Loss of PKGIβ/IRAG1 Signaling Causes Anemia-Associated Splenomegaly
Abstract
1. Introduction
2. Results
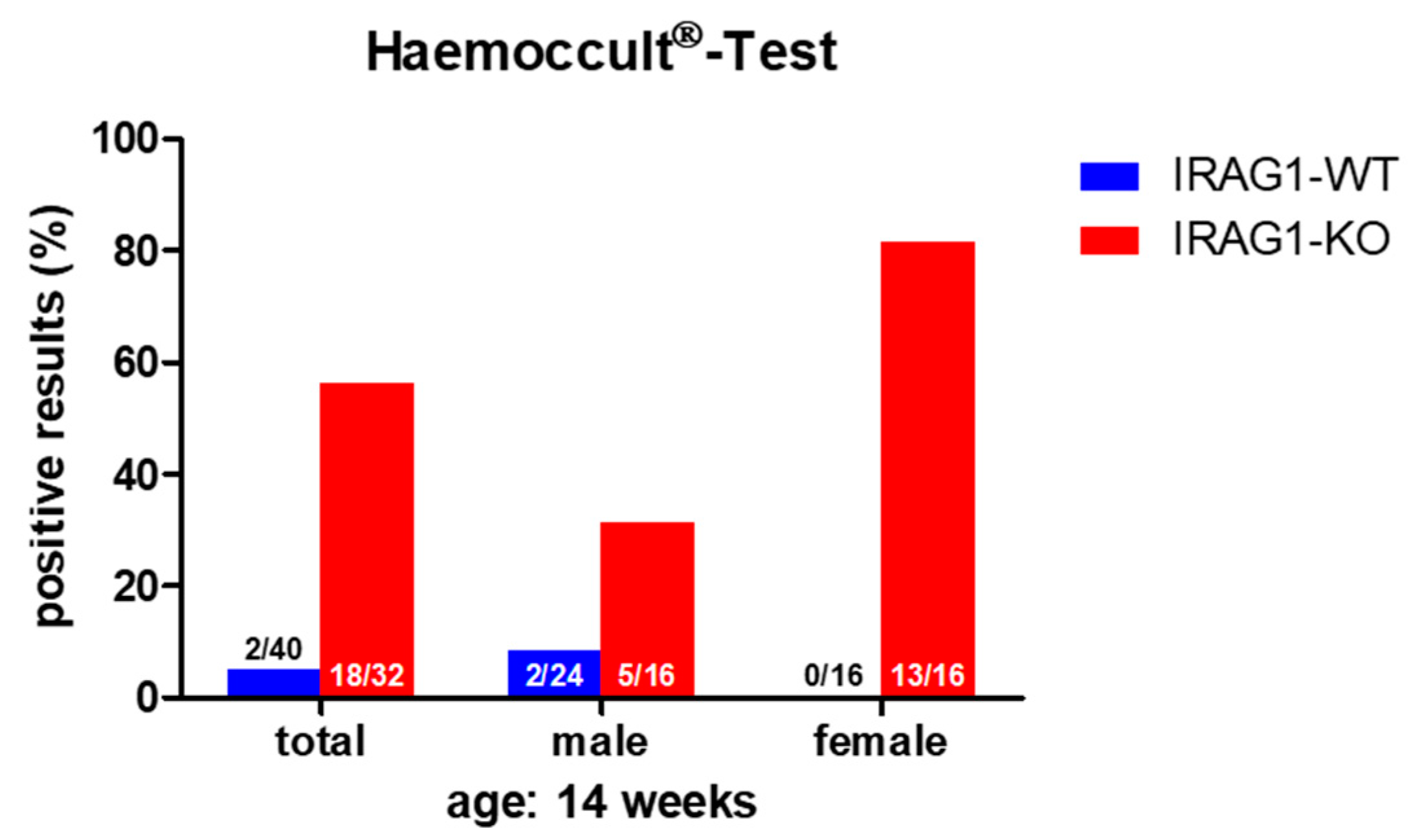
2.1. Detection of Occult Blood in IRAG1-WT and IRAG1-KO Mice
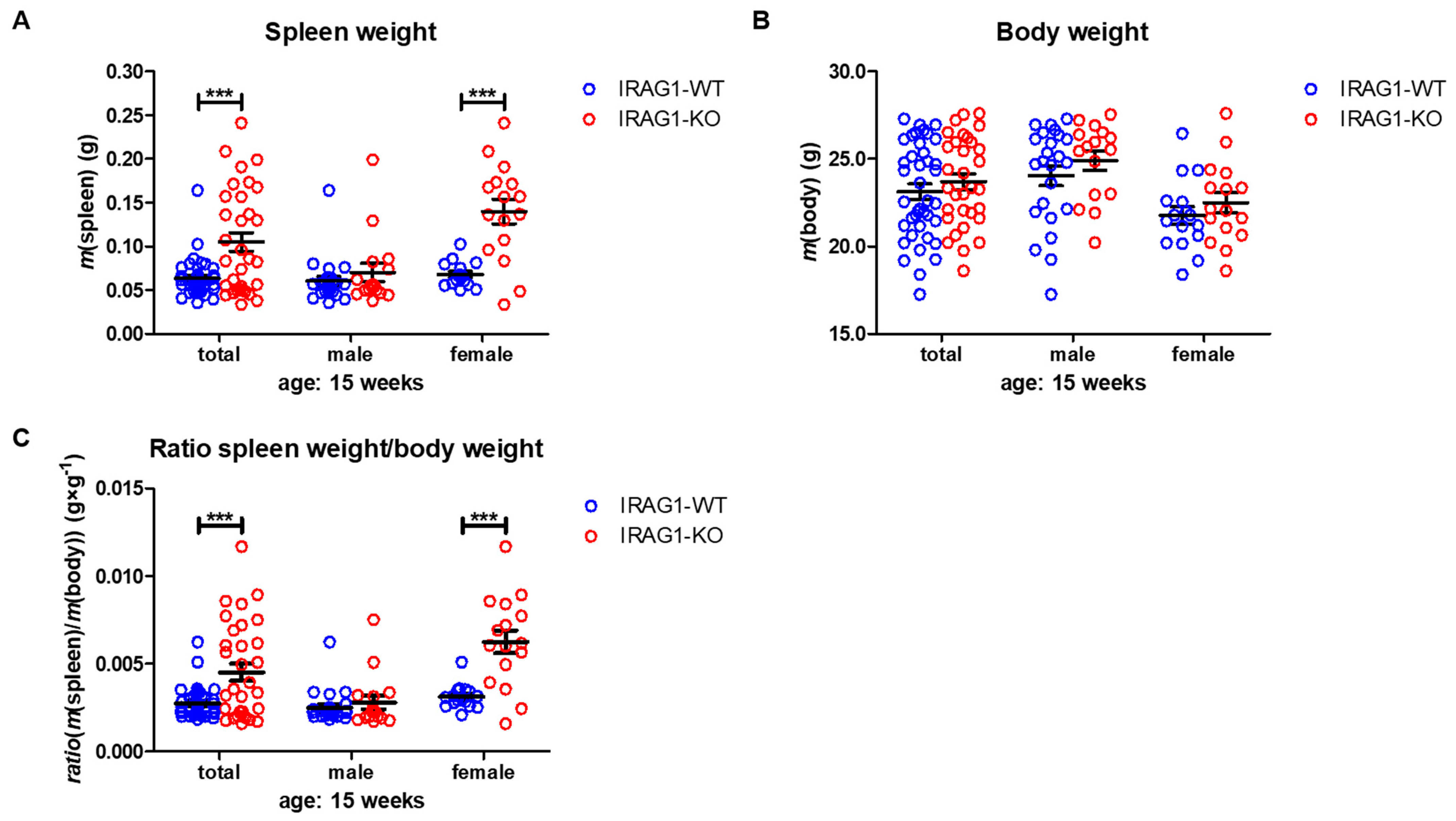
2.2. IRAG1-Deficient Mice Develop Splenomegaly
2.3. Analysis of Hematological Parameters
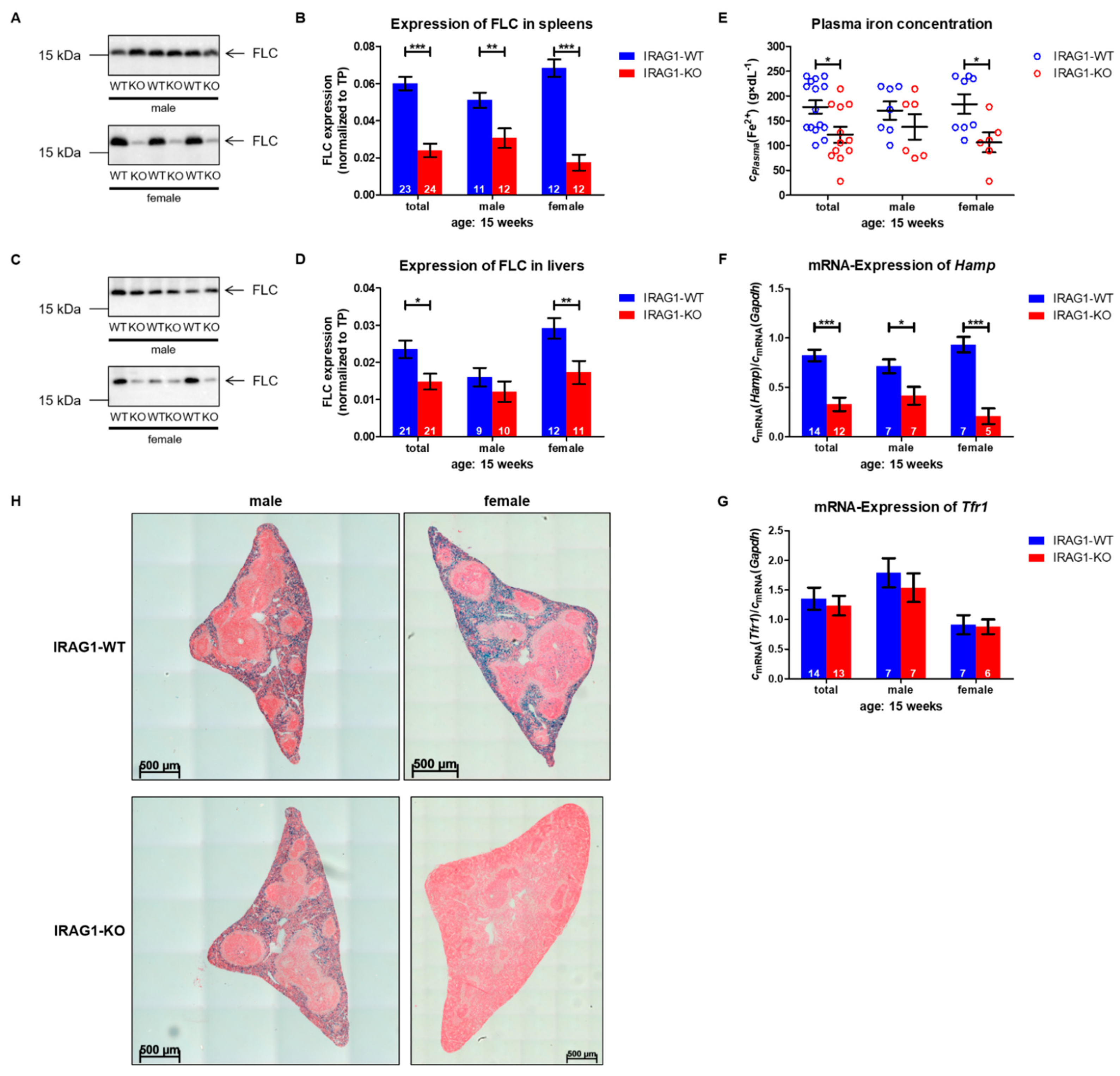
2.4. IRAG1-KO Mice Exhibit an Iron Deficiency
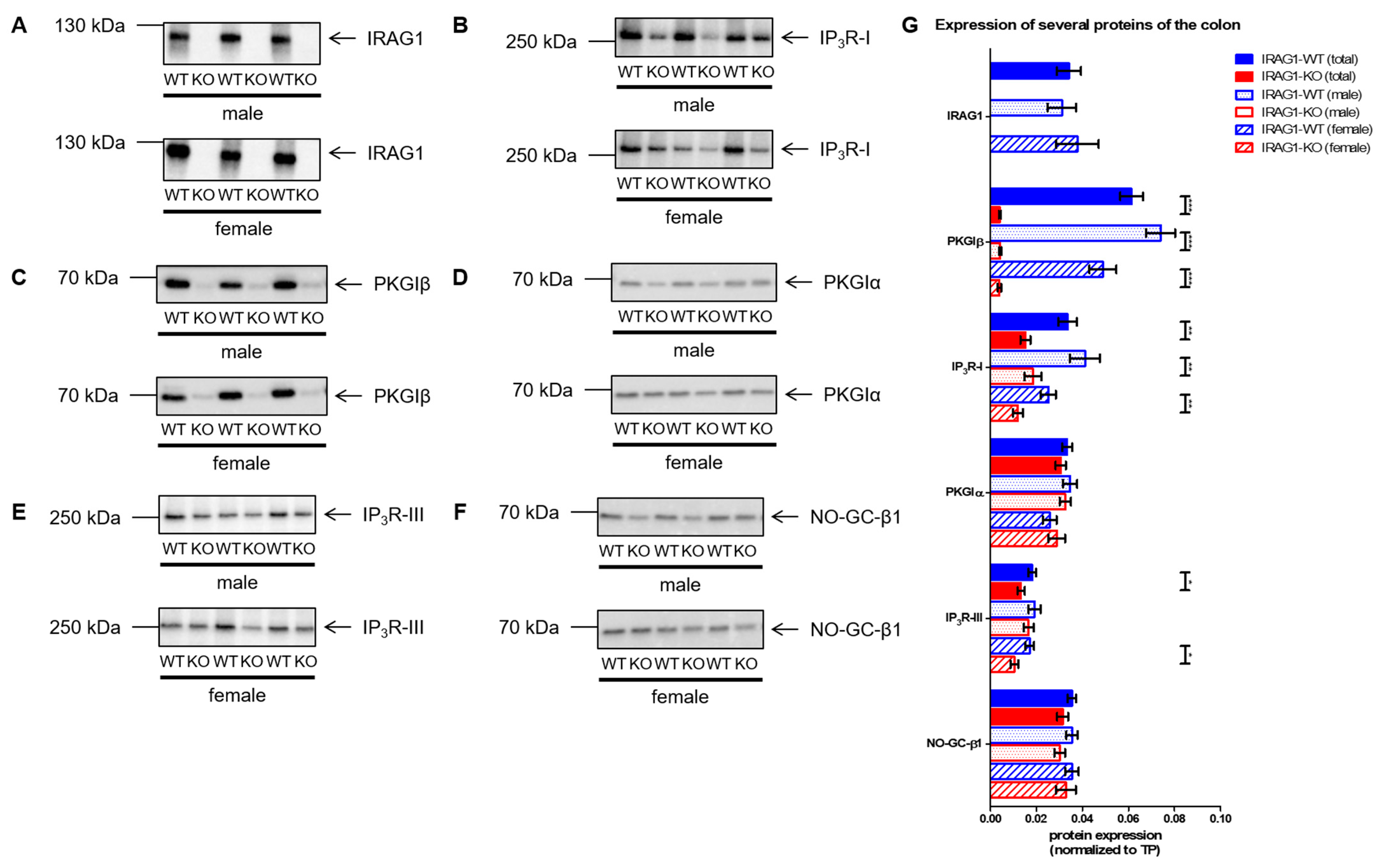
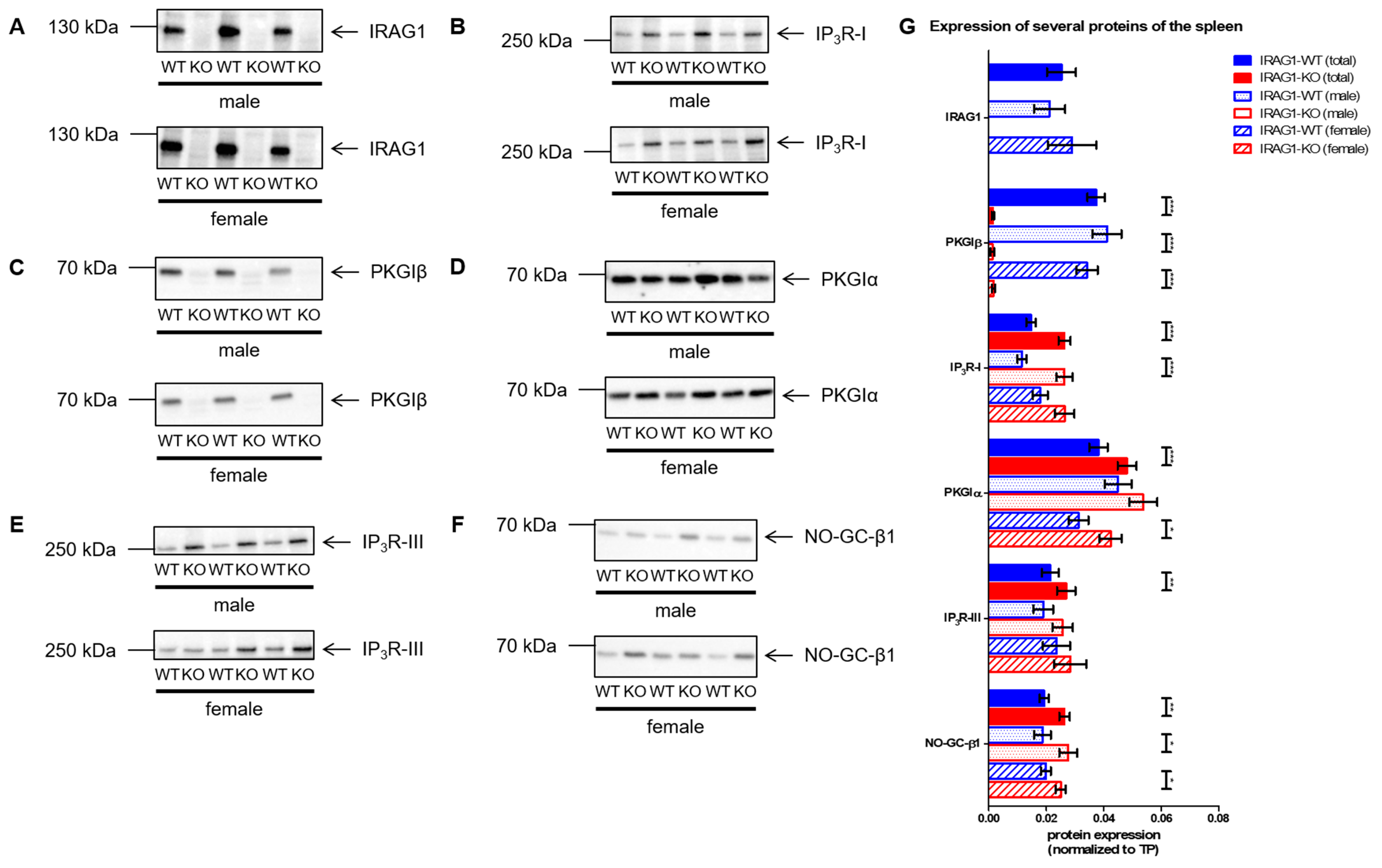
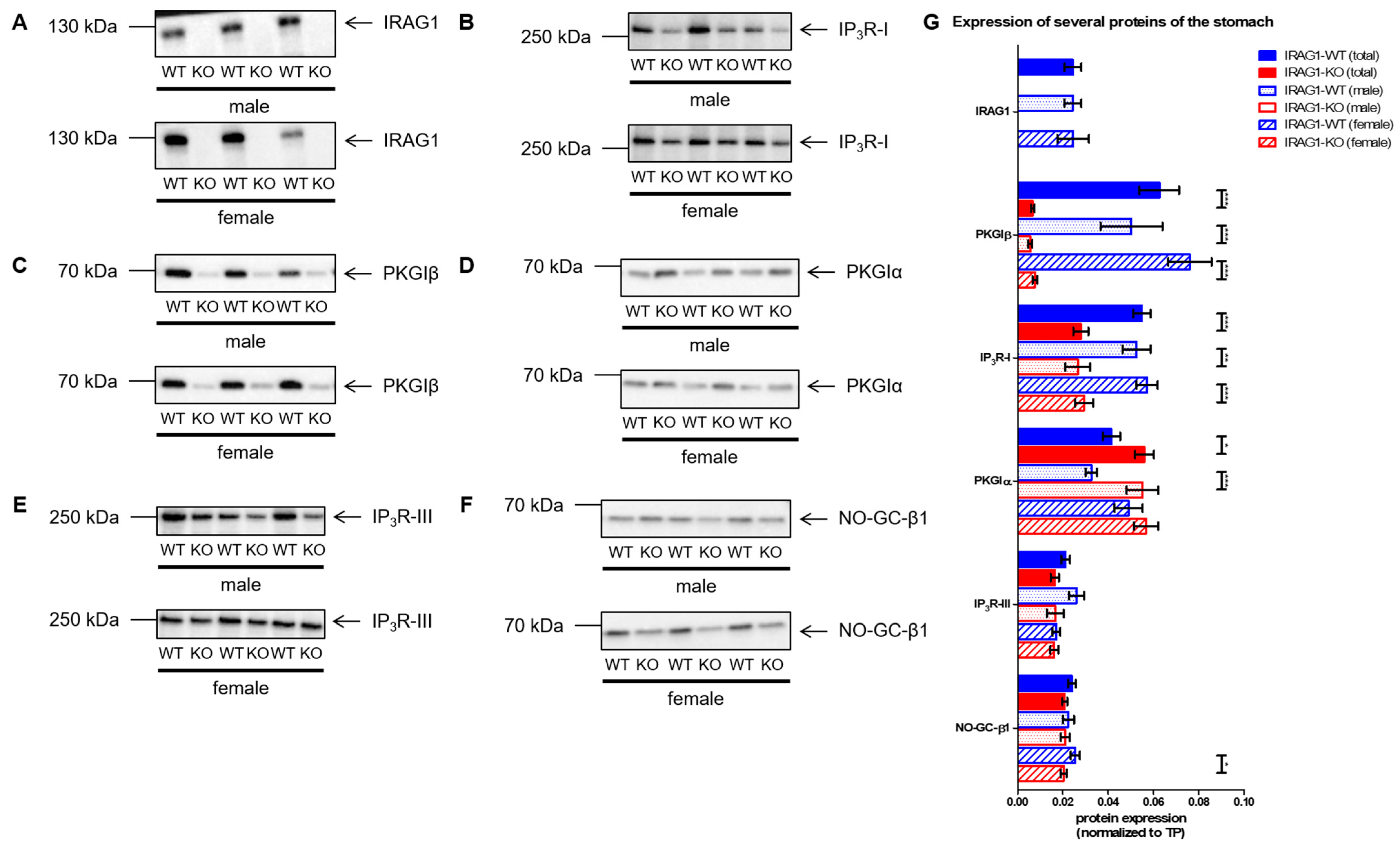
2.5. Analysis of the Expression of Several cGMP/PKGI Signaling Proteins in IRAG1-WT and IRAG1-KO Mice
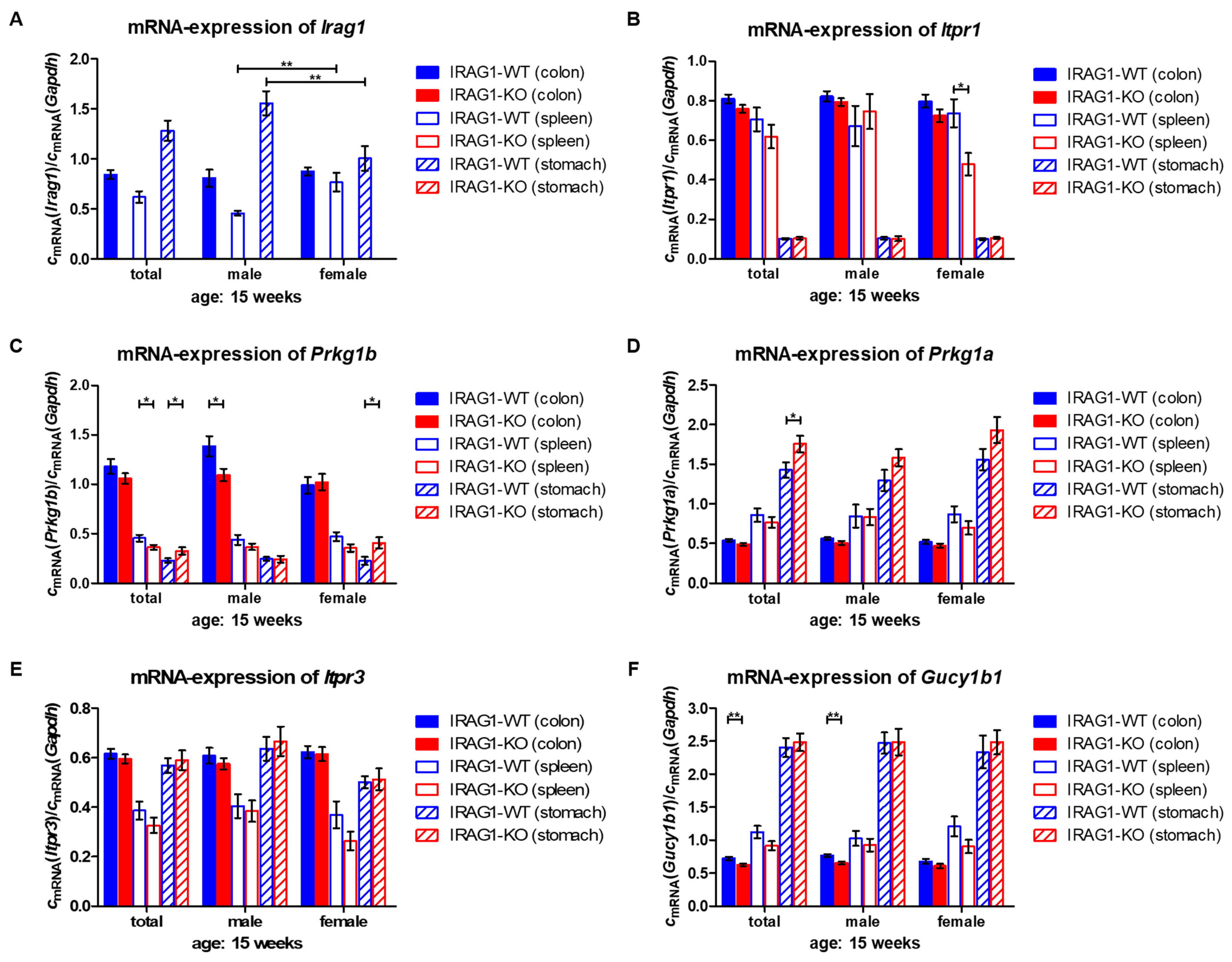
2.6. Analysis of mRNA Levels of cGMP/PKGI Signaling Genes in Colon, Spleen and Stomach
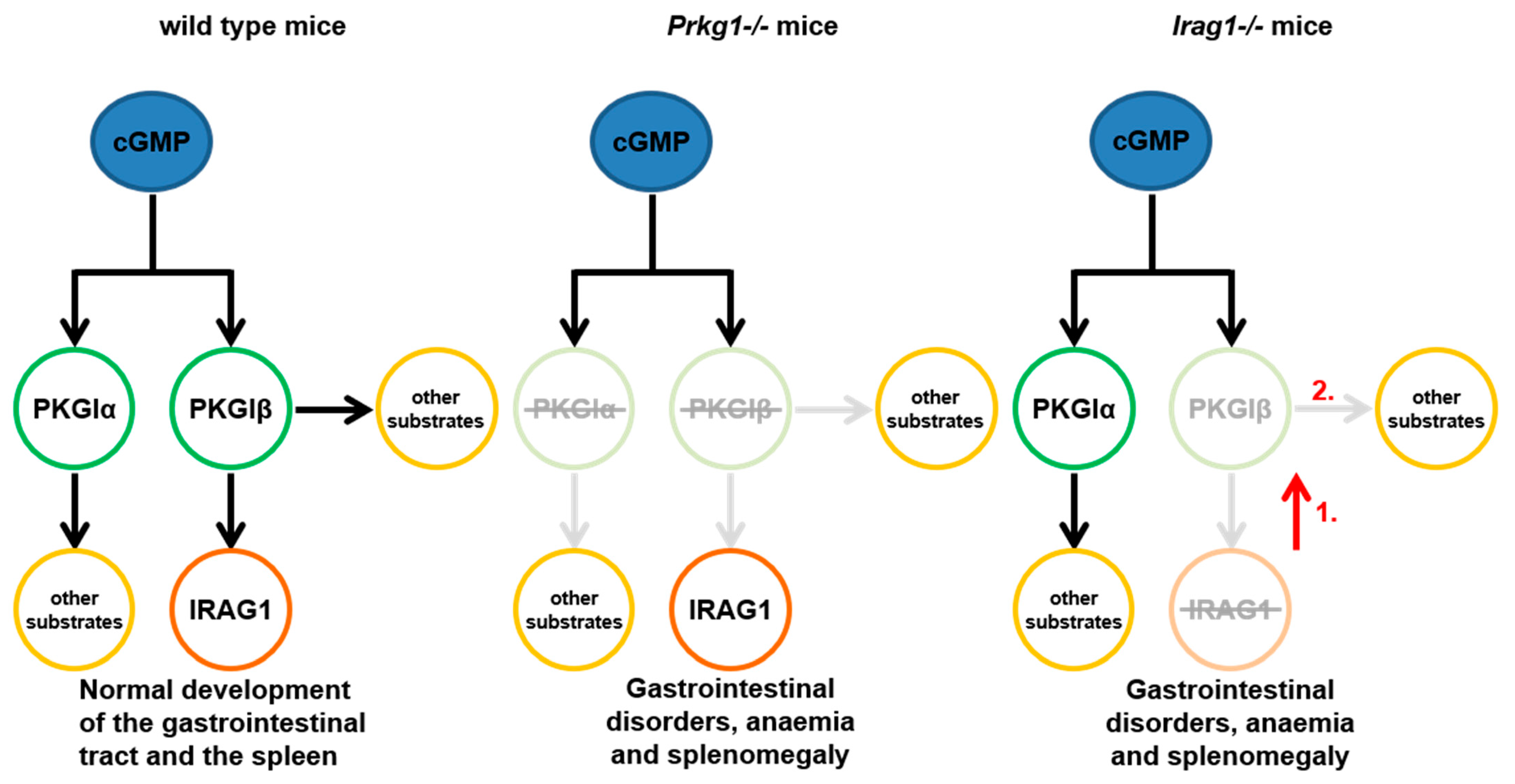
3. Discussion
- A loss of IRAG1 causes gastrointestinal bleedings, anemia, iron deficiency and splenomegaly, with a dominant phenotype in female IRAG1-KO mice. Unfortunately, it is at the moment not possible to explain the difference between the sexes.
- With this present work, we can demonstrate that IRAG1 is of prime importance for the stability of the PKGIβ protein and hence for PKGIβ/IRAG1 signaling.
- Presence of PKGIα in IRAG1-KO mice cannot compensate for the functional loss of PKGIβ. These findings suggest the hypothesis that loss of PKGIβ/IRAG1 signaling is mainly responsible for the development of gastrointestinal bleedings, anemia and splenomegaly in global Prkg1-deficient mice.
- IRAG1-KO mice are a kind of PKGIβ protein-deficient mouse; therefore, a possible model to study the pathophysiological function of PKGIβ in the presence of PKGIα.
4. Materials and Methods
4.1. Animal
