The Quorum Sensing Auto-Inducer 2 (AI-2) Stimulates Nitrogen Fixation and Favors Ethanol Production over Biomass Accumulation in Zymomonas mobilis
Abstract
1. Introduction
2. Results
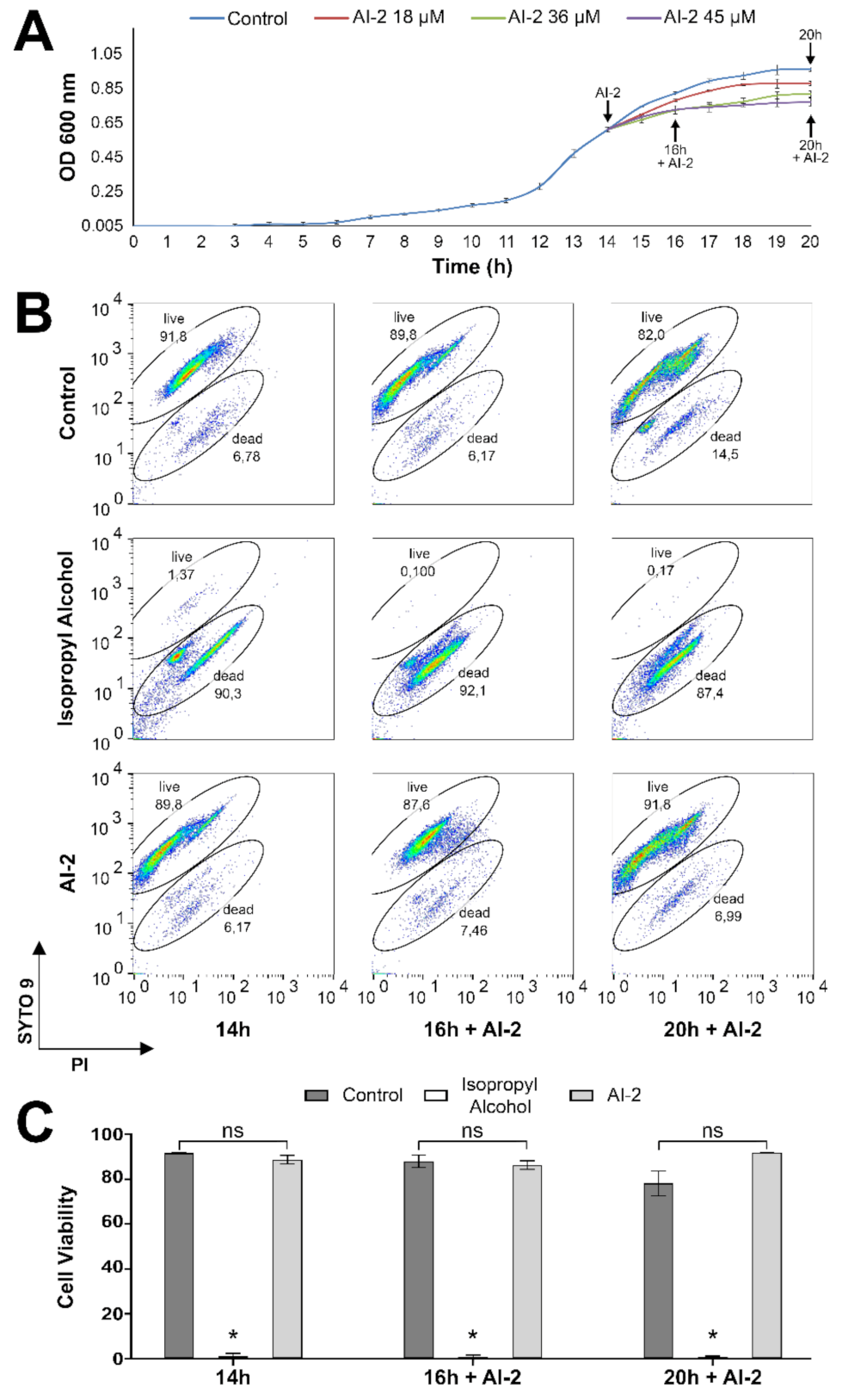
2.1. Z. mobilis Response to Increasing Concentrations of AI-2
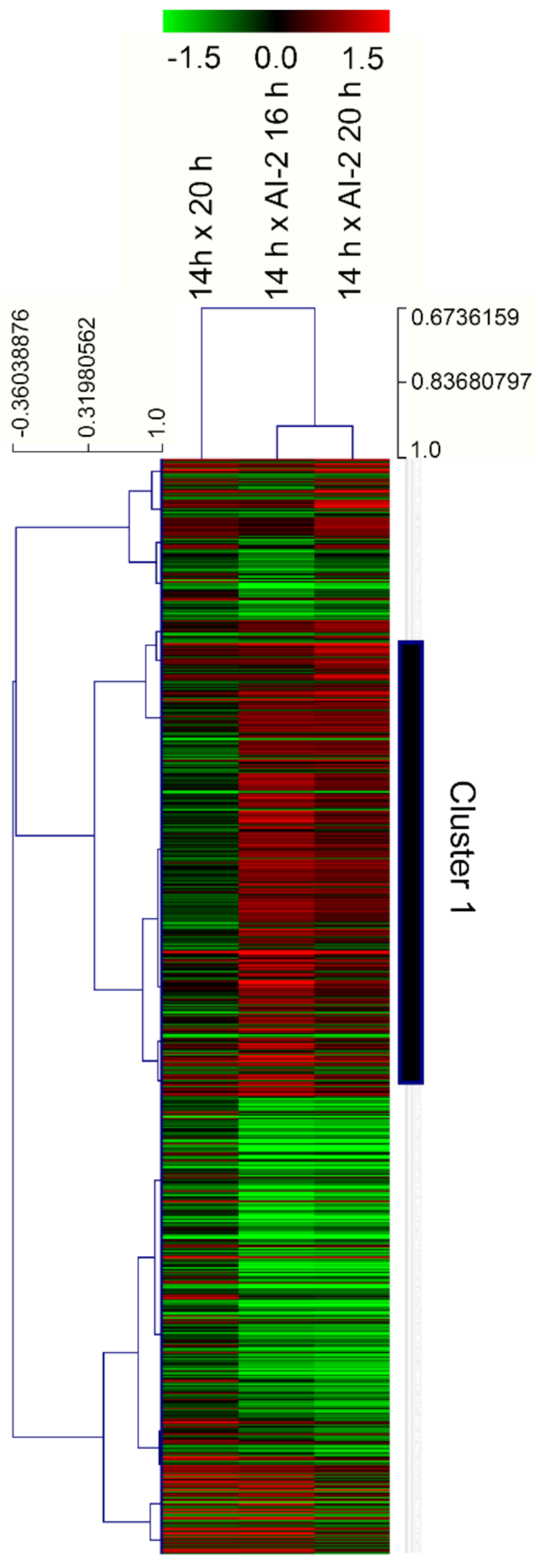
2.2. Characterization of Gene Modulation Patterns in Z. mobilis, in Response to Cell Growth and the Presence of AI-2
2.3. Metabolic and Structural Alterations Verified in Z. mobilis in Response to Cell Growth and the Presence of AI-2
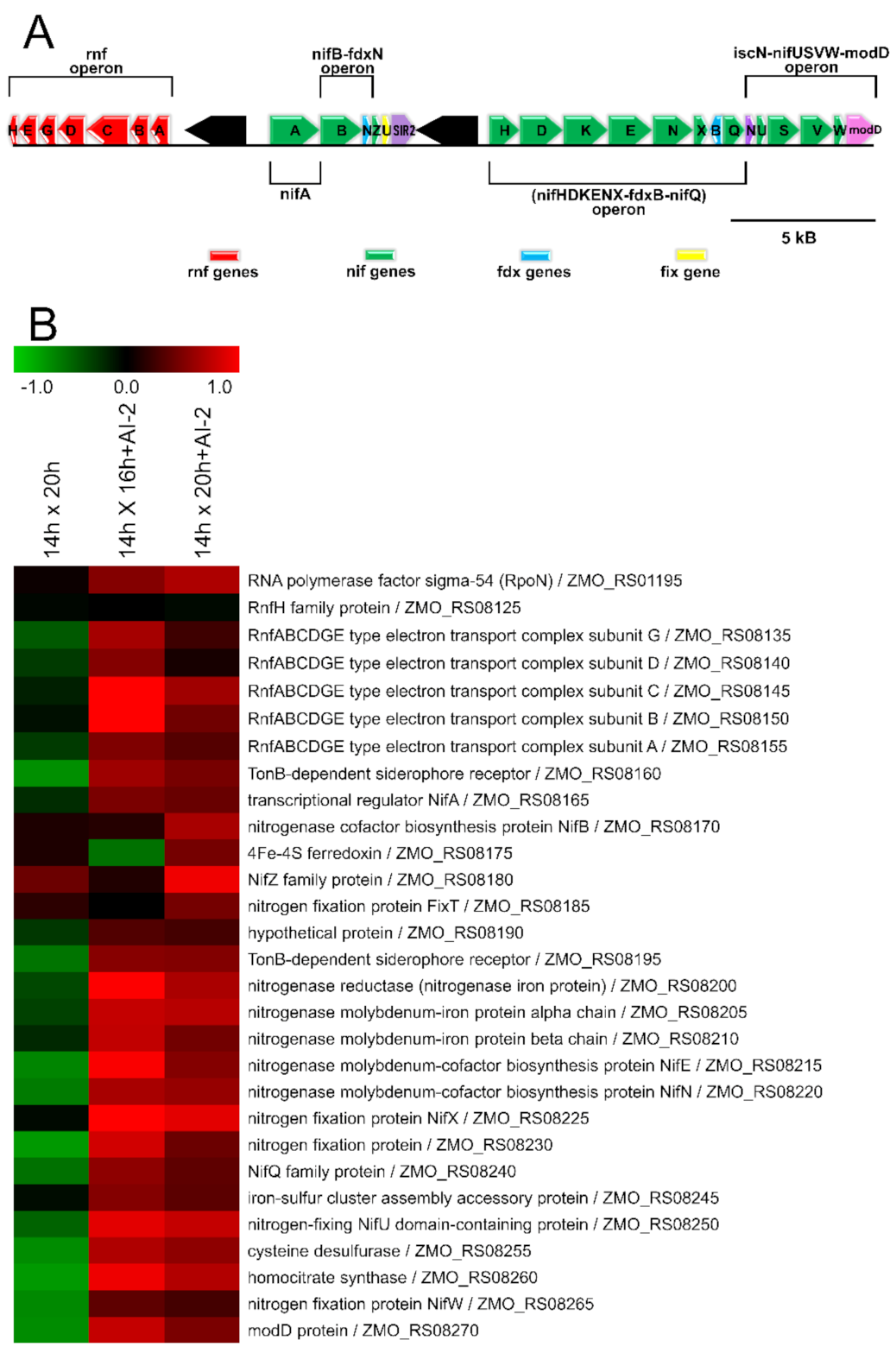
2.4. The Presence of AI-2 Increases N2 Fixation by Z. mobilis
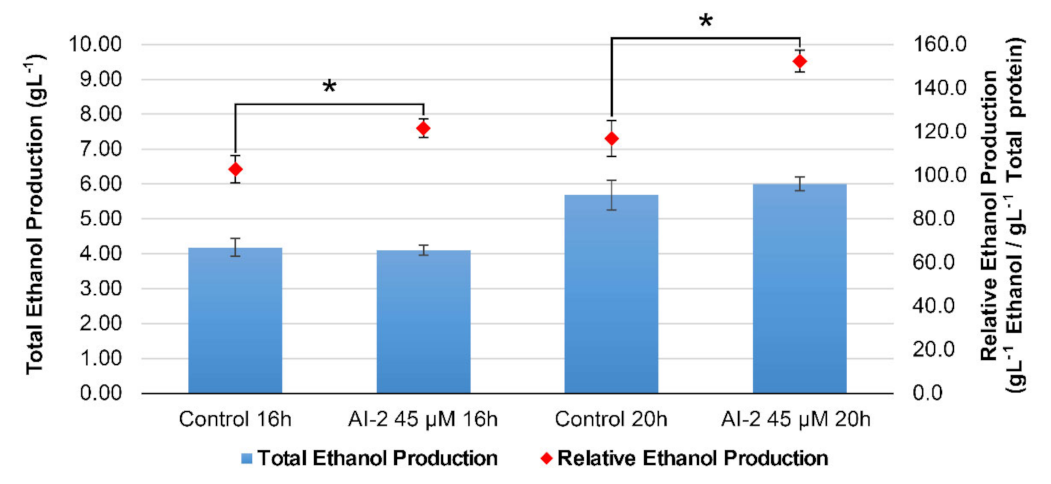
2.5. The Presence of AI-2 Favors Glucose-to-Ethanol Conversion, in Detriment of Biomass Accumulation in Z. mobilis Cells
3. Discussion
4. Materials and Methods
4.1. Bacterial Growth and Extraction of Genetic Material
4.2. Testing Z. mobilis Viability by Flow Cytometry Analyses
4.3. Transcriptomic Analysis of Z. mobilis in Response to the Presence of AI-2
4.4. Bioinformatics Analyses
4.5. Quantitative Real-Time PCR Experiments (qPCR)
4.6. Ethanol Quantification
4.7. Acetylene Reduction Assay (ARA)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319. [Google Scholar] [CrossRef]
- Bassler, B.L.; Losick, R. Bacterially speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal—Response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576. [Google Scholar] [CrossRef]
- Pereira, C.S.; Thompson, J.A.; Xavier, K.B. AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 2013, 37, 156–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Daniel, R.; Wagner-Döbler, I.; Zeng, A.P. Is autoinducer-2 a universal signal for interspecies communication: A comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol. Biol. 2004, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Xavier, K.B. Bacterial interspecies quorum sensing in the mammalian gut microbiota. Comptes Rendus Biol. 2018, 341, 297–299. [Google Scholar] [CrossRef]
