Novel (+)-Neoisopulegol-Based O-Benzyl Derivatives as Antimicrobial Agents
Abstract
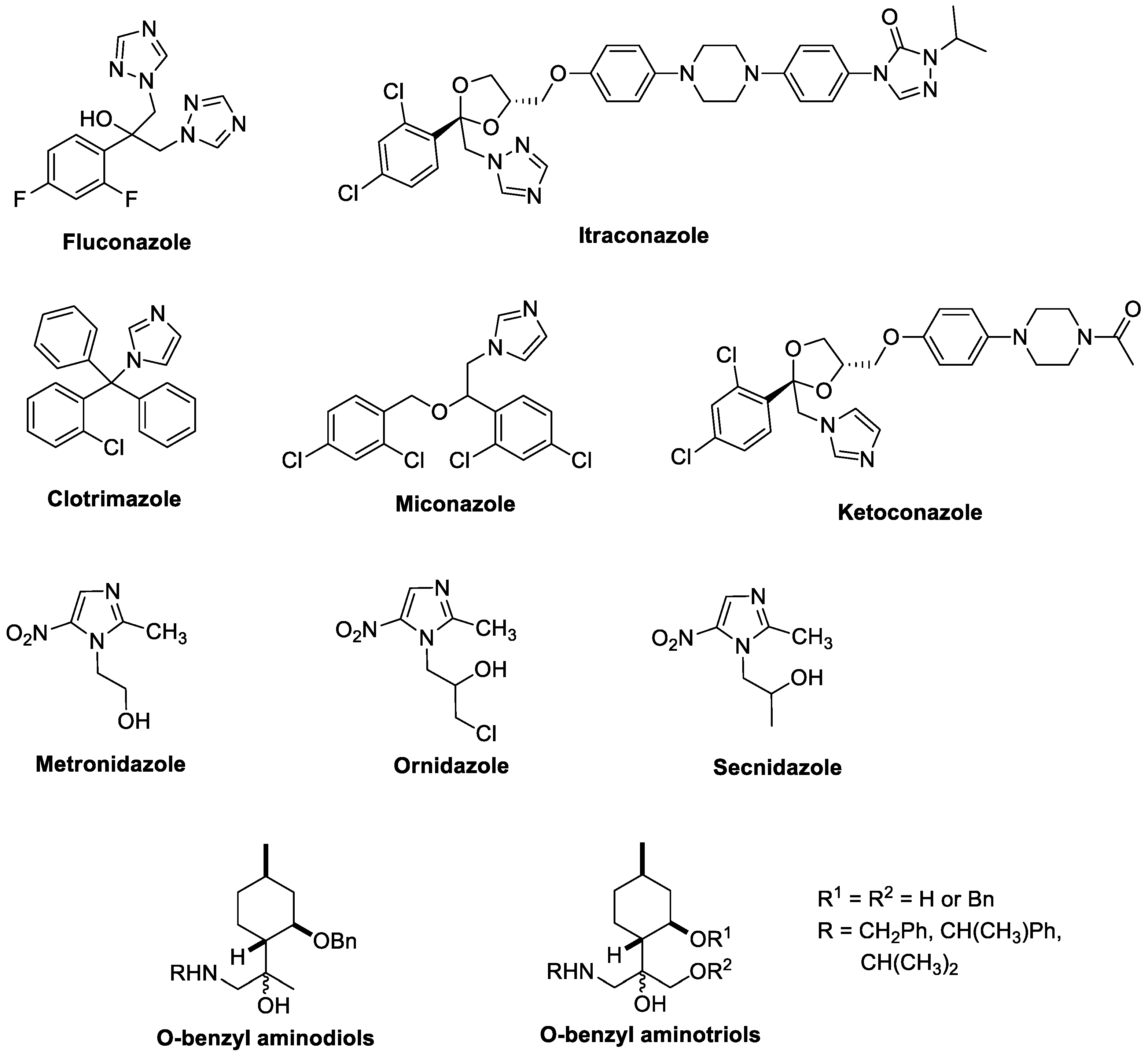
1. Introduction
2. Results
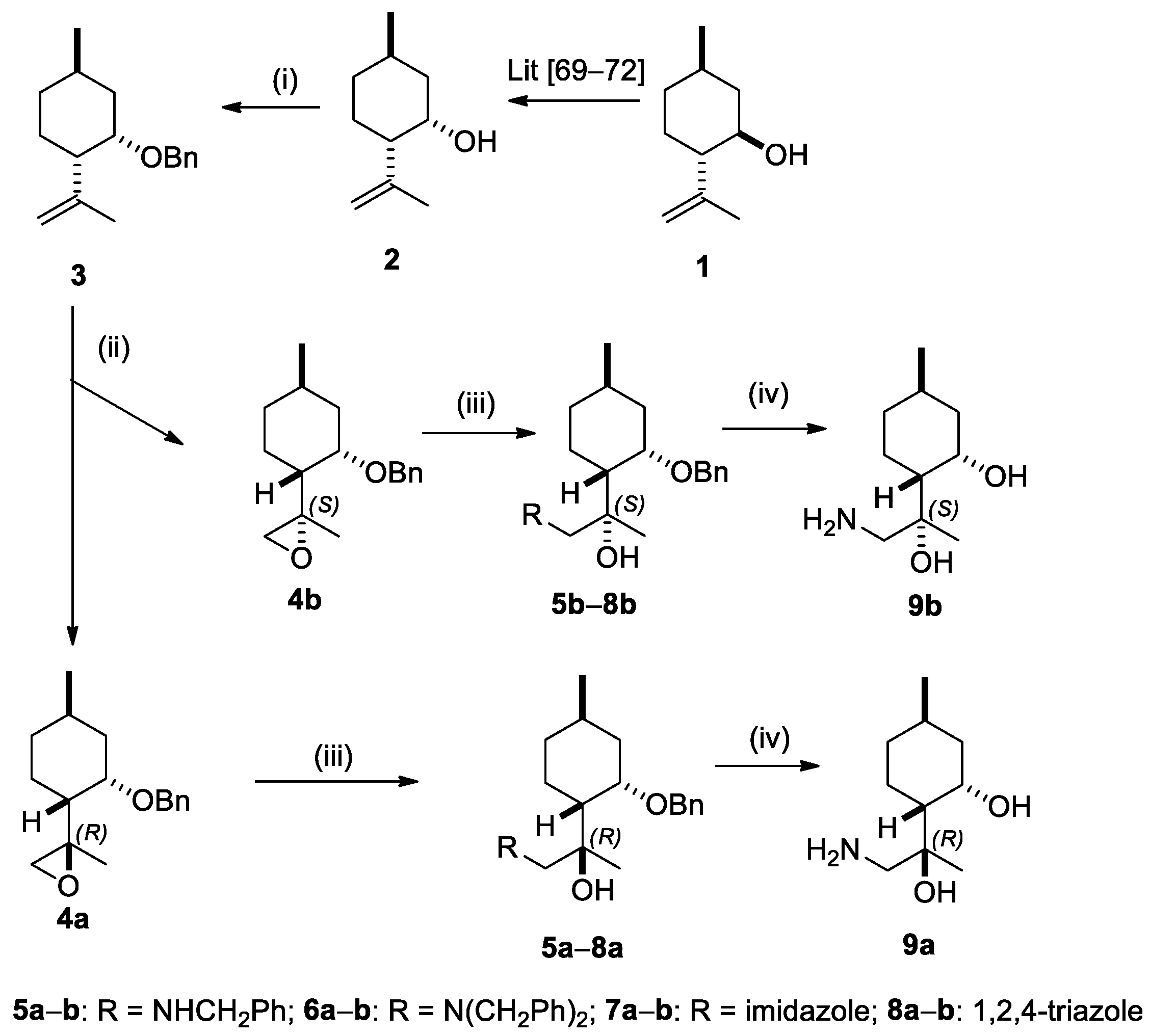
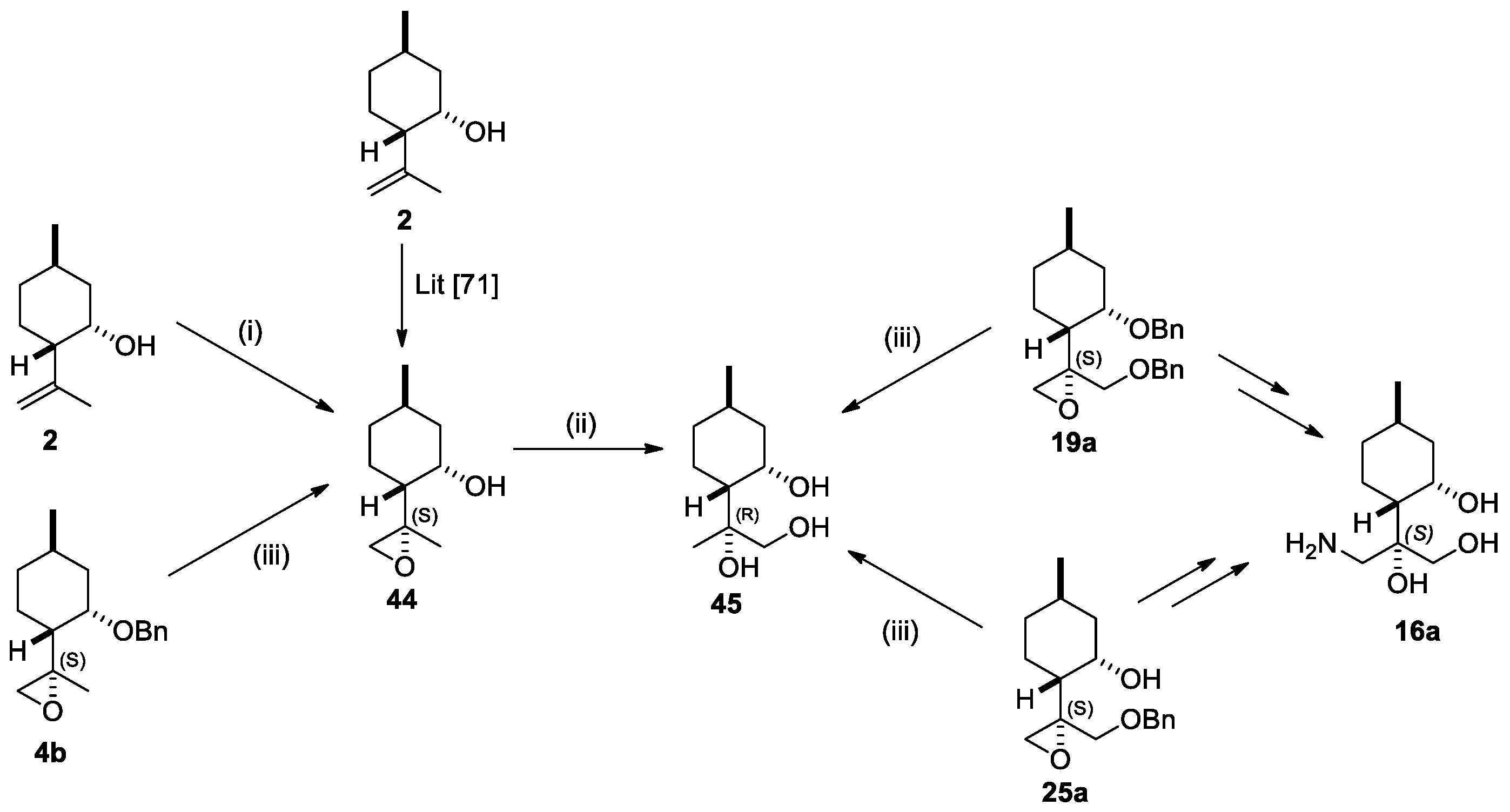
2.1. Synthesis of (+)-Neoisopulegol-Based O-Benzyl Derivatives
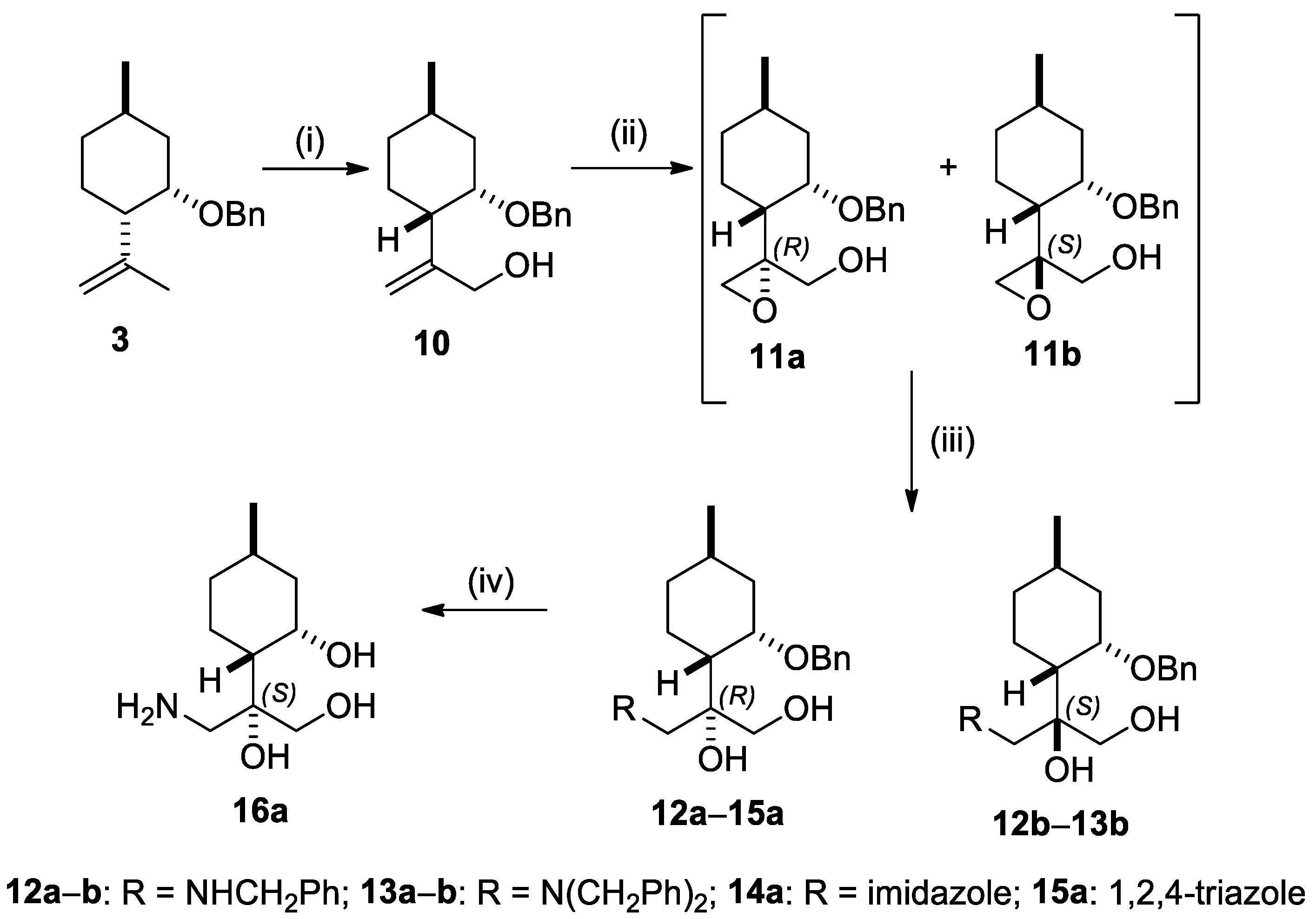
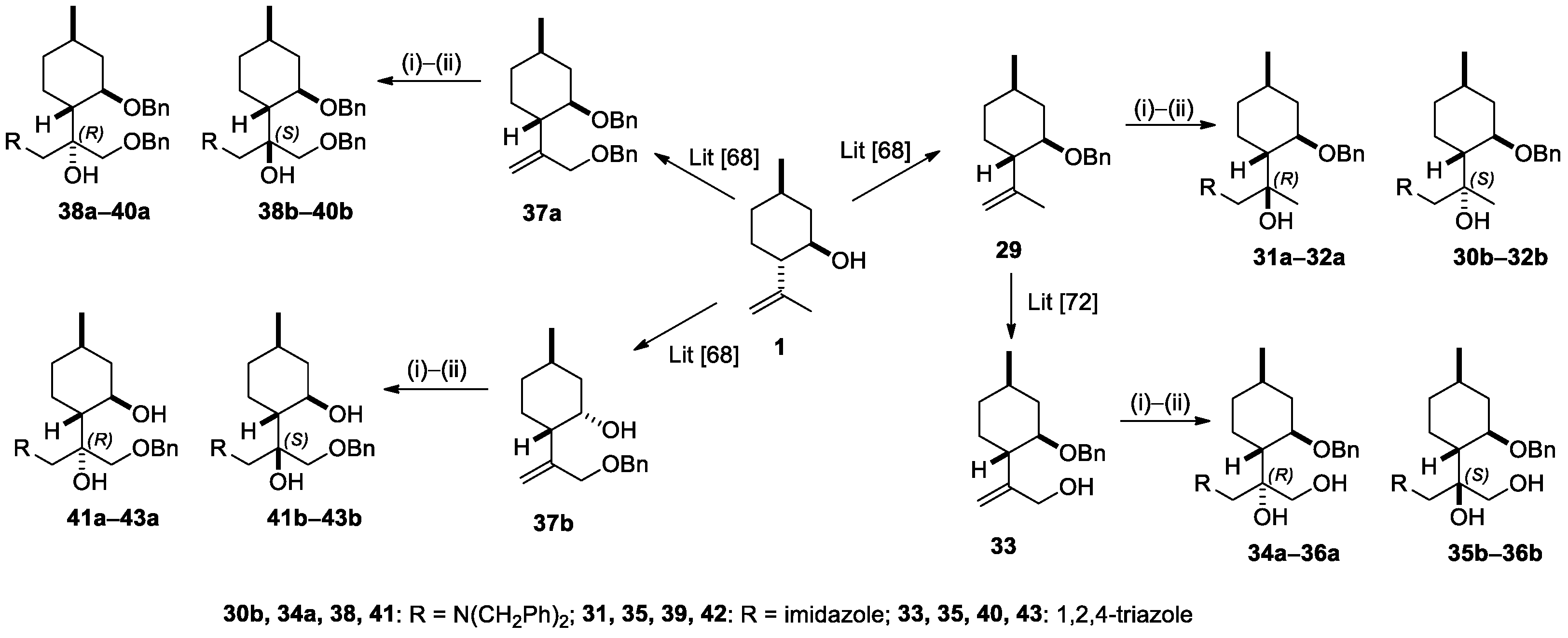
2.2. Synthesis of (−)-Isopulegol-Based O-Benzyl Derivatives
2.3. Determine Relative Configuration of (+)-Neoisopulegol-Based O-Benzyl Derivatives
2.4. Antimicrobial Effects
3. Discussion
3.1. Antimicrobial Activity
3.2. Structure-Activity Relationship
4. Materials and Methods
4.1. General Methods
4.2. Experimental Section and Compound Characterisations
4.2.1. (S)-2-((1R,2R,4R)-2-Hydroxy-4-methylcyclohexyl)propane-1,2-diol (45)
4.2.2. 2-((1S,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)prop-2-en-1-ol (10)
4.2.3. General Procedure for Benzylation
((((1S,2S,5R)-5-Methyl-2-(prop-1-en-2-yl)cyclohexyl)oxy)methyl)benzene (3)
(((2-((1S,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)allyl)oxy)methyl)benzene (18a)
(1S,2S,5R)-2-(3-(Benzyloxy)prop-1-en-2-yl)-5-methylcyclohexanol (18b)
4.2.4. General Procedure of Epoxidation
(R)-2-((1R,2S,4R)-2-(benzyloxy)-4-methylcyclohexyl)-2-methyloxirane (4a)
(S)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-2-methyloxirane (4b)
(S)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-2-((benzyloxy)methyl)oxirane (19a)
(R)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-2-((benzyloxy)methyl)oxirane (19b)
(1R,2R,5R)-2-((S)-2-((Benzyloxy)methyl)oxiran-2-yl)-5-methylcyclohexanol (24a)
(1R,2R,5R)-2-((R)-2-((Benzyloxy)methyl)oxiran-2-yl)-5-methylcyclohexanol (24b)
4.2.5. General Procedure for Ring-Opening of Epoxides with Different Amines
(R)-1-(Benzylamino)-2-((1R,2S,4R)-2-(benzyloxy)-4-methylcyclohexyl)propan-2-ol (5a)
(S)-1-(Benzylamino)-2-((1R,2S,4R)-2-(benzyloxy)-4-methylcyclohexyl)propan-2-ol (5b)
(R)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(dibenzylamino)propan-2-ol (6a)
(S)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(dibenzylamino)propan-2-ol (6b)
(S)-3-(Benzylamino)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)propane-1,2-diol (12a)
(R)-3-(Benzylamino)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)propane-1,2-diol (12b)
(S)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(dibenzylamino)propane-1,2-diol (13a)
(R)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(dibenzylamino)propane-1,2-diol (13b)
(S)-1-(Benzylamino)-3-(Benzyloxy)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)propan-2-ol (20a)
(R)-1-(Benzylamino)-3-(Benzyloxy)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)propan-2-ol (20b)
(S)-1-(Benzyloxy)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(dibenzylamino)propan-2-ol (21a)
(R)-1-(Benzyloxy)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(dibenzylamino)propan-2-ol (21b)
(1S,2R,5R)-2-((S)-1-(Benzylamino)-3-(Benzyloxy)-2-hydroxypropan-2-yl)-5-methylcyclohexanol (25a)
(1S,2R,5R)-2-((R)-1-(Benzylamino)-3-(Benzyloxy)-2-hydroxypropan-2-yl)-5-methylcyclohexanol (25b)
(1S,2R,5R)-2-((S)-1-(Benzyloxy)-3-(dibenzylamino)-2-hydroxypropan-2-yl)-5-methylcyclohexanol (26a)
(1S,2R,5R)-2-((R)-1-(Benzyloxy)-3-(dibenzylamino)-2-hydroxypropan-2-yl)-5-methylcyclohexanol (26b)
(S)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(dibenzylamino)propan-2-ol (30b)
(S)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(dibenzylamino)propane-1,2-diol (34a)
(S)-1-(Benzyloxy)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(dibenzylamino)propan-2-ol (38a)
(R)-1-(Benzyloxy)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(dibenzylamino)propan-2-ol (38b)
(1R,2R,5R)-2-((S)-1-(Benzyloxy)-3-(dibenzylamino)-2-hydroxypropan-2-yl)-5-methylcyclohexanol (41a)
(1R,2R,5R)-2-((R)-1-(Benzyloxy)-3-(dibenzylamino)-2-hydroxypropan-2-yl)-5-methylcyclohexanol (41b)
4.2.6. General Procedure for Ring-Opening of Epoxide with Azoles
(R)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(1H-imidazol-1-yl)propan-2-ol (7a)
(S)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(1H-imidazol-1-yl)propan-2-ol (7b)
(R)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(1H-1,2,4-triazol-1-yl)propan-2-ol (8a)
(S)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(1H-1,2,4-triazol-1-yl)propan-2-ol (8b)
