Acute and Chronic Pain from Facial Skin and Oral Mucosa: Unique Neurobiology and Challenging Treatment
Abstract
1. Introduction
2. Physiological Somatosensation and Pain from Oral Mucosa and Facial Skin
2.1. Somatosensation of Oral Mucosa and Facial Skin
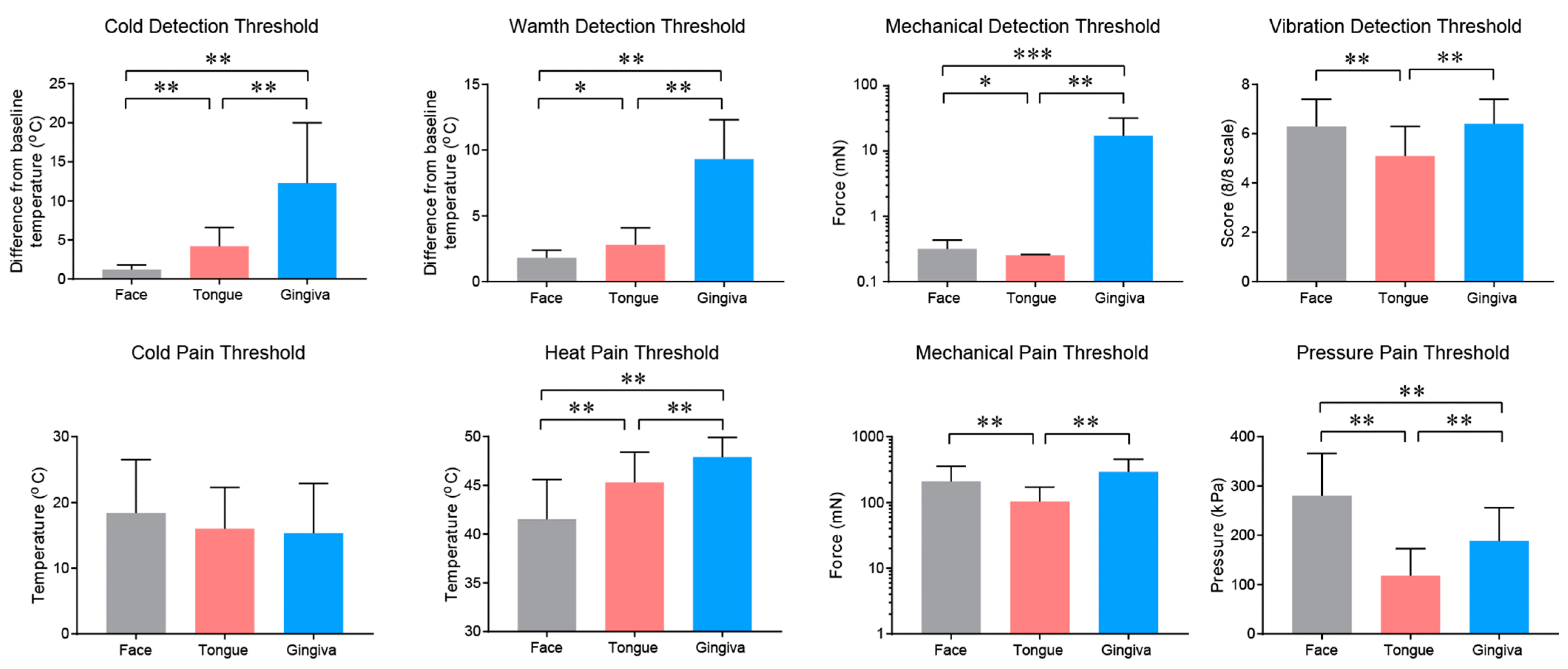
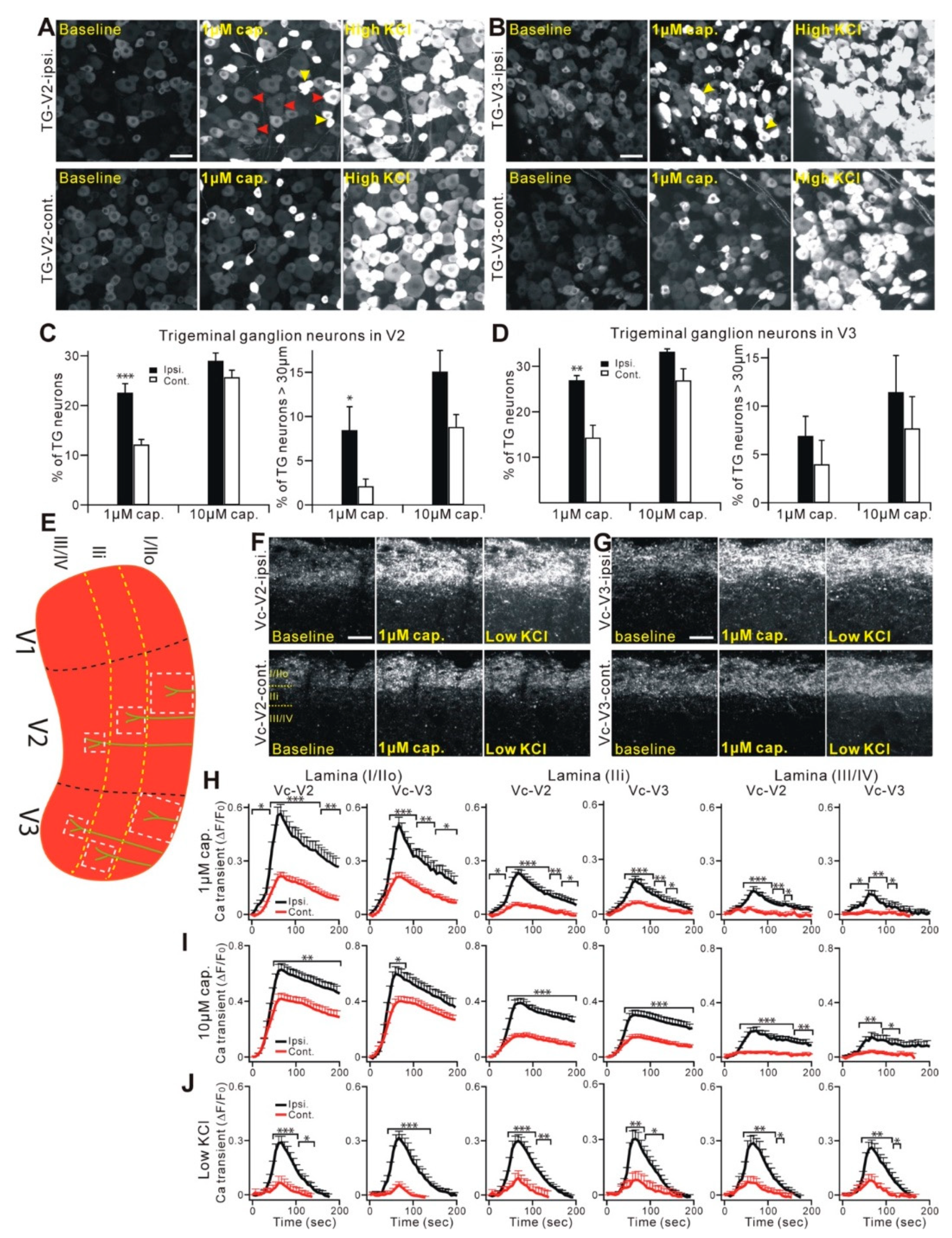
2.2. Properties and Projections of Primary Afferents in Oral Mucosa and Facial Skin
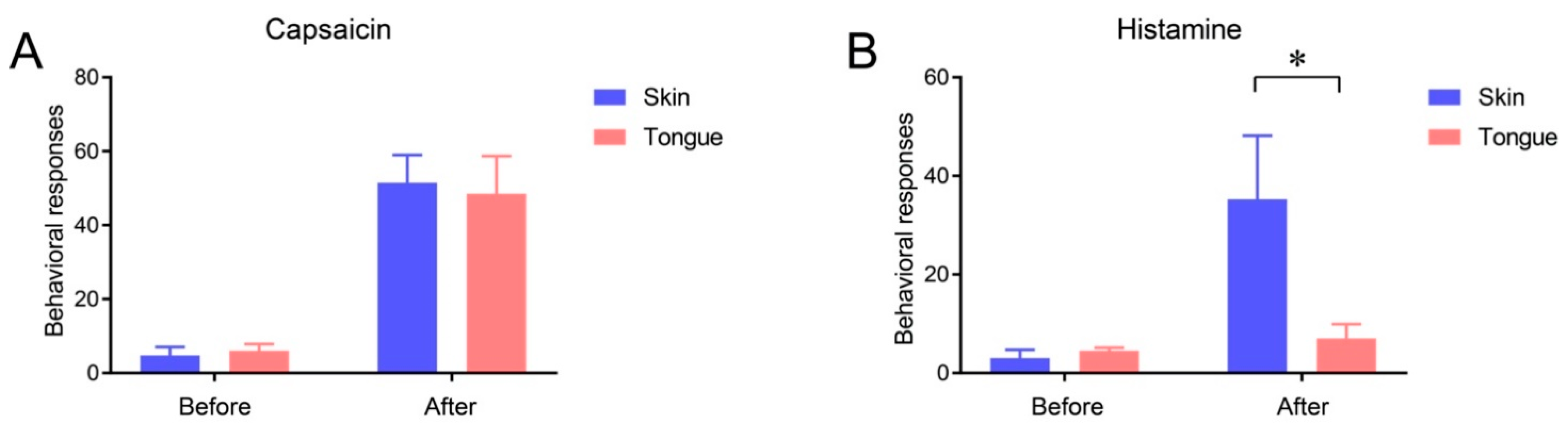
2.3. Itch Sensation of Oral Mucosa Is Weaker Than That of Facial Skin
3. Pathological Painful Conditions and Underlying Neurobiology
3.1. Ulcers and Injury of Oral Mucosa
3.2. Oral Cancer
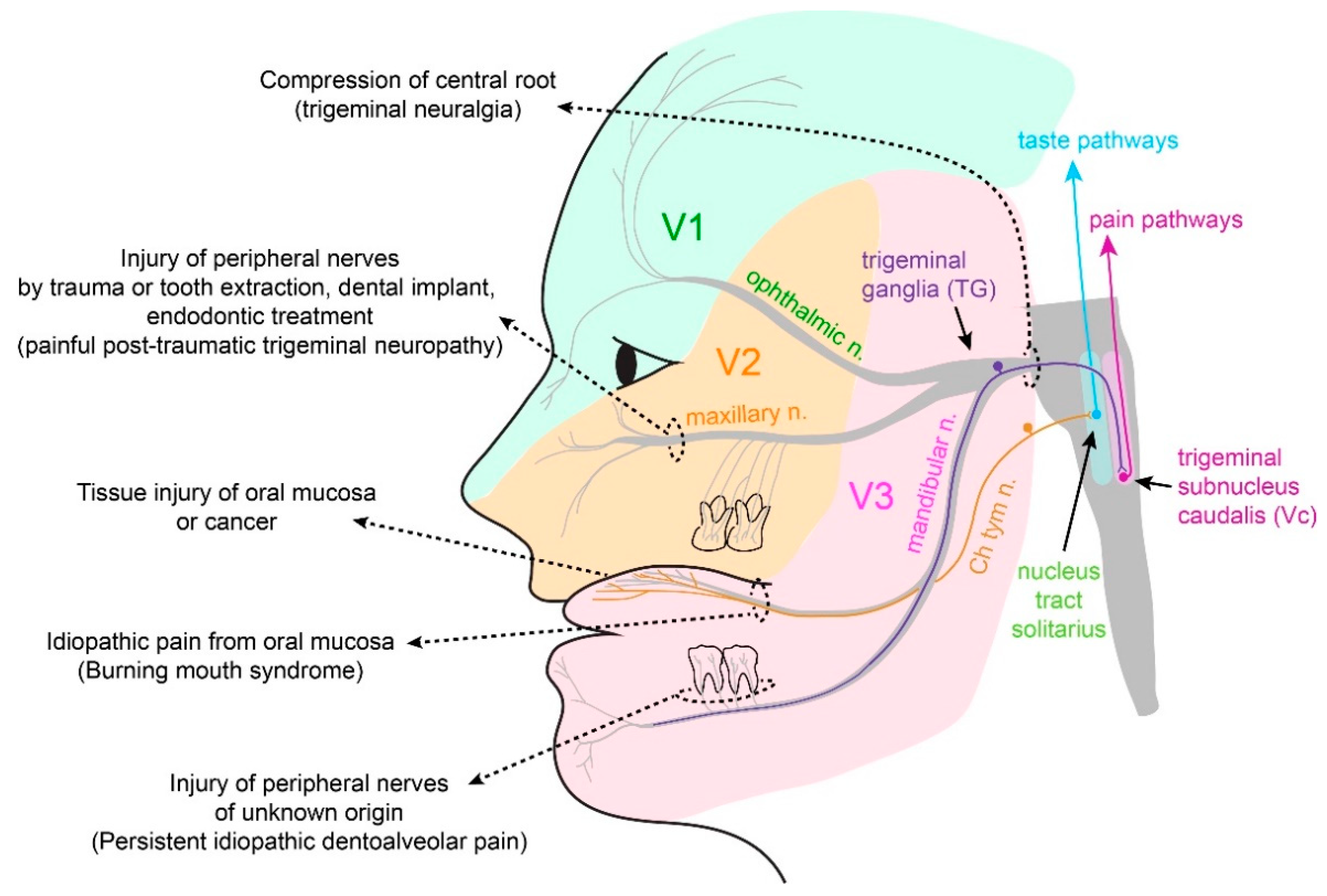
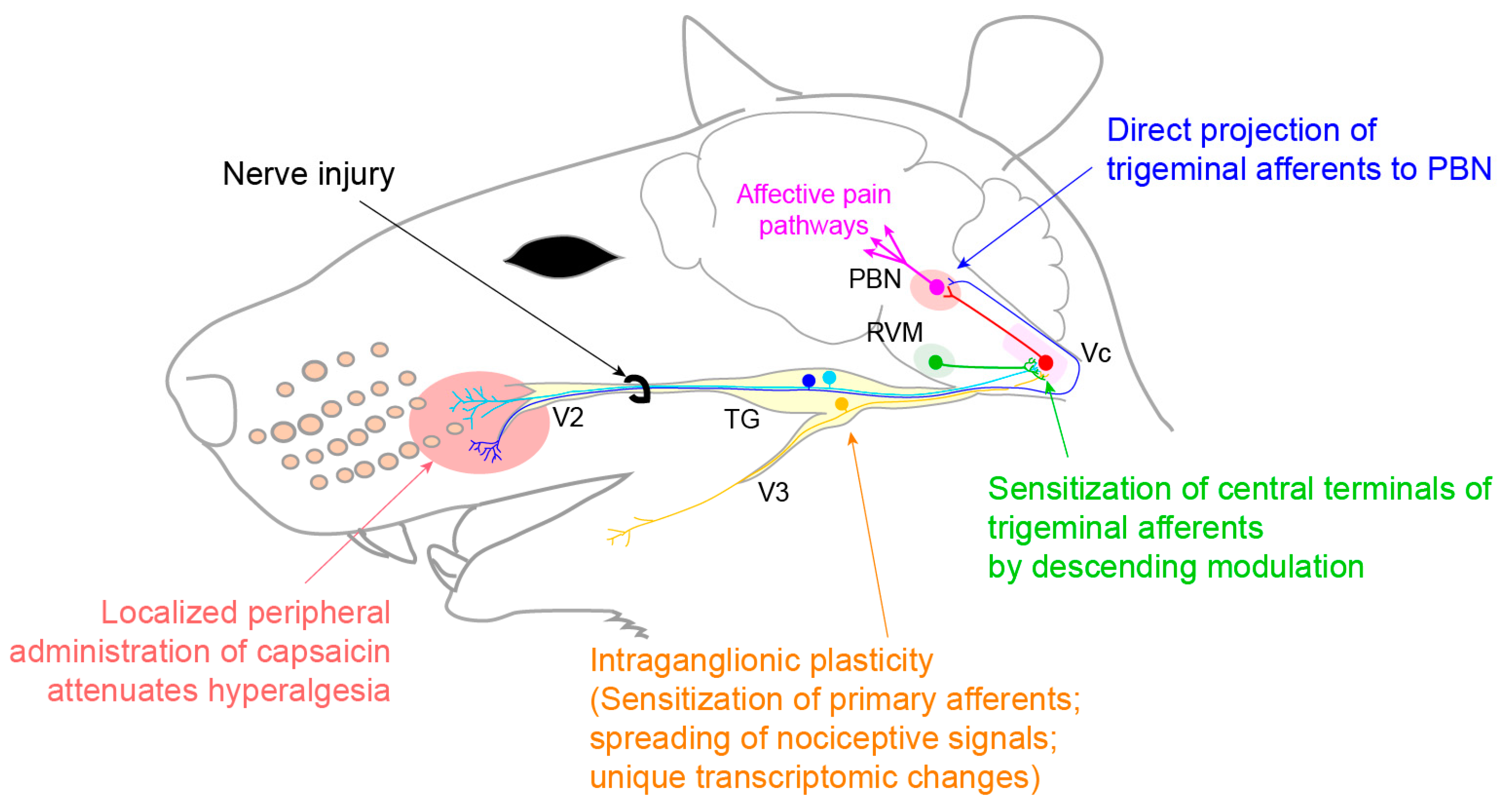
3.3. Neuropathic Pain
3.3.1. Trigeminal Neuralgia and Painful Post-Traumatic Trigeminal Neuropathy
3.3.2. Atypical Odontalgia
3.4. Burning Mouth Syndrome
4. Unique Contributors to Regulation of Oral Pain
4.1. Role of Gustatory Nerves in Pain Modulation
4.2. Chronic Periodontitis without Persistent Pain
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Targhotra, M.; Chauhan, M.K. An Overview on Various Approaches and Recent Patents on Buccal Drug Delivery Systems. Curr. Pharm. Des. 2020, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Glim, J.E.; Van Egmond, M.; Niessen, F.B.; Everts, V.; Beelen, R.H.J. Detrimental dermal wound healing: What can we learn from the oral mucosa? Wound Repair Regen. 2013, 21, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.M. Essentials of Oral Physiology; Mosby: St. Louis, MO, USA, 1995. [Google Scholar]
- Dubner, R. The Neural Basis of Oral and Facial Function; Springer: Boston, MA, USA, 1978. [Google Scholar]
- Zhao, N.N.; Whittle, T.; Murray, G.M.; Peck, C.C. The effects of capsaicin-induced intraoral mucosal pain on jaw movements in humans. J. Orofac. Pain 2012, 26, 277–287. [Google Scholar]
- Mirabile, A.; Airoldi, M.; Ripamonti, C.; Bolner, A.; Murphy, B.; Russi, E.; Numico, G.; Licitra, L.; Bossi, P. Pain management in head and neck cancer patients undergoing chemo-radiotherapy: Clinical practical recommendations. Crit. Rev. Oncol. 2016, 99, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Sessle, B.J. Acute and Chronic Craniofacial Pain: Brainstem Mechanisms of Nociceptive Transmission and Neuroplasticity, and Their Clinical Correlates. Crit. Rev. Oral Biol. Med. 2000, 11, 57–91. [Google Scholar] [CrossRef] [PubMed]
- Campillo, E.R.D.R.; López-López, J.; Chimenos-Küstner, E. Response to topical clonazepam in patients with burning mouth syndrome: A clinical study. Bulletin GIRSO 2010, 49, 19–29. [Google Scholar]
- Pigg, M.; Baad-Hansen, L.; Svensson, P.; Drangsholt, M.; List, T. Reliability of intraoral quantitative sensory testing (QST). Pain 2010, 148, 220–226. [Google Scholar] [CrossRef]
- Bearelly, S.; Cheung, S.W. Sensory Topography of Oral Structures. JAMA Otolaryngol. Neck Surg. 2017, 143, 73–80. [Google Scholar] [CrossRef]
- Cordeiro, P.G.; Schwartz, M.; Neves, R.; Tuma, R. A Comparison of Donor and Recipient Site Sensation in Free Tissue Reconstruction of the Oral Cavity. Ann. Plast. Surg. 1997, 39, 461–468. [Google Scholar] [CrossRef]
- McMillan, A.S. Pain-pressure threshold in human gingivae. J. Orofac. Pain 1995, 9, 44–50. [Google Scholar]
- Wang, Y.; Mo, X.; Zhang, J.; Fan, Y.; Wang, K.; Peter, S. Quantitative sensory testing (QST) in the orofacial region of healthy Chinese: Influence of site, gender and age. Acta Odontol. Scand. 2017, 76, 58–63. [Google Scholar] [CrossRef]
