A Single-Domain Antibody-Based Anti-PSMA Recombinant Immunotoxin Exhibits Specificity and Efficacy for Prostate Cancer Therapy
Abstract
1. Introduction
2. Results and Discussion
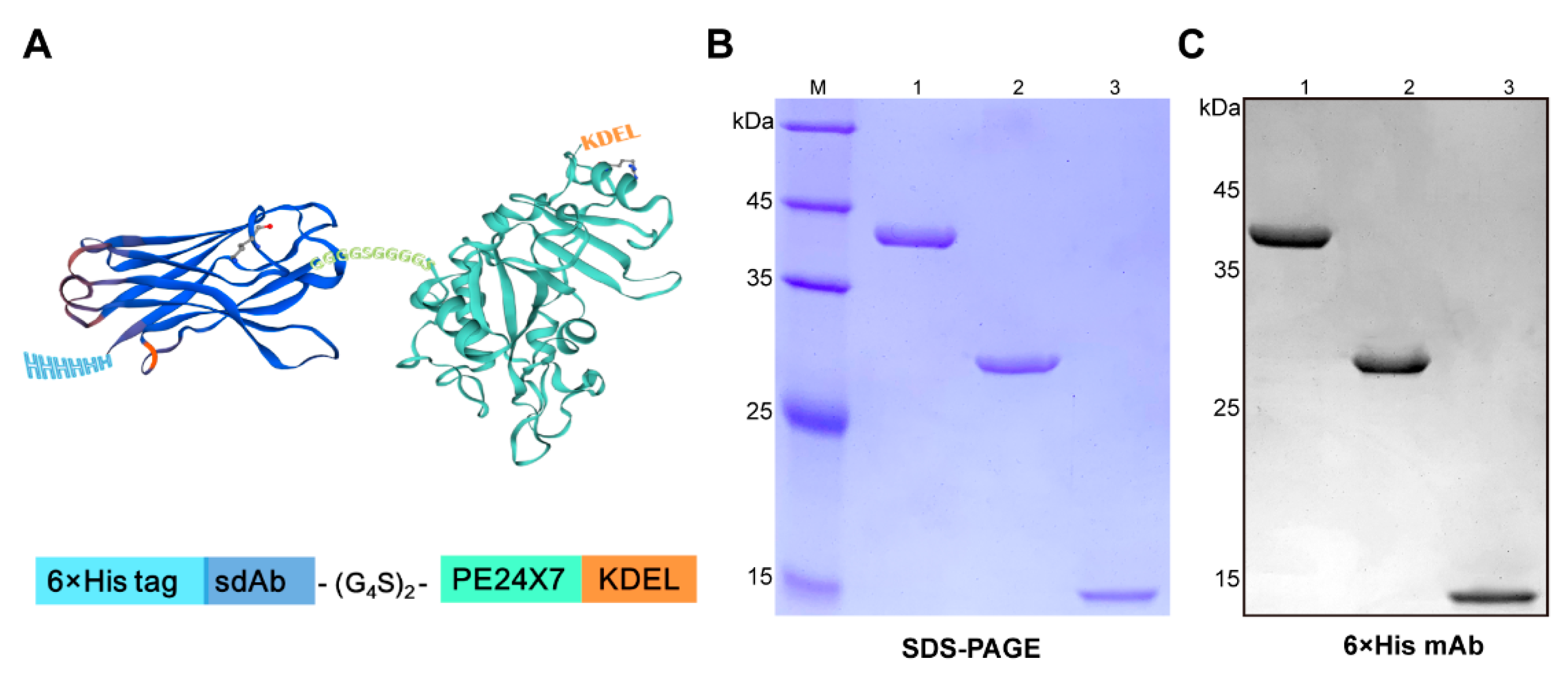
2.1. Expression and Characterization of the Immunotoxin JVM-PE24X7
2.2. Expression Levels of PSMA Receptors in PCa Cells
2.3. Binding Affinity Analysis of JVM-PE24X7 towards PSMA Receptors Determined by ELISA
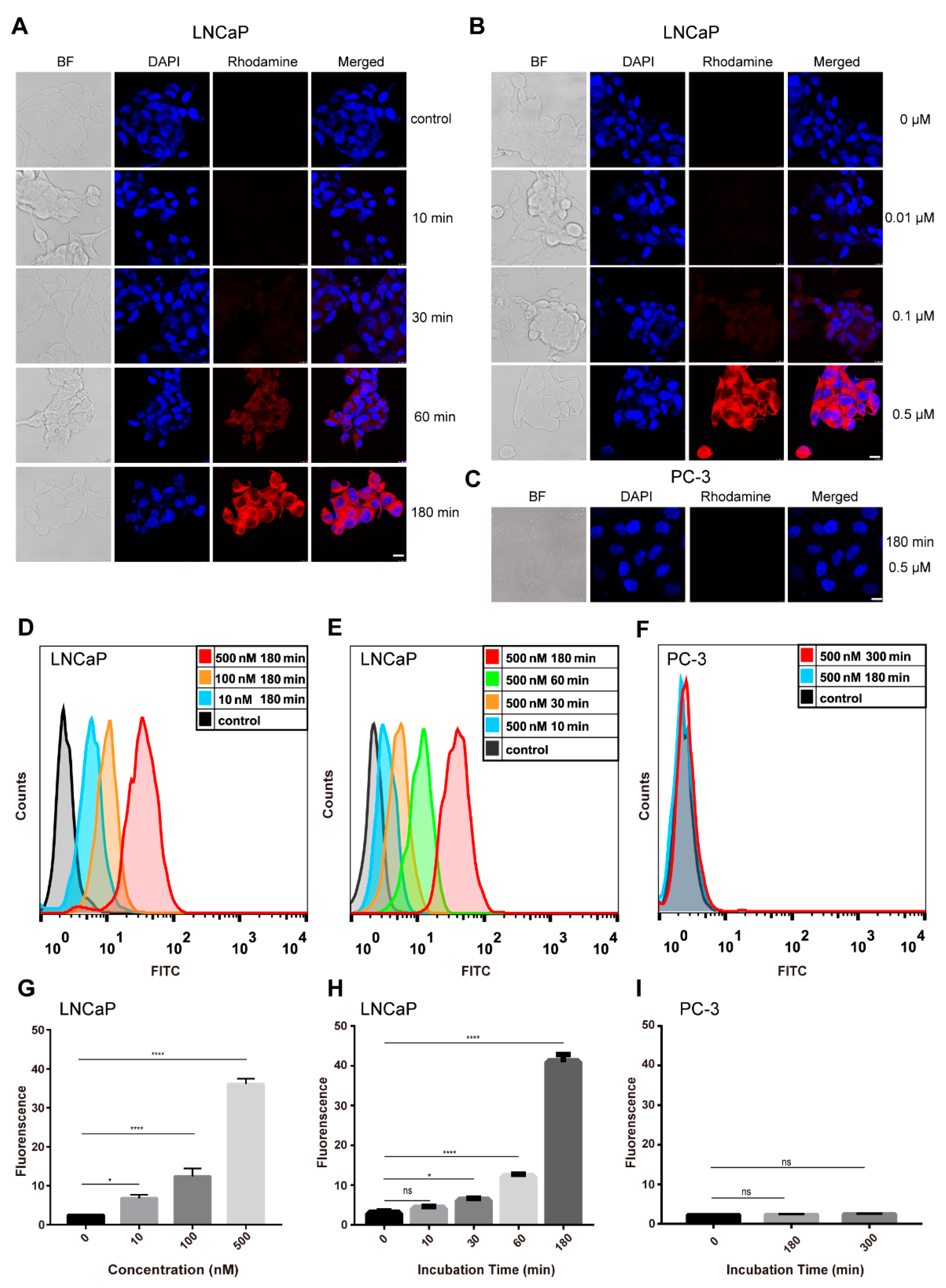
2.4. Cellular Internalization of JVM-PE24X7 into LNCaP and PC-3 Cells
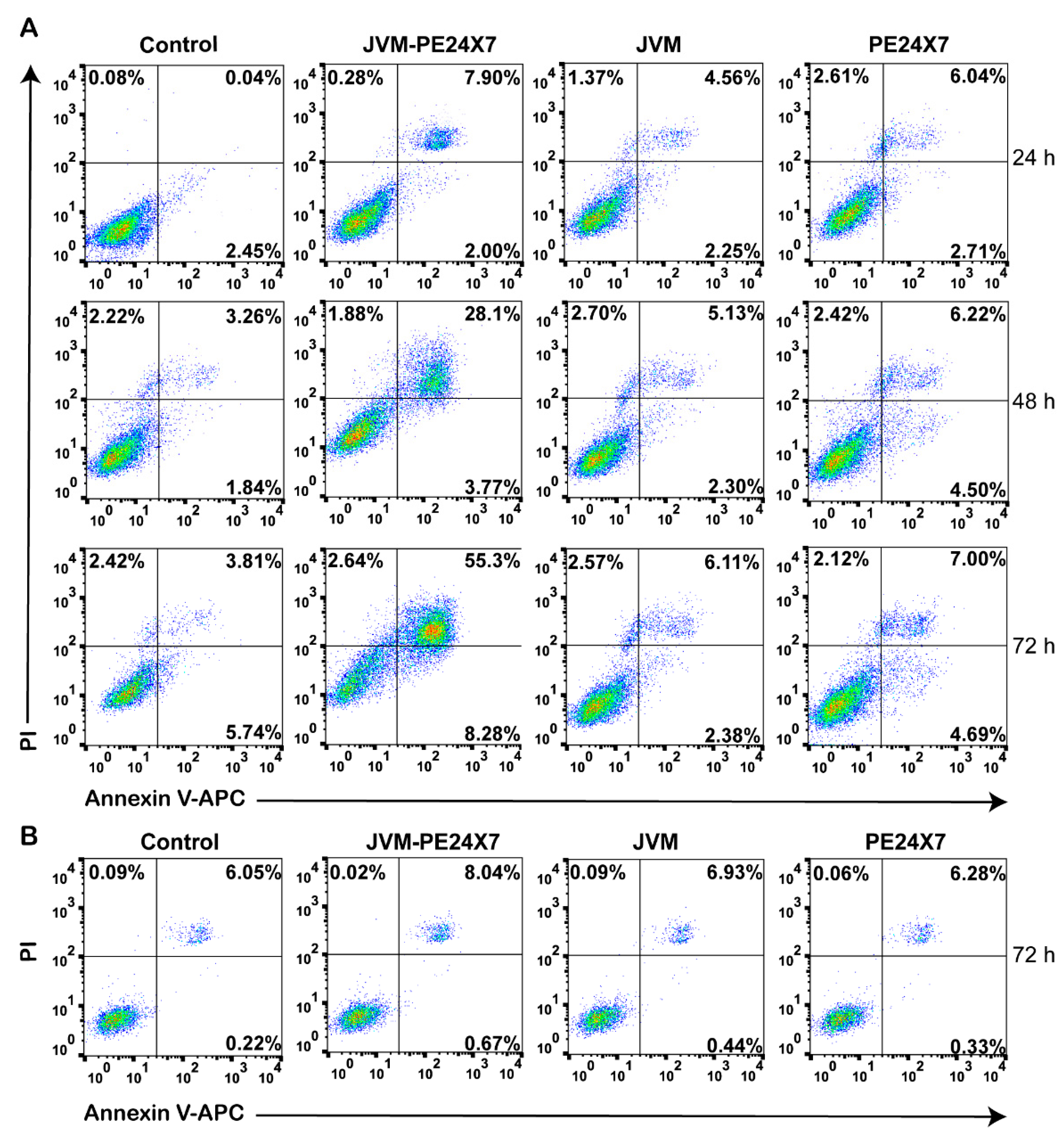
2.5. In Vitro Cytotoxicity of Immunotoxins
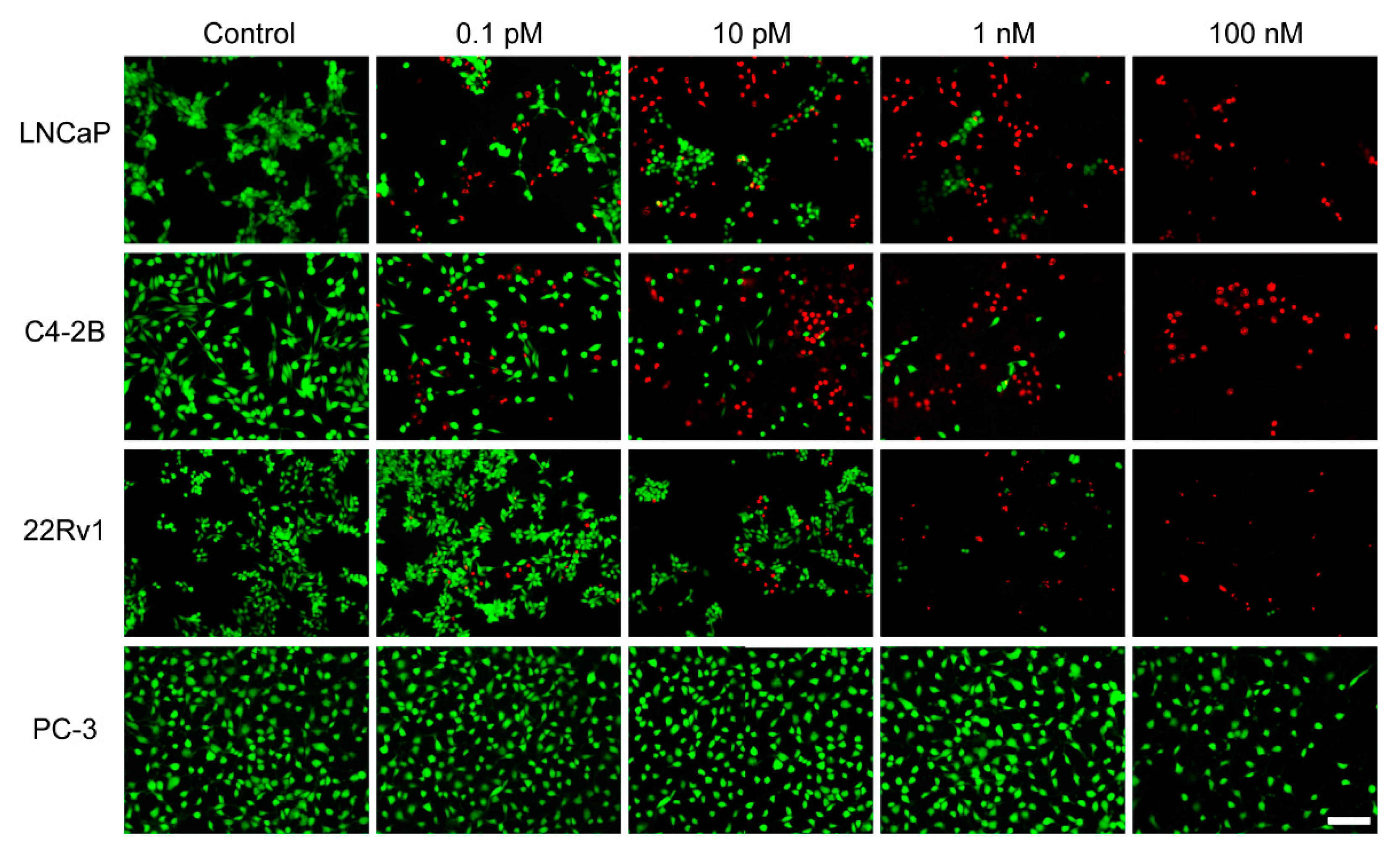
2.6. In Vitro Observations by Live/Dead Staining
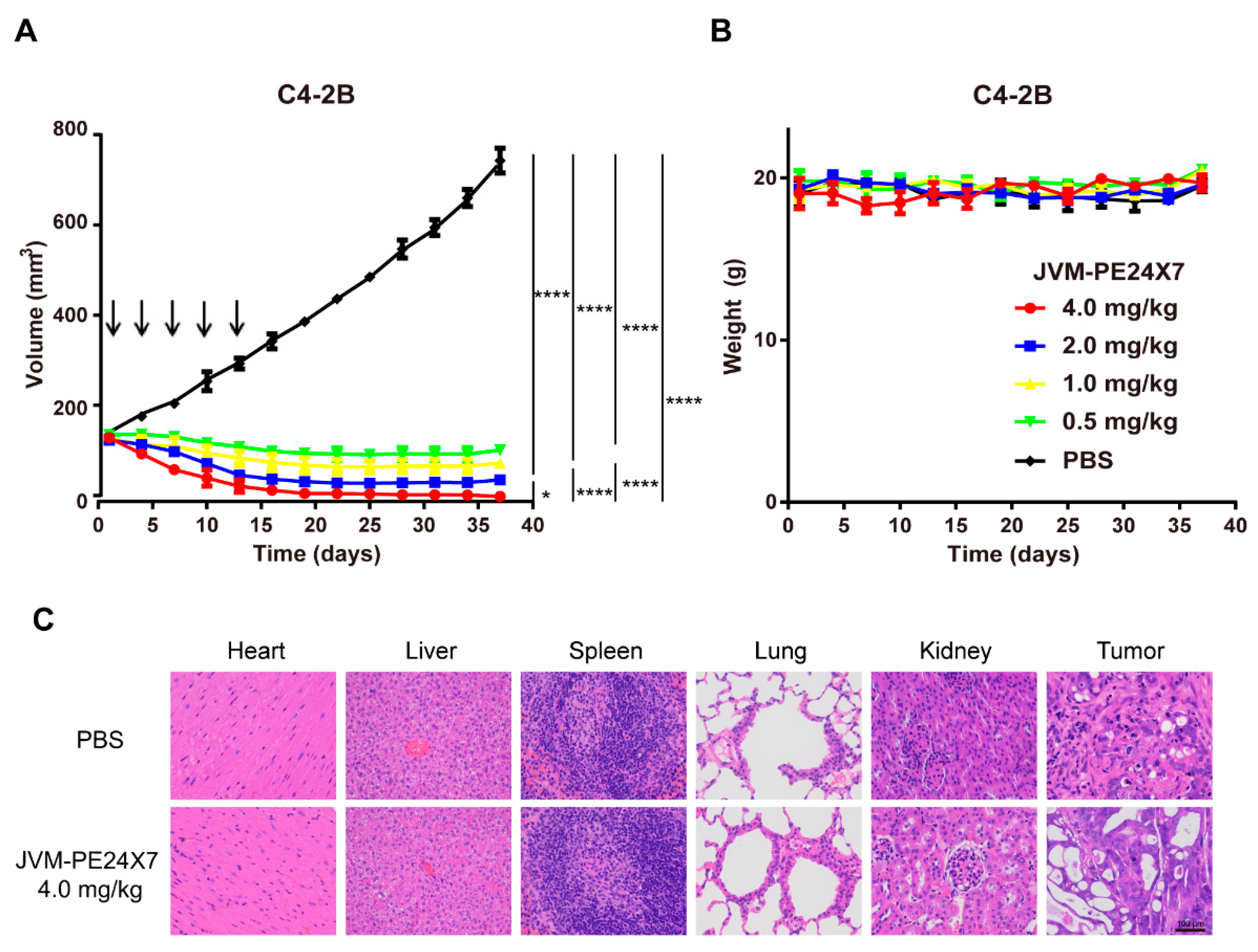
2.7. In Vivo Antitumor Activity of JVM-PE24X7 in a Xenograft Model
2.8. Off-Target Toxicity
3. Materials and Methods
3.1. Reagents
3.2. Cell Lines and Animals
3.3. Expression and Purification of the Anti-PSMA Immunotoxin JVM-PE24X7
3.4. Analysis of the Cellular Expression of PSMA by CLSM and Western Blot Analysis
3.5. Analysis of Binding Affinity Based on ELISA
3.6. In Vitro Cytotoxicity Assays
3.7. In Vitro Observation with Live/Dead Staining
3.8. Analysis of the Internalization of JVM-PE24X7 into Cells
3.9. In Vivo Antitumor Activity of JVM-PE24X7
