Acinetobacter baumannii K106 and K112: Two Structurally and Genetically Related 6-Deoxy-l-talose-Containing Capsular Polysaccharides
Abstract
1. Introduction
2. Results
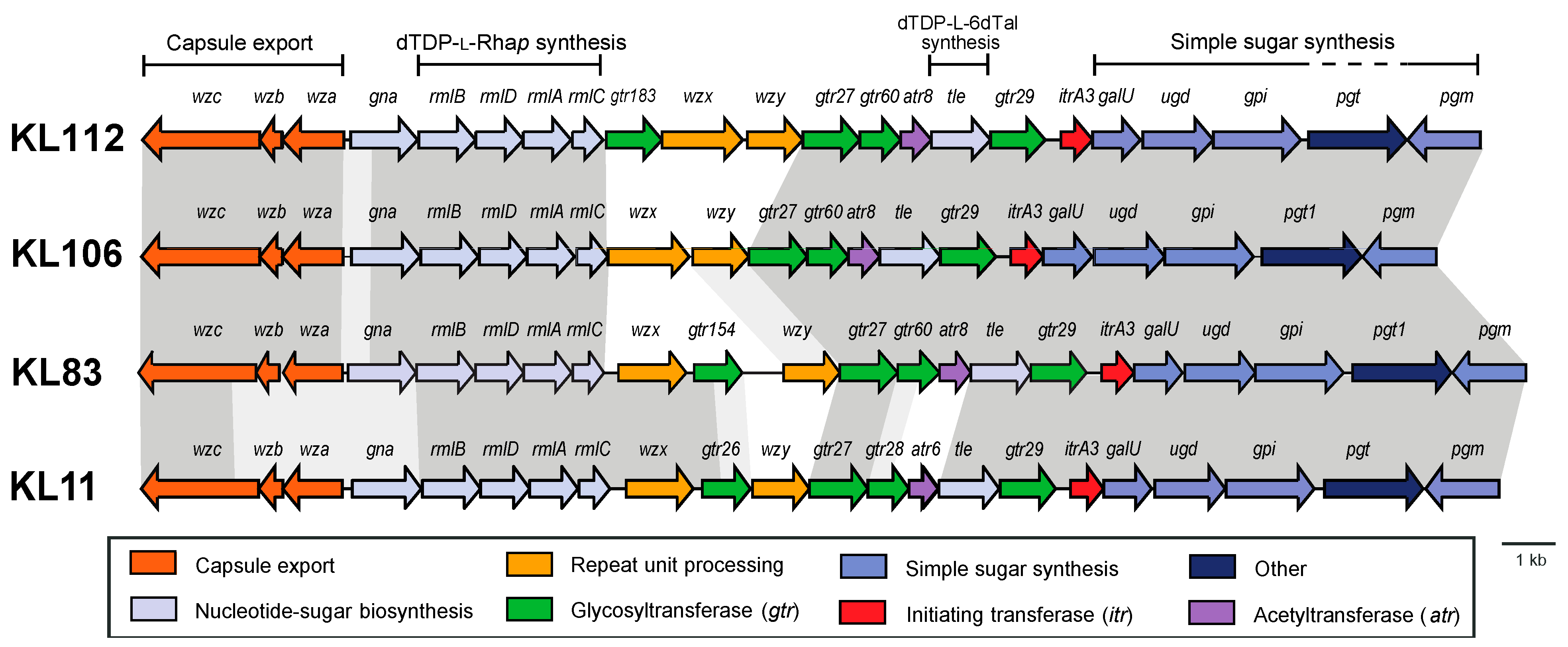
2.1. Characterization of the KL106 and KL112 CPS Biosynthesis Gene Clusters
2.2. Monosaccharide Composition of K106 and K112
2.3. The K106 Structure
2.4. The K112 Structure
2.5. Assignment of Glycosyltransferase Functions
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Cultivation, and Isolation of CPSs
4.2. Monosaccharide Analysis
4.3. Smith Degradation
4.4. NMR Spectroscopy
4.5. Mass Spectrometry
4.6. Sequencing and Bioinformatic Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6dTal | 6-Deoxy-l-talose |
| AcOH | Acetic acid |
| COSY | Correlation Spectroscopy |
| CPS | Capsular PolySaccharide |
| Glc | Glucose |
| GLC | Gas-Liquid Chromatography |
| GlcpNAc | N-acetyl Glucosamine |
| Gro-al | Glyceraldehyde |
| HMBC | Heteronuclear Multiple Bond Correlation |
| HR ESI MS | High Resolution Electrospray Ionization Mass Spectrometry |
| KL | (Chromosomal) K Locus |
| NMR | Nuclear Magnetic Resonance |
| OS | OligoSaccharide |
| Rha | Rhamnose |
| ROESY | Rotating-frame Overhauser Effect Spectroscopy |
| TFA | Trifluoroacetic Acid |
| TOCSY | Total Correlation Spectroscopy |
References
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 1 March 2021).