- Poulsen, C.E.; Bendixen, K.H.; Terkelsen, A.J.; May, A.; Hansen, J.; Svensson, P. Region-Specific Effects of Trigeminal Capsaicin Stimulation. J. Oral Facial Pain Headache 2019, 33, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, T.; Baad-Hansen, L.; Ando, T.; Svensson, P. Influence of topical application of capsaicin, menthol and local anesthetics on intraoral somatosensory sensitivity in healthy subjects: Temporal and spatial aspects. Exp. Brain Res. 2015, 233, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Albin, K.C.; Carstens, M.I.; Carstens, E. Modulation of Oral Heat and Cold Pain by Irritant Chemicals. Chem. Sens. 2008, 33, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Kalantzis, A.; Robinson, P.; Loescher, A. Effects of capsaicin and menthol on oral thermal sensory thresholds. Arch. Oral Biol. 2007, 52, 149–153. [Google Scholar] [CrossRef]
- Trulsson, M.; Essick, G.K. Sensations Evoked by Microstimulation of Single Mechanoreceptive Afferents Innervating the Human Face and Mouth. J. Neurophysiol. 2010, 103, 1741–1747. [Google Scholar] [CrossRef]
- Jacobs, R.; Wu, C.-H.; Goossens, K.; Van Loven, K.; Van Hees, J.; Van Steenberghe, D. Oral mucosal versus cutaneous sensory testing: A review of the literature. J. Oral Rehabil. 2002, 29, 923–950. [Google Scholar] [CrossRef]
- Moayedi, Y.; Duenas-Bianchi, L.F.; Lumpkin, E.A. Somatosensory innervation of the oral mucosa of adult and aging mice. Sci. Rep. 2018, 8, 9975. [Google Scholar] [CrossRef]
- Toda, K.; Ishii, N.; Nakamura, Y. Characteristics of mucosal nociceptors in the rat oral cavity: An in vitro study. Neurosci. Lett. 1997, 228, 95–98. [Google Scholar] [CrossRef]
- Grayson, M.; Furr, A.; Ruparel, S. Depiction of Oral Tumor-Induced Trigeminal Afferent Responses Using Single-Fiber Electrophysiology. Sci. Rep. 2019, 9, 4574. [Google Scholar] [CrossRef]
- Urata, K.; Shinoda, M.; Honda, K.; Lee, J.; Maruno, M.; Ito, R.; Gionhaku, N.; Iwata, K. Involvement of TRPV1 and TRPA1 in Incisional Intraoral and Extraoral Pain. J. Dent. Res. 2015, 94, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kichko, T.I.; Neuhuber, W.; Kobal, G.; Reeh, P.W. The roles of TRPV1, TRPA1 and TRPM8 channels in chemical and thermal sensitivity of the mouse oral mucosa. Eur. J. Neurosci. 2018, 47, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kovačič, U.; Tesovnik, B.; Molnar, N.; Cör, A.; Skalerič, U.; Gašperšič, R. Dental pulp and gingivomucosa in rats are innervated by two morphologically and neurochemically different populations of nociceptors. Arch. Oral Biol. 2013, 58, 788–795. [Google Scholar] [CrossRef]
- Wang, S.; Kim, M.; Ali, Z.; Ong, K.; Pae, E.K.; Chung, M.K. TRPV1 and TRPV1-Expressing Nociceptors Mediate Orofacial Pain Behaviors in a Mouse Model of Orthodontic Tooth Movement. Front. Physiol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Gašperšič, R.; Kovačič, U.; Cör, A.; Skaleric, U. Unilateral ligature-induced periodontitis influences the expression of neuropeptides in the ipsilateral and contralateral trigeminal ganglion in rats. Arch. Oral Biol. 2008, 53, 659–665. [Google Scholar] [CrossRef]
- Yajima, T.; Sato, T.; Hosokawa, H.; Kondo, T.; Saito, M.; Shimauchi, H.; Ichikawa, H. Distribution of transient receptor potential melastatin-8-containing nerve fibers in rat oral and craniofacial structures. Ann. Anat. Anat. Anz. 2015, 201, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jing, X.; DeBerry, J.J.; Schwartz, E.S.; Molliver, D.C.; Albers, K.M.; Davis, B.M. Neurturin Overexpression in Skin Enhances Expression of TRPM8 in Cutaneous Sensory Neurons and Leads to Behavioral Sensitivity to Cool and Menthol. J. Neurosci. 2013, 33, 2060–2070. [Google Scholar] [CrossRef]
- Wu, P.; Arris, D.; Grayson, M.; Hung, C.-N.; Ruparel, S. Characterization of sensory neuronal subtypes innervating mouse tongue. PLOS ONE 2018, 13, e0207069. [Google Scholar] [CrossRef]
- Chichorro, J.G.; Porreca, F.; Sessle, B. Mechanisms of craniofacial pain. Cephalalgia 2017, 37, 613–626. [Google Scholar] [CrossRef]
- Carstens, E.; Kuenzler, N.; Handwerker, H.O. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J. Neurophysiol. 1998, 80, 465–492. [Google Scholar] [CrossRef]