4.2. Detection of Occult Blood
4.3. Analysis of Haematological Parameters
4.4. Measurement of Plasma Iron Concentration
4.5. Removal of Spleen and Determination of Different Weights
4.6. Tissue Preparation for Western Blot
4.7. Western Blot Analysis
4.8. Histology
4.9. Isolation of RNA and qRT-PCR of Murine Tissues
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlossmann, J.; Ammendola, A.; Ashman, K.; Zong, X.; Huber, A.; Neubauer, G.; Wang, G.X.; Allescher, H.D.; Korth, M.; Wilm, M.; et al. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Iβ. Nature 2000, 404, 197–201. [Google Scholar] [CrossRef]
- Ammendola, A.; Geiselhöringer, A.; Hofmann, F.; Schlossmann, J. Molecular determinants of the interaction between the inositol 1,4,5-trisphosphate receptor-associated cGMP kinase substrate (IRAG) and cGMP kinase Iβ. J. Biol. Chem. 2001, 276, 24153–24159. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, R.M.; Saur, D.; Kurjak, M.; Oesterle, D.; Schlossmann, J.; Geiselhöringer, A.; Hofmann, F.; Allescher, H.-D. InsP3R-associated cGMP kinase substrate (IRAG) is essential for nitric oxide-induced inhibition of calcium signaling in human colonic smooth muscle. J. Biol. Chem. 2004, 279, 12551–12559. [Google Scholar] [CrossRef] [PubMed]
- Geiselhöringer, A.; Werner, M.; Sigl, K.; Smital, P.; Wörner, R.; Acheo, L.; Stieber, J.; Weinmeister, P.; Feil, R.; Feil, S.; et al. IRAG is essential for relaxation of receptor-triggered smooth muscle contraction by cGMP kinase. EMBO J. 2004, 23, 4222–4231. [Google Scholar] [CrossRef] [PubMed]