3.10. Off-Target Toxicity Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.; Dahut, W.L. Chemotherapy for prostate cancer: Finally an advance! Am. J. Ther. 2004, 11, 288–294. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zieren, R.C.; Xue, W.; de Reijke, T.M.; Pienta, K.J. Metastatic prostate cancer remains incurable, why? Asian J. Urol. 2019, 6, 26–41. [Google Scholar] [CrossRef]
- Israeli, R.S.; Powell, C.T.; Corr, J.G.; Fair, W.R.; Heston, W.D. Expression of the prostate-specific membrane antigen. Cancer Res. 1994, 54, 1807–1811. [Google Scholar]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Tsourlakis, M.C.; Klein, F.; Kluth, M.; Quaas, A.; Graefen, M.; Haese, A.; Simon, R.; Sauter, G.; Schlomm, T.; Minner, S. PSMA expression is highly homogenous in primary prostate cancer. Appl. Immunohistochem. Mol. Morphol. AIMM 2015, 23, 449–455. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilding, G.; Denmeade, S.; Sarantopoulas, J.; Cosgrove, D.; Cetnar, J.; Azad, N.; Bruce, J.; Kurman, M.; Allgood, V.E.; et al. Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: Results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br. J. Cancer 2016, 114, 986–994. [Google Scholar] [CrossRef]
- Flores, O.; Santra, S.; Kaittanis, C.; Bassiouni, R.; Khaled, A.S.; Khaled, A.R.; Grimm, J.; Perez, J.M. PSMA-targeted theranostic nanocarrier for prostate cancer. Theranostics 2017, 7, 2477–2494. [Google Scholar] [CrossRef]
