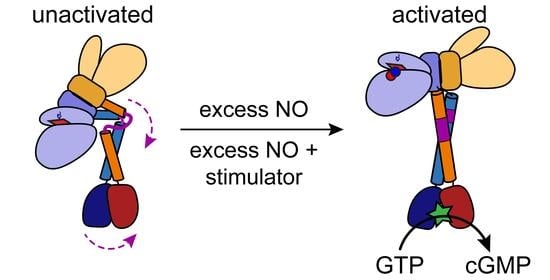
Structural Perspectives on the Mechanism of Soluble Guanylate Cyclase Activation
Abstract
1. Introduction
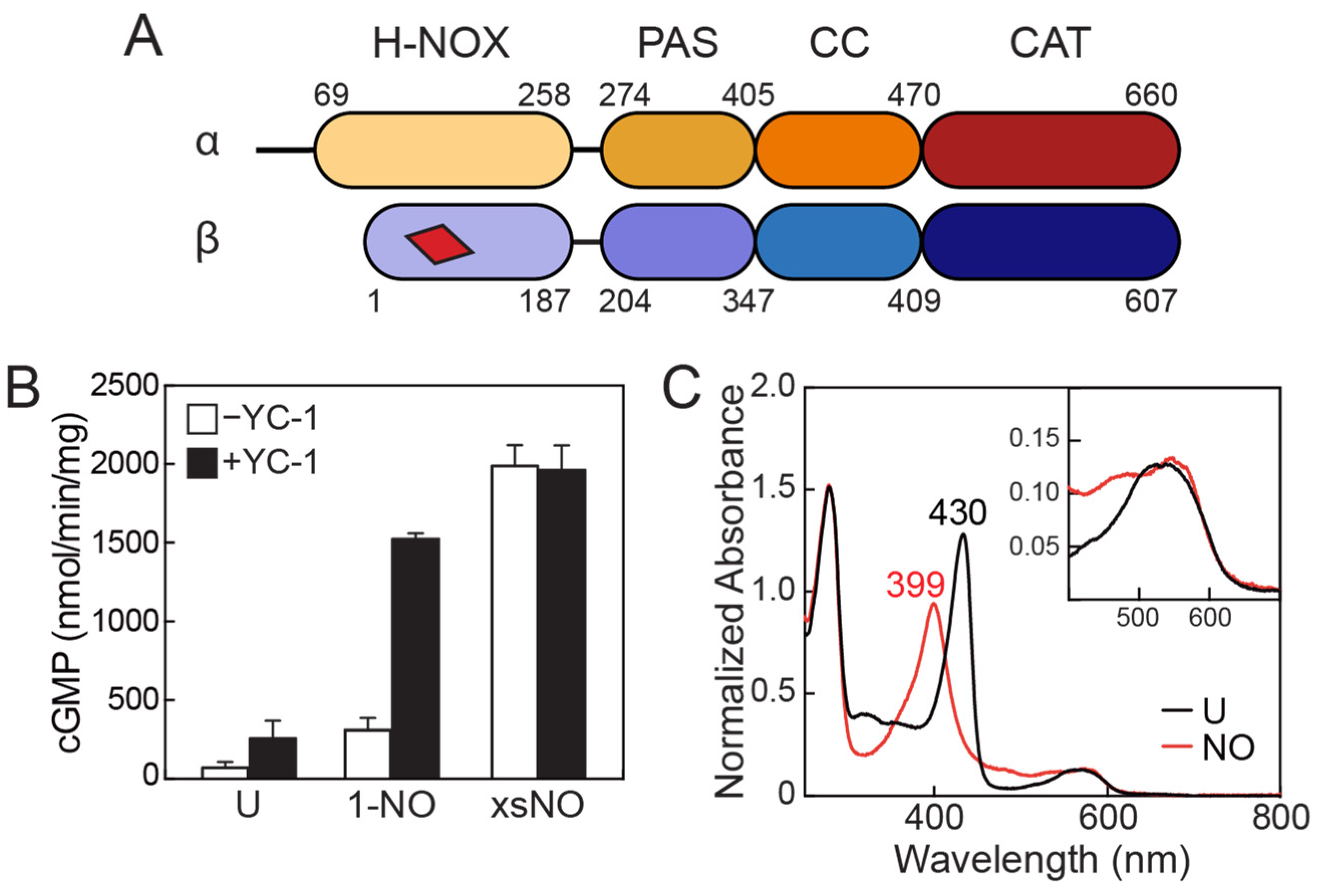
2. Domain Architecture of sGC
3. Activity Profile of sGC
4. Crystal Structures of Individual sGC Domains
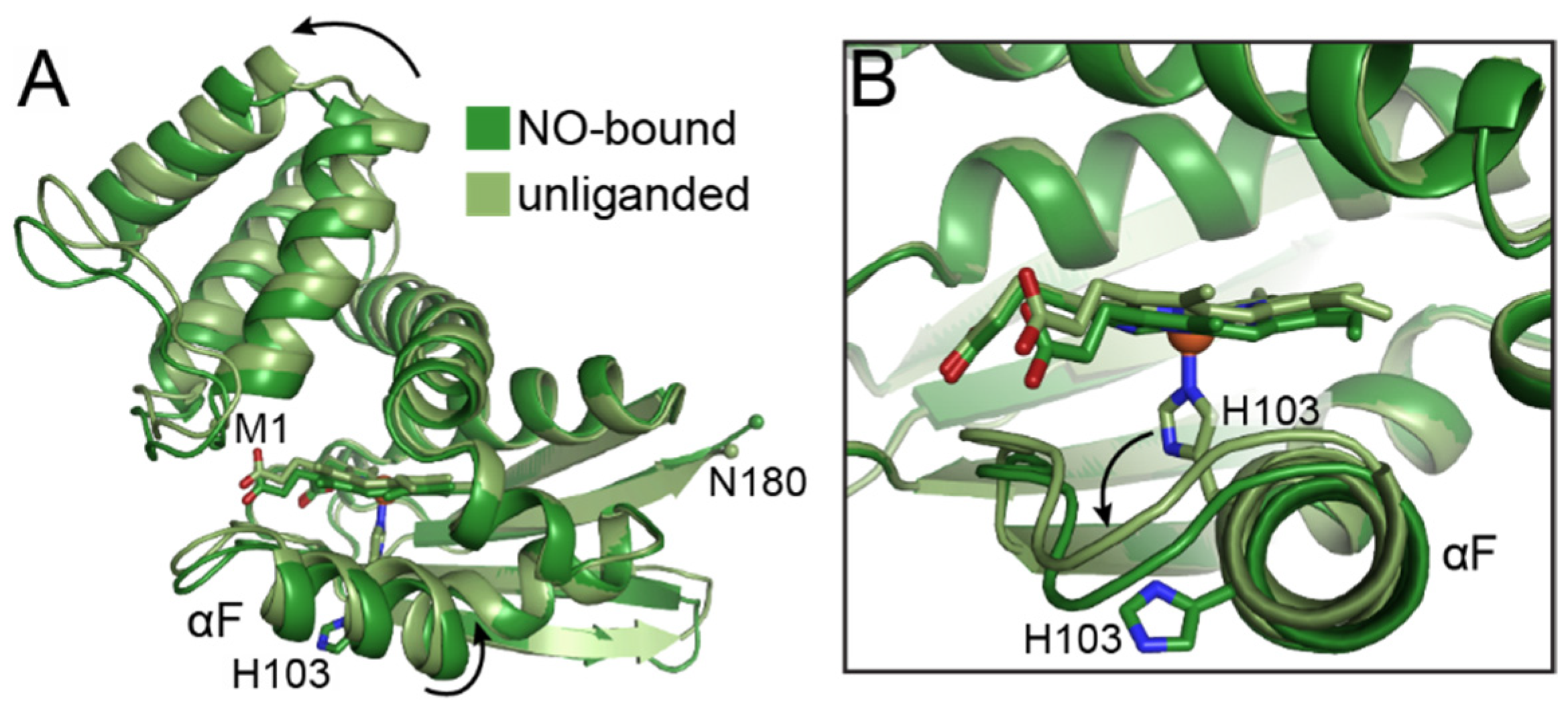
4.1. The H-NOX Domain
4.2. The PAS and CC Domains
4.3. The CAT Domain
5. Low-Resolution Structures of Full-Length sGC
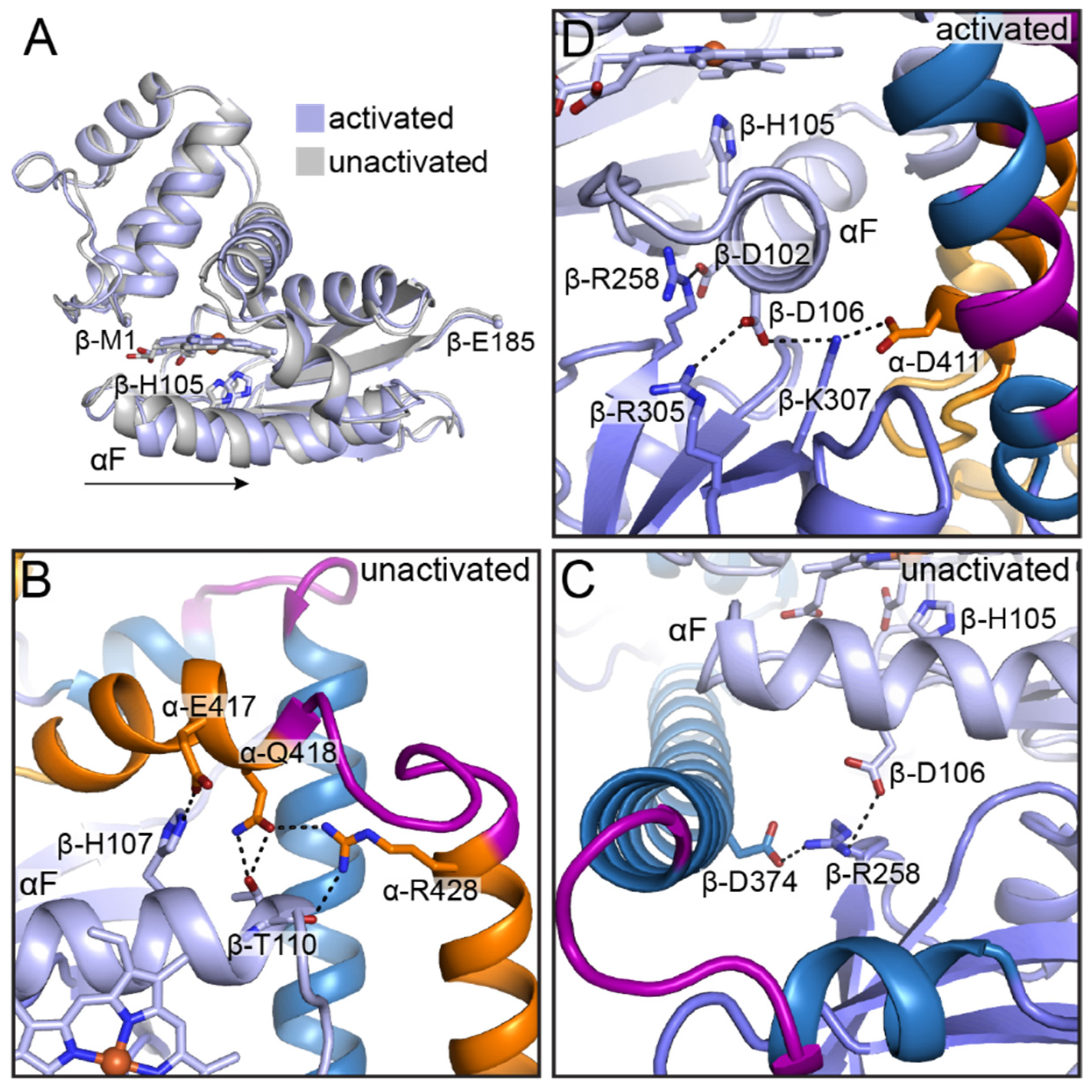
6. High-Resolution Cryo-EM Structures of Full-Length sGC
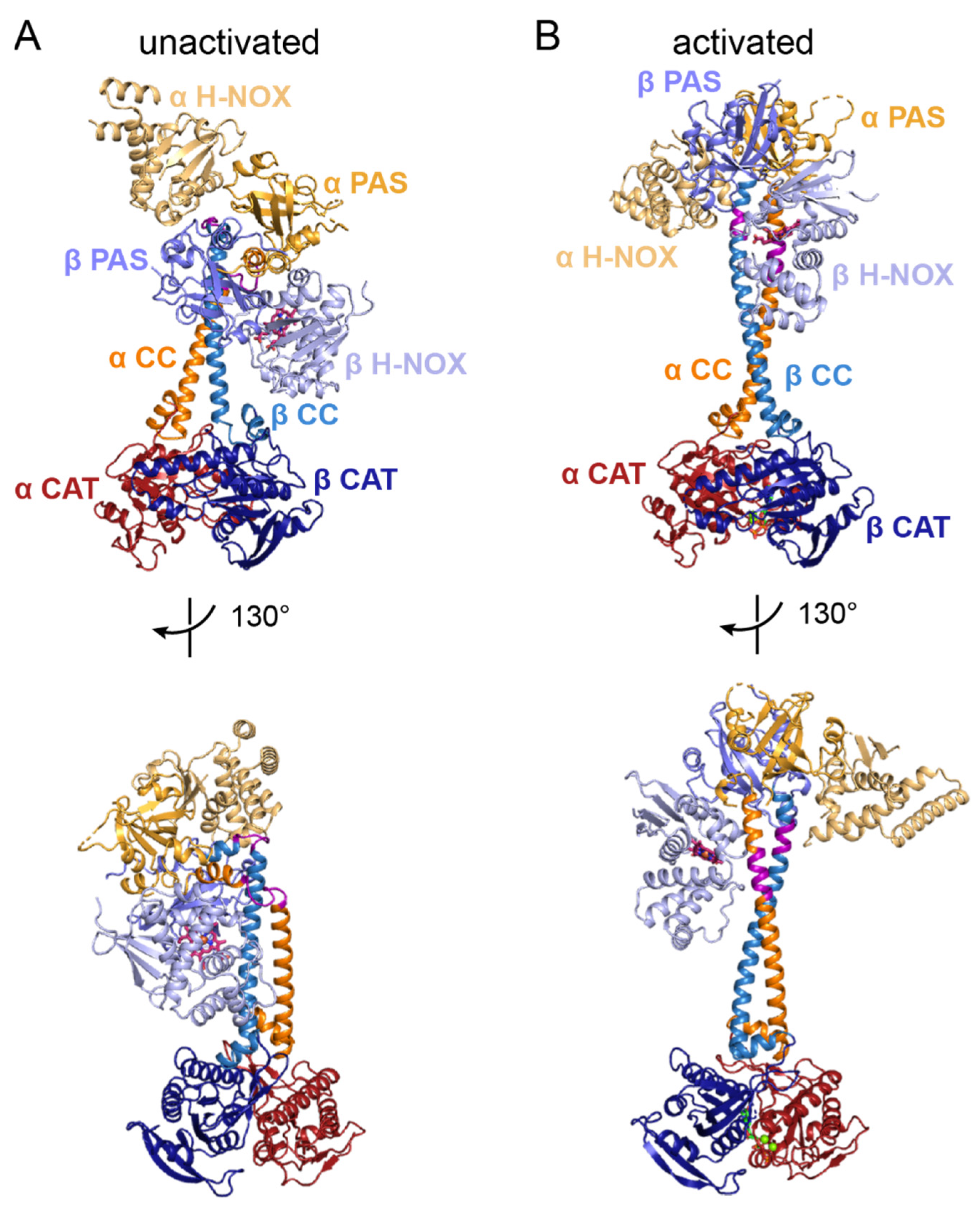
6.1. General Architecture of Full-Length sGC in the Unactivated and Activated States
6.2. The α H-NOX Domain
6.3. The β H-NOX Domain
6.4. The PAS Domains
6.5. The CC Domains
6.6. The CAT Domains
7. Insight into the 1-NO State from Small Angle X-Ray Scattering
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arnold, W.P.; Mittal, C.K.; Katsuki, S.; Murad, F. Nitric Oxide Activates Guanylate Cyclase and Increases Guanosine 3ʹ:5ʹ-Cyclic Monophosphate Levels in Various Tissue Preparations. Proc. Natl. Acad. Sci. USA 1977, 74, 3203–3207. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, H.G. Nitric Oxide and the Cardiovascular System. Compr. Physiol. 2015, 5, 803–828. [Google Scholar] [CrossRef]
- Steinert, J.R.; Chernova, T.; Forsythe, I.D. Nitric Oxide Signaling in Brain Function, Dysfunction, and Dementia. Neuroscientist 2010, 16, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Buys, E.; Sips, P. New Insights into the Role of Soluble Guanylate Cyclase in Blood Pressure Regulation. Curr. Opin. Nephrol. Hypertens. 2014, 23, 135–142. [Google Scholar] [CrossRef]
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble Guanylate Cyclase as an Emerging Therapeutic Target in Cardiopulmonary Disease. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Ben Aissa, M.; Lee, S.H.; Bennett, B.M.; Thatcher, G.R.J. Targeting NO/cGMP Signaling in the CNS for Neurodegeneration and Alzheimer’s Disease. Curr. Med. Chem. 2016, 23, 2770–2788. [Google Scholar] [CrossRef]
