Evolution and Application of Genome Editing Techniques for Achieving Food and Nutritional Security
Abstract
1. Introduction
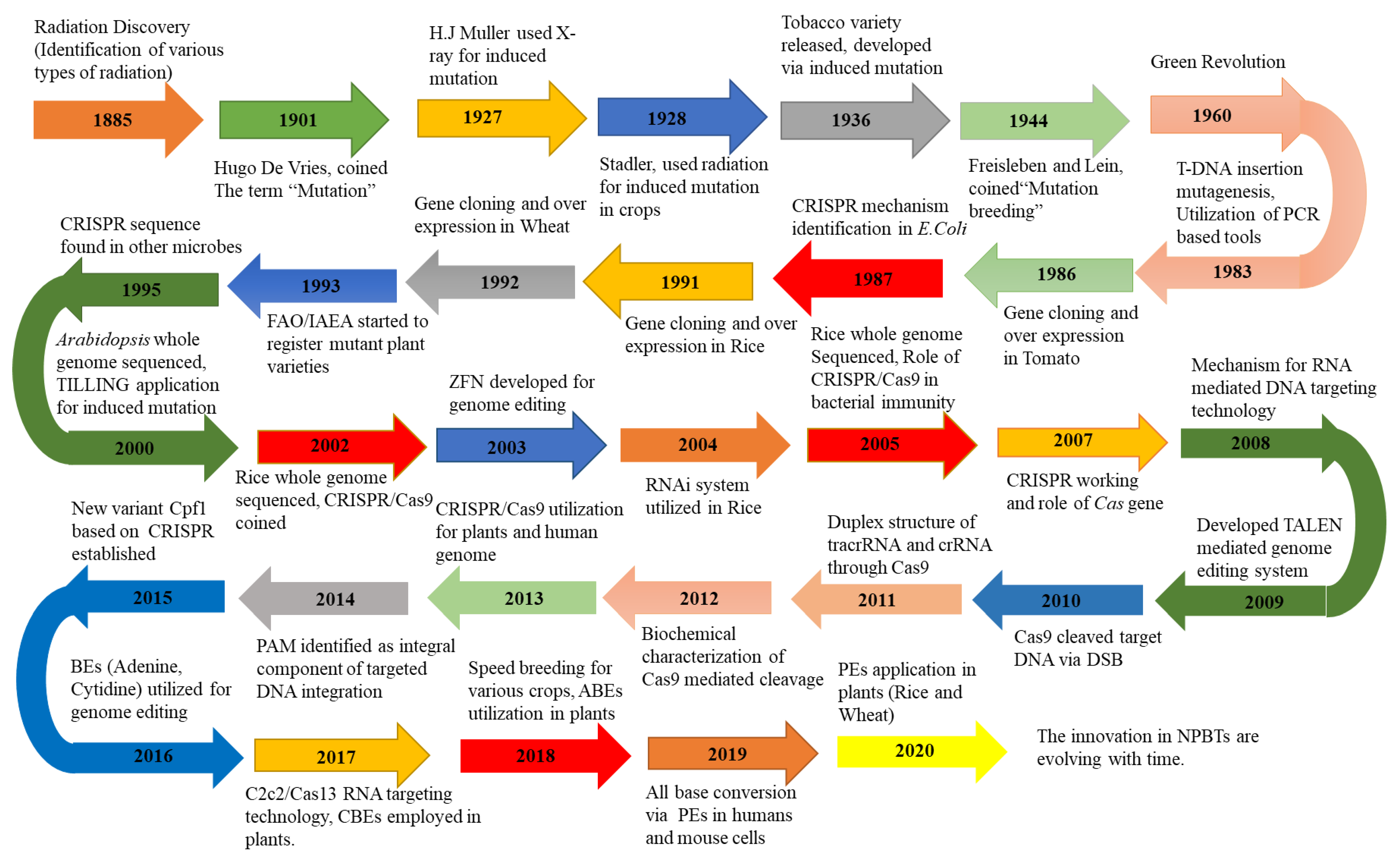
2. Evolution of GETs
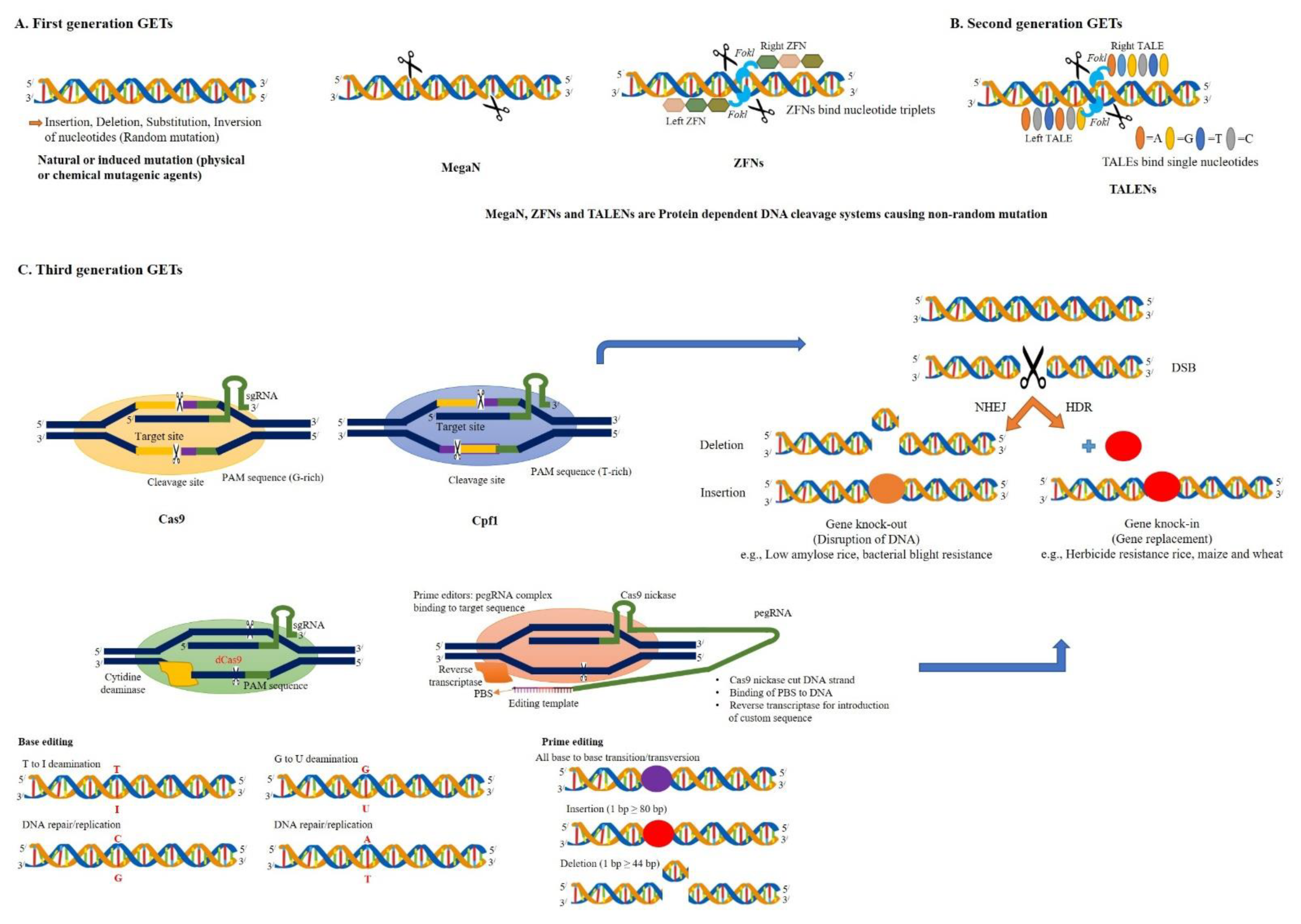
2.1. Third-Generation GETs
2.1.1. CRISPR/Cas9 System
2.1.2. CRISPR/Cpf1 System
2.1.3. BE System
2.1.4. PE System
3. Application of GETs in Agriculture to Ensure Food Security
3.1. GETs for Crop Improvements
3.1.1. The CRISPR/Cas9 System–Proof of Concept for Crop Improvement
3.1.2. The CRISPR/Cpf1 System–a Proof of Concept for Crop Improvement
3.1.3. The BE System–a Proof of Concept for Crop Improvement
3.1.4. The PE System–a Proof of Concept for Crop Improvement
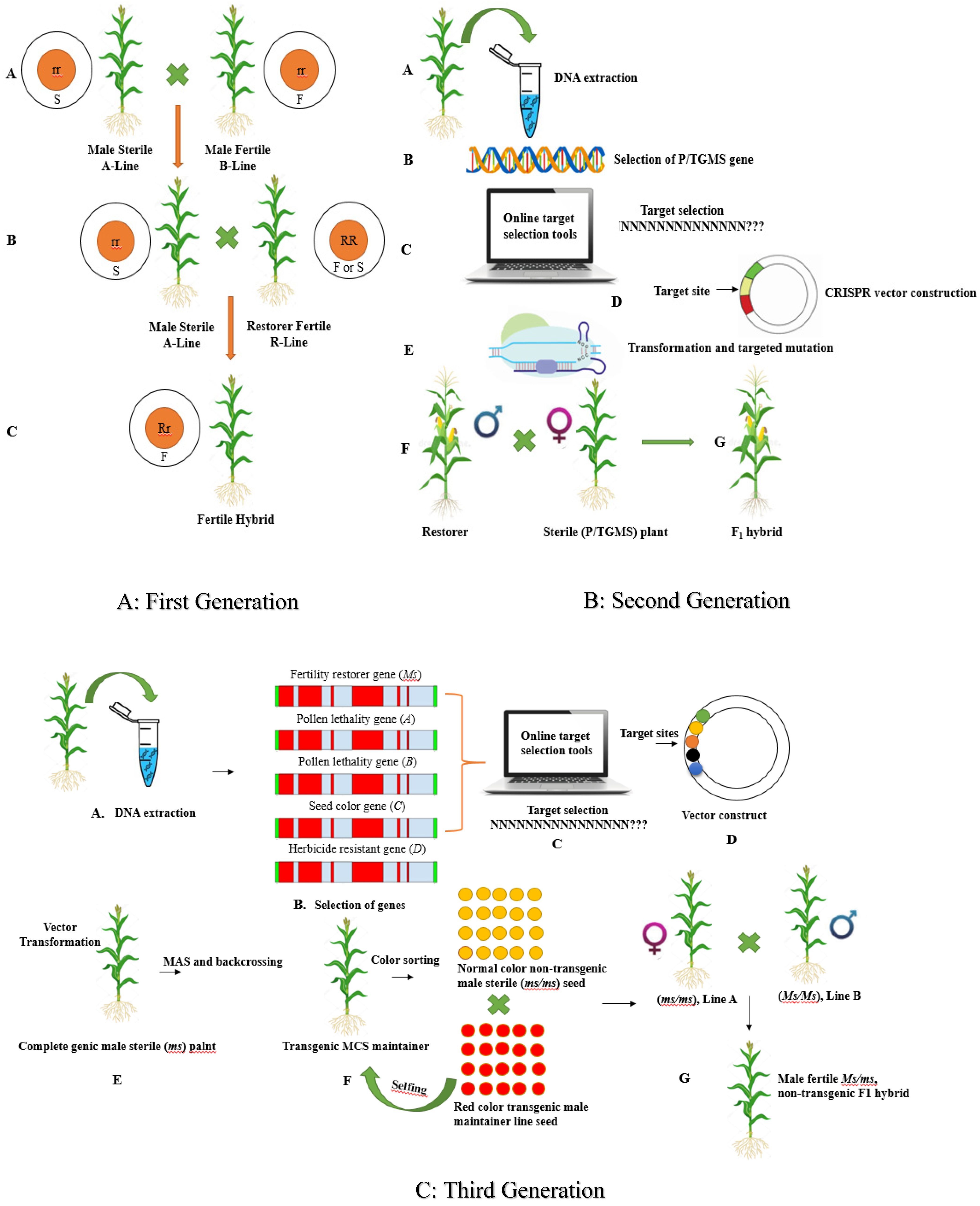
3.2. GETs for Hybrid Seed Production
3.2.1. First Generation Hybrid Development System
3.2.2. Second-Generation Hybrid Development System
3.2.3. Multi-Control Sterility (Third-Generation) Hybrid Development System
3.2.4. Induced Apomixis through Genome Editing to Preserve Heterosis
4. GETs for Improved Grain Quality
GETs Proof of Concept for Grain Quality Improvement
5. Multiplex Genome Editing for Complex Traits
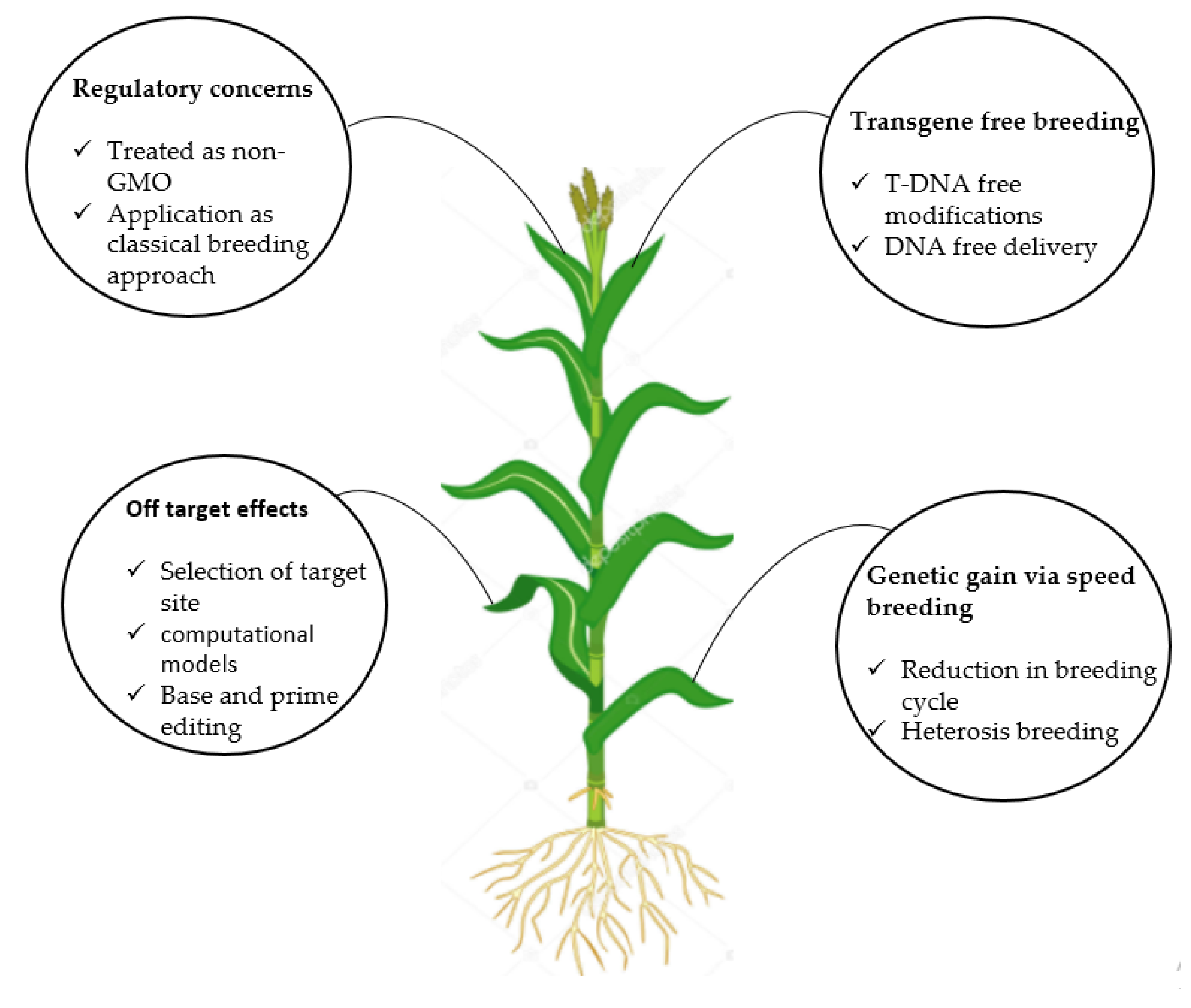
6. Challenges and Future Perspectives
6.1. Regulatory Concerns Regarding Genome Editing for Crops and Derived Products
6.2. Transgene-Free Breeding
6.3. Off-Target Effects
6.4. Genetic Gain Through Speed Breeding
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World: Safeguarding against Economic Slowdowns and Downturns; Food and Agriculture Organization: Quebec City, QC, Canada, 2019. [Google Scholar]
- Fanzo, J.; Hawkes, C.; Udomkesmalee, E.; Afshin, A.; Allemandi, L.; Assery, O.; Chen, K. 2018 Global Nutrition Report: Shining a Light to Spur Action on Nutrition; Development Initiatives Poverty Research Ltd.: Bristol, UK, 2018. [Google Scholar]
- UN. World Population Prospects; United Nations Publications: New York, NY, USA, 2012. [Google Scholar]
- Baulcombe, D. Reaping Benefits of Crop Research. Science 2010, 327, 761. [Google Scholar] [CrossRef] [PubMed]
- Meemken, E.-M.; Qaim, M. Organic Agriculture, Food Security, and the Environment. Annu. Rev. Resour. Econ. 2018, 10, 39–63. [Google Scholar] [CrossRef]
- Bhargava, A.; Srivastava, S. Participatory Plant Breeding: Concept and Applications; Springer: Berlin, Germany, 2019. [Google Scholar]
- Sedeek, K.E.M.; Mahas, A.; Mahfouz, M. Plant Genome Engineering for Targeted Improvement of Crop Traits. Front. Plant Sci. 2019, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Barman, H.N.; Sheng, Z.; Fiaz, S.; Zhong, M.; Wu, Y.; Cai, Y.; Wei, X. Generation of a new ther-mo-sensitive genic male sterile rice line by targeted mutagenesis of TMS5 gene through CRISPR/Cas9 system. BMC Plant Biol. 2019, 19, 109. [Google Scholar] [CrossRef]
- Voss-Fels, K.P.; Stahl, A.; Wittkop, B.; Lichthardt, C.; Nagler, S.; Rose, T.; Chen, T.-W.; Zetzsche, H.; Seddig, S.; Baig, M.M.; et al. Breeding improves wheat productivity under contrasting agrochemical input levels. Nat. Plants 2019, 5, 706–714. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chakraborty, N.; Agrawal, L.; Ghosh, S.; Narula, K.; Shekhar, S.; Datta, A. Next-generation protein-rich potato expressing the seed protein gene AmA1 is a result of proteome rebalancing in transgenic tuber. Proc. Nat. Acad. Sci. USA 2010, 107, 17533–17538. [Google Scholar] [CrossRef]
- Mugode, L.; Ha, B.; Kaunda, A.; Sikombe, T.; Phiri, S.; Mutale, R.; De Moura, F.F. Carotenoid re-tention of biofortified provitamin A maize (Zea mays L.) after Zambian traditional methods of milling, cooking and storage. J. Agric. Food Chem. 2014, 62, 6317–6325. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babili, S.; Klöti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef]
- Datta, A. Genetic engineering for improving quality and productivity of crops. Agric. Food Secur. 2013, 2, 1–3. [Google Scholar] [CrossRef]
- Fiaz, S.; Wang, X.; Younas, A.; Alharthi, B.; Riaz, A.; Ali, H. Apomixis and strategies to induce apomixis to preserve hybrid vigor for multiple generations. GM Crop. Food 2020, 12, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Fiaz, S.; Ahmad, S.; Noor, M.A.; Wang, X.; Younas, A.; Riaz, A.; Riaz, A.; Ali, F. Applications of the CRISPR/Cas9 System for Rice Grain Quality Improvement: Perspectives and Opportunities. Int. J. Mol. Sci. 2019, 20, 888. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Zhu, H. CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Hortic. Res. 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of Genetic Diversity Assessment in Crop Plants and Its Recent Advances: An Overview of Its Analytical Perspectives. Genet. Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hartung, F.; Schiemann, J. Precise plant breeding using new genome editing techniques: Opportunities, safety and regulation in the EU. Plant J. 2014, 78, 742–752. [Google Scholar] [CrossRef]
- Čermák, T.; Baltes, N.J.; Čegan, R.; Zhang, Y.; Voytas, D.F. High-frequency, precise modification of the tomato genome. Genome Biol. 2015, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Voytas, D.F.; Gao, C. Precision Genome Engineering and Agriculture: Opportunities and Regulatory Challenges. PLoS Biol. 2014, 12, e1001877. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 2014, 15, 321–334. [Google Scholar] [CrossRef]
- Ain, Q.U.; Chung, J.Y.; Kim, Y.H. Current and future delivery systems for engineered nucleases: ZFN, TALEN and RGEN. J. Control. Release 2015, 205, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Shen, X.Z.; Jiang, F.; Wu, Y.; Han, C. DNA-guided genome editing using the Natronobacterium gregoryi Argonaute. Nat. Biotechnol. 2016, 34, 768–773. [Google Scholar] [CrossRef]