- Nanus, D.M.; Milowsky, M.I.; Kostakoglu, L.; Smith-Jones, P.M.; Vallabahajosula, S.; Goldsmith, S.J.; Bander, N.H. Clinical use of monoclonal antibody HuJ591 therapy: Targeting prostate specific membrane antigen. J. Urol. 2003, 170, S84–S88, discussion S88–S89. [Google Scholar] [CrossRef] [PubMed]
- Milowsky, M.I.; Galsky, M.D.; Morris, M.J.; Crona, D.J.; George, D.J.; Dreicer, R.; Tse, K.; Petruck, J.; Webb, I.J.; Bander, N.H.; et al. Phase 1/2 multiple ascending dose trial of the prostate-specific membrane antigen-targeted antibody drug conjugate MLN2704 in metastatic castration-resistant prostate cancer. Urol. Oncol. 2016, 34, e515–e530. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Vogelzang, N.J.; Chatta, K.; Fleming, M.T.; Smith, D.C.; Appleman, L.J.; Hussain, A.; Modiano, M.; Singh, P.; Tagawa, S.T.; et al. PSMA ADC monotherapy in patients with progressive metastatic castration-resistant prostate cancer following abiraterone and/or enzalutamide: Efficacy and safety in open-label single-arm phase 2 study. Prostate 2020, 80, 99–108. [Google Scholar] [CrossRef]
- FDA Approves first PSMA-targeted PET drug. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 11n.