- Ghosh, A.; Koziol-White, C.J.; Asosingh, K.; Cheng, G.; Ruple, L.; Groneberg, D.; Friebe, A.; Comhair, S.A.A.; Stasch, J.-P.; Panettieri, R.A.; et al. Soluble Guanylate Cyclase as an Alternative Target for Bronchodilator Therapy in Asthma. Proc. Natl. Acad. Sci. USA 2016, 113, E2355–E2362. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Simoes, D.C.M.; Xanthou, G.; Roussos, C.; Gratziou, C. Soluble Guanylyl Cyclase Expression Is Reduced in Allergic Asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L179–L184. [Google Scholar] [CrossRef][Green Version]
- Conole, D.; Scott, L.J. Riociguat: First Global Approval. Drugs 2013, 73, 1967–1975. [Google Scholar] [CrossRef]
- Horst, B.G.; Marletta, M.A. Physiological Activation and Deactivation of Soluble Guanylate Cyclase. Nitric Oxide 2018, 77, 65–74. [Google Scholar] [CrossRef]
- Wedel, B.; Humbert, P.; Harteneck, C.; Foerster, J.; Malkewitz, J.; Bohme, E.; Schultz, G.; Koesling, D. Mutation of His-105 in the β1 Subunit Yields a Nitric Oxide-Insensitive Form of Soluble Guanylyl Cyclase. Proc. Natl. Acad. Sci. USA 1994, 91, 2592–2596. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Marletta, M.A. Localization of the Heme Binding Region in Soluble Guanylate Cyclase. Biochemistry 1997, 36, 15959–15964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Schelvis, J.P.M.; Babcock, G.T.; Marletta, M.A. Identification of Histidine 105 in the β1 Subunit of Soluble Guanylate Cyclase as the Heme Proximal Ligand. Biochemistry 1998, 37, 4502–4509. [Google Scholar] [CrossRef] [PubMed]
- Koglin, M.; Behrends, S. A Functional Domain of the α1 Subunit of Soluble Guanylyl Cyclase Is Necessary for Activation of the Enzyme by Nitric Oxide and YC-1 but Is Not Involved in Heme Binding. J. Biol. Chem. 2003, 278, 12590–12597. [Google Scholar] [CrossRef]
- Möglich, A.; Ayers, R.A.; Moffat, K. Structure and Signaling Mechanism of Per-ARNT-Sim Domains. Structure 2009, 17, 1282–1294. [Google Scholar] [CrossRef]
- Truebestein, L.; Leonard, T.A. Coiled-coils: The Long and Short of It. BioEssays 2016, 38, 903–916. [Google Scholar] [CrossRef]
- Mason, J.M.; Arndt, K.M. Coiled Coil Domains: Stability, Specificity, and Biological Implications. ChemBioChem 2004, 5, 170–176. [Google Scholar] [CrossRef]
- Winger, J.A.; Marletta, M.A. Expression and Characterization of the Catalytic Domains of Soluble Guanylate Cyclase: Interaction with the Heme Domain. Biochemistry 2005, 44, 4083–4090. [Google Scholar] [CrossRef]