(S)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(1H-imidazol-1-yl)propane-1,2-diol (14a)
(S)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(1H-1,2,4-triazol-1-yl)propane-1,2-diol (15a)
(S)-1-(Benzyloxy)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(1H-imidazol-1-yl)propan-2-ol (22a)
(R)-1-(Benzyloxy)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(1H-imidazol-1-yl)propan-2-ol (22b)
(S)-1-(Benzyloxy)-2-((1R,2S,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(1H-1,2,4-triazol-1-yl)propan-2-ol (23a)
(R)-1-(Benzyloxy)-2-((1R,2S,4R)-2-(benzyloxy)-4-methylcyclohexyl)-3-(1H-1,2,4-triazol-1-yl)propan-2-ol (23b)
(1S,2R,5R)-2-((S)-1-(Benzyloxy)-2-hydroxy-3-(1H-imidazol-1-yl)propan-2-yl)-5-methylcyclohexanol (27a)
(1S,2R,5R)-2-((R)-1-(Benzyloxy)-2-hydroxy-3-(1H-imidazol-1-yl)propan-2-yl)-5-methylcyclohexanol (27b)
(1S,2R,5R)-2-((S)-1-(Benzyloxy)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl)propan-2-yl)-5-methylcyclohexanol (28a)
(1S,2R,5R)-2-((R)-1-(Benzyloxy)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl)propan-2-yl)-5-methylcyclohexanol (28b)
(R)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(1H-imidazol-1-yl)propan-2-ol (31a)
(S)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(1H-imidazol-1-yl)propan-2-ol (31b)
(R)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(1H-1,2,4-triazol-1-yl)propan-2-ol (32a)
(S)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-1-(1H-1,2,4-triazol-1-yl)propan-2-ol (32b)
(S)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(1H-imidazol-1-yl)propane-1,2-diol (35a)
(R)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(1H-imidazol-1-yl)propane-1,2-diol (35b)
(S)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(1H-1,2,4-triazol-1-yl)propane-1,2-diol (36a)
(R)-2-((1R,2R,4R)-2-(Benzyloxy)-4-methylcyclohexyl)-3-(1H-1,2,4-triazol-1-yl)propane-1,2-diol (36b)
(S)-1-(Benzyloxy)-2-((1R,2R,4R)-2-(benzyloxy)-4-methylcyclohexyl)-3-(1H-imidazol-1-yl)propan-2-ol (39a)
(R)-1-(Benzyloxy)-2-((1R,2R,4R)-2-(benzyloxy)-4-methylcyclohexyl)-3-(1H-imidazol-1-yl)propan-2-ol (39b)
(S)-1-(Benzyloxy)-2-((1R,2R,4R)-2-(benzyloxy)-4-methylcyclohexyl)-3-(1H-1,2,4-triazol-1-yl)propan-2-ol (40a)
(R)-1-(Benzyloxy)-2-((1R,2R,4R)-2-(benzyloxy)-4-methylcyclohexyl)-3-(1H-1,2,4-triazol-1-yl)propan-2-ol (40b)
(1R,2R,5R)-2-((S)-1-(Benzyloxy)-2-hydroxy-3-(1H-imidazol-1-yl)propan-2-yl)-5-methylcyclohexanol (42a)
(1R,2R,5R)-2-((R)-1-(Benzyloxy)-2-hydroxy-3-(1H-imidazol-1-yl)propan-2-yl)-5-methylcyclohexanol (42b)
(1R,2R,5R)-2-((S)-1-(Benzyloxy)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl)propan-2-yl)-5-methylcyclohexanol (43a)
(1R,2R,5R)-2-((R)-1-(Benzyloxy)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl)propan-2-yl)-5-methylcyclohexanol (43b)
4.2.7. General Procedure for Debenzylation
(1S,2R,5R)-2-((R)-1-Amino-2-hydroxypropan-2-yl)-5-methylcyclohexanol (9a)
(1S,2R,5R)-2-((S)-1-Amino-2-hydroxypropan-2-yl)-5-methylcyclohexanol (9b)
(S)-3-Amino-2-((1R,2S,4R)-2-hydroxy-4-methylcyclohexyl)propane-1,2-diol (16a)
(R)-3-Amino-2-((1R,2S,4R)-2-hydroxy-4-methylcyclohexyl)propane-1,2-diol (16b)
4.3. General Procedure for Antimicrobial Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dabholkar, V.V.; Ansari, F.Y. Novel Pyrimidine Derivatives by Sonication and Traditional Thermal Methods. Green Chem. Lett. Rev. 2010, 3, 245–248. [Google Scholar] [CrossRef]
- Koparir, M.; Orek, C.; Parlak, A.E.; Söylemez, A.; Koparir, P.; Karatepe, M.; Dastan, S.D. Synthesis and Biological Activities of Some Novel Aminomethyl Derivatives of 4-Substituted-5-(2-Thienyl)-2,4-Dihydro-3H-1,2,4-Triazole-3-Thiones. Eur. J. Med. Chem. 2013, 63, 340–346. [Google Scholar] [CrossRef]
- Romero, D.H.; Heredia, V.E.T.; García-Barradas, O.; López, M.E.M.; Pavón, E.S. Synthesis of Imidazole Derivatives and Their Biological Activities. J. Chem. Biochem. 2014, 2. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kavitha, H.P. Synthesis and Biological Applications of Triazole Derivatives—A Review. Mini-Rev. Org. Chem. 2013, 10, 40–65. [Google Scholar] [CrossRef]