- Geiselhöringer, A.; Gaisa, M.; Hofmann, F.; Schlossmann, J. Distribution of IRAG and cGKI-isoforms in murine tissues. FEBS Lett. 2004, 575, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Antl, M.; von Brühl, M.-L.; Eiglsperger, C.; Werner, M.; Konrad, I.; Kocher, T.; Wilm, M.; Hofmann, F.; Massberg, S.; Schlossmann, J. IRAG mediates NO/cGMP-dependent inhibition of platelet aggregation and thrombus formation. Blood 2007, 109, 552–559. [Google Scholar] [CrossRef]
- Schinner, E.; Salb, K.; Schlossmann, J. Signaling via IRAG is essential for NO/cGMP-dependent inhibition of platelet activation. Platelets 2011, 22, 217–227. [Google Scholar] [CrossRef]
- Desch, M.; Sigl, K.; Hieke, B.; Salb, K.; Kees, F.; Bernhard, D.; Jochim, A.; Spießberger, B.; Höcherl, K.; Feil, R.; et al. IRAG determines nitric oxide- and atrial natriuretic peptide-mediated smooth muscle relaxation. Cardiovasc. Res. 2010, 86, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Klatt, P.; Massberg, S.; Ny, L.; Sausbier, M.; Hirneiß, C.; Wang, G.X.; Korth, M.; Aszódi, A.; Andersson, K.E.; et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998, 17, 3045–3051. [Google Scholar] [CrossRef]
- Föller, M.; Feil, S.; Ghoreschi, K.; Koka, S.; Gerling, A.; Thunemann, M.; Hofmann, F.; Schuler, B.; Vogel, J.; Pichler, B.; et al. Anemia and splenomegaly in cGKI-deficient mice. Proc. Natl. Acad. Sci. USA 2008, 105, 6771–6776. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Spießberger, B.; Zheng, W.; Xiao, F.; Lukowski, R.; Wegener, J.W.; Weinmeister, P.; Saur, D.; Klein, S.; Schemann, M.; et al. Neuronal cGMP kinase I is essential for stimulation of duodenal bicarbonate secretion by luminal acid. FASEB J. 2012, 26, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Angermeier, E.; Domes, K.; Lukowski, R.; Schlossmann, J.; Rathkolb, B.; Angelis, M.H.; Hofmann, F. Iron deficiency anemia in cyclic GMP kinase knockout mice. Haematologica 2016, 101, e48–e51. [Google Scholar] [CrossRef]
