Role of PEG35, Mitochondrial ALDH2, and Glutathione in Cold Fatty Liver Graft Preservation: An IGL-2 Approach
Abstract
1. Introduction
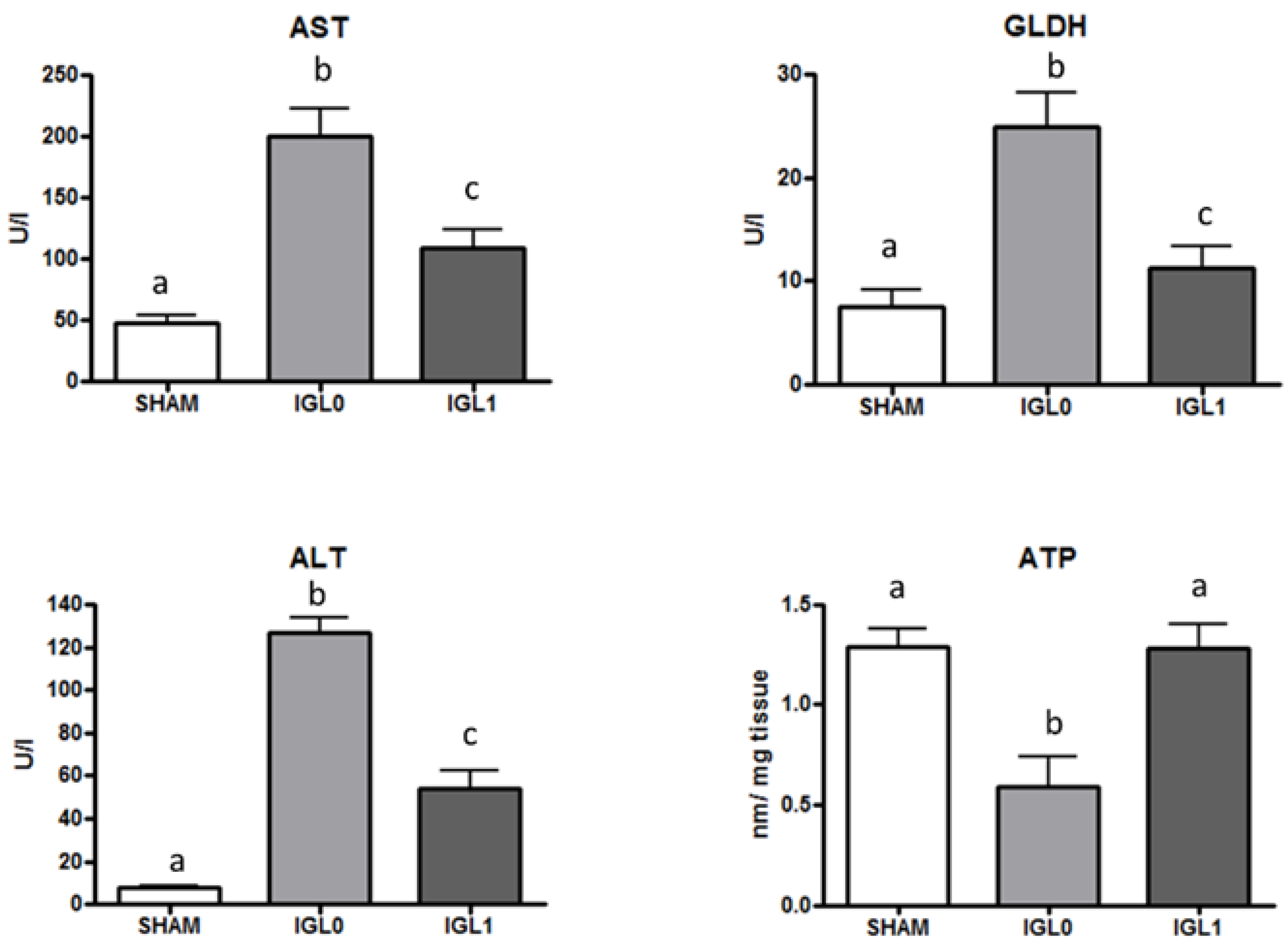
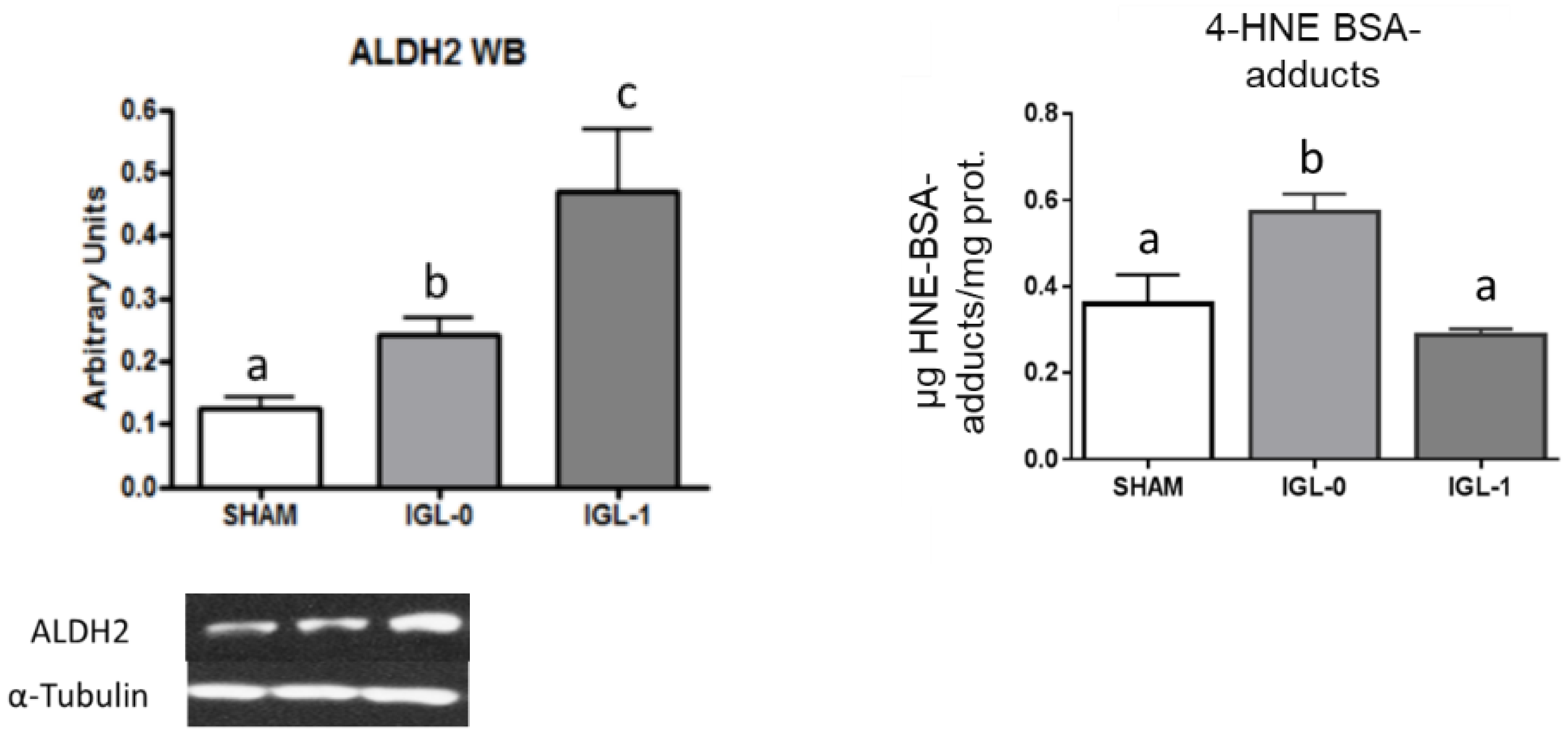