- Behrouz, S.; Rad, M.N.S.; Rostami, S.; Behrouz, M.; Zarehnezhad, E.; Zarehnezhad, A. Design, Synthesis, and Biological Activities of Novel Azole-Bonded β -Hydroxypropyl Oxime O-Ethers. Mol. Divers. 2014, 18, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Kosmalski, T.; Kutkowska, J.; Dwojak, I.; Studzińska, R.; Sikora, A.; Modzelewska-Banachiewicz, B.; Gzella, A. Novel O-Benzyl Oxime Ethers of 1-(Thiophen-2-Yl)Ethan-1-One—Synthesis, Structure and Antimicrobial Activity. Heterocycles 2017, 94, 523. [Google Scholar] [CrossRef]
- Padmavathi, V.; Thriveni, P.; Sudhakar Reddy, G.; Deepti, D. Synthesis and Antimicrobial Activity of Novel Sulfone-Linked Bis Heterocycles. Eur. J. Med. Chem. 2008, 43, 917–924. [Google Scholar] [CrossRef]
- Sztanke, K.; Tuzimski, T.; Rzymowska, J.; Pasternak, K.; Kandefer-Szerszeń, M. Synthesis, Determination of the Lipophilicity, Anticancer and Antimicrobial Properties of Some Fused 1,2,4-Triazole Derivatives. Eur. J. Med. Chem. 2008, 43, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Buzdar, A.U.; Robertson, J.F.R.; Eiermann, W.; Nabholtz, J.-M. An Overview of the Pharmacology and Pharmacokinetics of the Newer Generation Aromatase Inhibitors Anastrozole, Letrozole, and Exemestane. Cancer 2002, 95, 2006–2016. [Google Scholar] [CrossRef]
- Amir, M.; Kumar, H.; Javed, S.A. Condensed Bridgehead Nitrogen Heterocyclic System: Synthesis and Pharmacological Activities of 1,2,4-Triazolo-[3,4-b]-1,3,4-Thiadiazole Derivatives of Ibuprofen and Biphenyl-4-Yloxy Acetic Acid. Eur. J. Med. Chem. 2008, 43, 2056–2066. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüzel, Ş.G.; Çıkla-Süzgün, P. Recent Advances Bioactive 1,2,4-Triazole-3-Thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef] [PubMed]
- Kuş, C.; Ayhan-Kılcıgil, G.; Özbey, S.; Kaynak, F.B.; Kaya, M.; Çoban, T.; Can-Eke, B. Synthesis and Antioxidant Properties of Novel N-Methyl-1,3,4-Thiadiazol-2-Amine and 4-Methyl-2H-1,2,4-Triazole-3(4H)-Thione Derivatives of Benzimidazole Class. Bioorg. Med. Chem. 2008, 16, 4294–4303. [Google Scholar] [CrossRef] [PubMed]
- Boraei, A.T.A.; Gomaa, M.S.; El Ashry, E.S.H.; Duerkop, A. Design, Selective Alkylation and X-Ray Crystal Structure Determination of Dihydro-Indolyl-1,2,4-Triazole-3-Thione and Its 3-Benzylsulfanyl Analogue as Potent Anticancer Agents. Eur. J. Med. Chem. 2017, 125, 360–371. [Google Scholar] [CrossRef]
- Aouad, M.R.; Mayaba, M.M.; Naqvi, A.; Bardaweel, S.K.; Al-blewi, F.F.; Messali, M.; Rezki, N. Design, Synthesis, in Silico and in Vitro Antimicrobial Screenings of Novel 1,2,4-Triazoles Carrying 1,2,3-Triazole Scaffold with Lipophilic Side Chain Tether. Chem. Cent. J. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Vijesh, A.M.; Isloor, A.M.; Shetty, P.; Sundershan, S.; Fun, H.K. New Pyrazole Derivatives Containing 1,2,4-Triazoles and Benzoxazoles as Potent Antimicrobial and Analgesic Agents. Eur. J. Med. Chem. 2013, 62, 410–415. [Google Scholar] [CrossRef]
- Plech, T.; Kaproń, B.; Łuszczki, J.J.; Paneth, A.; Siwek, A.; Kołaczkowski, M.; Żołnierek, M.; Nowak, G. Studies on the Anticonvulsant Activity of 4-Alkyl-1,2,4-Triazole-3-Thiones and Their Effect on GABAergic System. Eur. J. Med. Chem. 2014, 86, 690–699. [Google Scholar] [CrossRef]
- Abuo-Rahma, G.E.-D.A.A.; Abdel-Aziz, M.; Beshr, E.A.M.; Ali, T.F.S. 1,2,4-Triazole/Oxime Hybrids as New Strategy for Nitric Oxide Donors: Synthesis, Anti-Inflammatory, Ulceroginicity and Antiproliferative Activities. Eur. J. Med. Chem. 2014, 71, 185–198. [Google Scholar] [CrossRef]
- Mohan Krishna, K.; Inturi, B.; Pujar, G.V.; Purohit, M.N.; Vijaykumar, G.S. Design, Synthesis and 3D-QSAR Studies of New Diphenylamine Containing 1,2,4-Triazoles as Potential Antitubercular Agents. Eur. J. Med. Chem. 2014, 84, 516–529. [Google Scholar] [CrossRef]
- Láinez, M.J. Rizatriptan in the Treatment of Migraine. Neuropsychiatr. Dis. Treat. 2006, 2, 247–259. [Google Scholar] [CrossRef]
- Hassan, G.S.; El-Messery, S.M.; Al-Omary, F.A.M.; Al-Rashood, S.T.; Shabayek, M.I.; Abulfadl, Y.S.; Habib, E.-S.E.; El-Hallouty, S.M.; Fayad, W.; Mohamed, K.M.; et al. Nonclassical Antifolates, Part 4. 5-(2-Aminothiazol-4-Yl)-4-Phenyl-4H-1,2,4-Triazole-3-Thiols as a New Class of DHFR Inhibitors: Synthesis, Biological Evaluation and Molecular Modeling Study. Eur. J. Med. Chem. 2013, 66, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüzel, İ.; Rollas, S.; Çevikbaş, A. Synthesis and Characterization of Certain Thiourea Derivatives Starting from 1,2,4-Triazoline-3-Thiones as Potential Antibacterial and Antifungal Agents. Drug Metabol. Drug Interact. 1995, 12. [Google Scholar] [CrossRef] [PubMed]