- Koehler, K.; Hmida, D.; Schlossmann, J.; Landgraf, D.; Reisch, N.; Schuelke, M.; Huebner, A. Homozygous mutation in murine retrovirus integration site 1 gene associated with a non-syndromic form of isolated familial achalasia. Neurogastroenterol. Motil. 2020, e13923. [Google Scholar] [CrossRef]
- Palmieri, O.; Mazza, T.; Bassotti, G.; Merla, A.; Tolone, S.; Biagini, T.; Cuttitta, A.; Bossa, F.; Martino, G.; Latiano, T.; et al. microRNA-mRNA network model in patients with achalasia. Neurogastroenterol. Motil. 2020, 32, e13764. [Google Scholar] [CrossRef]
- Friebe, A.; Mergia, E.; Dangel, O.; Lange, A.; Koesling, D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc. Natl. Acad. Sci. USA 2007, 104, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Mergia, E.; Friebe, A.; Dangel, O.; Russwurm, M.; Koesling, D. Spare guanylyl cyclase NO receptors ensure high NO sensitivity in the vascular system. J. Clin. Investig. 2006, 116, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kojonazarov, B.; Hadzic, S.; Majer, M.; Bajraktari, G.; Novoyatleva, T.; Ghofrani, H.A.; Grimminger, F.; Seeger, W.; Weissmann, N.; et al. IRAG1 deficient mice develop PKG1β dependent pulmonary hypertension. Cells 2020, 9, 2280. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.K.; Surks, H.K.; Wang, Y.; Zhu, Y.; Blanton, R.; Jamnongjit, M.; Aronovitz, M.; Baur, W.; Ohtani, K.; Wilkerson, M.K.; et al. High blood pressure arising from a defect in vascular function. Proc. Natl. Acad. Sci. USA 2008, 105, 6702–6707. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.F.; James, M.W.; McIntyre, A.S.; Scott, B.B. Guidelines for the management of iron deficiency anaemia. Gut 2011, 60, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Cotter, J.; Baldaia, C.; Ferreira, M.; Macedo, G.; Pedroto, I. Diagnosis and treatment of iron-deficiency anemia in gastrointestinal bleeding: A systematic review. World J. Gastroenterol. 2020, 26, 7242–7257. [Google Scholar] [CrossRef]
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2020, 188, 819–830. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Mergia, E.; Kramer, C.M.; Lückstädt, W.; Yang, J.; Wolff, G.; Panknin, C.; Bracht, T.; Sitek, B.; Pernow, J.; et al. Identification of a soluble guanylate cyclase in RBCs: Preserved activity in patients with coronary artery disease. Redox Biol. 2018, 14, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Grasso, J.A.; Sullivan, A.L.; Chan, S.C. Studies of the endoplasmic reticulum and plasma membrane-bound ribosomes in erythropoietic cells. J. Cell Sci. 1978, 31, 165–178. [Google Scholar] [CrossRef]
- Moras, M.; Lefevre, S.D.; Ostuni, M.A. From erythroblasts to mature red blood cells: Organelle clearance in mammals. Front. Physiol. 2017, 8, 1076. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.; Ferguson, D.J.P.; Edelmann, M.; Kessler, B.; Morten, K.J.; Komatsu, M.; Simon, A.K. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 832–837. [Google Scholar] [CrossRef]
- Shaughnessy, J.D.; Largaespada, D.A.; Tian, E.; Fletcher, C.F.; Cho, B.C.; Vyas, P.; Jenkins, N.A.; Copeland, N.G. Mrvi1, A common MRV integration site in BXH2 myeloid leukemias, encodes a protein with homology to a lymphoid-restricted membrane protein Jaw1. Oncogene 1999, 18, 2069–2084. [Google Scholar] [CrossRef][Green Version]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Öhrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef] [PubMed]