- Noma, N.; Tsuboi, Y.; Kondo, M.; Matsumoto, M.; Sessle, B.J.; Kitagawa, J.; Saito, K.; Iwata, K. Organization of pERK-immunoreactive cells in trigeminal spinal nucleus caudalis and upper cervical cord following capsaicin injection into oral and craniofacial regions in rats. J. Comp. Neurol. 2008, 507, 1428–1440. [Google Scholar] [CrossRef]
- Bay, B.; Hilliges, M.; Weidner, C.; Sandborgh-Englund, G. Response of human oral mucosa and skin to histamine provocation: Laser Doppler perfusion imaging discloses differences in the nociceptive nervous system. Acta Odontol. Scand. 2009, 67, 99–105. [Google Scholar] [CrossRef]
- Imamachi, N.; Park, G.H.; Lee, H.; Anderson, D.J.; Simon, M.I.; Basbaum, A.I.; Han, S.-K. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA 2009, 106, 11330–11335. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.M.; Liu, X.-T.; Liu, B.; Liu, X.-Y.; Gao, F.; Zeng, X.; Liu, J.; Yang, Q.; Wilhelm, S.; Yin, J.; et al. Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Martin Carreras-Presas, C.; Amaro Sanchez, J.; Lopez-Sanchez, A.F.; Jane-Salas, E.; Somacarrera Perez, M.L. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2021, 27, 710–712. [Google Scholar] [CrossRef]
- Raber-Durlacher, J.E.; Elad, S.; Barasch, A. Oral mucositis. Oral Oncol. 2010, 46, 452–456. [Google Scholar] [CrossRef]
- Brown, T.J.; Gupta, A. Management of Cancer Therapy–Associated Oral Mucositis. JCO Oncol. Pr. 2020, 16, 103–109. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Q.; Yu, W.; Chang, B.; Le, A. Oral Mucositis: An Update on Innate Immunity and New Interventional Targets. J. Dent. Res. 2020, 99, 1122–1130. [Google Scholar] [CrossRef]
- Nodai, T.; Hitomi, S.; Ono, K.; Masaki, C.; Harano, N.; Morii, A.; Sago-Ito, M.; Ujihara, I.; Hibino, T.; Terawaki, K.; et al. Endothelin-1 Elicits TRP-Mediated Pain in an Acid-Induced Oral Ulcer Model. J. Dent. Res. 2018, 97, 901–908. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ono, K.; Hitomi, S.; Yasuhito, U.; Nodai, T.; Goto, T.; Harano, N.; Watanabe, S.; Inoue, H.; Miyano, K.; et al. Distinct TRPV1- and TRPA1-based mechanisms underlying enhancement of oral ulcerative mucositis-induced pain by 5-fluorouracil. Pain 2016, 157, 1004–1020. [Google Scholar] [CrossRef]
- Ito, M.; Ono, K.; Hitomi, S.; Nodai, T.; Sago, T.; Yamaguchi, K.; Harano, N.; Gunjigake, K.; Hosokawa, R.; Kawamoto, T.; et al. Prostanoid-dependent spontaneous pain and PAR2-dependent mechanical allodynia following oral mucosal trauma: Involvement of TRPV1, TRPA1 and TRPV4. Mol. Pain 2017, 13, 1744806917704138. [Google Scholar] [CrossRef]
- Nolan, M.W.; Long, C.T.; Marcus, K.L.; Sarmadi, S.; Roback, D.M.; Fukuyama, T.; Baeumer, W.; Lascelles, B.D.X. Nocifensive Behaviors in Mice with Radiation-Induced Oral Mucositis. Radiat. Res. 2017, 187, 397–403. [Google Scholar] [CrossRef]
- Bagan, J.; Sarrion, G.; Jimenez, Y. Oral cancer: Clinical features. Oral Oncol. 2010, 46, 414–417. [Google Scholar] [CrossRef]
- Langlais, R.P.; Jainkittivong, A.; Swasdison, S.; Thangpisityotin, M. Oral Squamous Cell Carcinoma: A Clinicopathological Study of 342 Thai Cases. J. Contemp. Dent. Pr. 2009, 10, 33–41. [Google Scholar] [CrossRef]
- Lam, D.K.; Schmidt, B.L. Orofacial pain onset predicts transition to head and neck cancer. Pain 2011, 152, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, P.; Li, W. Comparison of orofacial pain of patients with different stages of precancer and oral cancer. Sci. Rep. 2017, 7, 203. [Google Scholar] [CrossRef]
- Nagamine, K.; Ozaki, N.; Shinoda, M.; Asai, H.; Nishiguchi, H.; Mitsudo, K.; Tohnai, I.; Ueda, M.; Sugiura, Y. Mechanical Allodynia and Thermal Hyperalgesia Induced by Experimental Squamous Cell Carcinoma of the Lower Gingiva in Rats. J. Pain 2006, 7, 659–670. [Google Scholar] [CrossRef]
- Schmidt, B.L. The neurobiology of cancer pain. Neuroscientist 2014, 20, 546–562. [Google Scholar] [CrossRef]