- Rauch, A.; Leipelt, M.; Russwurm, M.; Steegborn, C. Crystal Structure of the Guanylyl Cyclase Cya2. Proc. Natl. Acad. Sci. USA 2008, 105, 15720–15725. [Google Scholar] [CrossRef]
- Winger, J.A.; Derbyshire, E.R.; Lamers, M.H.; Marletta, M.A.; Kuriyan, J. The Crystal Structure of the Catalytic Domain of a Eukaryotic Guanylate Cyclase. BMC Struct. Biol. 2008, 8, 42. [Google Scholar] [CrossRef]
- Allerston, C.K.; von Delft, F.; Gileadi, O. Crystal Structures of the Catalytic Domain of Human Soluble Guanylate Cyclase. PLoS ONE 2013, 8, e57644. [Google Scholar] [CrossRef] [PubMed]
- Seeger, F.; Quintyn, R.; Tanimoto, A.; Williams, G.J.; Tainer, J.A.; Wysocki, V.H.; Garcin, E.D. Interfacial Residues Promote an Optimal Alignment of the Catalytic Center in Human Soluble Guanylate Cyclase: Heterodimerization Is Required but Not Sufficient for Activity. Biochemistry 2014, 53, 2153–2165. [Google Scholar] [CrossRef]
- Cary, S.P.L.; Winger, J.A.; Marletta, M.A. Tonic and Acute Nitric Oxide Signaling through Soluble Guanylate Cyclase Is Mediated by Nonheme Nitric Oxide, ATP, and GTP. Proc. Natl. Acad. Sci. USA 2005, 102, 13064–13069. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.R.; Fernhoff, N.B.; Deng, S.; Marletta, M.A. Nucleotide Regulation of Soluble Guanylate Cyclase Substrate Specificity. Biochemistry 2009, 48, 7519–7524. [Google Scholar] [CrossRef]
- Sürmeli, N.B.; Müskens, F.M.; Marletta, M.A. The Influence of Nitric Oxide on Soluble Guanylate Cyclase Regulation by Nucleotides: Role of the Pseudosymmetric Site. J. Biol. Chem. 2015, 290, 15570–15580. [Google Scholar] [CrossRef] [PubMed]
- Russwurm, M.; Koesling, D. NO Activation of Guanylyl Cyclase. EMBO J. 2004, 23, 4443–4450. [Google Scholar] [CrossRef] [PubMed]
- Fernhoff, N.B.; Derbyshire, E.R.; Marletta, M.A. A Nitric Oxide/Cysteine Interaction Mediates the Activation of Soluble Guanylate Cyclase. Proc. Natl. Acad. Sci. USA 2009, 106, 21602–21607. [Google Scholar] [CrossRef]
- Keefer, L.K.; Nims, R.W.; Davies, K.M.; Wink, D.A. “NONOates” (1-Substituted Diazen-1-Ium-1,2-Diolates) as Nitric Oxide Donors: Convenient Nitric Oxide Dosage Forms. Methods Enzymol. 1996, 268, 281–293. [Google Scholar] [CrossRef]