- Yang, S.; Fei, Q.; Zhang, Y.; Contreras, L.M.; Utturkar, S.M.; Brown, S.D.; Himmel, M.E.; Zhang, M. Zymomonas mobilis as a model system for production of biofuels and biochemicals. Microb. Biotechnol. 2016, 9, 699–717. [Google Scholar] [CrossRef]
- Kremer, T.A.; LaSarre, B.; Posto, A.L.; McKinlay, J.B. N2 gas is an effective fertilizer for bioethanol production by Zymomonas mobilis. Proc. Natl. Acad. Sci. USA 2015, 112, 2222–2226. [Google Scholar] [CrossRef] [PubMed]
- Palamae, S.; Choorit, W.; Chatsungnoen, T.; Chisti, Y. Simultaneous nitrogen fixation and ethanol production by Zymomonas mobilis. J. Biotechnol. 2020, 314–315, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Sánchez, J. Nuclease activities and cell death processes associated with the development of surface cultures of Streptomyces antibioticus ETH 7451. Microbiology 2002, 148, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Consortium. The Gene Ontology (GO) Database and Informatics Resource. Nucleic Acids Res. 2004, 32, D258–D261. [Google Scholar] [CrossRef] [PubMed]
- Tatli, M.; Hebert, A.S.; Coon, J.; Amador-Noguez, D. Genome wide phosphoproteome analysis of Zymomonas mobilis under anaerobic, aerobic, and N2- fixing conditions. Front. Microbiol. 2019, 10, 1986. [Google Scholar]
- Rice, W.A.; Paul, E.A. The acetylene reduction assay for measuring nitrogen fixation in waterlogged soil. Can. J. Microbiol. 1971, 17, 1049–1056. [Google Scholar] [CrossRef]
- Vessey, J.K.; Waterer, J. In search of the mechanism of nitrate inhibition of nitrogenase activity in legume nodules: Recent developments. Physiol. Plant. 1992, 84, 171–176. [Google Scholar]
- Fu, H.; Burris, R.H. Ammonium inhibition of nitrogenase activity in Herbaspirillum seropedicae. J. Bacteriol. 1989, 171, 3168–3175. [Google Scholar] [CrossRef]
- Weisser, P.; Krämer, R.; Sahm, H.; Sprenger, G.A. Functional expression of the glucose transporter of Zymomonas mobilis leads to restoration of glucose and fructose uptake in Escherichia coli mutants and provides evidence for its facilitator action. J. Bacteriol. 1995, 177, 3351–3354. [Google Scholar]
