Epstein, A.; Tran, L.B.; Bowman, N.N.; Wang, H.; Cabot, M.C.; Durden, D.L.; Vlckova, J.; Reynolds, C.P.; Stins, M.F.; Groshen, S.; et al. Ceramide signaling in fenretinide-induced endothelial cell apoptosis. J. Biol. Chem. 2002, 277, 49531–49537. [Google Scholar] [CrossRef]
- Wilschut, J.; Corver, J.; Nieva, J.L.; Bron, R.; Moesby, L.; Reddy, K.C.; Bittman, R. Fusion of Semliki Forest virus with cholesterol-containing liposomes at low pH: A specific requirement for sphingolipids. Mol. Membr. Biol. 1995, 12, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Nieva, J.L.; Bron, R.; Corver, J.; Wilschut, J. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 1994, 13, 2797–2804. [Google Scholar] [CrossRef]
- Corver, J.; Moesby, L.; Erukulla, R.K.; Reddy, K.C.; Bittman, R.; Wilschut, J. Sphingolipid-dependent fusion of Semliki Forest virus with cholesterol-containing liposomes requires both the 3-hydroxyl group and the double bond of the sphingolipid backbone. J. Virol. 1995, 69, 3220–3223. [Google Scholar] [CrossRef]
- Shivanna, V.; Kim, Y.; Chang, K.-O. Ceramide formation mediated by acid sphingomyelinase facilitates endosomal escape of caliciviruses. Virology 2015, 483, 218–228. [Google Scholar] [CrossRef]
- Heinrich, M.; Wickel, M.; Winoto-Morbach, S.; Schneider-Brachert, W.; Weber, T.; Brunner, J.; Saftig, P.; Peters, C.; Krönke, M.; Schütze, S. Ceramide as an activator lipid of cathepsin D. Adv. Exp. Med. Biol. 2000, 477, 305–315. [Google Scholar] [CrossRef]
- Shivanna, V.; Kim, Y.; Chang, K.-O. Endosomal acidification and cathepsin L activity is required for calicivirus replication. Virology 2014, 464–465, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, I.; Luyet, P.P.; Pons, V.; Ferguson, C.; Emans, N.; Petiot, A.; Mayran, N.; Demaurex, N.; Fauré, J.; Sadoul, R.; et al. Endosome-to-cytosol transport of viral nucleocapsids. Nat. Cell Biol. 2005, 7, 653–664. [Google Scholar] [CrossRef]
- Nour, A.M.; Li, Y.; Wolenski, J.; Modis, Y. Viral membrane fusion and nucleocapsid delivery into the cytoplasm are distinct events in some flaviviruses. PLoS Pathog. 2013, 9, e1003585. [Google Scholar] [CrossRef]
- Lang, J.; Bohn, P.; Bhat, H.; Jastrow, H.; Walkenfort, B.; Cansiz, F.; Fink, J.; Bauer, M.; Olszewski, D.; Ramos-Nascimento, A.; et al. Acid ceramidase of macrophages traps herpes simplex virus in multivesicular bodies and protects from severe disease. Nat. Commun. 2020, 11, 1338. [Google Scholar] [CrossRef]
- Guerrero, C.A.; Zárate, S.; Corkidi, G.; López, S.; Arias, C.F. Biochemical characterization of rotavirus receptors in MA104 cells. J. Virol. 2000, 74, 9362–9371. [Google Scholar] [CrossRef][Green Version]
- Han, Y.; Ventura, C.L.; Black, K.P.; Cummins, J.E., Jr.; Hall, S.D.; Jackson, S. Productive human immunodeficiency virus-1 infection of epithelial cell lines of salivary gland origin. Oral Microbiol. Immunol. 2000, 15, 82–88. [Google Scholar] [CrossRef]
- Garg, H.; Francella, N.; Tony, K.A.; Augustine, L.A.; Barchi, J.J.; Fantini, J.; Puri, A.; Mootoo, D.R.; Blumenthal, R. Glycoside analogs of β-galactosylceramide, a novel class of small molecule antiviral agents that inhibit HIV-1 entry. Antivir. Res. 2008, 80, 54–61. [Google Scholar] [CrossRef]
- Fantini, J.; Hammache, D.; Delézay, O.; Yahi, N.; André-Barrès, C.; Rico-Lattes, I.; Lattes, A. Synthetic Soluble Analogs of Galactosylceramide (GalCer) Bind to the V3 Domain of HIV-1 gp120 and Inhibit HIV-1-induced Fusion and Entry. J. Biol. Chem. 1997, 272, 7245–7252. [Google Scholar] [CrossRef]
- Blanzat, M.; Turrin, C.-O.; Aubertin, A.-M.; Couturier-Vidal, C.; Caminade, A.-M.; Majoral, J.-P.; Rico-Lattes, I.; Lattes, A. Dendritic Catanionic Assemblies: In vitro Anti-HIV Activity of Phosphorus-Containing Dendrimers Bearing Galβ1cer Analogues. ChemBioChem 2005, 6, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Fantini, J.; Hammache, D.; Delézay, O.; Piéroni, G.; Tamalet, C.; Yahi, N. Sulfatide Inhibits HIV-1 Entry into CD4−/CXCR4+Cells. Virology 1998, 246, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Lund, N.; Branch, D.R.; Mylvaganam, M.; Chark, D.; Ma, X.-Z.; Sakac, D.; Binnington, B.; Fantini, J.; Puri, A.; Blumenthal, R.; et al. A novel soluble mimic of the glycolipid, globotriaosyl ceramide inhibits HIV infection. AIDS 2006, 20, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Perino, J.; Foo, C.H.; Spehner, D.; Cohen, G.H.; Eisenberg, R.J.; Crance, J.-M.; Favier, A.-L. Role of sulfatide in vaccinia virus infection. Biol. Cell 2011, 103, 319–331. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef]
- Schloer, S.; Brunotte, L.; Goretzko, J.; Mecate-Zambrano, A.; Korthals, N.; Gerke, V.; Ludwig, S.; Rescher, U. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg. Microbes Infect. 2020, 9, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, A.; Gripp, B.; Hoffmann, M.; Pöhlmann, S.; Hoertel, N.; Edwards, M.J.; Kamler, M.; Kornhuber, J.; Becker, K.A.; Gulbins, E. Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. J. Biol. Chem. 2021, 296, 100701. [Google Scholar] [CrossRef]
- Tani, H.; Shiokawa, M.; Kaname, Y.; Kambara, H.; Mori, Y.; Abe, T.; Moriishi, K.; Matsuura, Y. Involvement of Ceramide in the Propagation of Japanese Encephalitis Virus. J. Virol. 2010, 84, 2798–2807. [Google Scholar] [CrossRef] [PubMed]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Darquennes, G.; Le Corre, P.; Le Moine, O.; Loas, G. Association between Functional Inhibitors of Acid Sphingomyelinase (FIASMAs) and Reduced Risk of Death in COVID-19 Patients: A Retrospective Cohort Study. Pharmaceuticals 2021, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, P.; Loas, G. Repurposing functional inhibitors of acid sphingomyelinase (fiasmas): An opportunity against SARS-CoV-2 infection? J. Clin. Pharm. Ther. 2021. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Upadhyay, D.; Bandapalli, O.R.; Jain, A.; Kleuser, B. Host sphingolipids: Perspective immune adjuvant for controlling SARS-CoV-2 infection for managing COVID-19 disease. Prostaglandins Other Lipid Mediat. 2021, 152, 106504. [Google Scholar] [CrossRef]