- Gerzer, R.; Böhme, E.; Hofmann, F.; Schultz, G. Soluble Guanylate Cyclase Purified from Bovine Lung Contains Heme and Copper. FEBS Lett. 1981, 132, 71–74. [Google Scholar] [CrossRef]
- Stone, J.R.; Marletta, M.A. Soluble Guanylate Cyclase from Bovine Lung: Activation with Nitric Oxide and Carbon Monoxide and Spectral Characterization of the Ferrous and Ferric States. Biochemistry 1994, 33, 5636–5640. [Google Scholar] [CrossRef]
- Ko, F.-N.; Wu, C.-C.; Kuo, S.-C.; Lee, F.-Y.; Teng, C.-M. YC-1, A Novel Activator of Platelet Guanylate Cyclase. Blood 1994, 84, 4226–4233. [Google Scholar] [CrossRef]
- Stasch, J.-P.; Becker, E.M.; Alonso-Alija, C.; Apeler, H.; Dembowsky, K.; Feurer, A.; Gerzer, R.; Minuth, T.; Perzborn, E.; Pleiß, U.; et al. NO-Independent Regulatory Site on Soluble Guanylate Cyclase. Nature 2001, 410, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Perl, N.R.; Barden, T.C.; Carvalho, A.; Fretzen, A.; Germano, P.; Im, G.-Y.J.; Jin, H.; Kim, C.; Lee, T.W.-H.; et al. Discovery of IWP-051, a Novel Orally Bioavailable sGC Stimulator with Once-Daily Dosing Potential in Humans. ACS Med. Chem. Lett. 2016, 7, 465–469. [Google Scholar] [CrossRef]
- Tobin, J.V.; Zimmer, D.P.; Shea, C.; Germano, P.; Bernier, S.G.; Liu, G.; Long, K.; Miyashiro, J.; Ranganath, S.; Jacobson, S.; et al. Pharmacological Characterization of IW-1973, a Novel Soluble Guanylate Cyclase Stimulator with Extensive Tissue Distribution, Antihypertensive, Anti-Inflammatory, and Antifibrotic Effects in Preclinical Models of Disease. J. Pharmacol. Exp. Ther. 2018, 365, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Buys, E.S.; Zimmer, D.P.; Chickering, J.; Graul, R.; Chien, Y.T.; Profy, A.; Hadcock, J.R.; Masferrer, J.L.; Milne, G.T. Discovery and Development of next Generation sGC Stimulators with Diverse Multidimensional Pharmacology and Broad Therapeutic Potential. Nitric Oxide 2018, 78, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Schultz, G.; Koesling, D. Sensitizing Soluble Guanylyl Cyclase to Become a Highly CO-Sensitive Enzyme. EMBO J. 1996, 15, 6863–6868. [Google Scholar] [CrossRef]