- Conway, T. The Entner-Doudoroff pathway: History, physiology and molecular biology. FEMS Microbiol. Rev. 1992, 9, 1–27. [Google Scholar]
- Federle, M.J. Autoinducer-2-based chemical communication in bacteria: Complexities of interspecies signaling. Contrib. Microbiol. 2009, 16, 18–32. [Google Scholar]
- Thompson, J.A.; Oliveira, R.A.; Djukovic, A.; Ubeda, C.; Xavier, K.B. Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota. Cell Rep. 2015, 10, 1861–1871. [Google Scholar]
- Thompson, J.A.; Oliveira, R.A.; Xavier, K.B. Chemical conversations in the gut microbiota. Gut Microbes 2016, 7, 163–170. [Google Scholar] [PubMed]
- Rader, B.A.; Wreden, C.; Hicks, K.G.; Sweeney, E.G.; Ottemann, K.M.; Guillemin, K. Helicobacter pylori perceives the quorum-sensing molecule AI-2 as a chemorepellent via the chemoreceptor TlpB. Microbiology 2011, 157, 2445–2455. [Google Scholar] [CrossRef]
- Quan, Y.; Meng, F.; Ma, X.; Song, X.; Liu, X.; Gao, W.; Dang, Y.; Meng, Y.; Cao, M.; Song, C. Regulation of bacteria population behaviors by AI-2 “consumer cells” and “supplier cells”. BMC Microbiol. 2017, 17, 198. [Google Scholar]
- Stephens, K.; Zargar, A.; Emamian, M.; Abutaleb, N.; Choi, E.; Quan, D.N.; Payne, G.; Bentley, W.E. Engineering Escherichia coli for enhanced sensitivity to the autoinducer-2 quorum sensing signal. Biotechnol. Prog. 2019, 35, e2881. [Google Scholar] [PubMed]
- Gu, Y.; Wu, J.; Tian, J.; Li, L.; Zhang, B.; Zhang, Y.; He, Y. Effects of exogenous synthetic autoinducer-2 on physiological behaviors and proteome of lactic acid bacteria. ACS Omega 2020, 5, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.E.; Smalley, D.J.; Conway, T. Gene expression profiling of Escherichia coli growth transitions: An expanded stringent response model. Mol. Microbiol. 2002, 45, 289–306. [Google Scholar] [CrossRef]
- Boles, B.R.; Singh, P.K. Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc. Natl. Acad. Sci. USA 2008, 105, 12503–12508. [Google Scholar] [PubMed]
- Navarro Llorens, J.M.; Tormo, A.; Martinez-Garcia, E. Stationary phase in gram—Negative bacteria. FEMS Microbiol. Rev. 2010, 34, 476–495. [Google Scholar] [CrossRef]