- Skerniškytė, J.; Krasauskas, R.; Péchoux, C.; Kulakauskas, S.; Armalytė, J.; Sužiedėlienė, E. Surface-related features and virulence among Acinetobacter baumannii clinical isolates belonging to International Clones I and II. Front. Microbiol. 2019, 9, 3116. [Google Scholar] [CrossRef] [PubMed]
- Talyansky, Y.; Nielsen, T.; Yan, J.; Carlino-Macdonald, U.; Di Venanzio, G.; Chakravorty, S.; Ulhaq, A.; Feldman, M.; Russo, T.; Vinogradov, E.; et al. Capsule carbohydrate structure determines virulence in Acinetobacter baumannii. PLoS Pathog. 2020, 17, e1009291. [Google Scholar]
- Crépin, S.; Ottosen, E.; Chandler, C.; Sintsova, A.; Ernst, R.; Mobley, H. The UDP-GalNAcA biosynthesis genes gna-gne2 are required to maintain cell envelope integrity and in vivo fitness in multi-drug resistant Acinetobacter baumannii. Mol. Microbiol. 2020, 113, 153–172. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef]
- Altamirano, F.L.G.; Forsyth, J.H.; Patwa, R.; Kostoulias, X.; Trim, M.; Subedi, D.; Archer, S.; Morris, F.C.; Oliveira, C.; Kielty, L.; et al. Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat. Microbiol. 2021, 6, 157–161. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Shneider, M.M.; Popova, A.V.; Kasimova, A.A.; Senchenkova, S.N.; Shashkov, A.S.; Chizhov, A.O. Mechanisms of Acinetobacter baumannii capsular polysaccharide cleavage by phage depolymerases. Biochemistry 2020, 85, 567–574. [Google Scholar]
- Cahill, S.M.; Arbatsky, N.P.; Shashkov, A.S.; Shneider, M.M.; Popova, A.V.; Hall, R.M.; Kenyon, J.J.; Knirel, Y.A. Elucidation of the K32 capsular polysaccharide structure and characterization of the KL32 gene cluster of Acinetobacter baumannii LUH5549. Biochemistry 2020, 85, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Arbatsky, N.; Kenyon, J.; Kasimova, A.; Shashkov, A.; Shneider, M.; Popova, A.; Knirel, Y.; Hall, R. K units of the K8 and K54 capsular polysaccharides produced by Acinetobacter baumannii BAL 097 and RCH52 have the same structure but contain different di-N-acyl derivatives of legionaminic acid and are linked differently. Carbohydr. Res. 2019, 483, 107745. [Google Scholar] [CrossRef]