- Safari, F.; Zare, K.; Negahdaripour, M.; Barekati-Mowahed, M.; Ghasemi, Y. CRISPR Cpf1 proteins: Structure, function and implications for genome editing. Cell Biosci. 2019, 9, 36. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Komor, A.C.; Levy, J.M.; Packer, M.S.; Zhao, K.T.; Liu, D.R. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol. 2017, 35, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Zafra, M.P.; Schatoff, E.M.; Katti, A.; Foronda, M.; Breinig, M.; Schweitzer, A.Y.; Simon, A.; Han, T.; Goswami, S.; Montgomery, E.; et al. Optimized base editors enable efficient editing in cells, organoids and mice. Nat. Biotechnol. 2018, 36, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nat. Cell Biol. 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Wolabu, T.W.; Park, J.J.; Chen, M.; Cong, L.; Ge, Y.; Jiang, Q.; Debnath, S.; Li, G.; Wen, J.; Wang, Z. Improving the genome editing efficiency of CRISPR/Cas9 in Arabidopsis and Medicago truncatula. Planta 2020, 252, 1–14. [Google Scholar] [CrossRef]
- Manghwar, H.; Li, B.; Ding, X.; Hussain, A.; Lindsey, K.; Zhang, X.; Jin, S. CRISPR/Cas Systems in Genome Editing: Methodologies and Tools for sgRNA Design, Off-Target Evaluation, and Strategies to Mitigate Off-Target Effects. Adv. Sci. 2020, 7, 1902312. [Google Scholar] [CrossRef]
- Jiang, W.; Bikard, D.; Cox, D.; Zhang, F.; Marraffini, L.A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, S.; Kim, J.M.; Kim, J.S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nature Biotechnol. 2013, 31, 230–232. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Lowder, L.G.; Zhang, D.; Baltes, N.J.; Paul, J.W.; Tang, X.; Zheng, X.; Voytas, D.F.; Hsieh, T.-F.; Zhang, Y.; Qi, Y. A CRISPR/Cas9 Toolbox for Multiplexed Plant Genome Editing and Transcriptional Regulation. Plant Physiol. 2015, 169, 971–985. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 Is a Single RNA-Guided Endonuclease of a Class 2 CRISPR-Cas System. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.B.; Lee, J.M.; Kang, J.G.; Lee, N.E.; Ha, D.I.; Kim, S.H.; Kim, Y.S. Highly efficient genome editing by CRISPR-Cpf1 using CRISPR RNA with a uridinylate-rich 3′-overhang. Nat. Commun. 2018, 9, 1–11. [Google Scholar]
- Liu, Y.; Han, J.; Chen, Z.; Wu, H.; Dong, H.; Nie, G. Engineering cell signaling using tunable CRISPR–Cpf1-based transcription factors. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Cheng, Q.; Zheng, X.; Zhao, G.; Wang, J. Multiplex gene regulation by CRISPR-ddCpf1. Cell Discov. 2017, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Hager, M.; Brant, E.; Budak, H. Efficient genome editing in wheat using Cas9 and Cpf1 (AsCpf1 and LbCpf1) nucleases. Funct. Integr. Genomics 2021. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nat. Cell Biol. 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Yin, K.; Gao, C.; Qiu, J.L. Progress and prospects in plant genome editing. Nat. Plants 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Marx, V. Base editing a CRISPR way. Nat. Chem. Biol. 2018, 15, 767–770. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A• T to G• C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef]
- Khanday, I.; Skinner, D.; Yang, B.; Mercier, R.; Sundaresan, V. A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nat. Cell Biol. 2019, 565, 91–95. [Google Scholar] [CrossRef]
- Eid, A.; AlShareef, S.; Mahfouz, M.M. CRISPR base editors: Genome editing without double-stranded breaks. Biochem. J. 2018, 475, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Fiaz, S.; Noor, M.A.; Aldosri, F.O. Achieving food security in the Kingdom of Saudi Arabia through innovation: Potential role of agricultural extension. J. Saudi Soc. Agric. Sci. 2018, 17, 365–375. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhou, Z.; Wu, P.; Fang, M.; Pan, X.; Lin, Q.; Luo, W.; Wu, G.; Li, H. Reassessment of the Four Yield-related Genes Gn1a, DEP1, GS3, and IPA1 in Rice Using a CRISPR/Cas9 System. Front. Plant Sci. 2016, 7, 377. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Y.; Qin, R.; Li, H.; Qiu, C.; Li, L.; Wei, P.; Yang, J. Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J. Genet. Genom. 2016, 43, 529–532. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.G.; Zhao, K. Enhanced Rice Blast Resistance by CRISPR/Cas9-Targeted Mutagenesis of the ERF Transcription Factor Gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, H.; Chern, M.; Yin, J.; Chen, Y.; Wang, J.; Zhu, X.; Chen, Z.; Yuan, C.; Zhao, W.; et al. Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 3174–3179. [Google Scholar] [CrossRef]
- Li, Z. Health risk characterization of maximum legal exposures for persistent organic pollutant (POP) pesticides in residential soil: An analysis. J. Environ. Manag. 2018, 205, 163–173. [Google Scholar] [CrossRef]
- Xu, R.; Li, H.; Qin, R.; Wang, L.; Li, L.; Wei, P.; Yang, J. Gene targeting using the Agrobacterium tumefaciens-mediated CRISPR-Cas system in rice. Rice 2014, 7, 5. [Google Scholar] [CrossRef]
- Tang, L.; Mao, B.; Li, Y.; Lv, Q.; Zhang, L.; Chen, C.; He, H.; Wang, W.; Zeng, X.; Shao, Y.; et al. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Gao, C. Genome editing in rice and wheat using the CRISPR/Cas system. Nat. Protoc. 2014, 9, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Gil-Humanes, J.; Wang, Y.; Liang, Z.; Shan, Q.; Ozuna, C.V.; León, S.S.; Baltes, N.J.; Starker, C.; Barro, F.; Gao, C.; et al. High-efficiency gene targeting in hexaploid wheat using DNA replicons and CRISPR/Cas9. Plant J. 2017, 89, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Alptekin, B.; Budak, H. CRISPR/Cas9 genome editing in wheat. Funct. Integr. Genom. 2017, 18, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, K.; Zhang, Y.; Liu, J.; Yin, K.; Qiu, J.-L.; Gao, C. Genome editing of bread wheat using biolistic delivery of CRISPR/Cas9 in vitro transcripts or ribonucleoproteins. Nat. Protoc. 2018, 13, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhang, K.; Chen, K.; Gao, C. Targeted Mutagenesis in Zea mays Using TALENs and the CRISPR/Cas System. J. Genet. Genom. 2014, 41, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS 8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef]