- Franklim, T.; Freire-de-Lima, L.; de Nazareth Sá Diniz, J.; Previato, J.; Castro, R.; Mendonça-Previato, L.; de Lima, M. Design, Synthesis and Trypanocidal Evaluation of Novel 1,2,4-Triazoles-3-Thiones Derived from Natural Piperine. Molecules 2013, 18, 6366–6382. [Google Scholar] [CrossRef] [PubMed]
- Kalluraya, B.; Isloor, A.M.; Shenoy, S. Synthesis and Biological Activity of 6-Substituted-3-[4-(3-Substituted Pyrazolidene) Hydrazino-4-Thiazolyl] Coumarins. Indian J. Heterocycl. Chem. 2001, 11, 159–162. [Google Scholar]
- Isloor, A.M.; Kalluraya, B.; Shetty, P. Regioselective Reaction: Synthesis, Characterization and Pharmacological Studies of Some New Mannich Bases Derived from 1,2,4-Triazoles. Eur. J. Med. Chem. 2009, 44, 3784–3787. [Google Scholar] [CrossRef]
- Enguehard, C.; Renou, J.-L.; Allouchi, H.; Leger, J.-M.; Gueiffier, A. Synthesis of Diaryl-Substituted Imidazo[1, 2-a]Pyridines Designed as Potential Aromatase Inhibitors. Chem. Pharm. Bull. 2000, 48, 935–940. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sánchez-Moreno, M.; Gómez-Contreras, F.; Navarro, P.; Marín, C.; Ramírez-Macías, I.; Olmo, F.; Sanz, A.M.; Campayo, L.; Cano, C.; Yunta, M.J.R. In Vitro Leishmanicidal Activity of Imidazole- or Pyrazole-Based Benzo[g]Phthalazine Derivatives against Leishmania Infantum and Leishmania Braziliensis Species. J. Antimicrob. Chemother. 2012, 67, 387–397. [Google Scholar] [CrossRef]
- Khabnadideh, S.; Rezaei, Z.; Motazedian, M.H.; Eskandari, M. Synthesis of Metronidazole Derivatives as Antigiardiasis Agents. DARU J. Pharm. Sci. 2007, 15, 17–20. [Google Scholar]
- Stover, C.K.; Warrener, P.; VanDevanter, D.R.; Sherman, D.R.; Arain, T.M.; Langhorne, M.H.; Anderson, S.W.; Towell, J.A.; Yuan, Y.; McMurray, D.N.; et al. A Small-Molecule Nitroimidazopyran Drug Candidate for the Treatment of Tuberculosis. Nature 2000, 405, 962–966. [Google Scholar] [CrossRef]
- Łażewska, D.; Więcek, M.; Ligneau, X.; Kottke, T.; Weizel, L.; Seifert, R.; Schunack, W.; Stark, H.; Kieć-Kononowicz, K. Histamine H3 and H4 Receptor Affinity of Branched 3-(1H-Imidazol-4-Yl)Propyl N-Alkylcarbamates. Bioorg. Med. Chem. Lett. 2009, 19, 6682–6685. [Google Scholar] [CrossRef]
- Galley, G.; Stalder, H.; Goergler, A.; Hoener, M.C.; Norcross, R.D. Optimisation of Imidazole Compounds as Selective TAAR1 Agonists: Discovery of RO5073012. Bioorg. Med. Chem. Lett. 2012, 22, 5244–5248. [Google Scholar] [CrossRef]
- Hancock, A.A.; Bennani, Y.L.; Bush, E.N.; Esbenshade, T.A.; Faghih, R.; Fox, G.B.; Jacobson, P.; Knourek-Segel, V.; Krueger, K.M.; Nuss, M.E.; et al. Antiobesity Effects of A-331440, a Novel Non-Imidazole Histamine H3 Receptor Antagonist. Eur. J. Pharmacol. 2004, 487, 183–197. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Hassanabad, Z.F.; Bamshad, M.; Poorsoghat, H.; Hassanabad, M.F. Synthesis and Antihypertensive Activity of New 1,4-Dihydropyridines. Indian J. Chem. 2005, 44B, 2343–2347. [Google Scholar] [CrossRef]
- Göker, H.; Kuş, C.; Boykin, D.W.; Yildiz, S.; Altanlar, N. Synthesis of Some New 2-Substituted-Phenyl-1H-Benzimidazole-5-Carbonitriles and Their Potent Activity against Candida Species. Bioorg. Med. Chem. 2002, 10, 2589–2596. [Google Scholar] [CrossRef]
- Insuasty, B.; Fernandez, F.; Quiroga, J.; Martinez, R.; Gavino, R.; Angeles, E. Reaction of 1,2-Diaminobenzimidazole with 1-Aryl-2-Bromo-3-Phenylpropanone. Synthesis of 2-Aryl-3-Benzyl-9-Aminoimidazo[1,2-a] Benzimidazoles. Heterocycl. Commun. 2002, 8. [Google Scholar] [CrossRef]
- Zhou, J.; Song, Y.; Yang, Y.; Zhu, Y.; Tu, S. One-Step Synthesis of 2-Aryl-4,5-diphenylimidazoles Under Microwave Irradiation. Synth. Commun. 2005, 35, 1369–1373. [Google Scholar] [CrossRef]
- Rastkari, N.; Sharifzadeh, M. Anticonvulsant Activities of New 1,4-Dihydropyridine Derivatives Containing 4-Nitroimidazolyl Substituents. Daru J. Pharm. Sci. 2004, 12, 81–86. [Google Scholar]
- Mishra, R.; Ganguly, S. Imidazole as an Anti-Epileptic: An Overview. Med. Chem. Res. 2012, 21, 3929–3939. [Google Scholar] [CrossRef]