- Eklund, J.W.; Kuzel, T.M. Denileukin diftitox: A concise clinical review. Expert Rev. Anticancer. Ther. 2005, 5, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Moxetumomab pasudotox: First global approval. Drugs 2018, 78, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Lane, A.A.; Sweet, K.L.; Stein, A.S.; Vasu, S.; Blum, W.; Rizzieri, D.A.; Wang, E.S.; Duvic, M.; Sloan, J.M.; et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N. Engl. J. Med. 2019, 380, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Yamaizumi, M.; Mekada, E.; Uchida, T.; Okada, Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 1978, 15, 245–250. [Google Scholar] [CrossRef]
- Thrush, G.R.; Lark, L.R.; Clinchy, B.C.; Vitetta, E.S. Immunotoxins: An update. Annu. Rev. Immunol. 1996, 14, 49–71. [Google Scholar] [CrossRef]
- Kreitman, R.J. Immunotoxins in cancer therapy. Curr. Opin. Immunol. 1999, 11, 570–578. [Google Scholar] [CrossRef]
- Henry, M.D.; Wen, S.; Silva, M.D.; Chandra, S.; Milton, M.; Worland, P.J. A prostate-specific membrane antigen-targeted monoclonal antibody-chemotherapeutic conjugate designed for the treatment of prostate cancer. Cancer Res. 2004, 64, 7995–8001. [Google Scholar] [CrossRef]
- Pastan, I.; Hassan, R.; Fitzgerald, D.J.; Kreitman, R.J. Immunotoxin therapy of cancer. Nat. Rev. Cancer 2006, 6, 559–565. [Google Scholar] [CrossRef]