- Blom, E.-J.; Ridder, A.N.J.A.; Lulko, A.T.; Roerdink, J.B.T.M.; Kuipers, O.P. Time-resolved transcriptomics and bioinformatic analyses reveal intrinsic stress responses during batch culture of Bacillus subtilis. PLoS ONE 2011, 6, e27160. [Google Scholar] [CrossRef][Green Version]
- Van Mooy, B.A.; Hmelo, L.R.; Sofen, L.E.; Campagna, S.R.; May, A.L.; Dyhrman, S.T.; Heithoff, A.; Webb, E.A.; Momper, L.; Mincer, T.J. Quorum sensing control of phosphorus acquisition in Trichodesmium consortia. ISME J. 2012, 6, 422–429. [Google Scholar]
- García-Contreras, R.; Nuñez-López, L.; Jasso-Chávez, R.; Kwan, B.W.; Belmont, J.A.; Rangel-Vega, A.; Maeda, T.; Wood, T.K. Quorum sensing enhancement of the stress response promotes resistance to quorum quenching and prevents social cheating. ISME J. 2015, 9, 115–125. [Google Scholar] [CrossRef]
- Lin, L.; Dai, S.; Tian, B.; Li, T.; Yu, J.; Liu, C.; Wang, L.; Xu, H.; Zhao, Y.; Hua, Y. DqsIR quorum sensing-mediated gene regulation of the extremophilic bacterium Deinococcus radiodurans in response to oxidative stress. Mol. Microbiol. 2016, 100, 527–541. [Google Scholar] [CrossRef]
- Lin, L.; Li, T.; Dai, S.; Yu, J.; Chen, X.; Wang, L.; Wang, Y.; Hua, Y.; Tian, B. Autoinducer-2 signaling is involved in regulation of stress-related genes of Deinococcus radiodurans. Arch. Microbiol. 2016, 198, 43–51. [Google Scholar] [CrossRef]
- Zhao, K.; Zhou, X.; Li, W.; Zhang, X.; Yue, B. Nutrient reduction induced stringent responses promote bacterial quorum-sensing divergence for population fitness. Sci. Rep. 2016, 6, 34925. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, J.; Srivastava, P. Molecular Basis of Stationary Phase Survival and Applications. Front. Microbiol. 2017, 8, 2000. [Google Scholar] [CrossRef] [PubMed]
- Prossliner, T.; Skovbo Winther, K.; Sorensen, M.A.; Gerdes, K. Ribosome hibernation. Annu. Rev. Genet. 2018, 52, 321–348. [Google Scholar] [CrossRef]