- Tamagawa, T.; Shinoda, M.; Honda, K.; Furukawa, A.; Kaji, K.; Nagashima, H.; Akasaka, R.; Chen, J.; Sessle, B.; Yonehara, Y.; et al. Involvement of Microglial P2Y12 Signaling in Tongue Cancer Pain. J. Dent. Res. 2016, 95, 1176–1182. [Google Scholar] [CrossRef]
- Eisenberg, E. Trigeminal Neuralgia Induced by Sour and Spicy Foods: What Is the Underlying Mechanism? A Case Report. J. Oral Facial Pain Headache 2016, 30, 267–270. [Google Scholar] [CrossRef]
- Benoliel, R.; Zadik, Y.; Eliav, E.; Sharav, Y. Peripheral painful traumatic trigeminal neuropathy: Clinical features in 91 cases and proposal of novel diagnostic criteria. J. Orofac. Pain 2012, 26, 49–58. [Google Scholar] [PubMed]
- Hillerup, S. Iatrogenic injury to oral branches of the trigeminal nerve: Records of 449 cases. Clin. Oral Investig. 2006, 11, 133–142. [Google Scholar] [CrossRef]
- Haviv, Y.; Zadik, Y.; Sharav, Y.; Benoliel, R. Painful Traumatic Trigeminal Neuropathy: An Open Study on the Pharmacotherapeutic Response to Stepped Treatment. J. Oral Facial Pain Headache 2014, 28, 52–60. [Google Scholar] [CrossRef]
- McQuay, H.J.; Tramér, M.; Nye, B.A.; Carroll, D.; Wiffen, P.J.; Moore, R.A. A systematic review of antidepressants in neuropathic pain. Pain 1996, 68, 217–227. [Google Scholar] [CrossRef]
- Niki, Y.; Kanai, A.; Hoshi, D.K.; Okamoto, H. Immediate Analgesic Effect of 8% Lidocaine Applied to the Oral Mucosa in Patients with Trigeminal Neuralgia. Pain Med. 2014, 15, 826–831. [Google Scholar] [CrossRef]
- Babiloni, A.H.; Kapos, F.P.; Nixdorf, D.R. Intraoral administration of botulinum toxin for trigeminal neuropathic pain. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. 2016, 121, e148–e153. [Google Scholar] [CrossRef]
- Wei, J.; Zhu, X.; Yang, G.; Shen, J.; Xie, P.; Zuo, X.; Xia, L.; Han, Q.; Zhao, Y. The efficacy and safety of botulinum toxin type A in treatment of trigeminal neuralgia and peripheral neuropathic pain: A meta-analysis of randomized controlled trials. Brain Behav. 2019, 9, e01409. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Marcoe, J.H. Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 135–140. [Google Scholar] [CrossRef]
- Gaul, C.; Resch, S. Application of the capsaicin 8% cutaneous patch in neuropathic pain of the head and face: A case series. Cephalalgia 2014, 35, 545–550. [Google Scholar] [CrossRef]
- Arora, V.; Campbell, J.N.; Chung, M.-K. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol. Ther. 2021, 220, 107743. [Google Scholar] [CrossRef]
- Wang, S.; Bian, C.; Yang, J.; Arora, V.; Gao, Y.; Wei, F.; Chung, M.-K. Ablation of TRPV1+ Afferent Terminals by Capsaicin Mediates Long-Lasting Analgesia for Trigeminal Neuropathic Pain. Eneuro 2020, 7. [Google Scholar] [CrossRef]
- Batbold, D.; Shinoda, M.; Honda, K.; Furukawa, A.; Koizumi, M.; Akasaka, R.; Yamaguchi, S.; Iwata, K. Macrophages in trigeminal ganglion contribute to ectopic mechanical hypersensitivity following inferior alveolar nerve injury in rats. J. Neuroinflamm. 2017, 14, 249. [Google Scholar] [CrossRef]
- Jeon, H.J.; Han, S.R.; Park, M.K.; Yang, K.Y.; Bae, Y.C.; Ahn, D.K. A novel trigeminal neuropathic pain model: Compression of the trigeminal nerve root produces prolonged nociception in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 149–158. [Google Scholar] [CrossRef]
- Iwata, K.; Imamura, Y.; Honda, K.; Shinoda, M. Physiological Mechanisms Of Neuropathic Pain: The Orofacial Region. Int. Rev. Neurobiol. 2011, 97, 227–250. [Google Scholar] [CrossRef]