- Akbari, B.; Farajnia, S.; Ahdi Khosroshahi, S.; Safari, F.; Yousefi, M.; Dariushnejad, H.; Rahbarnia, L. Immunotoxins in cancer therapy: Review and update. Int. Rev. Immunol. 2017, 36, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Alt, K.; Wetterauer, D.; Bühler, P.; Gierschner, D.; Katzenwadel, A.; Wetterauer, U.; Elsässer-Beile, U. Preclinical evaluation of a recombinant anti-prostate specific membrane antigen single-chain immunotoxin against prostate cancer. J. Immunother. 2010, 33, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, V.; Pegram, C.N.; Piao, H.; Szafranski, S.E.; Kuan, C.T.; Pastan, I.H.; Bigner, D.D. Production and quality control assessment of a GLP-grade immunotoxin, D2C7-(scdsFv)-PE38KDEL, for a phase I/II clinical trial. Appl. Microbiol. Biotechnol. 2017, 101, 2747–2766. [Google Scholar] [CrossRef]
- Linke, T.; Aspelund, M.T.; Thompson, C.; Xi, G.; Fulton, A.; Wendeler, M.; Pabst, T.M.; Wang, X.; Wang, W.K.; Ram, K.; et al. Development and scale-up of a commercial fed batch refolding process for an anti-CD22 two chain immunotoxin. Biotechnol. Prog. 2014, 30, 1380–1389. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Tijink, B.M.; Laeremans, T.; Budde, M.; Stigter-van Walsum, M.; Dreier, T.; de Haard, H.J.; Leemans, C.R.; van Dongen, G.A. Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: Taking advantage of modular Nanobody technology. Mol. Cancer Ther. 2008, 7, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef]