- Endo, A.; Masafumi, M.; Kaya, H.; Toki, S. Efficient targeted mutagenesis of rice and tobacco genomes using Cpf1 from Francisella novicida. Sci. Rep. 2016, 6, 38169. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.T.; Ryu, J.; Kang, B.C.; Kim, J.S. CRISPR/Cpf1-mediated DNA-free plant genome editing. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- Lee, K.; Zhang, Y.; Kleinstiver, B.P.; Guo, J.A.; Aryee, M.J.; Miller, J.; Joung, J.K. Activities and specificities of CRISPR/Cas9 and Cas12a nucleases for targeted mutagenesis in maize. Plant Biotechnol. J. 2019, 17, 362–372. [Google Scholar] [CrossRef]
- Tang, X.; Lowder, L.G.; Zhang, T.; Malzahn, A.A.; Zheng, X.; Voytas, D.F.; Zhong, Z.; Chen, Y.; Ren, Q.; Li, Q.; et al. A CRISPR–Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Wang, M.; Mao, Y.; Lu, Y.; Tao, X.; Zhu, J.-K. Multiplex Gene Editing in Rice Using the CRISPR-Cpf1 System. Mol. Plant 2017, 10, 1011–1013. [Google Scholar] [CrossRef]
- Xu, R.; Qin, R.; Li, H.; Li, J.; Yang, J.; Wei, P. Enhanced genome editing in rice using single transcript unit CRISPR-LbCpf1 systems. Plant Biotechnol. J. 2019, 17, 553. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Biswal, A.K.; Dionora, J.; Perdigon, K.M.; Balahadia, C.P.; Mazumdar, S.; Chater, C.; Lin, H.C.; Coe, R.A.; Kretzschmar, T.; et al. CRISPR-Cas9 and CRISPR-Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant Cell Rep. 2017, 36, 745–757. [Google Scholar] [CrossRef]
- Begemann, M.B.; Gray, B.N.; January, E.; Gordon, G.C.; He, Y.; Liu, H.; Oufattole, M. Precise insertion and guided editing of higher plant genomes using Cpf1 CRISPR nucleases. Sci. Rep. 2017, 7, 11606. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, J.K. Precise Editing of a Target Base in the Rice Genome Using a Modified CRISPR/Cas9 System. Mol. Plant 2017, 10, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Yan, F.; Kuang, Y.; Li, N.; Zhang, D.; Zhou, X.; Zhou, H. Improved base editor for effi-ciently inducing genetic variations in rice with CRISPR/Cas9-guided hyperactive hAID mutant. Mol. Plant 2018, 11, 623–626. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, Y.; You, Q.; Tang, X.; Ren, Q.; Liu, S.; Yang, L.; Wang, Y.; Liu, X.; Liu, B.; et al. Plant genome editing using FnCpf1 and LbCpf1 nucleases at redefined and altered PAM sites. Mol. Plant 2018, 11, 999–1002. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zong, M. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant. Cell Rep. 2018, 37, 1353–1356. [Google Scholar] [CrossRef]
- Zhang, Y.; Massel, K.; Godwin, I.D.; Gao, C. Correction to: Applications and potential of genome editing in crop improvement. Genome Biol. 2019, 19, 2l0. [Google Scholar] [CrossRef]
- Qin, R.; Li, J.; Li, H.; Zhang, Y.; Liu, X.; Miao, Y.; Wei, P. Developing a highly efficient and wildly adaptive CRISPR-SaCas9 toolset for plant genome editing. Plant. Biotechnol. J. 2019, 17, 706. [Google Scholar] [CrossRef]
- Li, C.; Zong, Y.; Wang, Y.; Jin, S.; Zhang, D.; Song, Q.; Zhang, R.; Gao, C. Expanded base editing in rice and wheat using a Cas9-adenosine deaminase fusion. Genome Biol. 2018, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Tao, X.; Zhu, J.K. Expanding the base editing scope in rice by using Cas9 variants. Plant Biotechnol. J. 2018, 17, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K.; Kaya, H.; Abe, K.; Hara, N.; Saika, H.; Toki, S. An adenine base editor with expanded targeting scope using SpCas9-NGv1 in rice. Plant Biotechnol. J. 2019, 17, 1476. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zong, Y.; Gao, Q.; Zhu, Z.; Wang, Y.; Qin, P.; Liang, C.; Wang, D.; Qiu, J.-L.; Zhang, F.; et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science 2019, 364, 292–295. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Mishra, R.; Joshi, R.K.; Zhao, K. Base editing in crops: Current advances, limitations and future implications. Plant Biotechnol. J. 2020, 18, 20–31. [Google Scholar] [CrossRef]
- Wang, X.; Ding, C.; Yu, W.; Wang, Y.; He, S.; Yang, B.; Liang, J. Cas12a Base Editors Induce Efficient and Specific Editing with Low DNA Damage Response. Cell Rep. 2020, 31, 107723. [Google Scholar] [CrossRef]
- Lin, Q.; Zong, Y.; Xue, C.; Wang, S.; Jin, S.; Zhu, Z.; Wang, Y.; Anzalone, A.V.; Raguram, A.; Doman, J.L.; et al. Prime genome editing in rice and wheat. Nat. Biotechnol. 2020, 38, 582–585. [Google Scholar] [CrossRef]
- Xu, R.; Li, J.; Lu, X.; Shan, T.; Qin, R.; Wei, P. Development of Plant Prime-Editing Systems for Precise Genome Editing. Plant Commun. 2020, 3, 100043. [Google Scholar] [CrossRef]
- Sheng, Z.; Fiaz, S.; Li, Q.; Chen, W.; Wei, X.; Xie, L.; Jiao, G.; Shao, G.; Tang, S.; Wang, J.; et al. Molecular breeding of fragrant early-season hybrid rice using the BADH2 gene. Pak. J. Bot. 2019, 51, 2089–2095. [Google Scholar] [CrossRef]
- Wright, H. Commercial Hybrid Seed Production. In Hybridization of Crop Plants; American Society of Agronomy Inc.: Madison, WI, USA, 2015; pp. 161–176. [Google Scholar]
- Ali, U.; Zhong, M.; Shar, T.; Fiaz, S.; Xie, L.; Jiao, G.; Ahmad, S.; Sheng, Z.; Tang, S.; Wei, X.; et al. The influence of Ph on Cadmium Accumulation in seedlings of Rice (Oryza sativa L.). J. Plant Gro. Reg. 2020, 39, 930–940. [Google Scholar] [CrossRef]