- Sharma, D.; Narasimhan, B.; Kumar, P.; Judge, V.; Narang, R.; De Clercq, E.; Balzarini, J. Synthesis, Antimicrobial and Antiviral Evaluation of Substituted Imidazole Derivatives. Eur. J. Med. Chem. 2009, 44, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, N.; Korba, B.E.; Hosmane, R.S. Synthesis and in Vitro Anti-Hepatitis B and C Virus Activities of Ring-Expanded (‘Fat’) Nucleobase Analogues Containing the Imidazo[4,5-e][1,3]Diazepine-4,8-Dione Ring System. Bioorg. Med. Chem. Lett. 2005, 15, 5397–5401. [Google Scholar] [CrossRef] [PubMed]
- Avram, S.; Svab, I.; Bologa, C.; Flonta, M.-L. Correlation between the Predicted and the Observed Biological Activity of the Symmetric and Nonsymmetric Cyclic Urea Derivatives Used as HIV-1 Protease Inhibitors. A 3D-QSAR-CoMFA Method for New Antiviral Drug Design. J. Cell. Mol. Med. 2003, 7, 287–296. [Google Scholar] [CrossRef]
- Chang, L.L.; Sidler, K.L.; Cascieri, M.A.; de Laszlo, S.; Koch, G.; Li, B.; MacCoss, M.; Mantlo, N.; O’Keefe, S.; Pang, M.; et al. Substituted Imidazoles as Glucagon Receptor Antagonists. Bioorg. Med. Chem. Lett. 2001, 11, 2549–2553. [Google Scholar] [CrossRef]
- Baroniya, S.; Anwer, Z.; Sharma, P.K.; Dudhe, R.; Kumar, N. Recent Advancement in Imidazole as Anti Cancer Agents: A Review. Der Pharm. Sin. 2010, 11, 172–182. [Google Scholar]
- Zhou, C.; Hassner, A. Synthesis and Anticancer Activity of Novel Chiral D-Glucose Derived Bis-Imidazoles and Their Analogs. Carbohydr. Res. 2001, 333, 313–326. [Google Scholar] [CrossRef]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The Biology and Chemistry of Antifungal Agents: A Review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Lin, X. Recent Progress on Antifungal Drug Development. Curr. Pharm. Biotechnol. 2011, 12, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.-L.; Lu, Y.-H.; Gan, L.-L.; Zhang, Y.-Y.; Zhou, C.-H. Recent Advance in the Research of Piperazine-Containing Compounds as Antimicrobial Agents. Chin. J. Antibiot. 2009, 34, 454–462. [Google Scholar]
- Peng, X.-M.; Cai, G.-X.; Zhou, C.-H. Recent Developments in Azole Compounds as Antibacterial and Antifungal Agents. Curr. Top. Med. Chem. 2013, 13, 1963–2010. [Google Scholar] [CrossRef] [PubMed]
- Hammond, N.L.; Choi, S.; Carvalho, P.; Liu, H.; Khan, S.; Avery, M.A. Synthesis and Biological Evaluation of a Novel Anti-Malarial Lead. Med. Chem. Res. 2011, 20, 401–407. [Google Scholar] [CrossRef]
- Glans, L.; Ehnbom, A.; de Kock, C.; Martínez, A.; Estrada, J.; Smith, P.J.; Haukka, M.; Sánchez-Delgado, R.A.; Nordlander, E. Ruthenium(Ii) Arene Complexes with Chelating Chloroquine Analogue Ligands: Synthesis, Characterization and in Vitro Antimalarial Activity. Dalton Trans. 2012, 41, 2764. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S. Synthesis and Anthelmintic Activity of Some Novel 2-Substituted-4,5-Diphenyl Imidazoles. Acta Pharm. 2010, 60, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Baliani, A.; Bueno, G.J.; Stewart, M.L.; Yardley, V.; Brun, R.; Barrett, M.P.; Gilbert, I.H. Design and Synthesis of a Series of Melamine-Based Nitroheterocycles with Activity against Trypanosomatid Parasites. J. Med. Chem. 2005, 48, 5570–5579. [Google Scholar] [CrossRef] [PubMed]
- Baliani, A.; Peal, V.; Gros, L.; Brun, R.; Kaiser, M.; Barrett, M.P.; Gilbert, I.H. Novel Functionalized Melamine-Based Nitroheterocycles: Synthesis and Activity against Trypanosomatid Parasites. Org. Biomol. Chem. 2009, 7, 1154. [Google Scholar] [CrossRef]
- Sánchez-Moreno, M.; Gómez-Contreras, F.; Navarro, P.; Marín, C.; Olmo, F.; Yunta, M.J.R.; Sanz, A.M.; Rosales, M.J.; Cano, C.; Campayo, L. Phthalazine Derivatives Containing Imidazole Rings Behave as Fe-SOD Inhibitors and Show Remarkable Anti- T. Cruzi Activity in Immunodeficient-Mouse Mode of Infection. J. Med. Chem. 2012, 55, 9900–9913. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, H.G.; Agrawal, Y.K.; Raval, H.G.; Manna, K.; Desai, P.R. Histamine H4 Receptor: A Novel Therapeutic Target for Immune and Allergic Responses. Mini-Rev. Med. Chem. 2010, 10, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Tiligada, E.; Zampeli, E.; Sander, K.; Stark, H. Histamine H3 and H4 Receptors as Novel Drug Targets. Expert Opin. Investig. Drugs 2009, 18, 1519–1531. [Google Scholar] [CrossRef]
- Geyer, R.; Buschauer, A. Synthesis and Histamine H3 and H4 Receptor Activity of Conformationally Restricted Cyanoguanidines Related to UR-PI376. Arch. Pharm. 2011, 344, 775–785. [Google Scholar] [CrossRef]