- Duggan, S. Caplacizumab: First global approval. Drugs 2018, 78, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I.; Muyldermans, S. The Therapeutic potential of nanobodies. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Guo, R.; Cao, L.; Guo, W.; Liu, H.; Xu, H.; Fang, Q.; Hong, Z. HER2-targeted immunotoxins with low nonspecific toxicity and immunogenicity. Biochem. Biophys. Res. Commun. 2016, 475, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Guo, W.; Cao, L.; Liu, H.; Liu, J.; Xu, H.; Huang, W.; Wang, F.; Hong, Z. Fusion of an albumin-binding domain extends the half-life of immunotoxins. Int. J. Pharm. 2016, 511, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Chatalic, K.L.; Veldhoven-Zweistra, J.; Bolkestein, M.; Hoeben, S.; Koning, G.A.; Boerman, O.C.; de Jong, M.; van Weerden, W.M. A novel ¹¹¹in-labeled anti-prostate-specific membrane antigen nanobody for targeted SPECT/CT imaging of prostate cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 1094–1099. [Google Scholar] [CrossRef]



| Dead/Total Mice | |||||
|---|---|---|---|---|---|
| Injected Dose (mg/kg) | 1.5 | 3.0 | 10 | 15 | 20 |
| JVM-PE24X7 | — | 0/5 | 0/5 | 0/5 | 3/5 |