- Becker, K.A.; Carpinteiro, A.; Hoffmann, M.; Pöhlmann, S.; Kornhuber, J.; Gulbins, E. Ex vivo assay to evaluate the efficacy of drugs targeting sphingolipids in preventing SARS-CoV-2 infection of nasal epithelial cells. STAR Protoc. 2021, 2, 100356. [Google Scholar] [CrossRef]
- Zhang, L.K.; Sun, Y.; Zeng, H.; Wang, Q.; Jiang, X.; Shang, W.J.; Wu, Y.; Li, S.; Zhang, Y.L.; Hao, Z.N.; et al. Calcium channel blocker amlodipine besylate therapy is associated with reduced case fatality rate of COVID-19 patients with hypertension. Cell Discov. 2020, 6, 96. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Beeker, N.; Jannot, A.S.; Neuraz, A.; Salamanca, E.; Paris, N.; Daniel, C.; Gramfort, A.; et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: Results from an observational study. Mol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Caterino, M.; Gelzo, M.; Sol, S.; Fedele, R.; Annunziata, A.; Calabrese, C.; Fiorentino, G.; D’Abbraccio, M.; Dell’Isola, C.; Fusco, F.M.; et al. Dysregulation of lipid metabolism and pathological inflammation in patients with COVID-19. Sci. Rep. 2021, 11, 2941. [Google Scholar] [CrossRef]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma Ceramides. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1933–1939. [Google Scholar] [CrossRef]
- Edwards, M.J.; Becker, K.A.; Gripp, B.; Hoffmann, M.; Keitsch, S.; Wilker, B.; Soddemann, M.; Gulbins, A.; Carpinteiro, E.; Patel, S.H.; et al. Sphingosine prevents binding of SARS-CoV-2 spike to its cellular receptor ACE2. J. Biol. Chem. 2020, 295, 15174–15182. [Google Scholar] [CrossRef]
- Gardner, A.I.; Haq, I.J.; Simpson, A.J.; Becker, K.A.; Gallagher, J.; Saint-Criq, V.; Verdon, B.; Mavin, E.; Trigg, A.; Gray, M.A.; et al. Recombinant Acid Ceramidase Reduces Inflammation and Infection in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 202, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Thanaraj, T.A.; Qaddoumi, M.G.; Hashem, A.; Abubaker, J.; Al-Mulla, F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int. J. Mol. Sci. 2020, 21, 3544. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.M.; Haddadi, N.; Nassif, N.T.; Lin, Y. Targeting the SphK-S1P-SIPR Pathway as a Potential Therapeutic Approach for COVID-19. Int. J. Mol. Sci. 2020, 21, 7189. [Google Scholar] [CrossRef] [PubMed]
- Fecchi, K.; Anticoli, S.; Peruzzu, D.; Iessi, E.; Gagliardi, M.C.; Matarrese, P.; Ruggieri, A. Coronavirus Interplay With Lipid Rafts and Autophagy Unveils Promising Therapeutic Targets. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Dadhich, R.; Kapoor, S. Various Facets of Pathogenic Lipids in Infectious Diseases: Exploring Virulent Lipid-Host Interactome and Their Druggability. J. Membr. Biol. 2020, 253, 399–423. [Google Scholar] [CrossRef]
- Sorice, M.; Misasi, R.; Riitano, G.; Manganelli, V.; Martellucci, S.; Longo, A.; Garofalo, T.; Mattei, V. Targeting Lipid Rafts as a Strategy Against Coronavirus. Front. Cell Dev. Biol. 2020, 8, 618296. [Google Scholar] [CrossRef]
- Steinhart, W.L.; Busch, J.S.; Oettgen, J.P.; Howland, J.L. Sphingolipid Metabolism during Infection of Human Fibroblasts by Herpes Simplex Virus Type 1. Intervirology 1984, 21, 70–76. [Google Scholar] [CrossRef]
- Steinharî, W.L.; Nicolet, C.M.; Howland, J.L. Incorporation of 32P-Phosphate into Membrane Phospholipids during Infection of Cultured Human Fibroblasts by Herpes Simplex Virus Type 1. Intervirology 1981, 16, 80–85. [Google Scholar] [CrossRef]
- Rivas, C.I.; Golde, D.W.; Vera, J.C.; Kolesnick, R.N. Involvement of the sphingomyelin pathway in autocrine tumor necrosis factor signaling for human immunodeficiency virus production in chronically infected HL-60 cells. Blood 1994, 83, 2191–2197. [Google Scholar] [CrossRef]
- Papp, B.; Zhang, D.; Groopman, J.E.; Byrn, R.A. Stimulation of human immunodeficiency virus type 1 expression by ceramide. AIDS Res. Hum. Retrovir. 1994, 10, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, R.M. The Human Cytomegalovirus Major Immediate-Early Gene. Intervirology 1996, 39, 343–349. [Google Scholar] [CrossRef]
- Machesky, N.J.; Zhang, G.; Raghavan, B.; Zimmerman, P.; Kelly, S.L.; Merrill, A.H.; Waldman, W.J.; Van Brocklyn, J.R.; Trgovcich, J. Human Cytomegalovirus Regulates Bioactive Sphingolipids. J. Biol. Chem. 2008, 283, 26148–26160. [Google Scholar] [CrossRef]
- Allan-Yorke, J.; Record, M.; de Préval, C.; Davrinche, C.; Davignon, J.-L. Distinct Pathways for Tumor Necrosis Factor Alpha and Ceramides in Human Cytomegalovirus Infection. J. Virol. 1998, 72, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Le Sage, V.; Cinti, A.; Amorim, R.; Mouland, A.J. Adapting the Stress Response: Viral Subversion of the mTOR Signaling Pathway. Viruses 2016, 8, 152. [Google Scholar] [CrossRef]
- Grafen, A.; Schumacher, F.; Chithelen, J.; Kleuser, B.; Beyersdorf, N.; Schneider-Schaulies, J. Use of Acid Ceramidase and Sphingosine Kinase Inhibitors as Antiviral Compounds Against Measles Virus Infection of Lymphocytes in vitro. Front. Cell Dev. Biol. 2019, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Leier, H.C.; Weinstein, J.B.; Kyle, J.E.; Lee, J.-Y.; Bramer, L.M.; Stratton, K.G.; Kempthorne, D.; Navratil, A.R.; Tafesse, E.G.; Hornemann, T.; et al. A global lipid map defines a network essential for Zika virus replication. Nat. Commun. 2020, 11, 3652. [Google Scholar] [CrossRef] [PubMed]