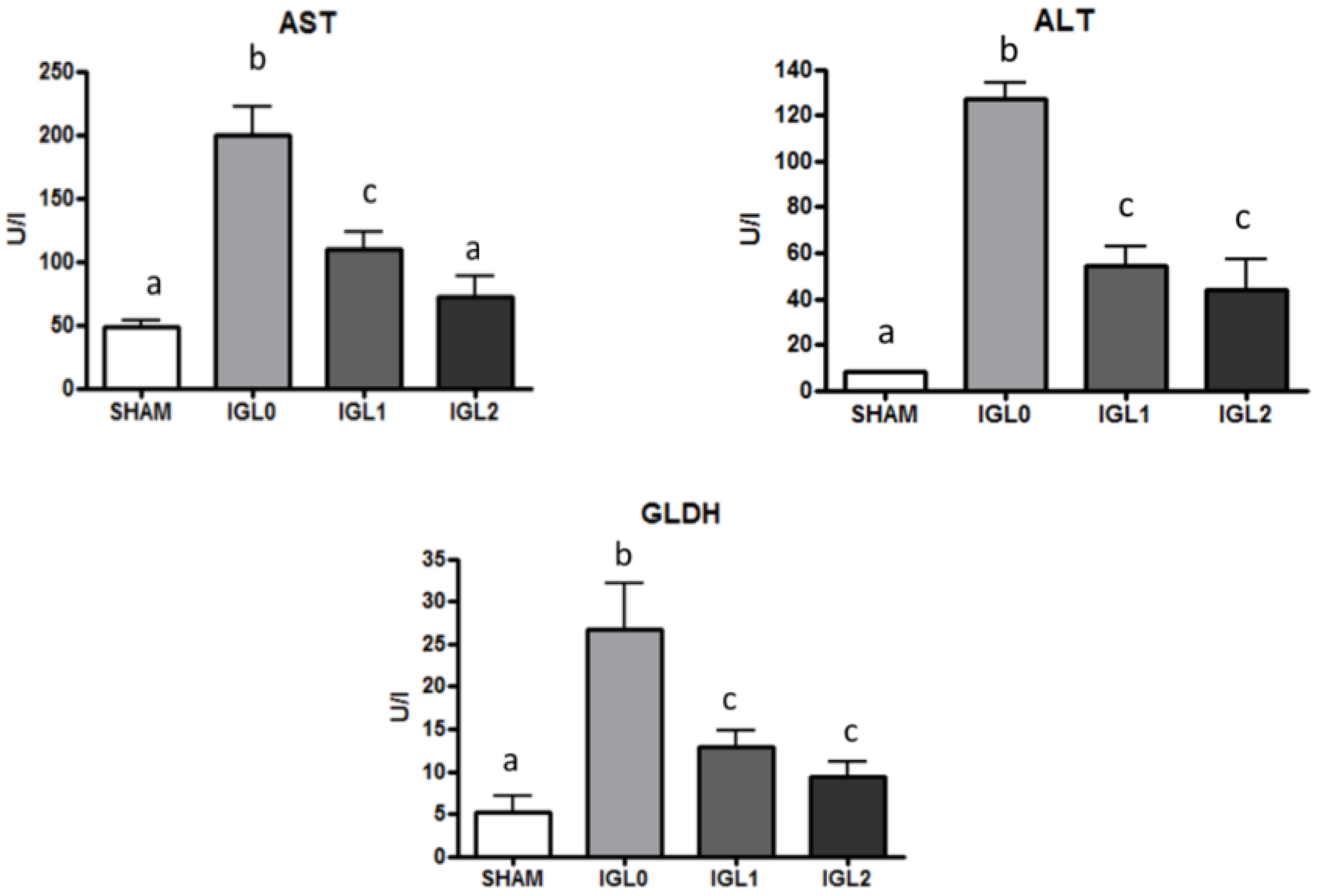