- Horner, H.T.; Palmer, R.G. Mechanisms of Genic Male Sterility. Crop. Sci. 1995, 35, 1527–1535. [Google Scholar] [CrossRef]
- Havey, M.J. The use of cytoplasmic male sterility for hybrid seed production. In Molecular Biology and Biotechnology of Plant Organelles; Springer: Berlin, Germany, 2004; pp. 623–634. [Google Scholar]
- Ma, G.H.; Yuan, L.P. Hybrid rice achievements, development and prospect in China. J. Integr. Agric. 2015, 14, 197–205. [Google Scholar] [CrossRef]
- Cheng, S.H.; Zhuang, J.Y.; Fan, Y.Y.; Du, J.H.; Cao, L.Y. Progress in Research and Development on Hybrid Rice: A Super-domesticate in China. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef]
- Williams, M.; Leemans, J. Maintenance of Male-Sterile Plants. Google Patents US5750867A, 12 May 1998. [Google Scholar]
- Zhang, Q.; Shen, B.Z.; Dai, X.K.; Mei, M.H.; Maroof, M.S.; Li, Z.B. Using bulked extremes and recessive class to map genes for photoperiod-sensitive genic male sterility in rice. Proc. Natl. Acad. Sci. USA 1994, 91, 8675–8679. [Google Scholar] [CrossRef]
- Ding, J.; Lu, Q.; Ouyang, Y.; Mao, H.; Zhang, P.; Yao, J.; Xu, C.; Li, X.; Xiao, J.; Zhang, Q. A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc. Natl. Acad. Sci. USA 2012, 109, 2654–2659. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, X.; Shao, Z.; Wei, Z.; Wang, Y.; Zhu, L.; Zhao, J.; Sun, M.; He, R.; He, G. Rice UDP-Glucose Pyrophosphorylase1 Is Essential for Pollen Callose Deposition and Its Cosuppression Results in a New Type of Thermosensitive Genic Male Sterility. Plant Cell 2007, 19, 847–861. [Google Scholar] [CrossRef]
- Zhang, H.L.; Chen, X.Y.; Huang, J.Z.; Zhi, E.G.; Gong, J.Y.; Shu, Q.Y. Identification and transition analysis of photo-/thermo-sensitive genic male sterile genes in two-line hybrid rice in China. Sci. Agric. Sin. 2015, 48, 1–9. [Google Scholar]
- Zhou, H.; He, M.; Li, J.; Chen, L.; Huang, Z.; Zheng, S.; Zhu, L.; Ni, E.; Jiang, D.; Zhao, B.; et al. Development of commercial thermo-sensitive genic male sterile rice accelerates hybrid rice breeding using the CRISPR/Cas9-mediated TMS5 editing system. Sci. Rep. 2016, 6, 37395. [Google Scholar] [CrossRef]
- Li, H.; Pinot, F.; Sauveplane, V.; Werck-Reichhart, D.; Diehl, P.; Schreiber, L.; Gao, Y. Cytochrome P450 family member CYP704B2 catalyzes the ω-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell 2010, 22, 173–190. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, Z.; Wang, N.; Xie, G.; Lu, J.; Yan, W.; Zhou, J.; Tang, X.; Deng, X.W. Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc. Natl. Acad. Sci. USA 2016, 113, 14145–14150. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, M.P.A.; Jolivet, S.; Ravi, M.; Pereira, L.; Davda, J.N.; Cromer, L.; Wang, L.; Nogué, F.; Chan, S.W.L.; Siddiqi, I.; et al. Synthetic Clonal Reproduction Through Seeds. Science 2011, 331, 876. [Google Scholar] [CrossRef] [PubMed]
- D’Erfurth, I.; Jolivet, S.; Froger, N.; Catrice, O.; Novatchkova, M.; Mercier, R. Turning Meiosis into Mitosis. PLoS Biol. 2009, 7, e1000124. [Google Scholar] [CrossRef] [PubMed]
- Mieulet, D.; Jolivet, S.; Rivard, M.; Cromer, L.; Vernet, A.; Mayonove, P.; Pereira, L.; Droc, G.; Courtois, B.; Guiderdoni, E.; et al. Turning rice meiosis into mitosis. Cell Res. 2016, 26, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. Fixation of hybrid vigor in rice: Synthetic apomixis generated by genome editing. aBIOTECH 2019, 1, 15–20. [Google Scholar] [CrossRef]
- Kelliher, T.; Starr, D.; Richbourg, L.; Chintamanani, S.; Delzer, B.; Nuccio, M.L.; Green, J.; Chen, Z.; McCuiston, J.; Wang, W.; et al. MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nat. Cell Biol. 2017, 542, 105–109. [Google Scholar] [CrossRef]
- Li, X.; Meng, D.; Chen, S.; Luo, H.; Zhang, Q.; Jin, W.; Yan, J. Single nucleus sequencing reveals spermatid chromosome fragmentation as a possible cause of maize haploid induction. Nat. Commun. 2017, 8, 991. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dusi, D.M.D.A.; Irsigler, A.; Gomes, A.C.M.M.; Mendes, M.A.; Colombo, L.; Carneiro, V.T.D.C. GID1 expression is associated with ovule development of sexual and apomictic plants. Plant Cell Rep. 2017, 37, 293–306. [Google Scholar] [CrossRef]
- Shar, T.; Sheng, Z.; Ali, U.; Fiaz, S.; Wei, X.; Xie, L.; Jiao, G.; Ali, F.; Shao, G.; Hu, S.; et al. Mapping quantitative trait loci associated with paste viscosity attributes in double haploid population of rice (Oryza sativa L.). J. Integra. Agri. 2019, 18, 2–14. [Google Scholar]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot Plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Botella, J.R.; Zhu, J.-K. Generation of new glutinous rice by CRISPR/Cas9-targeted mutagenesis of the Waxy gene in elite rice varieties. J. Integr. Plant Biol. 2018, 60, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Y.; Du, J.; Zhao, Y.; Xia, L. Generation of Targeted Point Mutations in Rice by a Modified CRISPR/Cas9 System. Mol. Plant 2017, 10, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Xie, L.; Jiao, G.; Wei, X.; Sheng, Z.; Tang, S.; Hu, P. CRISPR/CAS9-mediated editing of the fragrant gene Badh2 in rice. Chin. J. Rice Sci. 2017, 31, 216–222. [Google Scholar]
- Sánchez-León, S.; Gil-Humanes, J.; Ozuna, C.V.; Giménez, M.J.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Zhang, D.; Zhao, X.; Cao, X.; Dong, L.; Liu, J.; Chen, K.; Zhang, H.; Gao, C.; et al. Analysis of the functions of TaGW2 homoeologs in wheat grain weight and protein content traits. Plant J. 2018, 94, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Qi, W.; Wang, Q.; Feng, Y.; Yang, Q.; Zhang, N.; Wang, S.; Tang, Y.; Song, R. ZmMADS47 regulates zein gene transcription through interaction with opaque2. PLoS Genet. 2016, 12, e1005991. [Google Scholar]