- Chen, W.; Wang, B.; Gruber, J.D.; Zhang, Y.M.; Davies, C. Acyl carrier protein 3 is involved in oxidative stress response in Pseudomonas aeruginosa. Front. Microbiol. 2018, 9, 2244. [Google Scholar] [PubMed]
- Yadav, M.K.; Vidal, J.E.; Go, Y.Y.; Kim, S.H.; Chae, S.W.; Song, J.J. The LuxS/AI-2 quorum-sensing system of Streptococcus pneumoniae is required to cause disease, and to regulate virulence- and metabolism-related genes in a rat model of middle ear infection. Front. Cell. Infect. Microbiol. 2018, 8, 138. [Google Scholar] [CrossRef]
- Macintosh, M.; Eisenhardt, K.; Remes, B.; Konzer, A.; Klug, G. Adaptation of the Alphaproteobacterium Rhodobacter sphaeroides to stationary phase. Environ. Microbiol. 2019, 21, 4425–4445. [Google Scholar]
- Motib, A.S.; Al-Bayati, F.; Manzoor, I.; Shafeeq, S.; Kadam, A.; Kuipers, O.P.; Hiller, N.L.; Andrew, P.W.; Yesilkaya, H. TprA/PhrA quorum sensing system has a major effect on pneumococcal survival in respiratory tract and blood, and its activity is controlled by CcpA and GlnR. Front. Cell. Infect. Microbiol. 2019, 9, 326. [Google Scholar]
- Lu, R.; Tang, H.; Qiu, Y.; Yang, W.; Yang, H.; Zhou, D.; Huang, X.; Hu, L.; Zhang, Y. Quorum sensing regulates the transcription of lateral flagellar genes in Vibrio parahaemolyticus. Future Microbiol. 2019, 14, 1043–1053. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Turkina, M.V.; Vikström, E. Bacteria-Host Crosstalk: Sensing of the quorum in the context of Pseudomonas aeruginosa infections. J. Innate Immun. 2019, 11, 263–279. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, M.; Yin, W.; Dong, T.; Chang, C.; Miao, Y.; Jia, Y.; Deng, Y. Xanthomonas campestris promotes diffusible signal factor biosynthesis and pathogenicity by utilizing glucose and sucrose from host plants. Mol. Plant-Microbe Interact. MPMI 2019, 32, 157–166. [Google Scholar] [CrossRef]
- Shah, N.; Gislason, A.S.; Becker, M.; Belmonte, M.F.; Fernando, W.G.D.; de Kievit, T.R. Investigation of the quorum-sensing regulon of the biocontrol bacterium Pseudomonas chlororaphis strain PA23. PLoS ONE 2020, 15, e0226232. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.; Horng, Y.-T.; Shu, J.-C.; Soo, P.-C.; Liu, J.-H.; Yi, W.-C.; Lai, H.-C.; Luh, K.-T.; Ho, S.-W.; Swift, S. The role of RsmA in the regulation of swarming motility in Serratia marcescens. J. Biomed. Sci. 2001, 8, 160–169. [Google Scholar] [CrossRef]