- Kasimova, A.; Kenyon, J.; Arbatsky, N.; Shashkov, A.; Popova, A.; Knirel, Y.; Hall, R. Structure of the K82 capsular polysaccharide from Acinetobacter baumannii LUH5534 containing a D-galactose 4,6-pyruvic acid acetal. Biochemistry 2018, 83, 831–835. [Google Scholar] [CrossRef]
- Kenyon, J.; Senchenkova, S.; Shashkov, A.; Shneider, M.; Popova, A.; Knirel, Y.; Hall, R. K17 capsular polysaccharide produced by Acinetobacter baumannii isolate G7 contains an amide of 2-acetamido-2-deoxy-D-galacturonic acid with D-alanine. Int. J. Biol. Macromol. 2020, 144, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.J.; Arbatsky, N.P.; Shneider, M.M.; Popova, A.V.; Dmitrenok, A.S.; Kasimova, A.A.; Shashkov, A.S.; Hall, R.M.; Knirel, Y.A. The K46 and K5 capsular polysaccharides produced by Acinetobacter baumannii NIPH 329 and SDF have related structures and the side-chain non-ulosonic acids are 4-O-acetylated by phage-encoded O-acetyltransferases. PLoS ONE 2019, 14, e0218461. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.J.; Hall, R.M. Variation in the complex carbohydrate biosynthesis loci of Acinetobacter baumannii genomes. PLoS ONE 2013, 8, e62160. [Google Scholar] [CrossRef] [PubMed]
- Wyres, K.L.; Cahill, S.M.; Holt, K.E.; Hall, R.M.; Kenyon, J.J. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb. Genom. 2020, 6, e000339. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Van Calsteren, M. Bacterial Exopolysaccharides. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kenyon, J.J.; Marzaioli, A.M.; De Castro, C.; Hall, R.M. 5,7-Di-N-acetylacinetaminic acid—A novel non-2-ulosonic acid found in the capsule of an Acinetobacter baumannii isolate. Glycobiology 2015, 25, 644–654. [Google Scholar] [CrossRef]
- Kenyon, J.J.; Notaro, A.; Hsu, L.Y.; De Castro, C.; Hall, R.M. 5,7-Di-N-acetyl-8-epiacinetaminic acid: A new non-2-ulosonic acid found in the K73 capsule produced by an Acinetobacter baumannii isolate from Singapore. Sci. Rep. 2017, 7, 11357. [Google Scholar] [CrossRef]