- Waltz, E. Gene-edited CRISPR mushroom escapes US regulation. Nature 2016, 532, 293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Huang, J.; Lan, H.; Wang, C.; Yin, C.; Wu, Y.; Tang, H.; Qian, Q.; Li, J.; et al. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc. Nat. Acad. Sci. USA 2012, 109, 21534–21539. [Google Scholar] [CrossRef]
- Li, J.; Meng, X.; Zong, Y.; Chen, K.; Zhang, H.; Liu, J.; Li, J.; Gao, C. Gene replacements and insertions in rice by intron targeting using CRISPR–Cas9. Nat. Plants 2016, 2, 16139. [Google Scholar] [CrossRef]
- Sun, Y.; Jiao, G.; Liu, Z.; Zhang, X.; Li, J.; Guo, X.; Du, W.; Du, J.; Francis, F.; Zhao, Y.; et al. Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Front. Plant Sci. 2017, 8, 298. [Google Scholar] [CrossRef]
- Chao, S.; Cai, Y.; Feng, B.; Jiao, G.; Sheng, Z.; Luo, J.; Tang, S.; Wang, J.; Hu, P.; Wei, X. Editing of rice Isoamylase Gene ISA1 provides insights into its function in starch formation. Rice Sci. 2019, 26, 77–87. [Google Scholar]
- Jiang, M.; Liu, Y.; Liu, Y.; Tan, Y.; Huang, J.; Shu, Q. Mutation of inositol 1, 3, 4-trisphosphate 5/6-kinase6 impairs plant growth and phytic acid synthesis in rice. Plants 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, W.; Ren, Y.; Tian, X.; Lv, T.; Wang, Z.; Fang, J.; Chu, C.; Yang, J.; Bu, Q. High-efficiency breeding of early-maturing rice cultivars via CRISPR/Cas9-mediated genome editing. J. Genet. Genom. 2017, 44, 175–178. [Google Scholar] [CrossRef]
- Miao, C.; Xiao, L.; Hua, K.; Zou, C.; Zhao, Y.; Bressan, R.A.; Zhu, J.K. Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Nat. Acad. Sci. USA 2018, 115, 6058–6063. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Yang, Y. RNA-Guided Genome Editing in Plants Using a CRISPR–Cas System. Mol. Plant 2013, 6, 1975–1983. [Google Scholar] [CrossRef]
- Macovei, A.; Sevilla, N.R.; Cantos, C.; Jonson, G.B.; Slamet-Loedin, I.; Čermák, T.; Voytas, D.F.; Choi, I.R.; Chadha-Mohanty, P. Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotechnol. J. 2018, 16, 1918–1927. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Wu, C.; He, Y.; Ma, Y.; Hou, H.; Guo, X.; Du, W.; Zhao, Y.; Xia, L. Engineering herbicide-resistant rice plants through CRISPR/Cas9-mediated homologous recombination of acetolactate synthase. Mol. Plant 2016, 9, 628–631. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Wang, F.; Li, T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol. Breed. 2019, 39, 47. [Google Scholar] [CrossRef]
- Mao, X.; Zheng, Y.; Xiao, K.; Wei, Y.; Zhu, Y.; Cai, Q.; Chen, L.; Xie, H.; Zhang, J. OsPRX2 contributes to stomatal closure and improves potassium deficiency tolerance in rice. Biochem. Biophys. Res. Commun. 2018, 495, 461–467. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Mohamed, S.; Tanoi, K.; Kobayashi, N.I.; Takagi, K.; Vernet, A.; Guiderdoni, E.; Périn, C.; Sentenac, H.; Véry, A.-A. Production of low-Cs + rice plants by inactivation of the K + transporter OsHAK1 with the CRISPR -Cas system. Plant J. 2017, 92, 43–56. [Google Scholar] [CrossRef]
- Malzahn, A.; Lowder, L.; Qi, Y. Plant genome editing with TALEN and CRISPR. Cell Biosci. 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Li, X.; Huang, J.; Li, Y.; Cai, S.; Yu, W.; Li, Y.; Huang, Y.; Xie, X.; Gong, Q.; et al. Engineered Cas9 variant tools expand targeting scope of genome and base editing in rice. Plant Biotechnol. J. 2020, 18, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Tao, X.; Liang, W.; Zhang, Z.; Gou, R.; Zhu, J.K. Simplified adenine base editors improve adenine base editing efficiency in rice. Plant Biotech. J. 2020, 18, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, R.; Zhang, Y.; Xu, S.; Liu, X.; Yang, J.; Zhang, X.; Wei, P. Optimizing plant adenine base editor systems by modifying the transgene selection system. Plant Biotechnol. J. 2020, 18, 1495–1497. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Z.; Zong, Y.; Wang, Y.; Liu, J.; Chen, K.; Qiu, J.L.; Gao, C. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 2016, 7, 12617. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.L.; et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686688. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, Y.; Wu, G.; Zou, S.; Chen, Y.; Gao, C.; Tang, D. Simultaneous modification of three homoeologs ofTaEDR1by genome editing enhances powdery mildew resistance in wheat. Plant J. 2017, 91, 714–724. [Google Scholar] [CrossRef]
- Connorton, J.M.; Jones, E.R.; Rodríguez-Ramiro, I.; Fairweather-Tait, S.; Uauy, C.; Balk, J. Wheat vacuolar iron transporter TaVIT2 transports Fe and Mn and is effective for biofortification. Plant Physiol. 2017, 174, 2434–2444. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, Y.; Li, C.; Zhang, R.; Chen, K.; Ran, Y.; Qiu, J.L.; Wang, D.; Gao, C. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 438–440. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Chai, Z.; Chen, S.; Bai, Y.; Zong, Y.; Chen, K.; Li, J.; Jiang, L.; Gao, C. Generation of herbicide tolerance traits and a new selectable marker in wheat using base editing. Nat. Plants 2019, 5, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Svitashev, S.; Young, J.K.; Schwartz, C.; Gao, H.; Falco, S.C.; Cigan, A.M. Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol. 2015, 169, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Song, N.; Sun, S.; Yang, W.; Zhao, H.; Song, W.; Lai, J. Efficiency and inheritance of targeted mutagenesis in maize using CRISPR-Cas9. J. Genet. Genom. 2016, 43, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qing, R.; Liu, X.; Liao, S.; Xu, R.; Yang, J.; Wei, P. CRISPR/Cas9-Mediated Adenine Base Editing in Rice Genome. Rice Sci. 2019, 26, 125–128. [Google Scholar] [CrossRef]