- Aktepe, T.E.; Pham, H.; Mackenzie, J.M. Differential utilisation of ceramide during replication of the flaviviruses West Nile and dengue virus. Virology 2015, 484, 241–250. [Google Scholar] [CrossRef]
- Gewaid, H.; Aoyagi, H.; Arita, M.; Watashi, K.; Suzuki, R.; Sakai, S.; Kumagai, K.; Yamaji, T.; Fukasawa, M.; Kato, F.; et al. Sphingomyelin Is Essential for the Structure and Function of the Double-Membrane Vesicles in Hepatitis C Virus RNA Replication Factories. J. Virol. 2020, 94, e01080-20. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; Vázquez-Calvo, Á.; Saiz, J.-C. Lipids and flaviviruses, present and future perspectives for the control of dengue, Zika, and West Nile viruses. Prog. Lipid Res. 2016, 64, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Machamer, C.E. Ceramide Accumulation Uncovers a Cycling Pathway for the cis-Golgi Network Marker, Infectious Bronchitis Virus M Protein. J. Cell Biol. 1997, 139, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Tafesse, F.G.; Sanyal, S.; Ashour, J.; Guimaraes, C.P.; Hermansson, M.; Somerharju, P.; Ploegh, H.L. Intact sphingomyelin biosynthetic pathway is essential for intracellular transport of influenza virus glycoproteins. Proc. Natl. Acad. Sci. USA 2013, 110, 6406–6411. [Google Scholar] [CrossRef]
- Hidari, K.I.; Suzuki, Y.; Suzuki, T. Suppression of the biosynthesis of cellular sphingolipids results in the inhibition of the maturation of influenza virus particles in MDCK cells. Biol. Pharm. Bull. 2006, 29, 1575–1579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seo, Y.-J.; Pritzl, C.J.; Vijayan, M.; Bomb, K.; McClain, M.E.; Alexander, S.; Hahm, B. Sphingosine Kinase 1 Serves as a Pro-Viral Factor by Regulating Viral RNA Synthesis and Nuclear Export of Viral Ribonucleoprotein Complex upon Influenza Virus Infection. PLoS ONE 2013, 8, e75005. [Google Scholar] [CrossRef]
- Xia, C.; Seo, Y.-J.; Studstill, C.J.; Vijayan, M.; Wolf, J.J.; Hahm, B. Transient inhibition of sphingosine kinases confers protection to influenza A virus infected mice. Antivir. Res. 2018, 158, 171–177. [Google Scholar] [CrossRef]
- Soudani, N.; Hage-Sleiman, R.; Karam, W.; Dbaibo, G.; Zaraket, H. Ceramide Suppresses Influenza A Virus Replication In Vitro. J. Virol. 2019, 93, e00053-19. [Google Scholar] [CrossRef]
- Monick, M.M.; Cameron, K.; Powers, L.S.; Butler, N.S.; McCoy, D.; Mallampalli, R.K.; Hunninghake, G.W. Sphingosine Kinase Mediates Activation of Extracellular Signal–Related Kinase and Akt by Respiratory Syncytial Virus. Am. J. Respir. Cell Mol. Biol. 2004, 30, 844–852. [Google Scholar] [CrossRef]
- Galvan, V.; Roizman, B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 1998, 95, 3931. [Google Scholar] [CrossRef]
- Sawai, H.; Okazaki, T.; Yamamoto, H.; Okano, H.; Takeda, Y.; Tashima, M.; Sawada, H.; Okuma, M.; Ishikura, H.; Umehara, H.; et al. Requirement of AP-1 for ceramide-induced apoptosis in human leukemia HL-60 cells. J. Biol. Chem. 1995, 270, 27326–27331. [Google Scholar] [CrossRef]
- Richard, A.; Robichaud, G.; Lapointe, R.; Bourgoin, S.; Darveau, A.; Poulin, L. Interference of HIV-1 Nef in the sphingomyelin transduction pathway activated by tumour necrosis factor-α in human glial cells. AIDS 1997, 11, F1–F7. [Google Scholar] [CrossRef]
- Robichaud, G.A.; Poulin, L. HIV Type 1 nef Gene Inhibits Tumor Necrosis Factor α-Induced Apoptosis and Promotes Cell Proliferation through the Action of MAPK and JNK in Human Glial Cells. AIDS Res. Hum. Retrovir. 2000, 16, 1959–1965. [Google Scholar] [CrossRef]
- Miyake, Y.; Kozutsumi, Y.; Nakamura, S.; Fujita, T.; Kawasaki, T. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem. Biophys. Res. Commun. 1995, 211, 396–403. [Google Scholar] [CrossRef]
- Umehara, T.; Sudoh, M.; Yasui, F.; Matsuda, C.; Hayashi, Y.; Chayama, K.; Kohara, M. Serine palmitoyltransferase inhibitor suppresses HCV replication in a mouse model. Biochem. Biophys. Res. Commun. 2006, 346, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, F.; Maekawa, S.; Itakura, Y.; Kanayama, A.; Matsui, A.; Takano, S.; Yamaguchi, T.; Itakura, J.; Kitamura, T.; Inoue, T.; et al. Targeting lipid metabolism in the treatment of hepatitis C virus infection. J. Infect. Dis. 2008, 197, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Tatematsu, K.; Tanaka, Y.; Sugiyama, M.; Sudoh, M.; Mizokami, M. Host sphingolipid biosynthesis is a promising therapeutic target for the inhibition of hepatitis B virus replication. J. Med. Virol. 2011, 83, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Ciesek, S.; Steinmann, E.; Manns, M.P.; Wedemeyer, H.; Pietschmann, T. The suppressive effect that myriocin has on hepatitis C virus RNA replication is independent of inhibition of serine palmitoyl transferase. J. Infect. Dis. 2008, 198, 1091–1093. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Bochkov, Y.A.; Eickhoff, J.C.; Hu, T.; Zumwalde, N.A.; Tan, J.W.; Lopez, C.; Fichtinger, P.S.; Reddy, T.R.; Overmyer, K.A.; et al. Orosomucoid-like 3 Supports Rhinovirus Replication in Human Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2020, 62, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X.; Yang, Z.; Tan, R.X.; Chen, X.; Li, E. Fungal metabolite myriocin promotes human herpes simplex virus-2 infection. Life Sci. 2015, 120, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Norred, W.P.; Wang, E.; Yoo, H.; Riley, R.T.; Merrill, A.H., Jr. In vitro toxicology of fumonisins and the mechanistic implications. Mycopathologia 1992, 117, 73–78. [Google Scholar] [CrossRef]