- Sperandio, V.; Torres, A.G.; Girón, J.A.; Kaper, J.B. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157: H7. J. Bacteriol. 2001, 183, 5187–5197. [Google Scholar] [CrossRef]
- Sperandio, V.; Torres, A.G.; Kaper, J.B. Quorum sensing Escherichia coli regulators B and C (QS eBC): A novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 2002, 43, 809–821. [Google Scholar] [CrossRef]
- Van Houdt, R.; Givskov, M.; Michiels, C.W. Quorum sensing in Serratia. FEMS Microbiol. Rev. 2007, 31, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Tsou, A.M.; Frey, E.M.; Hsiao, A.; Liu, Z.; Zhu, J. Coordinated regulation of virulence by quorum sensing and motility pathways during the initial stages of Vibrio cholerae infection. Commun. Integr. Biol. 2008, 1, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.H.; Gurich, N.; González, J.E. Regulation of motility by the ExpR/Sin quorum-sensing system in Sinorhizobium meliloti. J. Bacteriol. 2008, 190, 861–871. [Google Scholar] [CrossRef]
- Shrout, J.D.; Chopp, D.L.; Just, C.L.; Hentzer, M.; Givskov, M.; Parsek, M.R. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006, 62, 1264–1277. [Google Scholar] [CrossRef]
- Verstraeten, N.; Braeken, K.; Debkumari, B.; Fauvart, M.; Fransaer, J.; Vermant, J.; Michiels, J. Living on a surface: Swarming and biofilm formation. Trends Microbiol. 2008, 16, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Alagely, A.; Krediet, C.J.; Ritchie, K.B.; Teplitski, M. Signaling-mediated cross-talk modulates swarming and biofilm formation in a coral pathogen Serratia marcescens. ISME J. 2011, 5, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Calatrava-Morales, N.; McIntosh, M.; Soto, M.J. Regulation mediated by N-acyl homoserine lactone quorum sensing signals in the rhizobium-legume symbiosis. Genes 2018, 9, 263. [Google Scholar] [CrossRef]