- Korczeniewska, O.A.; Khan, J.; Eliav, E.; Benoliel, R. Molecular mechanisms of painful traumatic trigeminal neuropathy—Evidence from animal research and clinical correlates. J. Oral Pathol. Med. 2020, 49, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Korczeniewska, O.A.; Rider, G.K.; Gajra, S.; Narra, V.; Ramavajla, V.; Chang, Y.; Tao, Y.; Soteropoulos, P.; Husain, S.; Khan, J.; et al. Differential gene expression changes in the dorsal root versus trigeminal ganglia following peripheral nerve injury in rats. Eur. J. Pain 2020, 24, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Michot, B.; Bourgoin, S.; Viguier, F.; Hamon, M.; Kayser, V. Differential effects of calcitonin gene-related peptide receptor blockade by olcegepant on mechanical allodynia induced by ligation of the infraorbital nerve vs the sciatic nerve in the rat. Pain 2012, 153, 1939–1948. [Google Scholar] [CrossRef]
- Kim, Y.S.; Chu, Y.; Han, L.; Li, M.; Li, Z.; LaVinka, P.C.; Sun, S.; Tang, Z.; Park, K.; Caterina, M.J.; et al. Central Terminal Sensitization of TRPV1 by Descending Serotonergic Facilitation Modulates Chronic Pain. Neuron 2014, 81, 873–887. [Google Scholar] [CrossRef]
- Rodriguez, E.; Sakurai, K.; Xu, J.; Chen, Y.; Toda, K.; Zhao, S.; Han, B.-X.; Ryu, D.; Yin, H.; Liedtke, W.; et al. A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat. Neurosci. 2017, 20, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Bongenhielm, U.; Boissonade, F.M.; Westermark, A.; Robinson, P.P.; Fried, K. Sympathetic nerve sprouting fails to occur in the trigeminal ganglion after peripheral nerve injury in the rat. Pain 1999, 82, 283–288. [Google Scholar] [CrossRef]
- Benoliel, R.; Eliav, E.; Tal, M. No sympathetic nerve sprouting in rat trigeminal ganglion following painful and non-painful infraorbital nerve neuropathy. Neurosci. Lett. 2001, 297, 151–154. [Google Scholar] [CrossRef]
- Green, B.G.; Schullery, M.T. Stimulation of bitterness by capsaicin and menthol: Differences between lingual areas innervated by the glossopharyngeal and chorda tympani nerves. Chem. Sens. 2003, 28, 45–55. [Google Scholar] [CrossRef]
- Baad-Hansen, L.; Pigg, M.; Ivanovic, S.E.; Faris, H.; List, T.; Drangsholt, M.; Svensson, P. Intraoral somatosensory abnormalities in patients with atypical odontalgia—a controlled multicenter quantitative sensory testing study. Pain 2013, 154, 1287–1294. [Google Scholar] [CrossRef]
- Baad-Hansen, L.; List, T.; Jensen, T.S.; Svensson, P. Increased pain sensitivity to intraoral capsaicin in patients with atypical odontalgia. J. Orofac. Pain 2006, 20, 107–114. [Google Scholar]
- List, T.; Leijon, G.; Helkimo, M.; Öster, A.; Svensson, P. Effect of local anesthesia on atypical odontalgia—A randomized controlled trial. Pain 2006, 122, 306–314. [Google Scholar] [CrossRef]
- Babiloni, A.H.; Nixdorf, D.R.; Moana-Filho, E.J. Persistent dentoalveolar pain disorder: A putative intraoral chronic overlapping pain condition. Oral Dis. 2020, 26, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Calbacho, V.; Torres, P.; Gremeau-Richard, C.; Dallel, R. Co-occurrence of Pain Symptoms and Somatosensory Sensitivity in Burning Mouth Syndrome: A Systematic Review. PLoS ONE 2016, 11, e0163449. [Google Scholar] [CrossRef]
- Honda, M.; Iida, T.; Kamiyama, H.; Masuda, M.; Kawara, M.; Svensson, P.; Komiyama, O. Mechanical sensitivity and psychological factors in patients with burning mouth syndrome. Clin. Oral Investig. 2019, 23, 757–762. [Google Scholar] [CrossRef]
- Beneng, K.; Yilmaz, Z.; Yiangou, Y.; McParland, H.; Anand, P.; Renton, T. Sensory purinergic receptor P2X3 is elevated in burning mouth syndrome. Int. J. Oral Maxillofac. Surg. 2010, 39, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Penza, P.; Majorana, A.; Lombardi, R.; Camozzi, F.; Bonadeo, S.; Sapelli, P.; Giuseppe, L. “Burning tongue” and “burning tip”: The diagnostic challenge of the burning mouth syndrome. Clin. J. Pain 2010, 26, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Z.; Renton, T.; Yiangou, Y.; Zakrzewska, J.; Chessell, I.; Bountra, C.; Anand, P. Burning mouth syndrome as a trigeminal small fibre neuropathy: Increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J. Clin. Neurosci. 2007, 14, 864–871. [Google Scholar] [CrossRef]