- Hall, C.N.; Garthwaite, J. What Is the Real Physiological NO Concentration in Vivo? Nitric Oxide 2009, 21, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Brandish, P.E.; Ballou, D.P.; Marletta, M.A. A Molecular Basis for Nitric Oxide Sensing by Soluble Guanylate Cyclase. Proc. Natl. Acad. Sci. USA 1999, 96, 14753–14758. [Google Scholar] [CrossRef]
- Schmidt, K.; Schrammel, A.; Koesling, D.; Mayer, B. Molecular Mechanisms Involved in the Synergistic Activation of Soluble Guanylyl Cyclase by YC-1 and Nitric Oxide in Endothelial Cells. Mol. Pharmacol. 2001, 59, 220–224. [Google Scholar] [CrossRef]
- Iyer, L.M.; Anantharaman, V.; Aravind, L. Ancient Conserved Domains Shared by Animal Soluble Guanylyl Cyclases and Bacterial Signaling Proteins. BMC Genom. 2003, 4, 5. [Google Scholar] [CrossRef]
- Plate, L.; Marletta, M.A. Nitric Oxide-Sensing H-NOX Proteins Govern Bacterial Communal Behavior. Trends Biochem. Sci. 2013, 38, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Karow, D.S.; Pan, D.; Tran, R.; Pellicena, P.; Presley, A.; Mathies, R.A.; Marletta, M.A. Spectroscopic Characterization of the Soluble Guanylate Cyclase-like Heme Domains from Vibrio Cholerae and Thermoanaerobacter Tengcongensis. Biochemistry 2004, 43, 10203–10211. [Google Scholar] [CrossRef] [PubMed]
- Pellicena, P.; Karow, D.S.; Boon, E.M.; Marletta, M.A.; Kuriyan, J. Crystal Structure of an Oxygen-Binding Heme Domain Related to Soluble Guanylate Cyclases. Proc. Natl. Acad. Sci. USA 2004, 101, 12854–12859. [Google Scholar] [CrossRef] [PubMed]
- Nioche, P.; Berka, V.; Vipond, J.; Minton, N.; Tsai, A.-L.; Raman, C.S. Femtomolar Sensitivity of a NO Sensor from Clostridium Botulinum. Science 2004, 306, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Herzik, M.A.; Jonnalagadda, R.; Kuriyan, J.; Marletta, M.A. Structural Insights into the Role of Iron–Histidine Bond Cleavage in Nitric Oxide-Induced Activation of H-NOX Gas Sensor Proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E4156–E4164. [Google Scholar] [CrossRef] [PubMed]
- Hespen, C.W.; Bruegger, J.J.; Phillips-Piro, C.M.; Marletta, M.A. Structural and Functional Evidence Indicates Selective Oxygen Signaling in Caldanaerobacter Subterraneus H-NOX. ACS Chem. Biol. 2016, 11, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Hespen, C.W.; Bruegger, J.J.; Guo, Y.; Marletta, M.A. Native Alanine Substitution in the Glycine Hinge Modulates Conformational