- Delgado, C.; Mora-Poblete, F.; Ahmar, S.; Chen, J.T.; Figueroa, C.R. Jasmonates and Plant Salt Stress: Molecular Players, Physiological Effects, and Improving Tolerance by Using Genome-Associated Tools. Int. J. Mol. Sci. 2021, 22, 3082. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Marraffini, L.A. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Akama-Garren, E.H.; Joshi, N.S.; Tammela, T.; Chang, G.P.; Wagner, B.L.; Lee, D.Y.; Rideout, W.M., 3rd; Papagiannakopoulos, T.; Xue, W.; Jacks, T. A Modular Assembly Platform for Rapid Generation of DNA Constructs. Sci. Rep. 2016, 18, 16836. [Google Scholar] [CrossRef]
- Xie, K.; Minkenberg, B.; Yang, Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc. Natl Acad. Sci. USA. 2015, 112, 3570–3575. [Google Scholar] [CrossRef]
- Fonfara, I.; Richter, H.; BratoviÄ, M.; Le Rhun, A.; Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 2016, 532, 517–521. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, S.; Tian, H.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Lycopene Is Enriched in Tomato Fruit by CRISPR/Cas9-Mediated Multiplex Genome Editing. Front. Plant Sci. 2018, 9, 559. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, B.; Ding, W.; Liu, X.; Yang, D.L.; Wei, P.; Cao, F.; Zhu, S.; Zhang, F.; Mao, Y.; et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013, 23, 1229–1232. [Google Scholar] [CrossRef]
- Fiaz, S.; Lv, S.; Barman, H.N.; Sahr, T.; Jiao, G.; Wei, X.; Sheng, Z.; Tang, S.; Hu, P. Analysis of Genomic regions governing cooking and eating quality traits using a Recombinant Inbred population in Rice (Oryza sativa L.). Int. J. Agri. Bio. 2019, 22, 611–619. [Google Scholar]
- El-Mounadi, K.; Morales-Floriano, M.L.; Garcia-Ruiz, H. Principles, Applications, and Biosafety of Plant Genome Editing Using CRISPR-Cas9. Front. Plant Sci. 2020, 11, 56. [Google Scholar] [CrossRef]
- Eckerstorfer, M.F.; Engelhard, M.; Heissenberger, A.; Simon, S.; Teichmann, H. Plants Developed by New Genetic Modification Techniques: Comparison of Existing Regulatory Frameworks in the EU and Non-EU Countries. Front. Bioeng. Biotechnol. 2019, 7, 26. [Google Scholar] [CrossRef]
- Van, V.T.; Sung, Y.W.; Kim, J.; Doan, D.T.H.; Tran, M.T.; Kim, J.-Y. Challenges and Perspectives in Homology-Directed Gene Targeting in Monocot Plants. Rice 2019, 12, 95. [Google Scholar] [CrossRef]
- Gleim, S.; Lubieniechi, S.; Smyth, S.J. CRISPR-Cas9 Application in Canadian Public and Private Plant Breeding. CRISPR J. 2020, 3, 44–51. [Google Scholar] [CrossRef]
- Spicer, A.; Molnar, A. Gene Editing of Microalgae: Scientific Progress and Regulatory Challenges in Europe. Biology 2018, 7, 21. [Google Scholar] [CrossRef]
- Gao, W.; Xu, W.T.; Huang, K.L.; Guo, M.Z.; Luo, Y.B. Risk analysis for genome editing-derived food safety in China. Food Control. 2018, 84, 128–137. [Google Scholar] [CrossRef]
- Chimata, M.K.; Bharti, G. Regulation of genome edited technologies in India. Transgenic Res. 2019, 28, 175–181. [Google Scholar] [CrossRef]
- Zannoni, L. Evolving Regulatory Landscape for Genome-Edited Plants. CRISPR J. 2019, 2, 3–8. [Google Scholar] [CrossRef]
- Malzahn, A.A.; Tang, X.; Lee, K.; Ren, Q.; Sretenovic, S.; Zhang, Y.; Chen, H.; Kang, M.; Bao, Y.; Zheng, X.; et al. Application of CRISPR-Cas12a temperature sensitivity for improved genome editing in rice, maize, and Arabidopsis. BMC Biol. 2019, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wolter, F.; Puchta, H. Knocking out consumer concerns and regulator’s rules: Efficient use of CRISPR/Cas ribonucleoprotein complexes for genome editing in cereals. Genome Biol. 2017, 18, 43. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, F.J.; Kopittke, P.M. Engineering crops without genome integration using nanotechnology. Trends Plant Sci. 2019, 24, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.B.; Zhang, S.; Kos, P.; Xiong, H.; Zhou, K.; Perelman, S.S.; Zhu, H.; Siegwart, D.J. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle codelivery of Cas9 mRNA and sgRNA. Angew. Chem. Int. Ed. 2017, 56, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, X.; Liu, H.; Li, Z. Highly efficient DNA-free plant genome editing using virally delivered CRISPR-Cas9. Nat. Plants 2020, 6, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Cody, W.B.; Scholthof, H.B. Native processing of single guide RNA transcripts to create catalytic Cas9/Single guide RNA complexes in planta. Plant Physiol. 2020, 184, 1194–1206. [Google Scholar] [CrossRef]
- Metje-Sprink, J.; Menz, J.; Modrzejewski, D.; Sprink, T. DNA-free genome editing: Past, present and future. Front. Plant Sci. 2019, 9, 1957. [Google Scholar] [CrossRef]
- Andersson, M.; Turesson, H.; Olsson, N.; Fält, A.S.; Ohlsson, P.; Gonzalez, M.N.; Samuelsson, M.; Hofvander, P. Genome editing in potato via CRISPR-Cas9 ribonucleoprotein delivery. Physiol Plant. 2018, 164, 378–384. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.; Li, Z.; Li, H.; Song, W.; Zhao, H.; Lai, J.; Xia, L.; Li, D.; Zhang, Y. A barley stripe mosaic virus-based guide RNA delivery system for targeted mutagenesis in wheat and maize. Mol. Plant Pathol. 2019, 20, 1463–1474. [Google Scholar] [CrossRef]
- Sant’Ana, R.R.A.; Caprestano, C.A.; Nodari, R.O.; Agapito-Tenfen, S.Z. PEG-delivered CRISPR-Cas9 ribonucleoproteins system for gene-editing screening of maize protoplasts. Genes 2020, 11, 1029. [Google Scholar] [CrossRef]
- Murovec, J.; Guček, K.; Bohanec, B.; Avbelj, M.; Jerala, R. DNA-free genome editing of Brassica oleracea and B. rapa protoplasts using CRISPR-Cas9 ribonucleoprotein complexes. Front. Plant Sci. 2018, 9, 1594. [Google Scholar] [CrossRef] [PubMed]