- Izquierdo-Useros, N.; Naranjo-Gómez, M.; Archer, J.; Hatch, S.C.; Erkizia, I.; Blanco, J.; Borràs, F.E.; Puertas, M.C.; Connor, J.H.; Fernández-Figueras, M.T.; et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 2009, 113, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Hatch, S.C.; Archer, J.; Gummuluru, S. Glycosphingolipid composition of human immunodeficiency virus type 1 (HIV-1) particles is a crucial determinant for dendritic cell-mediated HIV-1 trans-infection. J. Virol. 2009, 83, 3496–3506. [Google Scholar] [CrossRef]
- Amtmann, E. The antiviral, antitumoural xanthate D609 is a competitive inhibitor of phosphatidylcholine-specific phospholipase C. Drugs Exp. Clin. Res. 1996, 22, 287–294. [Google Scholar] [PubMed]
- Luberto, C.; Hannun, Y.A. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C? J. Biol. Chem. 1998, 273, 14550–14559. [Google Scholar] [CrossRef]
- Meng, A.; Luberto, C.; Meier, P.; Bai, A.; Yang, X.; Hannun, Y.A.; Zhou, D. Sphingomyelin synthase as a potential target for D609-induced apoptosis in U937 human monocytic leukemia cells. Exp. Cell Res. 2004, 292, 385–392. [Google Scholar] [CrossRef]
- Sauer, G.; Amtmann, E.; Melber, K.; Knapp, A.; Müller, K.; Hummel, K.; Scherm, A. DNA and RNA virus species are inhibited by xanthates, a class of antiviral compounds with unique properties. Proc. Natl. Acad. Sci. USA 1984, 81, 3263–3267. [Google Scholar] [CrossRef]
- Müller-Decker, K.; Amtmann, E.; Sauer, G. Inhibition of the phosphorylation of the regulatory non-structural protein of vesicular stomatitis virus by an antiviral xanthate compound. J. Gen. Virol. 1987, 68 Pt 12, 3045–3056. [Google Scholar] [CrossRef]
- Mellert, W.; Amtmann, E.; Erfle, V.; Sauer, G. Inhibition of HIV-1 replication by an antiviral xanthate compound in vitro. AIDS Res. Hum. Retroviruses 1988, 4, 71–81. [Google Scholar] [CrossRef]
- Waldeck, W. Antiviral xanthate causes conformational changes in simian virus 40 DNA and chromatin. Oncology 1990, 47, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, N.; Navarro, J.; Cubero, E. Antiviral effects of xanthate D609 on the human respiratory syncytial virus growth cycle. Virology 1991, 181, 101–108. [Google Scholar] [CrossRef]
- Nguyen, A.; Guedán, A.; Mousnier, A.; Swieboda, D.; Zhang, Q.; Horkai, D.; Le Novere, N.; Solari, R.; Wakelam, M.J.O. Host lipidome analysis during rhinovirus replication in HBECs identifies potential therapeutic targets. J. Lipid Res. 2018, 59, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Walro, D.G.; Rosenthal, K.S. The antiviral xanthate compound D609 inhibits herpes simplex virus type 1 replication and protein phosphorylation. Antiviral Res. 1997, 36, 63–72. [Google Scholar] [CrossRef]
- Wang, H.; Maurer, B.J. Fenretinide increased ceramides through progressive de novo synthesis and inhibition of sphingomyelin synthesis in a neuroblastoma cell line. Cancer Res. 2006, 66, 1093. [Google Scholar]
- Carocci, M.; Hinshaw, S.M.; Rodgers, M.A.; Villareal, V.A.; Burri, D.J.; Pilankatta, R.; Maharaj, N.P.; Gack, M.U.; Stavale, E.J.; Warfield, K.L.; et al. The bioactive lipid 4-hydroxyphenyl retinamide inhibits flavivirus replication. Antimicrob. Agents Chemother. 2015, 59, 85–95. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.N.Y.; Smith, K.; Forwood, J.K.; Jans, D.A. Nuclear import inhibitor N-(4-hydroxyphenyl) retinamide targets Zika virus (ZIKV) nonstructural protein 5 to inhibit ZIKV infection. Biochem. Biophys. Res. Commun. 2017, 493, 1555–1559. [Google Scholar] [CrossRef]
- Pitts, J.D.; Li, P.C.; de Wispelaere, M.; Yang, P.L. Antiviral activity of N-(4-hydroxyphenyl) retinamide (4-HPR) against Zika virus. Antiviral. Res. 2017, 147, 124–130. [Google Scholar] [CrossRef]
- Graziano, F.; Desdouits, M.; Garzetti, L.; Podini, P.; Alfano, M.; Rubartelli, A.; Furlan, R.; Benaroch, P.; Poli, G. Extracellular ATP induces the rapid release of HIV-1 from virus containing compartments of human macrophages. Proc. Natl. Acad. Sci. USA 2015, 112, E3265–E3273. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; Merino-Ramos, T.; Blázquez, A.-B.; Casas, J.; Escribano-Romero, E.; Sobrino, F.; Saiz, J.-C. The Composition of West Nile Virus Lipid Envelope Unveils a Role of Sphingolipid Metabolism in Flavivirus Biogenesis. J. Virol. 2014, 88, 12041–12054. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Zhang, H.; Zhao, R.; Jing, R.; Xu, Y.; He, M.; Peer, J.; Kim, Y.C.; Luo, J.; et al. Zika virus propagation and release in human fetal astrocytes can be suppressed by neutral sphingomyelinase-2 inhibitor GW4869. Cell Discov. 2018, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Okamoto, K.; Aoki, M.; Kato, H.; Katsume, A.; Ohta, A.; Tsukuda, T.; Shimma, N.; Aoki, Y.; Arisawa, M.; et al. Host sphingolipid biosynthesis as a target for hepatitis C virus therapy. Nat. Chem. Biol. 2005, 1, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Kanj, S.S.; Dandashi, N.; El-Hed, A.; Harik, H.; Maalouf, M.; Kozhaya, L.; Mousallem, T.; Tollefson, A.E.; Wold, W.S.; Chalfant, C.E.; et al. Ceramide regulates SR protein phosphorylation during adenoviral infection. Virology 2006, 345, 280–289. [Google Scholar] [CrossRef]
- Laevskaya, A.; Borovjagin, A.; Timashev, P.S.; Lesniak, M.S.; Ulasov, I. Metabolome-Driven Regulation of Adenovirus-Induced Cell Death. Int. J. Mol. Sci. 2021, 22, 464. [Google Scholar] [CrossRef]