- Kempe, D.S.; Lang, P.A.; Duranton, C.; Akel, A.; Lang, K.S.; Huber, S.M.; Wieder, T.; Lang, F. Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 2006, 20, 368–370. [Google Scholar] [CrossRef]
- Tonelli, A.R.; Yadav, R.; Gupta, A.; Arrossi, A.V.; Heresi, G.A.; Dweik, R.A. Spleen size in idiopathic and heritable pulmonary arterial hypertension. Respiration 2013, 85, 391–399. [Google Scholar] [CrossRef]
- Ramchandran, R.; Raghavan, A.; Geenen, D.; Sun, M.; Bach, L.; Yang, Q.; Raj, J.U. PKG-1α leucine zipper domain defect increases pulmonary vascular tone: Implications in hypoxic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L537–L544. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.B.; Busch, J.L.; Francis, S.H.; Corbin, J.D.; Lincoln, T.M. Cyclic GMP specifically suppresses Type-Iα cGMP-dependent protein kinase expression by ubiquitination. Cell. Signal. 2009, 21, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Al-Shboul, O.A.; Nazzal, M.S.; Mustafa, A.G.; Al-Dwairi, A.N.; Alqudah, M.A.; Abu Omar, A.; Alfaqih, M.A.; Alsalem, M.I. Estrogen relaxes gastric muscle cells via a nitric oxide- and cyclic guanosine monophosphate-dependent mechanism: A sex-associated differential effect. Exp. Ther. Med. 2018, 16, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.S.; Shah, A.; Stanworth, S.J.; Frise, C.J.; Spiby, H.; Lax, S.J.; Murray, J.; Klein, A.A. The effect of iron deficiency and anaemia on women’s health. Anaesthesia 2021, 76 (Suppl. 4), 84–95. [Google Scholar] [CrossRef]
- Conran, N.; Torres, L. cGMP modulation therapeutics for sickle cell disease. Exp. Biol. Med. 2019, 244, 132–146. [Google Scholar] [CrossRef]