- Toda, E.; Koiso, N.; Takebayashi, A.; Ichikawa, M.; Kiba, T.; Osakabe, K.; Osakabe, Y.; Sakakibara, H.; Kato, N.; Okamoto, T. An efficient DNA- and selectable-marker-free genome-editing system using zygotes in rice. Nat. Plants 2019, 5, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, H.; Liu, J.; Yang, Q.; Shao, X.; Bi, F.; Hu, C.; Huo, H.; Chen, K.; Yi, G. Establishment of a PEG-mediated protoplast transformation system based on DNA and CRISPR/Cas9 ribonucleoprotein complexes for banana. BMC Plant Biol. 2020, 20, 425. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, S.; Park, S.; Yoon, J.; Park, A.Y.; Choe, S. DNA-free genome editing via ribonucleoprotein (RNP) delivery of CRISPR/Cas in lettuce. Methods Mol. Biol. 2019, 1917, 337–354. [Google Scholar]
- Kim, H.; Choi, J.; Won, K.-H. A stable DNA-free screening system for CRISPR/RNPs-mediated gene editing in hot and sweet cultivars of Capsicum annuum. BMC Plant Biol. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Veillet, F.; Perrot, L.; Chauvin, L.; Kermarrec, M.P.; Guyon-Debast, A.; Chauvin, J.E.; Nogué, F.; Mazier, M. Transgene-free genome editing in tomato and potato plants using Agro-bacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int. J. Mol. Sci. 2019, 20, 402. [Google Scholar] [CrossRef]
- Zhang, X.H.; Tee, L.Y.; Wang, X.G.; Huang, Q.S.; Yang, S.H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef]
- Pattanayak, V.; Lin, S.; Guilinger, J.P.; Ma, E.; Doudna, J.A.; Liu, D.R. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat. Biotechnol. 2013, 31, 839–843. [Google Scholar] [CrossRef]
- Li, H.; Rasheed, A.; Hickey, L.; He, Z. Fast-Forwarding Genetic Gain. Trends Plant Sci. 2018, 23, 184–186. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.D.; Hatta, M.A.M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Yu, S.; Ali, J.; Zhang, C.; Li, Z.; Zhang, Q. Genomic breeding of green super rice varieties and their deployment in Asia and Africa. Theor. Appl. Genet. 2020, 133, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Ahmar, S.; Saeed, S.; Khan, M.H.; Ullah Khan, S.; Mora-Poblete, F.; Kamran, M.; Faheem, A.; Maqsood, A.; Rauf, M.; Saleem, S.; et al. A Revolution toward Gene-Editing Technology and Its Application to Crop Improvement. Int. J. Mol. Sci. 2020, 21, 5665. [Google Scholar] [CrossRef] [PubMed]
- Ahmar, S.; Mahmood, T.; Fiaz, S.; Mora-Poblete, F.; Shafiq, M.S.; Chattha, M.S.; Jung, K.H. Genome editing with nanomaterials: A cutting edge strategy for crop improvement. Front. Plant Sci. 2021, 12, 943. [Google Scholar]
| Property | MegaN | ZFN | TALEN | CRISPR/Cas9 | CRISPR/Cpf1 | BE | PE |
|---|---|---|---|---|---|---|---|
| DNA binding determinant | Meganuclease | Zinc finger protein | Transcription-activator-like effector | CrRNA/sgRNA | CrRNA/Cpf1 | dCas/nCas | nCas9/pegRNA |
| Recognition | Protein-DNA | Protein-DNA | Protein-DNA | RNA-DNA | RNA-DNA-Protein | RNA-DNA-Protein | RNA-DNA-Protein |
| Endonuclease | Meganuclease | FokI | FokI | Cas9 | Cpf1 | dCas | pegRNA |
| Mutation rate | High | Medium | Medium | Low | High | High | Very High |
| Target size length (bp) | 14–40 | 18–36 | 30–40 | 22 | 20–24 | 4–6 | 8–15 |
| Off-target effects | High | High | Low | Variable | Low | Low | Very low |
| Mechanism of action | Able to induce double-strand breaks (DSB) with two possibilities of Non-homology end joining (NHEJ) and homology-directed repair (HDR), depends on the designing tool | No DSBs | |||||
| Design feasibility | Difficult, may require substantial efforts to design engineered protein | Required customized protein for each gene sequence. Oligomerized pool engineering (OPEN) used to select for new zinc finger assays | Technical challenging due to repeating sequence. Golden gate molecular cloning used to produce a TALE array | Easy to clone, only 20nt to targeting each gene expressed in a plasmid. | Easy | Easy | Easy |
| Multiplexing | Not possible | Difficult | Difficult | Easier | Easier | Easier | Not tested yet |
| Methylation sensitivity | High | High | High | Low | |||
| Target recognition efficiency | Low | High | High | High | High | Very high | Very high |
| Cost-effectiveness | No | No | Moderate | High | High | Very high | Very high |
| Application | Human, Animals, and Plants | Human, Animals, and Plants | Human, Animals, and Plants | Human, Animals, and Plants | Human, Animals, and Plants | Human, Animals, and Plants | Human, Plants (rice and wheat) |
| References | [21] | [22,23] | [22,23] | [22,23] | [24] | [25,26,27] | [28] |
| Specie. | GET System | Trait of Interest | Gene Function | Target Gene | Transformation Method | Reference |
|---|---|---|---|---|---|---|
| Oryza sativa L. | CRISPR/Cas 9 | Yield and quality improvement | Increases length and yield | OsPPKL1 | Agrobacterium | [115] |
| A key enzyme of aromatic amino acids biosynthesis | EPSPS | Biolistic transformation | [116] | |||
| Regulators of inflorescence Architecture of plant height | DEP1 | Agrobacterium | [48] | |||
| High amylose | SBEIIb | Electroporation | [117] | |||
| Amylose content | Waxy | Agrobacterium | [108] | |||
| Isoamylase-type debranching enzyme | ISA1 | Agrobacterium | [118] | |||
| Negative regulator of thermosensitive genicmale sterility | TMS5 | Agrobacterium | [96] | |||
| Low phytic acid | OsITPK6 | Agrobacterium | [119] | |||
| Enhanced fragrance | Badh2 | Agrobacterium | [110] | |||
| Grain weight | GW2, | Agrobacterium | [49] | |||
| Grain weight | TGW6 | Agrobacterium | [49] | |||
| Grain weight | GW5, | Agrobacterium | [49] | |||
| Early maturity of rice varieties | Hd2, | Agrobacterium | [120] | |||
| Early maturity of rice varieties | Hd4 | Agrobacterium | [120] | |||