- Hack, S.; Wörlein, B.; Höfner, G.; Pabel, J.; Wanner, K.T. Development of Imidazole Alkanoic Acids as MGAT3 Selective GABA Uptake Inhibitors. Eur. J. Med. Chem. 2011, 46, 1483–1498. [Google Scholar] [CrossRef]
- Seo, H.J.; Park, E.-J.; Kim, M.J.; Kang, S.Y.; Lee, S.H.; Kim, H.J.; Lee, K.N.; Jung, M.E.; Lee, M.; Kim, M.-S.; et al. Design and Synthesis of Novel Arylpiperazine Derivatives Containing the Imidazole Core Targeting 5-HT2A Receptor and 5-HT Transporter. J. Med. Chem. 2011, 54, 6305–6318. [Google Scholar] [CrossRef]
- Gonçalves, A.E.; Bürger, C.; Amoah, S.K.S.; Tolardo, R.; Biavatti, M.W.; de Souza, M.M. The Antidepressant-like Effect of Hedyosmum Brasiliense and Its Sesquiterpene Lactone, Podoandin in Mice: Evidence for the Involvement of Adrenergic, Dopaminergic and Serotonergic Systems. Eur. J. Pharmacol. 2012, 674, 307–314. [Google Scholar] [CrossRef]
- Lakatos, A.; Gyurcsik, B.; Nagy, N.V.; Csendes, Z.; Wéber, E.; Fülöp, L.; Kiss, T. Histidine-Rich Branched Peptides as Cu(Ii) and Zn(Ii) Chelators with Potential Therapeutic Application in Alzheimer’s Disease. Dalton Trans. 2012, 41, 1713–1726. [Google Scholar] [CrossRef]
- Tyagarajan, S.; Chakravarty, P.K.; Zhou, B.; Fisher, M.H.; Wyvratt, M.J.; Lyons, K.; Klatt, T.; Li, X.; Kumar, S.; Williams, B.; et al. Substituted Biaryl Oxazoles, Imidazoles, and Thiazoles as Sodium Channel Blockers. Bioorg. Med. Chem. Lett. 2010, 20, 5536–5540. [Google Scholar] [CrossRef]
- Hanna-Elias, A.; Manallack, D.T.; Berque-Bestel, I.; Irving, H.R.; Coupar, I.M.; Iskander, M.N. Synthesis and Preliminary Screening of Novel Tryptamines as 5-HT4 Receptor Ligands. Curr. Med. Chem. 2010, 17, 2775–2787. [Google Scholar] [CrossRef] [PubMed]
- Galambos, J.; Wágner, G.; Nógrádi, K.; Bielik, A.; Molnár, L.; Bobok, A.; Horváth, A.; Kiss, B.; Kolok, S.; Nagy, J.; et al. Carbamoyloximes as Novel Non-Competitive MGlu5 Receptor Antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 4371–4375. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.; Pathak, M.B.; Panday, S.K. An Efficient and Straight Forward Synthesis of (5S)-1-Benzyl-5- (1H-Imidazol-1-Ylmethyl)-2-Pyrrolidinone (MM1): A Novel Antihypertensive Agent. Med. Chem. Res. 2012, 21, 321–324. [Google Scholar] [CrossRef]
- Agelis, G.; Resvani, A.; Durdagi, S.; Spyridaki, K.; Tůmová, T.; Slaninová, J.; Giannopoulos, P.; Vlahakos, D.; Liapakis, G.; Mavromoustakos, T.; et al. The Discovery of New Potent Non-Peptide Angiotensin II AT1 Receptor Blockers: A Concise Synthesis, Molecular Docking Studies and Biological Evaluation of N-Substituted 5-Butylimidazole Derivatives. Eur. J. Med. Chem. 2012, 55, 358–374. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, K.; Srinivas, N.; Shiva Keshava, G.B.; Shukla, P.K. Tetrahydronaphthyl Azole Oxime Ethers: The Conformationally Rigid Analogues of Oxiconazole as Antibacterials. Eur. J. Med. Chem. 2009, 44, 437–447. [Google Scholar] [CrossRef]
- Le, T.M.; Huynh, T.; Endre, G.; Szekeres, A.; Fülöp, F.; Szakonyi, Z. Stereoselective Synthesis and Application of Isopulegol-Based Bi- and Trifunctional Chiral Compounds. RSC Adv. 2020, 10, 38468–38477. [Google Scholar] [CrossRef]
- Nazimova, E.; Pavlova, A.; Mikhalchenko, O.; Il’ina, I.; Korchagina, D.; Tolstikova, T.; Volcho, K.; Salakhutdinov, N. Discovery of Highly Potent Analgesic Activity of Isopulegol-Derived (2R,4aR,7R,8aR)-4,7-Dimethyl-2-(Thiophen-2-Yl)Octahydro-2H-Chromen-4-Ol. Med. Chem. Res. 2016, 25, 1369–1383. [Google Scholar] [CrossRef]
- Moreira, J.A.; Corrêa, A.G. Enantioselective Synthesis of Three Stereoisomers of 5,9-Dimethylpentadecane, Sex Pheromone Component of Leucoptera Coffeella, from (−)-Isopulegol. Tetrahedron Asymmetry 2003, 14, 3787–3795. [Google Scholar] [CrossRef]
- Rigamonti, M.G.; Gatti, F.G. Stereoselective Synthesis of Hernandulcin, Peroxylippidulcine A, Lippidulcines A, B and C and Taste Evaluation. Beilstein J. Org. Chem. 2015, 11, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, D.; Bohlmann, F. Total Synthesis of Various Elemanolides. Tetrahedron 1988, 44, 1369–1392. [Google Scholar] [CrossRef]