- Kenyon, J.J.; Kasimova, A.; Shashkov, A.S.; Hall, R.M.; Knirel, Y.A. Acinetobacter baumannii isolate BAL_212 from Vietnam produces the K57 capsular polysaccharide containing a rarely occurring amino sugar N-acetylviosamine. Microbiology 2018, 164, 217–220. [Google Scholar] [CrossRef]
- Kenyon, J.J.; Shashkov, A.S.; Senchenkova, S.N.; Shneider, M.M.; Liu, B.; Popova, A.V.; Arbatsky, N.P.; Miroshnikov, K.A.; Wang, L.; Knirel, Y.A.; et al. Acinetobacter baumannii K11 and K83 capsular polysaccharides have the same 6-deoxy-L-talose-containing pentasaccharide K units but different linkages between the K units. Int. J. Biol. Macromol. 2017, 103, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Shashkov, A.S.; Paramonov, N.; Veremeychenko, S.; Grosskurth, H.; Zdorovenko, G.; Knirel, Y.A.; Kochetkov, N. Somatic antigens of pseudomonads: Structure of the O-specific polysaccharide of Pseudomonas fluorescens biovar B, strain IMV 247. Carbohydr. Res. 1998, 306, 297–303. [Google Scholar] [CrossRef]
- Lipkind, G.M.; Shashkov, A.S.; Knirel, Y.A.; Vinogradov, E.V.; Kochetkov, N.K. A computer-assisted structural analysis of regular polysaccharides on the basis of 13C-n.m.r. data. Carbohydr. Res. 1988, 175, 59–75. [Google Scholar] [CrossRef]
- Harding, C.; Nasr, M.; Knisella, R.; Scott, N.; Foster, L.; Weber, B.; Fiester, S.; Actis, L.; Tracy, E.; Munson, R.; et al. Acinetobacter strains carry two functional oligosaccharyltransferases, one devoted exclusively to type IV pilin, and the other one dedicated to O-glycosylation of multiple proteins. Mol. Microbiol. 2015, 96, 1023–1041. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.; Dvorkin, M.; Kulikov, A.; Lesin, V.; Nikolenko, S.; Pham, S.; Prjibelski, A.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
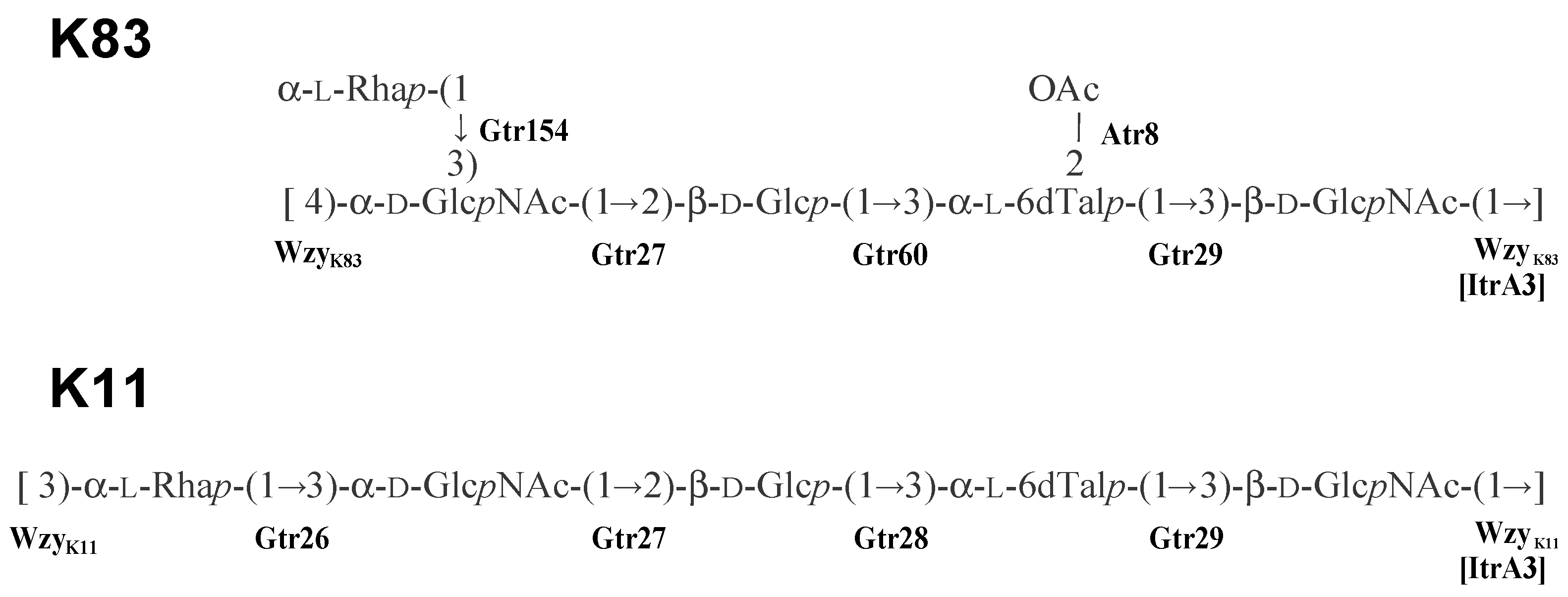

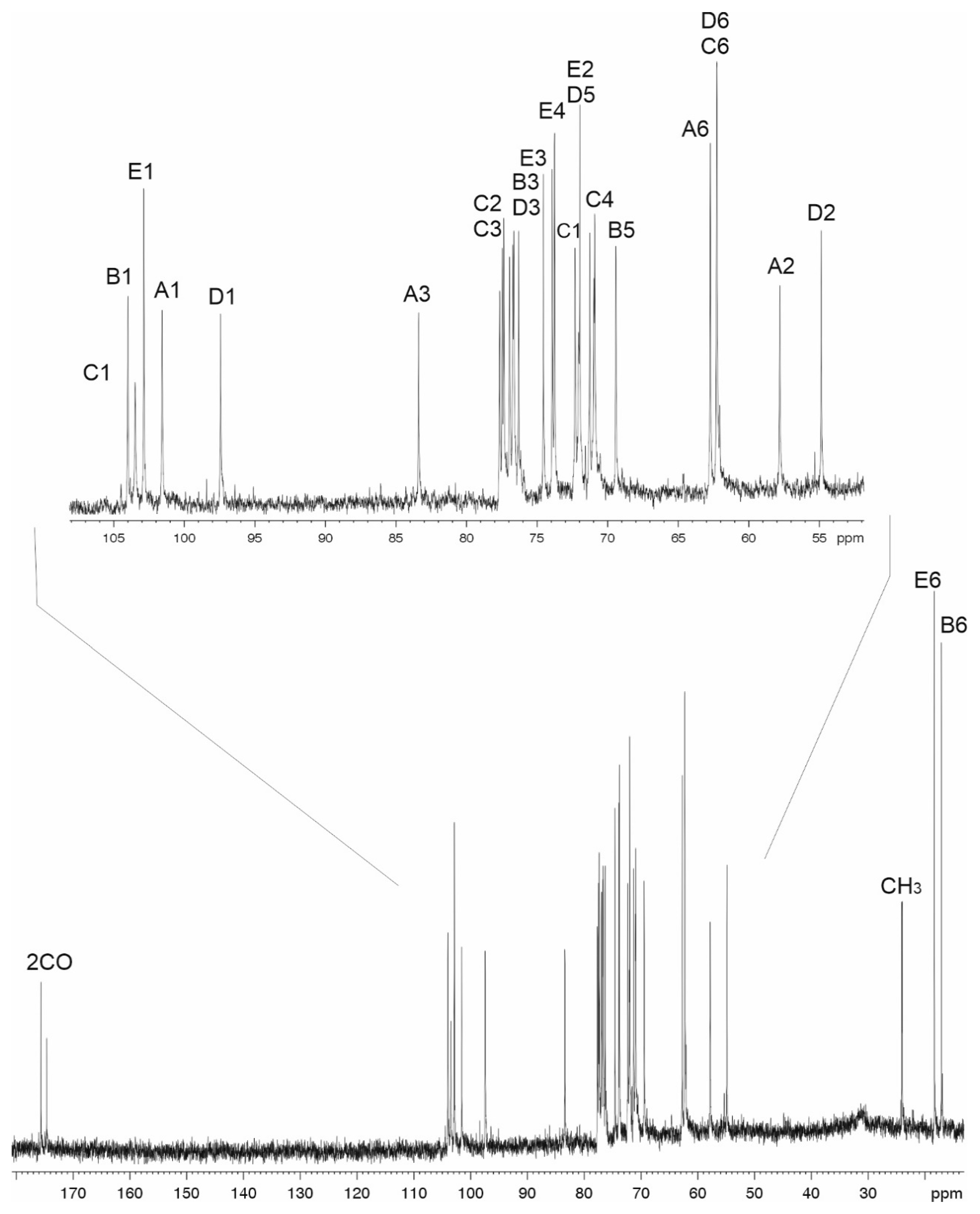

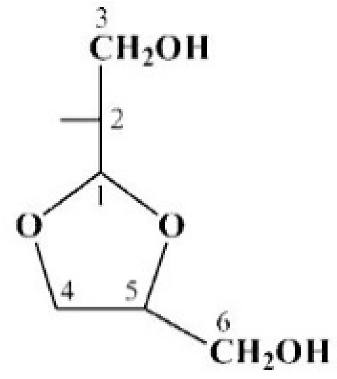