Flexibility of Heme Nitric Oxide/Oxygen (H-NOX) Sensing Proteins. ACS Chem. Biol. 2018, 13, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Suess, D.L.M.; Herzik, M.A.; Iavarone, A.T.; Britt, R.D.; Marletta, M.A. Regulation of Nitric Oxide Signaling by Formation of a Distal Receptor–Ligand Complex. Nat. Chem. Biol. 2017, 13, 1216–1221. [Google Scholar] [CrossRef]
- Nambu, J.R.; Lewis, J.O.; Wharton, K.A.; Crews, S.T. The Drosophila Single-Minded Gene Encodes a Helix-Loop-Helix Protein That Acts as a Master Regulator of CNS Midline Development. Cell 1991, 67, 1157–1167. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The Protein Families Database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Ma, X.; Sayed, N.; Baskaran, P.; Beuve, A.; van den Akker, F. PAS-Mediated Dimerization of Soluble Guanylyl Cyclase Revealed by Signal Transduction Histidine Kinase Domain Crystal Structure. J. Biol. Chem. 2008, 283, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Hefti, M.; Purcell, E.B.; Moffat, K. Structure of the Redox Sensor Domain of Azotobacter Vinelandii NifL at Atomic Resolution: Signaling, Dimerization, and Mechanism. Biochemistry 2007, 46, 3614–3623. [Google Scholar] [CrossRef] [PubMed]
- Purohit, R.; Weichsel, A.; Montfort, W.R. Crystal Structure of the Alpha Subunit PAS Domain from Soluble Guanylyl Cyclase. Protein Sci. 2013, 22, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Underbakke, E.S.; Iavarone, A.T.; Chalmers, M.J.; Pascal, B.D.; Novick, S.; Griffin, P.R.; Marletta, M.A. Nitric Oxide-Induced Conformational Changes in Soluble Guanylate Cyclase. Structure 2014, 22, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Beuve, A.; van den Akker, F. Crystal Structure of the Signaling Helix Coiled-Coil Domain of the β1 Subunit of the Soluble Guanylyl Cyclase. BMC Struct. Biol. 2010, 10, 1–13. [Google Scholar] [CrossRef]
- Fritz, B.G.; Roberts, S.A.; Ahmed, A.; Breci, L.; Li, W.; Weichsel, A.; Brailey, J.L.; Wysocki, V.H.; Tama, F.; Montfort, W.R. Molecular Model of a Soluble Guanylyl Cyclase Fragment Determined by Small-Angle X-Ray Scattering and Chemical Cross-Linking. Biochemistry 2013, 52, 1568–1582. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Ruoho, A.E.; Hurley, J.H. Structure of the Adenylyl Cyclase Catalytic Core. Nature 1997, 386, 247–253. [Google Scholar] [CrossRef]