- Pereira, C.S.; McAuley, J.R.; Taga, M.E.; Xavier, K.B.; Miller, S.T. Sinorhizobium meliloti, a bacterium lacking the autoinducer-2 (AI-2) synthase, responds to AI-2 supplied by other bacteria. Mol. Microbiol. 2008, 70, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Torcato, I.M.; Kasal, M.R.; Brito, P.H.; Miller, S.T.; Xavier, K.B. Identification of novel autoinducer-2 receptors in Clostridia reveals plasticity in the binding site of the LsrB receptor family. J. Biol. Chem. 2019, 294, 4450–4463. [Google Scholar] [CrossRef]
- González, J.E.; Marketon, M.M. Quorum sensing in nitrogen-fixing rhizobia. Microbiol. Mol. Biol. Rev. MMBR 2003, 67, 574–592. [Google Scholar] [CrossRef]
- Rodelas, B.; Lithgow, J.K.; Wisniewski-Dye, F.; Hardman, A.; Wilkinson, A.; Economou, A.; Williams, P.; Downie, J.A. Analysis of quorum-sensing-dependent control of rhizosphere-expressed (rhi) genes in Rhizobium leguminosarum bv. viciae. J. Bacteriol. 1999, 181, 3816–3823. [Google Scholar] [CrossRef]
- Daniels, R.; De Vos, D.E.; Desair, J.; Raedschelders, G.; Luyten, E.; Rosemeyer, V.; Verreth, C.; Schoeters, E.; Vanderleyden, J.; Michiels, J. The cin quorum sensing locus of Rhizobium etli cnpaf512 affects growth and symbiotic nitrogen fixation. J. Biol. Chem. 2002, 277, 462–468. [Google Scholar] [CrossRef]
- Goodman, A.E.; Rogers, P.L.; Skotnicki, M.L. Minimal medium for isolation of auxotrophic Zymomonas mutants. Appl. Environ. Microbiol. 1982, 44, 496–498. [Google Scholar] [CrossRef]
- Croucher, N.J.; Thomson, N.R. Studying bacterial transcriptomes using RNA-seq. Curr. Opin. Microbiol. 2010, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- McClure, R.; Balasubramanian, D.; Sun, Y.; Bobrovskyym, M.; Sumbym, P.; Genco, C.A.; Vanderpool, C.K.; Tjaden, B. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res. 2013, 41, e140. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Chong, H.; Park, H.S.; Yoon, K.O.; Jung, C.; Kim, J.J.; Hong, J.H.; Kim, H.; Kim, J.H.; Kil, J.I.; et al. The genome sequence of the ethanologenic bacterium Zymomonas mobilis ZM4. Nat. Biotechnol. 2005, 23, 63–68. [Google Scholar] [CrossRef]