- Ghofrani, H.-A.; Sanchez, M.-A.G.; Humbert, M.; Pittrow, D.; Simonneau, G.; Gall, H.; Grünig, E.; Klose, H.; Halank, M.; Langleben, D.; et al. Riociguat treatment in patients with chronic thromboembolic pulmonary hypertension: Final safety data from the EXPERT registry. Respir. Med. 2021, 178, 106220. [Google Scholar] [CrossRef]
- Jakstaite, A.-M.; Luedike, P.; Schmack, B.; Pizanis, N.; Riebisch, M.; Weymann, A.; Kamler, M.; Ruhparwar, A.; Rassaf, T.; Papathanasiou, M. Increased bleeding risk with phosphodiesterase-5 inhibitors after left ventricular assist device implantation. ESC Heart Fail. 2021, 1–9. [Google Scholar] [CrossRef]
- Rick, W. Klinische Chemie und Mikroskopie, Sechste, Überarbeitete und Erweiterte Auflage; Springer: Berlin/Heidelberg, Germany, 1990; ISBN 9783642840388. [Google Scholar]
- Schinner, E.; Schramm, A.; Kees, F.; Hofmann, F.; Schlossmann, J. The cyclic GMP-dependent protein kinase Iα suppresses kidney fibrosis. Kidney Int. 2013, 84, 1198–1206. [Google Scholar] [CrossRef]
- Schramm, A.; Schweda, F.; Sequeira-Lopez, M.L.S.; Hofmann, F.; Sandner, P.; Schlossmann, J. Protein kinase G is involved in acute but not in long-term regulation of renin secretion. Front. Pharmacol. 2019, 10, 800. [Google Scholar] [CrossRef]

| Parameter | Total | Male | Female | |||
|---|---|---|---|---|---|---|
| IRAG1-WT | IRAG1-KO | IRAG1-WT | IRAG1-KO | IRAG1-WT | IRAG1-KO | |
| spleen weight (g) | 0.0634 ± 0.0034 | 0.1051 ± 0.0106 a | 0.0605 ± 0.0050 | 0.0702 ± 0.0103 | 0.0678 ± 0.0035 | 0.1399 ± 0.0140 a |
| body weight (g) | 23.13 ± 0.43 | 23.70 ± 0.45 | 24.04 ± 0.57 | 24.91 ± 0.55 | 21.77 ± 0.51 | 22.49 ± 0.58 |
| spleen/body-ratio (g × g−1) | 0.0028 ± 0.0001 | 0.0045 ± 0.0005 a | 0.0025 ± 0.0002 | 0.0028 ± 0.0004 | 0.0031 ± 0.0002 | 0.0062 ± 0.0007 a |