- Travis, B.R.; Narayan, R.S.; Borhan, B. Osmium Tetroxide-Promoted Catalytic Oxidative Cleavage of Olefins: An Organometallic Ozonolysis. J. Am. Chem. Soc. 2002, 124, 3824–3825. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.N.; Carrilho, R.M.B.; Dias, L.D.; Viana, J.C.; Aquino, G.L.B.; Pineiro, M.; Pereira, M.M. Highly Efficient Rh(I)/Tris-Binaphthyl Monophosphite Catalysts for Hydroformylation of Sterically Hindered Alkyl Olefins. J. Mol. Catal. Chem. 2016, 416, 73–80. [Google Scholar] [CrossRef]
- Ren, B.; Wang, M.; Liu, J.; Ge, J.; Dong, H. Enhanced Basicity of Ag2O by Coordination to Soft Anions. ChemCatChem 2015, 7, 761–765. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Harrasi, A.; Green, I.R.; Ahmed, I.; Abbas, G.; Rehman, N.U. Meta-Chloroperbenzoic Acid (MCPBA): A Versatile Reagent in Organic Synthesis. RSC Adv. 2014, 4, 12882–12917. [Google Scholar] [CrossRef]
- Katz, S.J.; Bergmeier, S.C. Convenient Methods for the Hydrolysis of Oxazolidinones to Vicinal Aminoalcohols. Tetrahedron Lett. 2002, 43, 557–559. [Google Scholar] [CrossRef]
- Shivani, P.B.; Pujala, B.; Chakraborti, A.K. Zinc(II) Perchlorate Hexahydrate Catalyzed Opening of Epoxide Ring by Amines: Applications to Synthesis of (RS)/(R)-Propranolols and (RS )/(R)/(S)-Naftopidils. J. Org. Chem. 2007, 72, 3713–3722. [Google Scholar] [CrossRef]
- Azizi, N.; Mehrazma, S.; Saidi, M.R. A Simple, Highly Regioselective and Efficient Reaction of Indole with Epoxides under Solvent-Free Conditions. Can. J. Chem. 2006, 84, 800–803. [Google Scholar] [CrossRef]
- Azizi, N.; Mirmashhori, B.; Saidi, M.R. Lithium Perchlorate Promoted Highly Regioselective Ring Opening of Epoxides under Solvent-Free Conditions. Catal. Commun. 2007, 8, 2198–2203. [Google Scholar] [CrossRef]
- Upadhayaya, R.S.; Lahore, S.V.; Sayyed, A.Y.; Dixit, S.S.; Shinde, P.D.; Chattopadhyaya, J. Conformationally-Constrained Indeno[2,1-c]Quinolines—A New Class of Anti-Mycobacterial Agents. Org. Biomol. Chem. 2010, 8, 2180. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, Z.; Li, M.; Wang, C. K2CO3-promoted Ring-opening/Cyclization Reactions of Multi-substituted Donor-acceptor Cyclopropanes with Thiourea: Access to 2-amino-4,6-diarylnicotinonitrile Derivatives. ChemistrySelect 2020, 5, 6011–6015. [Google Scholar] [CrossRef]
- Gonda, T.; Szakonyi, Z.; Csámpai, A.; Haukka, M.; Fülöp, F. Stereoselective Synthesis and Application of Tridentate Aminodiols Derived from (+)-Pulegone. Tetrahedron Asymmetry 2016, 27, 480–486. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Csőr, Á.; Csámpai, A.; Fülöp, F. Stereoselective Synthesis and Modelling-Driven Optimisation of Carane-Based Aminodiols and 1,3-Oxazines as Catalysts for the Enantioselective Addition of Diethylzinc to Benzaldehyde. Chem. Eur. J. 2016, 22, 7163–7173. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Velasco, R.F.; Álvarez, J.A.; Castellano, D.; Galindo, J.C.G. Synthesis of Melampolides and Cis,Cis-Germacranolides as Natural Herbicide Models. Tetrahedron 2004, 60, 8477–8488. [Google Scholar] [CrossRef]
- Chen, K.; Baran, P.S. Total Synthesis of Eudesmane Terpenes by Site-Selective C–H Oxidations. Nature 2009, 459, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Sajiki, H.; Hattori, K.; Hirota, K. Highly Chemoselective Hydrogenation with Retention of the Epoxide Function Using a Heterogeneous Pd/C-Ethylenediamine Catalyst and THF. Chem. Eur. J. 2000, 6, 2200–2204. [Google Scholar] [CrossRef]
- Ley, S.V.; Stewart-Liddon, A.J.P.; Pears, D.; Perni, R.H.; Treacher, K. Hydrogenation of Aromatic Ketones, Aldehydes, and Epoxides with Hydrogen and Pd(0)EnCatTM 30NP. Beilstein J. Org. Chem. 2006, 2. [Google Scholar] [CrossRef]
- Rani, N.; Sharma, A.; Singh, R. Imidazoles as Promising Scaffolds for Antibacterial Activity: A Review. Mini-Rev. Med. Chem. 2013, 13, 1812–1835. [Google Scholar] [CrossRef]
- Borgers, M. Mechanism of Action of Antifungal Drugs, with Special Reference to the Imidazole Derivatives. Clin. Infect. Dis. 1980, 2, 520–534. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Zampieri, D.; Falagiani, V.; Vio, L.; Fermeglia, M.; Ferrone, M.; Pricl, S.; Banfi, E.; Scialino, G. Antifungal and Antimycobacterial Activity of New N1-[1-Aryl-2-(1H-Imidazol-1-Yl and 1H-1,2,4-Triazol-1-Yl)-Ethylidene]-Pyridine-2-Carboxamidrazone Derivatives: A Combined Experimental and Computational Approach. Arkivoc 2004, 2004, 231–250. [Google Scholar] [CrossRef]
- Weinstein, M.P. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 2 February 2021).