- Menck, K.; Sönmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef]
- Chan, R.; Uchil, P.D.; Jin, J.; Shui, G.; Ott, D.E.; Mothes, W.; Wenk, M.R. Retroviruses Human Immunodeficiency Virus and Murine Leukemia Virus Are Enriched in Phosphoinositides. J. Virol. 2008, 82, 11228–11238. [Google Scholar] [CrossRef]
- Lindenbach, B.D. Virion assembly and release. Curr. Top. Microbiol. Immunol. 2013, 369, 199–218. [Google Scholar] [CrossRef]
- Amako, Y.; Syed, G.H.; Siddiqui, A. Protein Kinase D Negatively Regulates Hepatitis C Virus Secretion through Phosphorylation of Oxysterol-binding Protein and Ceramide Transfer Protein. J. Biol. Chem. 2011, 286, 11265–11274. [Google Scholar] [CrossRef]
- Amako, Y.; Sarkeshik, A.; Hotta, H.; Yates, J.; Siddiqui, A. Role of Oxysterol Binding Protein in Hepatitis C Virus infection. J. Virol. 2009, 83, 9237–9246. [Google Scholar] [CrossRef] [PubMed]
- Bishé, B.; Syed, G.; Siddiqui, A. Phosphoinositides in the Hepatitis C Virus Life Cycle. Viruses 2012, 4, 2340–2358. [Google Scholar] [CrossRef] [PubMed]
- Roussel, É.; Lippé, R. Cellular Protein Kinase D Modulators Play a Role during Multiple Steps of Herpes Simplex Virus 1 Egress. J. Virol. 2018, 92, e01486-18. [Google Scholar] [CrossRef] [PubMed]
- Scheiffele, P.; Roth, M.G.; Simons, K. Interaction of influenza virus haemagglutinin with sphingolipid–cholesterol membrane domains via its transmembrane domain. EMBO J. 1997, 16, 5501–5508. [Google Scholar] [CrossRef] [PubMed]
- Pickl, W.F.; Pimentel-Muiños, F.X.; Seed, B. Lipid Rafts and Pseudotyping. J. Virol. 2001, 75, 7175–7183. [Google Scholar] [CrossRef] [PubMed]
- Manié, S.N.; Debreyne, S.; Vincent, S.; Gerlier, D. Measles Virus Structural Components Are Enriched into Lipid Raft Microdomains: A Potential Cellular Location for Virus Assembly. J. Virol. 2000, 74, 305–311. [Google Scholar] [CrossRef]
- Vincent, S.; Gerlier, D.; Manié, S.N. Measles Virus Assembly within Membrane Rafts. J. Virol. 2000, 74, 9911–9915. [Google Scholar] [CrossRef] [PubMed]
- Bavari, S.; Bosio, C.M.; Wiegand, E.; Ruthel, G.; Will, A.B.; Geisbert, T.W.; Hevey, M.; Schmaljohn, C.; Schmaljohn, A.; Aman, M.J. Lipid Raft Microdomains: A Gateway for Compartmentalized Trafficking of Ebola and Marburg Viruses. J. Exp. Med. 2002, 195, 593–602. [Google Scholar] [CrossRef]
- Barklis, E.; Alfadhli, A.; Kyle, J.E.; Bramer, L.M.; Bloodsworth, K.J.; Barklis, R.L.; Leier, H.C.; Petty, R.M.; Zelnik, I.D.; Metz, T.O.; et al. Ceramide synthase 2 deletion decreases the infectivity of HIV-1. J. Biol. Chem. 2021, 296, 100340. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Sato, K.; Kiso, M.; Hasegawa, A. New ganglioside analogs that inhibit influenze virus sialidase. Glycoconj. J. 1990, 7, 349–356. [Google Scholar] [CrossRef]
- Imre, G. The involvement of regulated cell death forms in modulating the bacterial and viral pathogenesis. Int. Rev. Cell Mol. Biol. 2020, 353, 211–253. [Google Scholar] [CrossRef]
- Henderson, G.; Peng, W.; Jin, L.; Perng, G.C.; Nesburn, A.B.; Wechsler, S.L.; Jones, C. Regulation of caspase 8- and caspase 9-induced apoptosis by the herpes simplex virus type 1 latency-associated transcript. J. Neurovirol. 2002, 8 (Suppl. 2), 103–111. [Google Scholar] [CrossRef] [PubMed]
- Danthi, P. Viruses and the Diversity of Cell Death. Annu. Rev. Virol. 2016, 3, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Jan, J.-T.; Chatterjee, S.; Griffin, D.E. Sindbis Virus Entry into Cells Triggers Apoptosis by Activating Sphingomyelinase, Leading to the Release of Ceramide. J. Virol. 2000, 74, 6425–6432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Griffin, D.E. Neuronal Cell Death in Alphavirus Encephalomyelitis. In Role of Apoptosis in Infection; Griffin, D.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 57–77. [Google Scholar]
- Sol, N.; Le Junter, J.; Vassias, I.; Freyssinier, J.M.; Thomas, A.; Prigent, A.F.; Rudkin, B.B.; Fichelson, S.; Morinet, F. Possible Interactions between the NS-1 Protein and Tumor Necrosis Factor Alpha Pathways in Erythroid Cell Apoptosis Induced by Human Parvovirus B19. J. Virol. 1999, 73, 8762–8770. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Hamilton, J.A.; Tjoelker, L.W.; Dbaibo, G.; Dzenko, K.A.; Epstein, L.G.; Hannun, Y.; Whittaker, J.S.; Dewhurst, S.; Gelbard, H.A. Platelet-activating factor receptor activation. An initiator step in HIV-1 neuropathogenesis. J. Biol. Chem. 1998, 273, 17660–17664. [Google Scholar] [CrossRef]
- Haughey, N.J.; Cutler, R.G.; Tamara, A.; McArthur, J.C.; Vargas, D.L.; Pardo, C.A.; Turchan, J.; Nath, A.; Mattson, M.P. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann. Neurol. 2004, 55, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Pahan, K. Human Immunodeficiency Virus Type 1 gp120 Induces Apoptosis in Human Primary Neurons through Redox-Regulated Activation of Neutral Sphingomyelinase. J. Neurosci. 2004, 24, 9531–9540. [Google Scholar] [CrossRef]
- Fujinami, R.S.; Zurbriggen, A.; Powell, H.C. Monoclonal antibody defines determinant between Theiler’s virus and lipid-like structures. J. Neuroimmunol. 1988, 20, 25–32. [Google Scholar] [CrossRef]
- Yamada, M.; Zurbriggen, A.; Fujinami, R.S. Monoclonal antibody to Theiler’s murine encephalomyelitis virus defines a determinant on myelin and oligodendrocytes, and augments demyelination in experimental allergic encephalomyelitis. J. Exp. Med. 1990, 171, 1893–1907. [Google Scholar] [CrossRef]