- Tesmer, J.J.G.; Sunahara, R.K.; Gilman, A.G.; Sprang, S.R. Crystal Structure of the Catalytic Domains of Adenylyl Cyclase in a Complex with Gsα·GTPγS. Science 1997, 278, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Tesmer, J.J.G.; Sunahara, R.K.; Johnson, R.A.; Gosselin, G.; Gilman, A.G.; Sprang, S.R. Two-Metal-Ion Catalysis in Adenylyl Cyclase. Science 1999, 285, 756–760. [Google Scholar] [CrossRef]
- Liu, Y.; Ruoho, A.E.; Rao, V.D.; Hurley, J.H. Catalytic Mechanism of the Adenylyl and Guanylyl Cyclases: Modeling and Mutational Analysis. Proc. Natl. Acad. Sci. USA 1997, 94, 13414–13419. [Google Scholar] [CrossRef]
- Sunahara, R.K.; Beuve, A.; Tesmer, J.J.G.; Sprang, S.R.; Garbers, D.L.; Gilman, A.G. Exchange of Substrate and Inhibitor Specificities between Adenylyl and Guanylyl Cyclases. J. Biol. Chem. 1998, 273, 16332–16338. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Underbakke, E.S.; Potter, C.S.; Carragher, B.; Marletta, M.A. Single-Particle EM Reveals the Higher-Order Domain Architecture of Soluble Guanylate Cyclase. Proc. Natl. Acad. Sci. USA 2014, 111, 2960–2965. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. The Resolution Revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef] [PubMed]
- Horst, B.G.; Yokom, A.L.; Rosenberg, D.J.; Morris, K.L.; Hammel, M.; Hurley, J.H.; Marletta, M.A. Allosteric Activation of the Nitric Oxide Receptor Soluble Guanylate Cyclase Mapped by Cryo-Electron Microscopy. eLife 2019, 8, e50634. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, R.; Wu, J.-X.; Chen, L. Structural Insights into the Mechanism of Human Soluble Guanylate Cyclase. Nature 2019, 574, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Murata, L.B.; Weichsel, A.; Brailey, J.L.; Roberts, S.A.; Nighorn, A.; Montfort, W.R. Allostery in Recombinant Soluble Guanylyl Cyclase from Manduca sexta. J. Biol. Chem. 2008, 283, 20968–20977. [Google Scholar] [CrossRef] [PubMed]
- Zabel, U.; Weeger, M.; La, M.; Schmidt, H.H.H.W. Human Soluble Guanylate Cyclase: Functional Expression and Revised Isoenzyme Family. Biochem. J. 1998, 335, 51–57. [Google Scholar] [CrossRef]