- Béni, Z.; Dékány, M.; Kovács, B.; Csupor-Löffler, B.; Zomborszki, Z.; Kerekes, E.; Szekeres, A.; Urbán, E.; Hohmann, J.; Ványolós, A. Bioactivity-Guided Isolation of Antimicrobial and Antioxidant Metabolites from the Mushroom Tapinella Atrotomentosa. Molecules 2018, 23, 1082. [Google Scholar] [CrossRef] [PubMed]


| Inhibitory Effect (%) ± RSD (%) | |||||||
|---|---|---|---|---|---|---|---|
| Gram Positive | Gram Negative | Yeast | |||||
| Analogue | Conc. (µg/mL) | B. subtilis SZMC0209 | S. aureus SZMC14611 | E. coli SZMC6271 | P. aeruginosa SZMC23290 | C. albicans SZMC1533 | C. krusei SZMC1352 |
| Nystatin | 100 | - | - | - | - | 93.38 ± 2.13 (100 µg/mL) | 92.01 ± 3.64 (100 µg/mL) |
| 10 | - | - | - | - | 92.88 ± 10.18 | 58.00 ± 9.21 | |
| Ampicillin | 100 | 95.22 ± 8.40 (<0.78 µg/mL) | 81.88 ± 8.99 (<0.78 µg/mL) | 94.07 ± 3.61 (100 µg/mL) | 29.03 ± 2.06 | - | - |
| 10 | 93.00 ± 3.20 | 70.37 ± 6.15 | 89.37 ± 0.39 | - | - | - | |
| 3 | 100 | 97.60 ± 6.42 (100 µg/mL) | 57.57 ± 9.93 | - | 49.10 ± 7.52 | - | - |
| 10 | 59.58 ± 8.06 | - | - | - | - | - | |
| 5a | 100 | 92.82 ± 4.69 (25 µg/mL) | 80.07 ± 2.21 (50 µg/mL) | - | 54.06 ± 9.08 | 91.56 ± 1.27 (>100 µg/mL) | 94.88 ± 2.18 (100 µg/mL) |
| 10 | 48.25 ± 6.16 | - | - | - | - | - | |
| 5b | 100 | 86.35 ± 1.88 (50 µg/mL) | 71.48 ± 1.28 | - | 46.66 ± 1.37 | 92.88 ± 2.63 (>100 µg/mL) | 93.62 ± 0.80 |
| 10 | 23.23 ± 3.15 | - | - | - | - | - | |
| 7a | 100 | 81.51 ± 4.73 (50 µg/mL) | 70.66 ± 0.91 | - | - | 87.90 ± 10.46 (>100 µg/mL) | - |
| 10 | - | - | - | - | - | - | |
| 7b | 100 | 95.34 ± 4.81 (50 µg/mL) | 92.34 ± 1.32 (100 µg/mL) | - | 41.59 ± 3.53 | - | - |
| 10 | 50.00 ± 7.21 | - | - | - | - | - | |
| 10 | 100 | 95.16 ± 2.81 (100 µg/mL) | 90.71 ± 3.27 (100 µg/mL) | - | 50.87 ± 9.72 | 95.91 ± 16.31 (>100 µg/mL) | - |
| 10 | 55.43 ± 15.48 | - | - | 44.05 ± 7.57 | - | - | |
| 12a | 100 | 95.16 ± 6.46 (100 µg/mL) | - | - | 70.85 ± 6.49 | 95.83 ± 11.18 (>100 µg/mL) | - |
| 10 | 73.41 ± 5.45 | - | - | 47.81 ± 7.92 | - | - | |
| 12b | 100 | 91.84 ± 6.01 (100 µg/mL) | 83.11 ± 2.61 (100 µg/mL) | 50.07 ± 10.97 | 75.84 ± 7.14 | 94.50 ± 0.97 (>100 µg/mL) | 67.59 ± 16.45 |
| 10 | 32.17 ± 11.19 | - | - | 58.24 ± 4.20 | - | - | |
| 14a | 100 | 92.67 ± 3.90 (100 µg/mL) | 82.35 ± 3.19 (100 µg/mL) | - | 52.97 ± 7.47 | - | - |
| 10 | - | - | - | 44.00 ± 1.32 | - | - | |
| 20a | 100 | 84.57 ± 3.18 (6.25 µg/mL) | 70.13 ± 0.90 | - | - | 91.35 ± 1.07 (>100 µg/mL) | - |
| 10 | 89.70 ± 1.32 | 65.81 ± 0.51 | - | - | - | - | |
| 20b | 100 | 78.34 ± 2.51 | 69.49 ± 0.57 | - | - | 90.74 ± 2.90 (>100 µg/mL) | 79.88 ± 3.39 |
| 10 | 78.43 ± 5.39 | 61.84 ± 0.27 | - | - | 80.54 ± 17.23 | - | |
| 22a | 100 | 83.44 ± 20.97 | 76.39 ± 1.13 | - | - | - | - |
| 10 | 81.63 ± 1.22 (25 µg/mL) | 70.02 ± 1.01 | - | - | - | - | |
| 22b | 100 | 78.43 ± 10.14 (<0.78 µg/mL) | 60.32 ± 1.11 | - | - | 81.97 ± 4.00 (>100 µg/mL) | - |
| 10 | 81.01 ± 1.08 | 62.77 ± 0.27 | - | - | 61.02 ± 6.51 | - | |
| 23a | 100 | 73.83 ± 4.14 (<0.78 µg/mL) | 73.99 ± 5.15 | - | 47.92 ± 1.67 | - | - |
| 10 | 83.29 ± 5.94 | - | - | 47.78 ± 3.40 | - | - | |
| 23b | 100 | 75.64 ± 0.21 | 71.95 ± 4.38 | - | 46.03 ± 2.10 | - | - |
| 10 | 77.54 ± 5.94 | - | - | 42.22 ± 1.49 | - | - | |
| 25a | 100 | 78.96 ± 0.88 | - | - | - | - | - |
| 10 | - | - | - | - | - | - | |
| 27a | 100 | 71.13 ± 4.78 | - | - | 43.48 ± 3.42 | - | - |
| 10 | - | - | - | 38.95 ± 9.32 | - | - | |
| 27b | 100 | - | - | - | 34.19 ± 6.00 | 80.58 ± 12.34 (>100 µg/mL) | - |
| 10 | - | - | - | 33.16 ± 8.01 | - | - | |
| 31a | 100 | 95.13 ± 9.21 (100 µg/mL) | 82.58 ± 10.08 (>100 µg/mL) | - | 48.38 ± 1.94 | - | - |
| 10 | 12.76 ± 9.95 | - | - | 32.10 ± 3.98 | - | - | |
| 31b | 100 | 93.89 ± 5.51 (21 µg/mL) | 86.85 ± 4.00 (50 µg/mL) | - | 53.31 ± 4.84 | 95.21 ± 3.59 (100 µg/mL) | - |
| 10 | 47.83 ± 9.92 | - | - | 47.81 ± 6.60 | - | - | |
| 39a | 100 | 79.38 ± 4.19 (3.13 µg/mL) | 63.47 ± 4.90 | - | - | 88.22 ± 3.96 (>100 µg/mL) | - |
| 10 | 82.73 ± 0.52 | 69.84 ± 0.00 | - | - | - | - | |
| 39b | 100 | 87.80 ± 7.04 (1.56 µg/mL) | 79.66 ± 2.59 (3.13 µg/mL) | - | 48.09 ± 1.38 | 90.89 ± 13.31 (>100 µg/mL) | 91.08 ± 4.90 (>100 µg/mL) |
| 10 | 92.94 ± 1.46 | 83.69 ± 38.18 | - | 33.59 ± 6.43 | 85.10 ± 9.56 | - | |
| 40a | 100 | 72.88 ± 1.68 | 68.26 ± 1.66 | - | 37.66 ± 2.39 | - | - |
| 10 | 55.43 ± 5.07 | - | - | 38.89 ± 1.13 | - | - | |
| 40b | 100 | 71.39 ± 3.84 | 69.37 ± 1.44 | - | 42.13 ± 2.25 | - | - |
| 10 | 65.82 ± 4.56 | - | - | 39.58 ± 0.73 | - | - | |
| 42a | 100 | 69.05 ± 10.02 | - | - | - | - | - |
| 10 | 51.75 ± 11.13 | - | - | - | - | - | |
| 42b | 100 | 86.62 ± 8.48 (>100 µg/mL) | 66.22 ± 4.03 | - | - | - | - |
| 10 | 43.95 ± 5.65 | - | - | - | - | - | |
| 43b | 100 | - | - | - | - | 80.90 ± 4.76 (>100 µg/mL) | - |
| 10 | - | - | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.M.; Huynh, T.; Bamou, F.Z.; Szekeres, A.; Fülöp, F.; Szakonyi, Z. Novel (+)-Neoisopulegol-Based O-Benzyl Derivatives as Antimicrobial Agents. Int. J. Mol. Sci. 2021, 22, 5626. https://doi.org/10.3390/ijms22115626
Le TM, Huynh T, Bamou FZ, Szekeres A, Fülöp F, Szakonyi Z. Novel (+)-Neoisopulegol-Based O-Benzyl Derivatives as Antimicrobial Agents. International Journal of Molecular Sciences. 2021; 22(11):5626. https://doi.org/10.3390/ijms22115626
Chicago/Turabian StyleLe, Tam Minh, Thu Huynh, Fatima Zahra Bamou, András Szekeres, Ferenc Fülöp, and Zsolt Szakonyi. 2021. "Novel (+)-Neoisopulegol-Based O-Benzyl Derivatives as Antimicrobial Agents" International Journal of Molecular Sciences 22, no. 11: 5626. https://doi.org/10.3390/ijms22115626
APA StyleLe, T. M., Huynh, T., Bamou, F. Z., Szekeres, A., Fülöp, F., & Szakonyi, Z. (2021). Novel (+)-Neoisopulegol-Based O-Benzyl Derivatives as Antimicrobial Agents. International Journal of Molecular Sciences, 22(11), 5626. https://doi.org/10.3390/ijms22115626