- Pathak, S.; Illavia, S.J.; Khalili-Shirazi, A.; Webb, H.E. Immunoelectron microscopical labelling of a glycolipid in the envelopes of brain cell-derived budding viruses, Semliki Forest, influenza and measles, using a monoclonal antibody directed chiefly against galactocerebroside resulting from Semliki Forest virus infection. J. Neurol. Sci. 1990, 96, 293–302. [Google Scholar] [CrossRef]
- Webb, H.E.; Mehta, S.; Gregson, N.A.; Leibowitz, S. Immunological reaction of the demyelinating Semliki Forest virus with immune serum to glycolipids and its possible importance to central nervous system viral auto-immune disease. Neuropathol. Appl. Neurobiol. 1984, 10, 77–84. [Google Scholar]
- Atkins, G.J.; Mooney, D.A.; Fahy, D.A.; Ng, S.H.; Sheahan, B.J. Multiplication of rubella and measles viruses in primary rat neural cell cultures: Relevance to a postulated triggering mechanism for multiple sclerosis. Neuropathol. Appl. Neurobiol. 1991, 17, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Webb, H.E. CNS pathogenesis following a dual viral infection with Semliki Forest (alphavirus) and Langat (flavivirus). Br. J. Exp. Pathol 1988, 69, 197–208. [Google Scholar] [PubMed]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Dubois, M.; Plisson-Chastang, C.; Bonnefois, T.; Lhomme, S.; Bertrand-Michel, J.; You, B.; Simoneau, S.; Gleizes, P.-E.; Flan, B.; et al. Characterization of the lipid envelope of exosome encapsulated HEV particles protected from the immune response. Biochimie 2017, 141, 70–79. [Google Scholar] [CrossRef]
- Sanada, T.; Hirata, Y.; Naito, Y.; Yamamoto, N.; Kikkawa, Y.; Ishida, Y.; Yamasaki, C.; Tateno, C.; Ochiya, T.; Kohara, M. Transmission of HBV DNA Mediated by Ceramide-Triggered Extracellular Vesicles. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 272–283. [Google Scholar] [CrossRef]
- Avota, E.; Gassert, E.; Schneider-Schaulies, S. Cytoskeletal Dynamics: Concepts in Measles Virus Replication and Immunomodulation. Viruses 2011, 3, 102–117. [Google Scholar] [CrossRef]
- Gassert, E.; Avota, E.; Harms, H.; Krohne, G.; Gulbins, E.; Schneider-Schaulies, S. Induction of Membrane Ceramides: A Novel Strategy to Interfere with T Lymphocyte Cytoskeletal Reorganisation in Viral Immunosuppression. PLoS Pathog. 2009, 5, e1000623. [Google Scholar] [CrossRef]
- Mueller, N.; Avota, E.; Collenburg, L.; Grassmé, H.; Schneider-Schaulies, S. Neutral Sphingomyelinase in Physiological and Measles Virus Induced T Cell Suppression. PLoS Pathog. 2014, 10, e1004574. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.J.; Brigl, M.; Brenner, M.B. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 2013, 13, 101–117. [Google Scholar] [CrossRef]
- Juno, J.A.; Keynan, Y.; Fowke, K.R. Invariant NKT cells: Regulation and function during viral infection. PLoS Pathog. 2012, 8, e1002838. [Google Scholar] [CrossRef]
- Saroha, A.; Pewzner-Jung, Y.; Ferreira, N.S.; Sharma, P.; Jouan, Y.; Kelly, S.L.; Feldmesser, E.; Merrill, A.H.; Trottein, F.; Paget, C.; et al. Critical Role for Very-Long Chain Sphingolipids in Invariant Natural Killer T Cell Development and Homeostasis. Front. Immunol. 2017, 8, 1386. [Google Scholar] [CrossRef]
- Fernandez, C.S.; Kelleher, A.D.; Finlayson, R.; Godfrey, D.I.; Kent, S.J. NKT cell depletion in humans during early HIV infection. Immunol. Cell Biol. 2014, 92, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Tessmer, M.S.; Fatima, A.; Paget, C.; Trottein, F.; Brossay, L. NKT cell immune responses to viral infection. Expert Opin. Ther. Targets 2009, 13, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Raftery, M.J.; Winau, F.; Giese, T.; Kaufmann, S.H.E.; Schaible, U.E.; Schönrich, G. Viral danger signals control CD1d de novo synthesis and NKT cell activation. Eur. J. Immunol. 2008, 38, 668–679. [Google Scholar] [CrossRef]
- Paquin-Proulx, D.; Gibbs, A.; Bächle, S.M.; Checa, A.; Introini, A.; Leeansyah, E.; Wheelock, C.E.; Nixon, D.F.; Broliden, K.; Tjernlund, A.; et al. Innate Invariant NKT Cell Recognition of HIV-1–Infected Dendritic Cells Is an Early Detection Mechanism Targeted by Viral Immune Evasion. J. Immunol. 2016, 197, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Opasawatchai, A.; Matangkasombut, P. iNKT Cells and Their Potential Lipid Ligands during Viral Infection. Front. Immunol. 2015, 6, 378. [Google Scholar] [CrossRef] [PubMed]
- Brutkiewicz, R.R.; Yunes-Medina, L.; Liu, J. Immune evasion of the CD1d/NKT cell axis. Curr. Opin. Immunol. 2018, 52, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Bosnjak, L.; Sahlström, P.; Paquin-Proulx, D.; Leeansyah, E.; Moll, M.; Sandberg, J.K. Contact-Dependent Interference with Invariant NKT Cell Activation by Herpes Simplex Virus-Infected Cells. J. Immunol. 2012, 188, 6216–6224. [Google Scholar] [CrossRef]
- Reilly, E.C.; Thompson, E.A.; Aspeslagh, S.; Wands, J.R.; Elewaut, D.; Brossay, L. Activated iNKT cells promote memory CD8+ T cell differentiation during viral infection. PLoS ONE 2012, 7, e37991. [Google Scholar] [CrossRef] [PubMed]
- Amador-Molina, A.; Trejo-Moreno, C.; Romero-Rodríguez, D.; Sada-Ovalle, I.; Pérez-Cárdenas, E.; Lamoyi, E.; Moreno, J.; Lizano, M. Vaccination with human papillomavirus-18 E1 protein plus α-galactosyl-ceramide induces CD8(+) cytotoxic response and impairs the growth of E1-expressing tumors. Vaccine 2019, 37, 1219–1228. [Google Scholar] [CrossRef]
- Anderson, R.J.; Li, J.; Kedzierski, L.; Compton, B.J.; Hayman, C.M.; Osmond, T.L.; Tang, C.-w.; Farrand, K.J.; Koay, H.-F.; Almeida, C.F.D.S.S.E.; et al. Augmenting Influenza-Specific T Cell Memory Generation with a Natural Killer T Cell-Dependent Glycolipid–Peptide Vaccine. ACS Chem. Biol. 2017, 12, 2898–2905. [Google Scholar] [CrossRef]