| Early maturity of rice varieties | Hd5 | Agrobacterium | [120] | |||
| Improved growth and productivity | PYLs | Agrobacterium | [121] | |||
| Biotic stresses | Various abiotic stress tolerance and disease resistance | OsMPK5 | Agrobacterium | [122] | ||
| Rice blast resistance negative regulator | ERF922 | Electroporation | [50] | |||
| Resistance to rice tungrospherical virus | eIF4G | Agrobacterium | [123] | |||
| A key enzyme for the biosynthesis of branched-chain amino acids (major targets for herbicides) | ALS | Agrobacterium | [124] | |||
| Salinity tolerance | OsRR22 | Agrobacterium | [125] | |||
| Various abiotic stress tolerance and disease resistance | OsMPK5 | Agrobacterium | [122] | |||
| Nutritional improvement | Low Cd-accumulation | OsNramp5 | Agrobacterium | [54] | ||
| Potassium deficiency tolerance | OsPRX2 | Agrobacterium | [126] | |||
| Low cesium accumulation | OsHAK-1 | Agrobacterium | [127] | |||
| CPf1 | Yield and quality | Grain length-yield | OsGS3 | Agrobacterium | [66] | |
| Leaf and yield | OsDEP1 | Agrobacterium | [64] | |||
| Grain yield | OsNAL | Agrobacterium | [66] | |||
| Floral organ identity | OsDL | Agrobacterium | [61] | |||
| Negatively modulates bulliform cells | OsROC5 | Agrobacterium | [64,128] | |||
| Abiotic stress | Carotenoid biosynthetic pathway | OsPDS, | Agrobacterium | [109] | ||
| Herbicide resistance | OsALS | Agrobacterium | [78] | |||
| Abscisic acid regulation-stress tolerance | OsNCED1 | Agrobacterium | [61] | |||
| Caroteniod catabolism and abscisic acid metabolism-stress tolerance | OsAO1 | Agrobacterium | [61] | |||
| Abiotic stress tolerance | EPFL9 | Agrobacterium | [67] | |||
| Herbicide resistance | OsBEL | Agrobacterium | [65] | |||
| Herbicide resistance | OsRLK | Agrobacterium | [65] | |||
| BEs | Yield and quality | Amylose content | OsWaxy, | Agrobacterium | [129] | |
| Spikelet and floral organ | SNB | Agrobacterium | [130] | |||
| Grain shape | SLR1, | Agrobacterium | [130] | |||
| Male fertility | Tms9-1, | Agrobacterium | [130] | |||
| Grain weight | OsSPL14, | Agrobacterium | [130] | |||
| Grain size | OsSPL17, | Agrobacterium | [130] | |||
| Biotic stress | Rice blast resistance gene | Pid3 | Agrobacterium | [131] | ||
| Nitrogen transport and leaf death | Nitrogen transport | OsACC1, | Agrobacterium | [130] | ||
| Nitrogen transport | OsNRT1, | Agrobacterium | [78] | |||
| Leaf senescence | OsCDC48, | Agrobacterium | [78] | |||
| Triticum aestivum | CRISPR/CAS9 | Yield and quality | Grain weight negative Regulator | TaGW2 | Biolistic transformation | [58] |
| Low-gluten | Alpha-gliadin | Biolistic transformation | [111] | |||
| Control grain length and weight | TaGASR7 | Biolistic bombardment | [132] | |||
| Biotic stress | Mildew-resistance locus | TaMLO | Agrobacterium | [133] | ||
| Powdery mildew-resistance negative regulator | TaMLO-A1 | Biolistic bombardment | [134] | |||
| Disease resistance against powdery mildew | TaEDR1 | Biolistic transformation | [135] | |||
| Abiotic stress | Fe content | TaVIT2 | Biolistic bombardment | [136] | ||
| BEs | Yield and quality | Control grain size and weight | TaGW2 | Agrobacterium | [75] | |
| Inflorescence architecture and affects panicle growth and grain yield | TaDEP1, | Agrobacterium | [75] | |||
| Biotic and Abiotic stress | repress resistance pathway to powdery mildew | TaLOX2 | Particle bombardment | [137] | ||
| Herbicides resistance | TaALS, | Particle bombardment | [138] | |||
| PEs | Yield and quality | Control grain length and weight | TaGW2 | Agrobacterium | [82] | |
| A gibberellin regulated gene that controls grain length | TaGASR7 | Agrobacterium | [82] | |||
| Biotic stress | Repress resistance pathway to powdery mildew | TaLOX2 | Agrobacterium | [82] | ||
| Mildew-resistance locus | TaMLO, | Agrobacterium | [82] | |||
| Zea mays | CRISPR/Cas9 | Yield and quality | 45 (male sterility) | MS45 | Biolistic-mediated transformation | [139] |
| Increased grain yield under drought stress | ARGOS8 | Agrobacterium | [60] | |||
| Phytoene synthase | PSY1 | Agrobacterium | [140] | |||
| Seed and leaves traits | ZmIPK1A, | Agrobacterium | [59] | |||
| Seed and leaves traits | ZmIPK | Agrobacterium | [59] | |||
| Seed and leaves traits | ZmMRP4 | Agrobacterium | [59] | |||
| Abiotic stress | A key enzyme for the biosynthesis of branched-chain amino acids (major targets for herbicides) | ALS2 | Agrobacterium | [139] | ||
| CPf1 | Yield and Quality | Cuticular lipids | Maize glossy2 gene | Agrobacterium | [63] | |
| BEs | ZmCENH3 | Agrobacterium | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiaz, S.; Ahmar, S.; Saeed, S.; Riaz, A.; Mora-Poblete, F.; Jung, K.-H. Evolution and Application of Genome Editing Techniques for Achieving Food and Nutritional Security. Int. J. Mol. Sci. 2021, 22, 5585. https://doi.org/10.3390/ijms22115585
Fiaz S, Ahmar S, Saeed S, Riaz A, Mora-Poblete F, Jung K-H. Evolution and Application of Genome Editing Techniques for Achieving Food and Nutritional Security. International Journal of Molecular Sciences. 2021; 22(11):5585. https://doi.org/10.3390/ijms22115585
Chicago/Turabian StyleFiaz, Sajid, Sunny Ahmar, Sajjad Saeed, Aamir Riaz, Freddy Mora-Poblete, and Ki-Hung Jung. 2021. "Evolution and Application of Genome Editing Techniques for Achieving Food and Nutritional Security" International Journal of Molecular Sciences 22, no. 11: 5585. https://doi.org/10.3390/ijms22115585
APA StyleFiaz, S., Ahmar, S., Saeed, S., Riaz, A., Mora-Poblete, F., & Jung, K.-H. (2021). Evolution and Application of Genome Editing Techniques for Achieving Food and Nutritional Security. International Journal of Molecular Sciences, 22(11), 5585. https://doi.org/10.3390/ijms22115585