- Lauria, G.; Majorana, A.; Borgna, M.; Lombardi, R.; Penza, P.; Padovani, A.; Sapelli, P. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain 2005, 115, 332–337. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Egbuniwe, O.; Renton, T. The Detection of Small-Fiber Neuropathies in Burning Mouth Syndrome and Iatrogenic Lingual Nerve Injuries: Use of Quantitative Sensory Testing. J. Oral Facial Pain Headache 2016, 30, 87–98. [Google Scholar] [CrossRef]
- Madariaga, V.I.; Tanaka, H.; Ernberg, M. Psychophysical characterisation of burning mouth syndrome—A systematic review and meta-analysis. J. Oral Rehabil. 2020, 47, 1590–1605. [Google Scholar] [CrossRef]
- Azzi, L.; Croveri, F.; Pasina, L.; Porrini, M.; Vinci, R.; Manfredini, M.; Tettamanti, L.; Tagliabue, A.; Silvestre-Rangil, J.; Spadari, F. A “burning” therapy for burning mouth syndrome: Preliminary results with the administration of topical capsaicin. J. Boil. Regul. Homeost. Agents 2017, 31, 89–95. [Google Scholar]
- Just, T.; Steiner, S.; Pau, H.W. Oral pain perception and taste in Burning Mouth Syndrome. J. Oral Pathol. Med. 2010, 39, 22–27. [Google Scholar] [CrossRef]
- Jääskeläinen, S.K. Pathophysiology of primary burning mouth syndrome. Clin. Neurophysiol. 2012, 123, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, S.K. Is burning mouth syndrome a neuropathic pain condition? Pain 2018, 159, 610–613. [Google Scholar] [CrossRef]
- Shinoda, M.; Takeda, M.; Honda, K.; Maruno, M.; Katagiri, A.; Satoh-Kuriwada, S.; Shoji, N.; Tsuchiya, M.; Iwata, K. Involvement of peripheral artemin signaling in tongue pain: Possible mechanism in burning mouth syndrome. Pain 2015, 156, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Elitt, C.M.; Malin, S.A.; Koerber, H.R.; Davis, B.M.; Albers, K.M. Overexpression of artemin in the tongue increases expression of TRPV1 and TRPA1 in trigeminal afferents and causes oral sensitivity to capsaicin and mustard oil. Brain Res. 2008, 1230, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Yamada, J.; Ohlsson, A.; Haliburton, S.; Shorkey, A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst. Rev. 2016, 7, CD001069. [Google Scholar] [CrossRef]
- E Mercer, M.; Holder, M.D. Antinociceptive Effects of Palatable Sweet Ingesta on Human Responsivity to Pressure Pain. Physiol. Behav. 1997, 61, 311–318. [Google Scholar] [CrossRef]
- Eggleston, K.; White, T.L.; Sheehe, P.R. Adding Cocoa to Sucrose: The Effect on Cold Pain Tolerance. Chem. Sens. 2010, 35, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kakeda, T.; Ogino, Y.; Moriya, F.; Saito, S. Sweet taste-induced analgesia: An fMRI study. Neuroreport 2010, 21, 427–431. [Google Scholar] [CrossRef]
- Kakeda, T.; Ito, M.; Matsui, T.; Ishikawa, T. The evidence for sweet substance-induced analgesia in adult human. PAIN Res. 2008, 23, 159–166. [Google Scholar] [CrossRef][Green Version]
- Riello, M.; Cecchini, M.P.; Zanini, A.; Di Chiappari, M.; Tinazzi, M.; Fiorio, M. Perception of phasic pain is modulated by smell and taste. Eur. J. Pain 2019, 23, 1790–1800. [Google Scholar] [CrossRef]
- Nixdorf, N.R.; John, M.T.; Schierz, O.; A Bereiter, D.; Hellekant, G.; Berieter, D.A. Self-reported severity of taste disturbances correlates with dysfunctional grade of TMD pain. J. Oral Rehabil. 2009, 36, 792–800. [Google Scholar] [CrossRef]
- Eliav, E.; Kamran, B.; Schaham, R.; Czerninski, R.; Gracely, R.H.; Benoliel, R. Evidence of chorda tympani dysfunction in patients with burning mouth syndrome. J. Am. Dent. Assoc. 2007, 138, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Maeda, E.; Katsura, H.; Nin, T.; Sakaguchi-Fukunaga, A.; Mishiro, Y.; Sakagami, M. Change of somatosensory function of the tongue caused by chorda tympani nerve disorder after stapes surgery. Laryngoscope 2018, 128, 701–706. [Google Scholar] [CrossRef]