- Fotouhi, F.; Shaffifar, M.; Farahmand, B.; Shirian, S.; Saeidi, M.; Tabarraei, A.; Gorji, A.; Ghaemi, A. Adjuvant use of the NKT cell agonist alpha-galactosylceramide leads to enhancement of M2-based DNA vaccine immunogenicity and protective immunity against influenza A virus. Arch. Virol. 2017, 162, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Artiaga, B.L.; Yang, G.; Hutchinson, T.E.; Loeb, J.C.; Richt, J.A.; Lednicky, J.A.; Salek-Ardakani, S.; Driver, J.P. Rapid control of pandemic H1N1 influenza by targeting NKT-cells. Sci. Rep. 2016, 6, 37999. [Google Scholar] [CrossRef]
- Dwivedi, V.; Manickam, C.; Dhakal, S.; Binjawadagi, B.; Ouyang, K.; Hiremath, J.; Khatri, M.; Hague, J.G.; Lee, C.W.; Renukaradhya, G.J. Adjuvant effects of invariant NKT cell ligand potentiates the innate and adaptive immunity to an inactivated H1N1 swine influenza virus vaccine in pigs. Vet. Microbiol. 2016, 186, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Artiaga, B.L.; Yang, G.; Hackmann, T.J.; Liu, Q.; Richt, J.A.; Salek-Ardakani, S.; Castleman, W.L.; Lednicky, J.A.; Driver, J.P. α-Galactosylceramide protects swine against influenza infection when administered as a vaccine adjuvant. Sci. Rep. 2016, 6, 23593. [Google Scholar] [CrossRef]
- Li, K.; Luo, J.; Wang, C.; He, H. α-Galactosylceramide potently augments M2e-induced protective immunity against highly pathogenic H5N1 avian influenza virus infection in mice. Vaccine 2011, 29, 7711–7717. [Google Scholar] [CrossRef] [PubMed]
- Kamijuku, H.; Nagata, Y.; Jiang, X.; Ichinohe, T.; Tashiro, T.; Mori, K.; Taniguchi, M.; Hase, K.; Ohno, H.; Shimaoka, T.; et al. Mechanism of NKT cell activation by intranasal coadministration of α-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunol. 2008, 1, 208–218. [Google Scholar] [CrossRef]
- Youn, H.-J.; Ko, S.-Y.; Lee, K.-A.; Ko, H.-J.; Lee, Y.-S.; Fujihashi, K.; Boyaka, P.N.; Kim, S.-H.; Horimoto, T.; Kweon, M.-N.; et al. A single intranasal immunization with inactivated influenza virus and α-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine 2007, 25, 5189–5198. [Google Scholar] [CrossRef]
- Kopecky-Bromberg, S.A.; Fraser, K.A.; Pica, N.; Carnero, E.; Moran, T.M.; Franck, R.W.; Tsuji, M.; Palese, P. Alpha-C-galactosylceramide as an adjuvant for a live attenuated influenza virus vaccine. Vaccine 2009, 27, 3766–3774. [Google Scholar] [CrossRef]
- Miller, D.S.; Finnie, J.; Bowden, T.R.; Scholz, A.C.; Oh, S.; Kok, T.; Burrell, C.J.; Trinidad, L.; Boyle, D.B.; Li, P. Preclinical efficacy studies of influenza A haemagglutinin precursor cleavage loop peptides as a potential vaccine. J. Gen. Virol. 2011, 92, 1152–1161. [Google Scholar] [CrossRef]
- Lindqvist, M.; Persson, J.; Thörn, K.; Harandi, A.M. The Mucosal Adjuvant Effect of α-Galactosylceramide for Induction of Protective Immunity to Sexually Transmitted Viral Infection. J. Immunol. 2009, 182, 6435–6443. [Google Scholar] [CrossRef]
- Iversen, M.B.; Jensen, S.K.; Hansen, A.L.; Winther, H.; Issazadeh-Navikas, S.; Reinert, L.S.; Holm, C.K. NKT cell activation by local α-galactosylceramide administration decreases susceptibility to HSV-2 infection. Immunobiology 2015, 220, 762–768. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, A.; Li, X.; Chen, Z.; Zhang, W.; Song, Y.; Gurner, D.; Gardiner, D.; Basu, S.; Ho, D.D.; et al. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, α-galactosylceramide. Vaccine 2008, 26, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Yang, G.; Byrareddy, S.N.; Barry, M.A.; Sastry, K.J. Natural killer T cell and TLR9 agonists as mucosal adjuvants for sublingual vaccination with clade C HIV-1 envelope protein. Vaccine 2014, 32, 6934–6940. [Google Scholar] [CrossRef]
- Cox, R.G.; Erickson, J.J.; Hastings, A.K.; Becker, J.C.; Johnson, M.; Craven, R.E.; Tollefson, S.J.; Boyd, K.L.; Williams, J.V. Human Metapneumovirus Virus-Like Particles Induce Protective B and T Cell Responses in a Mouse Model. J. Virol. 2014, 88, 6368–6379. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Han, S.-H.; Kang, H.-W.; Lee, J.-M.; Kim, Y.-S.; Seo, J.-H.; Seong, Y.-K.; Ko, H.-J.; Choi, T.H.; Moon, C.; et al. NKT ligand-loaded, antigen-expressing B cells function as long-lasting antigen presenting cells in vivo. Cell. Immunol. 2011, 270, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.O.; Shin, H.Y.; Kang, C.-Y.; Kang, H.J. Generation of antigen-specific cytotoxic T lymphocytes with activated B cells. Cytotherapy 2017, 19, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kakimi, K.; Guidotti, L.G.; Koezuka, Y.; Chisari, F.V. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 2000, 192, 921–930. [Google Scholar] [CrossRef]
- Ito, H.; Ando, K.; Ishikawa, T.; Nakayama, T.; Taniguchi, M.; Saito, K.; Imawari, M.; Moriwaki, H.; Yokochi, T.; Kakumu, S.; et al. Role of Vα14+ NKT cells in the development of Hepatitis B virus-specific CTL: Activation of Vα14+ NKT cells promotes the breakage of CTL tolerance. Int. Immunol. 2008, 20, 869–879. [Google Scholar] [CrossRef]
- Ho, L.-P.; Denney, L.; Luhn, K.; Teoh, D.; Clelland, C.; McMichael, A.J. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza. A virus infection. Eur. J. Immunol. 2008, 38, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, A.; Ivanov, S.; Hassane, M.; Fontaine, J.; Heurtault, B.; Frisch, B.; Faveeuw, C.; Paget, C.; Trottein, F. Exogenous Activation of Invariant Natural Killer T Cells by α-Galactosylceramide Reduces Pneumococcal Outgrowth and Dissemination Postinfluenza. mBio 2016, 7, e01440-16. [Google Scholar] [CrossRef]