| Parameter | Total | Male | Female | |||
|---|---|---|---|---|---|---|
| IRAG1-WT | IRAG1-KO | IRAG1-WT | IRAG1-KO | IRAG1-WT | IRAG1-KO | |
| HCT (%) | 49.9 ± 0.9 | 45.1 ± 1.4 b | 49.5 ± 1.0 | 46.9 ± 1.7 | 50.3 ± 1.5 | 43.3 ± 2.2 a |
| RBC (106 × µL−1) | 9.27 ± 0.30 | 7.28 ± 0.41 c | 9.60 ± 0.46 | 8.58 ± 0.47 | 8.92 ± 0.38 | 5.90 ± 0.43 c |
| Hb (g × dL−1) | 17.6 ± 0.2 | 14.5 ± 0.6 c | 17.9 ± 0.3 | 15.5 ± 0.6c | 17.2 ± 0.3 | 13.2 ± 1.0 c |
| MCH (fL) | 55.7 ± 2.1 | 65.8 ± 3.0 | 53.0 ± 2.2 | 56.6 ± 3.4 | 58.6 ± 3.7 | 75.7 ± 3.6 a |
| MCV (pg) | 18.8 ± 0.6 | 19.1 ± 0.8 b | 19.2 ± 4.0 | 17.5 ± 0.5 | 18.2 ± 0.6 | 21.3 ± 1.1 b |
| WBC (µL−1) | 1807 ± 189 | 1811 ± 199 | 1617 ± 393 | 2600 ± 582 | 1933 ± 188 | 1586 ± 162 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majer, M.; Prueschenk, S.; Schlossmann, J. Loss of PKGIβ/IRAG1 Signaling Causes Anemia-Associated Splenomegaly. Int. J. Mol. Sci. 2021, 22, 5458. https://doi.org/10.3390/ijms22115458
Majer M, Prueschenk S, Schlossmann J. Loss of PKGIβ/IRAG1 Signaling Causes Anemia-Associated Splenomegaly. International Journal of Molecular Sciences. 2021; 22(11):5458. https://doi.org/10.3390/ijms22115458
Chicago/Turabian StyleMajer, Michael, Sally Prueschenk, and Jens Schlossmann. 2021. "Loss of PKGIβ/IRAG1 Signaling Causes Anemia-Associated Splenomegaly" International Journal of Molecular Sciences 22, no. 11: 5458. https://doi.org/10.3390/ijms22115458
APA StyleMajer, M., Prueschenk, S., & Schlossmann, J. (2021). Loss of PKGIβ/IRAG1 Signaling Causes Anemia-Associated Splenomegaly. International Journal of Molecular Sciences, 22(11), 5458. https://doi.org/10.3390/ijms22115458