- Schöbel, N.; Kyereme, J.; Minovi, A.; Dazert, S.; Bartoshuk, L.; Hatt, H. Sweet taste and chorda tympani transection alter capsaicin-induced lingual pain perception in adult human subjects. Physiol. Behav. 2012, 107, 368–373. [Google Scholar] [CrossRef]
- Ren, K.; Blass, E.M.; Zhou, Q.-Q.; Dubner, R. Suckling and sucrose ingestion suppress persistent hyperalgesia and spinal Fos expression after forepaw inflammation in infant rats. Proc. Natl. Acad. Sci. USA 1997, 94, 1471–1475. [Google Scholar] [CrossRef]
- Anseloni, V.; Ren, K.; Dubner, R.; Ennis, M. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience 2005, 133, 231–243. [Google Scholar] [CrossRef]
- Segato, F.; Castro-Souza, C.; Segato, E.; Morato, S.; Coimbra, N. Sucrose ingestion causes opioid analgesia. Braz. J. Med Biol. Res. 1997, 30, 981–984. [Google Scholar] [CrossRef][Green Version]
- Irusta, A.; Savoldi, M.; Kishi, R.; Resende, G.; Freitas, R.L.; Carvalho, A.D.; Coimbra, N.C. Psychopharmacological evidences for the involvement of muscarinic and nicotinic cholinergic receptors on sweet substance-induced analgesia in Rattus norvegicus. Neurosci. Lett. 2001, 305, 115–118. [Google Scholar] [CrossRef]
- Reboucas, E.C.; Segato, E.N.; Kishi, R.; Freitas, R.L.; Savoldi, M.; Morato, S.; Coimbra, N.C. Effect of the blockade of mu1-opioid and 5HT2A-serotonergic/alpha1-noradrenergic receptors on sweet-substance-induced analgesia. Psychopharmacology 2005, 179, 349–355. [Google Scholar] [CrossRef]
- Davies, A.J.; Kim, D.; Park, J.; Lee, J.-Y.; Vang, H.; Pickering, A.E.; Oh, S.B. Hedonic drinking engages a supraspinal inhibition of thermal nociception in adult rats. Pain 2019, 160, 1059–1069. [Google Scholar] [CrossRef]
- Boucher, Y.; Felizardo, R.; Klein, A.H.; Carstens, M.I.; Carstens, E. Gustatory modulation of the responses of trigeminal subnucleus caudalis neurons to noxious stimulation of the tongue in rats. Eur. J. Neurosci. 2013, 38, 2812–2822. [Google Scholar] [CrossRef]
- Boucher, Y.; Simons, C.T.; Carstens, M.I.; Carstens, E. Effects of gustatory nerve transection and/or ovariectomy on oral capsaicin avoidance in rats. Pain 2014, 155, 814–820. [Google Scholar] [CrossRef]
- Katsura, H.; Tsuzuki, K.; Noguchi, K.; Sakagami, M. Differential Expression of Capsaicin-, Menthol-, and Mustard Oil-Sensitive Receptors in Naive Rat Geniculate Ganglion Neurons. Chem. Sens. 2006, 31, 681–688. [Google Scholar] [CrossRef][Green Version]
- Tatsumi, E.; Katsura, H.; Kobayashi, K.; Yamanaka, H.; Tsuzuki, K.; Noguchi, K.; Sakagami, M. Changes in transient receptor potential channels in the rat geniculate ganglion after chorda tympani nerve injury. Neuroreport 2015, 26, 856–861. [Google Scholar] [CrossRef]
- Brunsvold, M.A.; Nair, P.; Oates, T.W. Chief complaints of patients seeking treatment for periodontitis. J. Am. Dent. Assoc. 1999, 130, 359–364. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence 2015, 6, 236–243. [Google Scholar] [CrossRef]
- Nagashima, H.; Shinoda, M.; Honda, K.; Kamio, N.; Watanabe, M.; Suzuki, T.; Sugano, N.; Sato, S.; Iwata, K. CXCR4 signaling in macrophages contributes to periodontal mechanical hypersensitivity inPorphyromonas gingivalis-induced periodontitis in mice. Mol. Pain 2017, 13, 1744806916689269. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; McIntosh, M.L.; Nishiyama, S.-I.; Yoshimura, F. Mechanism and implications of CXCR4-mediated integrin activation byPorphyromonas gingivalis. Mol. Oral Microbiol. 2013, 28, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Puchimada, B.; Kadouri, D.; Zusman, T.; Javed, F.; Eliav, E. The anti-nociceptive effects of Porphyromonas gingivalis lipopolysaccharide. Arch. Oral Biol. 2019, 102, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Calil, I.L.; Zarpelon, A.C.; Guerrero, A.T.G.; Alves-Filho, J.C.; Ferreira, S.H.; Cunha, F.Q.; Cunha, T.M.; Verri, W.A., Jr. Lipopolysaccharide Induces Inflammatory Hyperalgesia Triggering a TLR4/MyD88-Dependent Cytokine Cascade in the Mice Paw. PLoS ONE 2014, 9, e90013. [Google Scholar] [CrossRef] [PubMed]