- Livak, K.J.; Schimittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-(Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alencar, V.C.; Silva, J.d.F.d.S.; Vilas Boas, R.O.; Farnézio, V.M.; de Maria, Y.N.L.F.; Aciole Barbosa, D.; Almeida, A.T.; de Souza, E.M.; Müller-Santos, M.; Jabes, D.L.; et al. The Quorum Sensing Auto-Inducer 2 (AI-2) Stimulates Nitrogen Fixation and Favors Ethanol Production over Biomass Accumulation in Zymomonas mobilis. Int. J. Mol. Sci. 2021, 22, 5628. https://doi.org/10.3390/ijms22115628
Alencar VC, Silva JdFdS, Vilas Boas RO, Farnézio VM, de Maria YNLF, Aciole Barbosa D, Almeida AT, de Souza EM, Müller-Santos M, Jabes DL, et al. The Quorum Sensing Auto-Inducer 2 (AI-2) Stimulates Nitrogen Fixation and Favors Ethanol Production over Biomass Accumulation in Zymomonas mobilis. International Journal of Molecular Sciences. 2021; 22(11):5628. https://doi.org/10.3390/ijms22115628
Chicago/Turabian StyleAlencar, Valquíria Campos, Juliana de Fátima dos Santos Silva, Renata Ozelami Vilas Boas, Vinícius Manganaro Farnézio, Yara N. L. F. de Maria, David Aciole Barbosa, Alex Tramontin Almeida, Emanuel Maltempi de Souza, Marcelo Müller-Santos, Daniela L. Jabes, and et al. 2021. "The Quorum Sensing Auto-Inducer 2 (AI-2) Stimulates Nitrogen Fixation and Favors Ethanol Production over Biomass Accumulation in Zymomonas mobilis" International Journal of Molecular Sciences 22, no. 11: 5628. https://doi.org/10.3390/ijms22115628
APA StyleAlencar, V. C., Silva, J. d. F. d. S., Vilas Boas, R. O., Farnézio, V. M., de Maria, Y. N. L. F., Aciole Barbosa, D., Almeida, A. T., de Souza, E. M., Müller-Santos, M., Jabes, D. L., Menegidio, F. B., Costa de Oliveira, R., Rodrigues, T., Tersariol, I. L. d. S., Walmsley, A. R., & Nunes, L. R. (2021). The Quorum Sensing Auto-Inducer 2 (AI-2) Stimulates Nitrogen Fixation and Favors Ethanol Production over Biomass Accumulation in Zymomonas mobilis. International Journal of Molecular Sciences, 22(11), 5628. https://doi.org/10.3390/ijms22115628