- Haase, T.; Haase, N.; Kraehling, J.R.; Behrends, S. Fluorescent Fusion Proteins of Soluble Guanylyl Cyclase Indicate Proximity of the Heme Nitric Oxide Domain and Catalytic Domain. PLoS ONE 2010, 5, e11617. [Google Scholar] [CrossRef]
- Baskaran, P.; Heckler, E.J.; van den Akker, F.; Beuve, A. Identification of Residues in the Heme Domain of Soluble Guanylyl Cyclase That Are Important for Basal and Stimulated Catalytic Activity. PLoS ONE 2011, 6, e26976. [Google Scholar] [CrossRef][Green Version]
- Underbakke, E.S.; Iavarone, A.T.; Marletta, M.A. Higher-Order Interactions Bridge the Nitric Oxide Receptor and Catalytic Domains of Soluble Guanylate Cyclase. Proc. Natl. Acad. Sci. USA 2013, 110, 6777–6782. [Google Scholar] [CrossRef]
- Denninger, J.W.; Schelvis, J.P.M.; Brandish, P.E.; Zhao, Y.; Babcock, G.T.; Marletta, M.A. Interaction of Soluble Guanylate Cyclase with YC-1: Kinetic and Resonance Raman Studies. Biochemistry 2000, 39, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Meisburger, S.P.; Thomas, W.C.; Watkins, M.B.; Ando, N. X-Ray Scattering Studies of Protein Structural Dynamics. Chem. Rev. 2017, 117, 7615–7672. [Google Scholar] [CrossRef] [PubMed]
- Zhong, E.D.; Bepler, T.; Berger, B.; Davis, J.H. CryoDRGN: Reconstruction of Heterogeneous Cryo-EM Structures Using Neural Networks. Nat. Methods 2021, 18, 176–185. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittenborn, E.C.; Marletta, M.A. Structural Perspectives on the Mechanism of Soluble Guanylate Cyclase Activation. Int. J. Mol. Sci. 2021, 22, 5439. https://doi.org/10.3390/ijms22115439
Wittenborn EC, Marletta MA. Structural Perspectives on the Mechanism of Soluble Guanylate Cyclase Activation. International Journal of Molecular Sciences. 2021; 22(11):5439. https://doi.org/10.3390/ijms22115439
Chicago/Turabian StyleWittenborn, Elizabeth C., and Michael A. Marletta. 2021. "Structural Perspectives on the Mechanism of Soluble Guanylate Cyclase Activation" International Journal of Molecular Sciences 22, no. 11: 5439. https://doi.org/10.3390/ijms22115439
APA StyleWittenborn, E. C., & Marletta, M. A. (2021). Structural Perspectives on the Mechanism of Soluble Guanylate Cyclase Activation. International Journal of Molecular Sciences, 22(11), 5439. https://doi.org/10.3390/ijms22115439