| Table | Subtype |
|---|---|
| 1. Orofacial pain attributed to disorders of dentoalveolar and anatomically related structures | 1.1 Dental pain 1.2 Oral mucosal, salivary gland, and jawbone pains |
| 2. Myofascial orofacial pain | 2.1 Primary myofascial orofacial pain 2.2 Secondary myofascial orofacial pain |
| 3. Temporomandibular joint (TMJ) pain | 3.1 Primary temporomandibular joint pain 3.2 Secondary temporomandibular joint pain |
| 4. Orofacial pain attributed to lesion or disease of the cranial nerves | 4.1Pain attributed to lesion or diseaseofthetrigeminalnerve 4.2 Pain attributed to lesion or disease of the glossopharyngeal nerve |
| 5. Orofacial pains resembling presentations of primary headaches | 5.1 Orofacial migraine 5.2 Tension-type orofacial pain 5.3 Trigeminal autonomic orofacial pain 5.4 Neurovascular orofacial pain |
| 6. Idiopathic orofacial pain | 6.1 Burning mouth syndrome (BMS) 6.2 Persistent idiopathic facial pain (PIFP) 6.3 Persistent idiopathic dentoalveolar pain 6.4 Constant unilateral facial pain with additional attacks (CUFPA) |
| TN | PTTN | |
|---|---|---|
| Average Age of Onset | 59 | 49 |
| Etiology | Classical TN: Spontaneous, neurovascular compression Secondary TN: underlying disease (e.g., multiple sclerosis) | Trauma to facial skeleton Iatrogenic: 3rd molar extraction, injection of local anesthetic, dentoalveolar surgery, implant, endodontic treatment, orthognathic surgery |
| Pain | Side: Unilateral | Unilateral or bilateral (10%) |
| Area involved: V3 (50%), V2 (30%), V2 + V3 (20%) | Injury-related area | |
| Quality: Electric shock-like pain (sometimes combined with shooting and stabbing pain) | Burning, stabbing, pressure, and throbbing | |
| Duration: Occurs continuously. Paroxysms lasting from a second to 2 min followed by a refractory period. Abrupt in onset and termination. Sometimes superimposed on background pain between attacks | Heterogeneous with respect to frequency and duration and often occurs continuously throughout day (50%), short attack 1–4 min (25%; longer than TN), constant, or intermediate. | |
| Intensity: Severe (VAS 9.1; greater than PTTN) | Moderate to severe (VAS 7.7) | |
| Trigger: Often triggered by innocuous stimuli within the affected region | Trigger is rarely identifiable | |
| QST | No sensory dysfunction | Hypoalgesia, allodynia, hyperalgesia, dysesthesia, paresthesia |
| First line of treatment | Carbamazepine | Tricyclic antidepressant (Amitriptyline) alone or in combination with gabapentin or an SNRI (duloxetine) |
| Response to therapy | Pain relief greater than 50% in 74% of patients | Pain relief greater than 50% in 11% of patients |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, M.-K.; Wang, S.; Oh, S.-L.; Kim, Y.S. Acute and Chronic Pain from Facial Skin and Oral Mucosa: Unique Neurobiology and Challenging Treatment. Int. J. Mol. Sci. 2021, 22, 5810. https://doi.org/10.3390/ijms22115810
Chung M-K, Wang S, Oh S-L, Kim YS. Acute and Chronic Pain from Facial Skin and Oral Mucosa: Unique Neurobiology and Challenging Treatment. International Journal of Molecular Sciences. 2021; 22(11):5810. https://doi.org/10.3390/ijms22115810
Chicago/Turabian StyleChung, Man-Kyo, Sheng Wang, Se-Lim Oh, and Yu Shin Kim. 2021. "Acute and Chronic Pain from Facial Skin and Oral Mucosa: Unique Neurobiology and Challenging Treatment" International Journal of Molecular Sciences 22, no. 11: 5810. https://doi.org/10.3390/ijms22115810
APA StyleChung, M.-K., Wang, S., Oh, S.-L., & Kim, Y. S. (2021). Acute and Chronic Pain from Facial Skin and Oral Mucosa: Unique Neurobiology and Challenging Treatment. International Journal of Molecular Sciences, 22(11), 5810. https://doi.org/10.3390/ijms22115810