| Sugar Residue | C-1 H-1 | C-2 H-2 | C-3 H-3 | C-4 H-4 | C-5 H-5 | C-6 H-6 (6a,6b) |
|---|---|---|---|---|---|---|
| CPS a | ||||||
| →3)-β-d-GlcpNAc-(1→ A | 102.1 | 56.6 | 83.3 | 69.7 | 77.3 | 61.9 |
| 4.65 | 3.85 | 3.65 | 3.49 | 3.51 | 3.78, 3.93 | |
| →3)-α-l-6dTalp-(1→ B | 103.3 | 70.976.4 | 71.6 | 69.0 | 16.7 | |
| 4.98 | 3.88 | 3.86 | 3.66 | 4.23 | 1.22 | |
| →2)-β-d-Glcp-(1→ C | 103.8 | 77.2 | 75.9 | 70.6 | 77.3 | 61.8 |
| 4.73 | 3.51 | 3.57 | 3.48 | 3.51 | 3.78, 3.93 | |
| →4)-α-d-GlcpNAc-(1→ D | 96.8 | 54.3 | 71.0 | 80.7 | 71.5 | 61.2 |
| 5.45 | 3.96 | 3.91 | 3.68 | 4.12 | 3.67, 3.81 | |
| OS1 b | ||||||
| α-l-6dTalp-(1→ B | 103.4 | 71.7 | 66.9 | 73.4 | 69.1 | 16.8 |
| 4.99 | 3.69 | 3.86 | 3.75 | 4.27 | 1.22 | |
| →3)-β-d-GlcpNAc-(1→ A | 102.1 | 56.8 | 82.7 | 69.9 | 77.3 | 62.0 |
| 4.67 | 3.86 | 3.68 | 3.53 | 3.53 | 3.76, 3.94 | |
| α-d-GlcpNAc-(1→ D | 98.1 | 54.6 | 70.8 | 81.0 | 78.1 | 61.4 |
| 5.21 | 3.93 | 3.93 | 3.67 | 4.28 | 3.69, 3.80 | |
| →2)-Gro-al C’ | 90.8 | 74.9 | 62.3 | |||
| 5.10 | 3.60 | 3.77 | ||||
| Sugar Residue | C-1 H-1 | C-2 H-2 | C-3 H-3 | C-4 H-4 | C-5 H-5 | C-6 H-6 (6a,6b) | |
|---|---|---|---|---|---|---|---|
| CPS a | |||||||
| →3)-β-d-GlcpNAc-(1→ A | 101.1 | 57.4 | 83.0 | 70.6 | 76.9 | 62.7 | |
| 4.61 | 3.67 | 3.71 | 3.41 | 3.42 | 3.76, 3.97 | ||
| →3)-α-l-6dTalp-(1→ B | 103.1 | 70.9 | 76.3 | 71.7 | 69.0 | 16.7 | |
| 5.00 | 3.88 | 3.83 | 3.64 | 4.22 | 1.22 | ||
| →2)-β-d-Glcp-(1→ C | 103.6 | 77.3 | 75.9 | 70.5 | 76.9 | 62.3 | |
| 4.73 | 3.52 | 3.57 | 3.48 | 3.44 | 3.83, 3.88 | ||
| →3,4)-α-d-GlcpNAc-(1→ D | 97.0 | 54.5 | 76.2 | 76.5 | 71.9 | 62.2 | |
| 5.38 | 4.05 | 4.22 | 3.74 | 4.13 | 3.76, 3.85 | ||
| β-l-Rhap-(1→ E | 102.5 | 71.5 | 74.2 | 73.4 | 73.6 | 17.9 | |
| 4.86 | 4.19 | 3.57 | 3.39 | 3.42 | 1.31 | ||
| OS2, OS3 b | |||||||
| α-l-6dTalp-(1→ B | 103.2 | 71.6 | 66.7 | 73.4 | 68.9 | 16.7 | |
| 4.98 | 3.68 | 3.86 | 3.74 | 4.27 | 1.19 | ||
| →3)-β-d-GlcpNAc-(1→ A d | 101.6 | 56.9 | 82.4 | 69.7 | 76.9 | 62.2 | |
| 4.66 | 3.79 | 3.70 | 3.53 | 3.49 | 3.78, 3.82 | ||