| JVM-PE38 | 0/5 | 5/5 | — | — | — |
| PE24X7 | — | — | — | 0/5 | 0/5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Xu, K.; Li, S.; Cao, L.; Nan, Y.; Li, Q.; Li, W.; Hong, Z. A Single-Domain Antibody-Based Anti-PSMA Recombinant Immunotoxin Exhibits Specificity and Efficacy for Prostate Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 5501. https://doi.org/10.3390/ijms22115501
Xing Y, Xu K, Li S, Cao L, Nan Y, Li Q, Li W, Hong Z. A Single-Domain Antibody-Based Anti-PSMA Recombinant Immunotoxin Exhibits Specificity and Efficacy for Prostate Cancer Therapy. International Journal of Molecular Sciences. 2021; 22(11):5501. https://doi.org/10.3390/ijms22115501
Chicago/Turabian StyleXing, Yutong, Keyuan Xu, Shixiong Li, Li Cao, Yue Nan, Qiyu Li, Wenjing Li, and Zhangyong Hong. 2021. "A Single-Domain Antibody-Based Anti-PSMA Recombinant Immunotoxin Exhibits Specificity and Efficacy for Prostate Cancer Therapy" International Journal of Molecular Sciences 22, no. 11: 5501. https://doi.org/10.3390/ijms22115501
APA StyleXing, Y., Xu, K., Li, S., Cao, L., Nan, Y., Li, Q., Li, W., & Hong, Z. (2021). A Single-Domain Antibody-Based Anti-PSMA Recombinant Immunotoxin Exhibits Specificity and Efficacy for Prostate Cancer Therapy. International Journal of Molecular Sciences, 22(11), 5501. https://doi.org/10.3390/ijms22115501