- Wu, C.Y.; Feng, Y.; Qian, G.C.; Wu, J.H.; Luo, J.; Wang, Y.; Chen, G.J.; Guo, X.K.; Wang, Z.J. α-Galactosylceramide protects mice from lethal Coxsackievirus B3 infection and subsequent myocarditis. Clin. Exp. Immunol. 2010, 162, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.A.; Roberts, B.; Moussawi, M.; Boyson, J.E. Slam Haplotype 2 Promotes NKT But Suppresses Vγ4+ T-Cell Activation in Coxsackievirus B3 Infection Leading to Increased Liver Damage But Reduced Myocarditis. Am. J. Pathol. 2013, 182, 401–409. [Google Scholar] [CrossRef]
- Exley, M.A.; Bigley, N.J.; Cheng, O.; Tahir, S.M.; Smiley, S.T.; Carter, Q.L.; Stills, H.F.; Grusby, M.J.; Koezuka, Y.; Taniguchi, M.; et al. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J. Leukoc. Biol. 2001, 69, 713–718. [Google Scholar]
- Mehta, A.S.; Gu, B.; Conyers, B.; Ouzounov, S.; Wang, L.; Moriarty, R.M.; Dwek, R.A.; Block, T.M. alpha-Galactosylceramide and novel synthetic glycolipids directly induce the innate host defense pathway and have direct activity against hepatitis B and C viruses. Antimicrob. Agents Chemother. 2004, 48, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Tripal, P.; Reichel, M.; Mühle, C.; Rhein, C.; Muehlbacher, M.; Groemer, T.W.; Gulbins, E. Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): A novel pharmacological group of drugs with broad clinical applications. Cell Physiol. Biochem. 2010, 26, 9–20. [Google Scholar] [CrossRef]
- Phillips, N. The coronavirus is here to stay—Here’s what that means. Nature 2021, 590, 382–384. [Google Scholar] [CrossRef]
- Wu, B.; Huang, Y.; Braun, A.L.; Tong, Z.; Zhao, R.; Li, Y.; Liu, F.; Zheng, J.C. Glutaminase-containing microvesicles from HIV-1-infected macrophages and immune-activated microglia induce neurotoxicity. Mol. Neurodegener. 2015, 10, 61. [Google Scholar] [CrossRef] [PubMed]
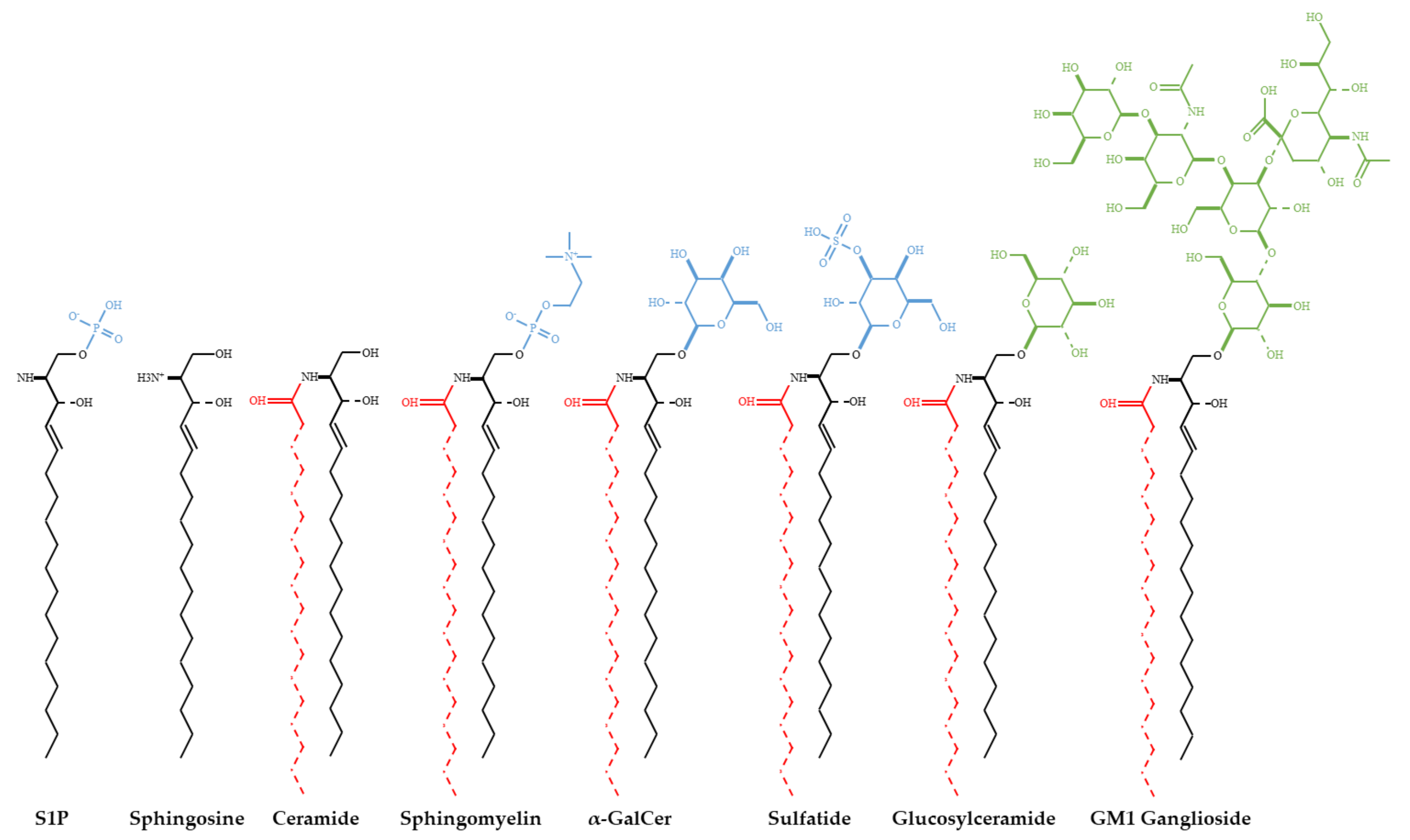
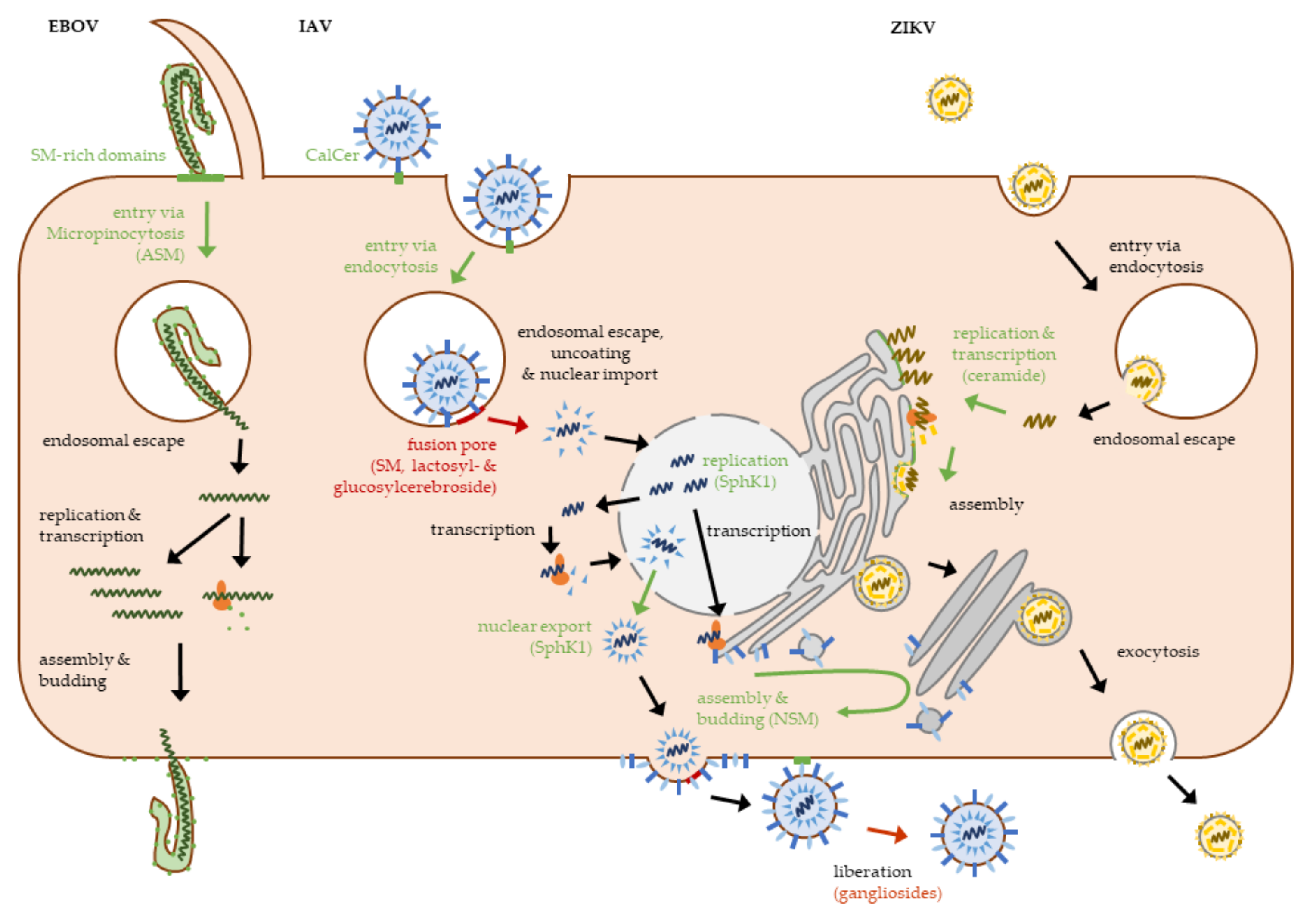
| Virus | Receptor/Pathway | Reference |
|---|---|---|
| ebola virus (EBOV) | SM-rich regions | [39] |
| hepatitis C virus (HCV) | viral sphingomyelin required for internalization | [40] |
| human immunodeficiency virus type I (HIV-1) | GalCer, Gb3, GM3, SMS2 | [13,15,16,17,18,19,20,21,22,23,41,42,43] |
| human parvovirus B19 (B19V) | Gb4Cer | [37,38] |
| influenza A virus (IAV) | CalCer | [26,27] |
| measles virus (MV) | ASM-dependent CD150 surface localization | [44,45] |
| murine norovirus | serine palmitoyltransferase-dependent conformation of CD300lf | [36] |
| norovirus GII.4 | CalCer | [28] |
| rhinovirus | ceramide-enriched platforms | [30,31,32] |
| rotavirus | GA1, GA2, pentaosylceramides | [11,12] |
| rubella virus | SM and cholesterol | [29] |
| severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) | ACE2 in lipid rafts | [34] |
| simian virus 40 (SV40) | GM1, N-glycolyl GM1 | [46,47] |
| Substance | Target Molecule (effect) | Virus | Reference |
|---|---|---|---|
| D609 | SMS (−) | HIV-1 Rhinovirus RSV SV40 VSV | [142] [145] [144] [143] [141] |
| FIASMAs | ASM (−) | Adenovirus EBOV HIV-1 IAV JEV MV Norovirus Rhinovirus SARS-CoV-2 | [57] [39] [151] [84] [86] [44] [58,69] [30] [83,84,85,92,93] |
| Fenretinide | SPT (+) dihydroceramide desaturase (−) | DEN HIV-1 ZIKV | [148] [55] [149,150] |
| Fumosin B | CerS (−) | IAV WNV | [118] [113] |
| GW4869 | NSM (−) | WNV ZIKV | [152] [112,153] |
| 12-HPA | CERT (−) | HCV | [154] |
| Myriocin | SPT (−) | HBV HCV IAV WNV | [130] [128,129] [117,118] [113] |
| SKI-II | SphK (−) | HCMV IAV MV | [108] [120] [111] |
| Virus | Inhibitor (Target Molecule) | Effect | Reference |
|---|---|---|---|
| adenovirus | Fluoxetine, Amitriptyline (ASM) | block endosomal escape | [57] |
| EBOV | Imipramine, Desipramine (ASM) | prevent entry | [39] |
| HIV-1 | Imipramine (ASM) | decreases release | [151] |
| GW4869 (NSM) | protects from neuronal cell death | [231] | |
| IAV | Fluoxetine, Amiodarone, Imipramine (ASM) | reduce viral titers | [84] |
| Desipramine (ASM)) | no effect | [64] | |
| JEV | Amitriptyline (ASM) | reduces infection | [86] |
| MV | GW4869 (NSM), Amitriptyline (ASM) | mitigate T cell suppression | [191] |
| Amitriptyline (ASM) | inhibits uptake | [44] | |
| norovirus | AY9944, Fluoxetine, Desipramine, Chlorpromazine, Amitriptyline (ASM) | reduce viral titers | [58,69] |
| Desipramine | blocks endosomal escape | [69] | |
| GW4869 (NSM) | no effect | [58] | |
| rhinovirus | Amitriptyline, Imipramine (ASM) | inhibit uptake | [30] |
| SARS-CoV-2 | Amitriptyline (ASM), Ambroxol (ASM) | prevent entry | [83,85] |
| Fluoxetine, Amiodarone, Imipramine (ASM) | reduce viral titers | [84] | |
| Amlodipine (ASM) | reduces mortality | [88,92] | |
| Fluoxetine (ASM) | lowers risk of intubation and reduces mortality | [93] | |
| WNV | GW4869 (NSM) | decreases release | [152] |
| ZIKV | GW4869 (NSM) | decreases production and shedding | [112,153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beckmann, N.; Becker, K.A. Ceramide and Related Molecules in Viral Infections. Int. J. Mol. Sci. 2021, 22, 5676. https://doi.org/10.3390/ijms22115676
Beckmann N, Becker KA. Ceramide and Related Molecules in Viral Infections. International Journal of Molecular Sciences. 2021; 22(11):5676. https://doi.org/10.3390/ijms22115676
Chicago/Turabian StyleBeckmann, Nadine, and Katrin Anne Becker. 2021. "Ceramide and Related Molecules in Viral Infections" International Journal of Molecular Sciences 22, no. 11: 5676. https://doi.org/10.3390/ijms22115676
APA StyleBeckmann, N., & Becker, K. A. (2021). Ceramide and Related Molecules in Viral Infections. International Journal of Molecular Sciences, 22(11), 5676. https://doi.org/10.3390/ijms22115676