| →3)-α-d-GlcpNAc-(1→ D d | 99.3 c, 98.4 | 53.9 | 80.5 | 69.7 | 73.0 | 61.8 | |
| 5.18c, 5.08 | 4.01 | 3.95 | 3.58 | 3.91 | 3.84, 3.93 | ||
| →2)-Gro-al C’ | 90.5 | 82.2 | 67.4 | ||||
| 5.11 | 3.60 | 3.84,4.03 | |||||
 | C” | 104.2 5.06 | 79.0 3.78 | 67.4 3.84, 4.03 | 62.8 3.62, 3.72 | 77.9 4.28 | 61.7 not found |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasimova, A.A.; Arbatsky, N.P.; Tickner, J.; Kenyon, J.J.; Hall, R.M.; Shneider, M.M.; Dzhaparova, A.A.; Shashkov, A.S.; Chizhov, A.O.; Popova, A.V.; et al. Acinetobacter baumannii K106 and K112: Two Structurally and Genetically Related 6-Deoxy-l-talose-Containing Capsular Polysaccharides. Int. J. Mol. Sci. 2021, 22, 5641. https://doi.org/10.3390/ijms22115641
Kasimova AA, Arbatsky NP, Tickner J, Kenyon JJ, Hall RM, Shneider MM, Dzhaparova AA, Shashkov AS, Chizhov AO, Popova AV, et al. Acinetobacter baumannii K106 and K112: Two Structurally and Genetically Related 6-Deoxy-l-talose-Containing Capsular Polysaccharides. International Journal of Molecular Sciences. 2021; 22(11):5641. https://doi.org/10.3390/ijms22115641
Chicago/Turabian StyleKasimova, Anastasiya A., Nikolay P. Arbatsky, Jacob Tickner, Johanna J. Kenyon, Ruth M. Hall, Michael M. Shneider, Alina A. Dzhaparova, Alexander S. Shashkov, Alexander O. Chizhov, Anastasiya V. Popova, and et al. 2021. "Acinetobacter baumannii K106 and K112: Two Structurally and Genetically Related 6-Deoxy-l-talose-Containing Capsular Polysaccharides" International Journal of Molecular Sciences 22, no. 11: 5641. https://doi.org/10.3390/ijms22115641
APA StyleKasimova, A. A., Arbatsky, N. P., Tickner, J., Kenyon, J. J., Hall, R. M., Shneider, M. M., Dzhaparova, A. A., Shashkov, A. S., Chizhov, A. O., Popova, A. V., & Knirel, Y. A. (2021). Acinetobacter baumannii K106 and K112: Two Structurally and Genetically Related 6-Deoxy-l-talose-Containing Capsular Polysaccharides. International Journal of Molecular Sciences, 22(11), 5641. https://doi.org/10.3390/ijms22115641