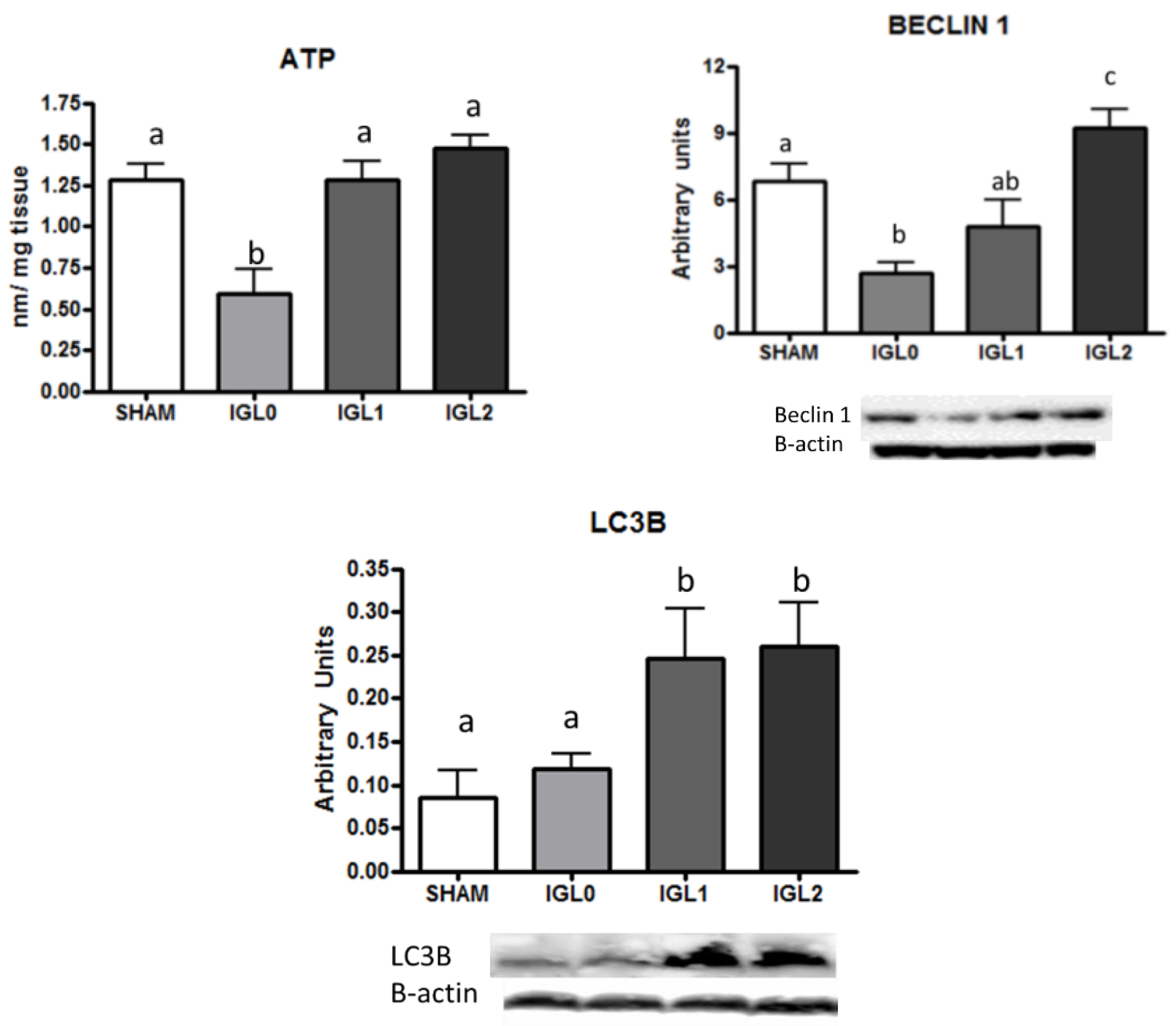
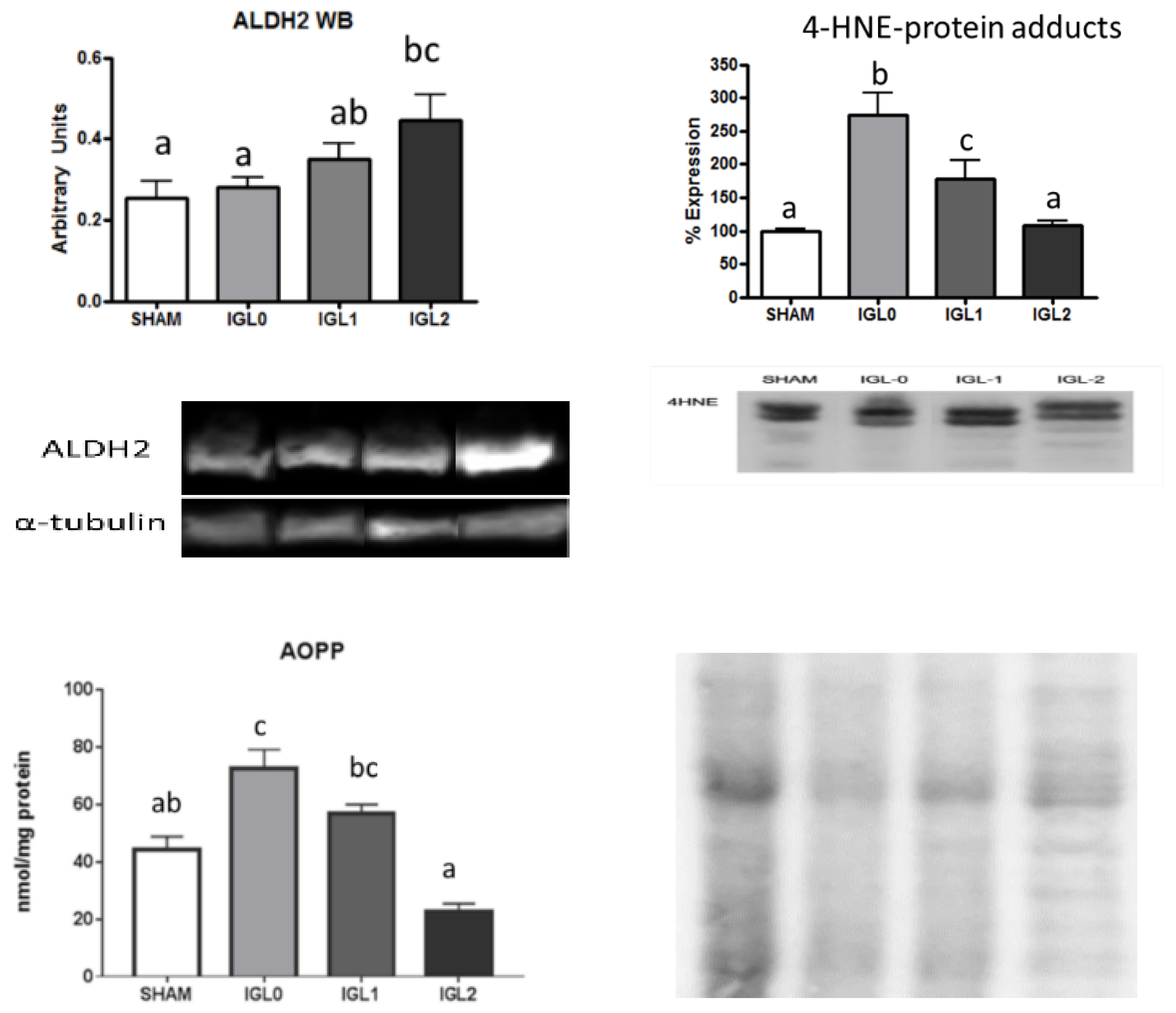
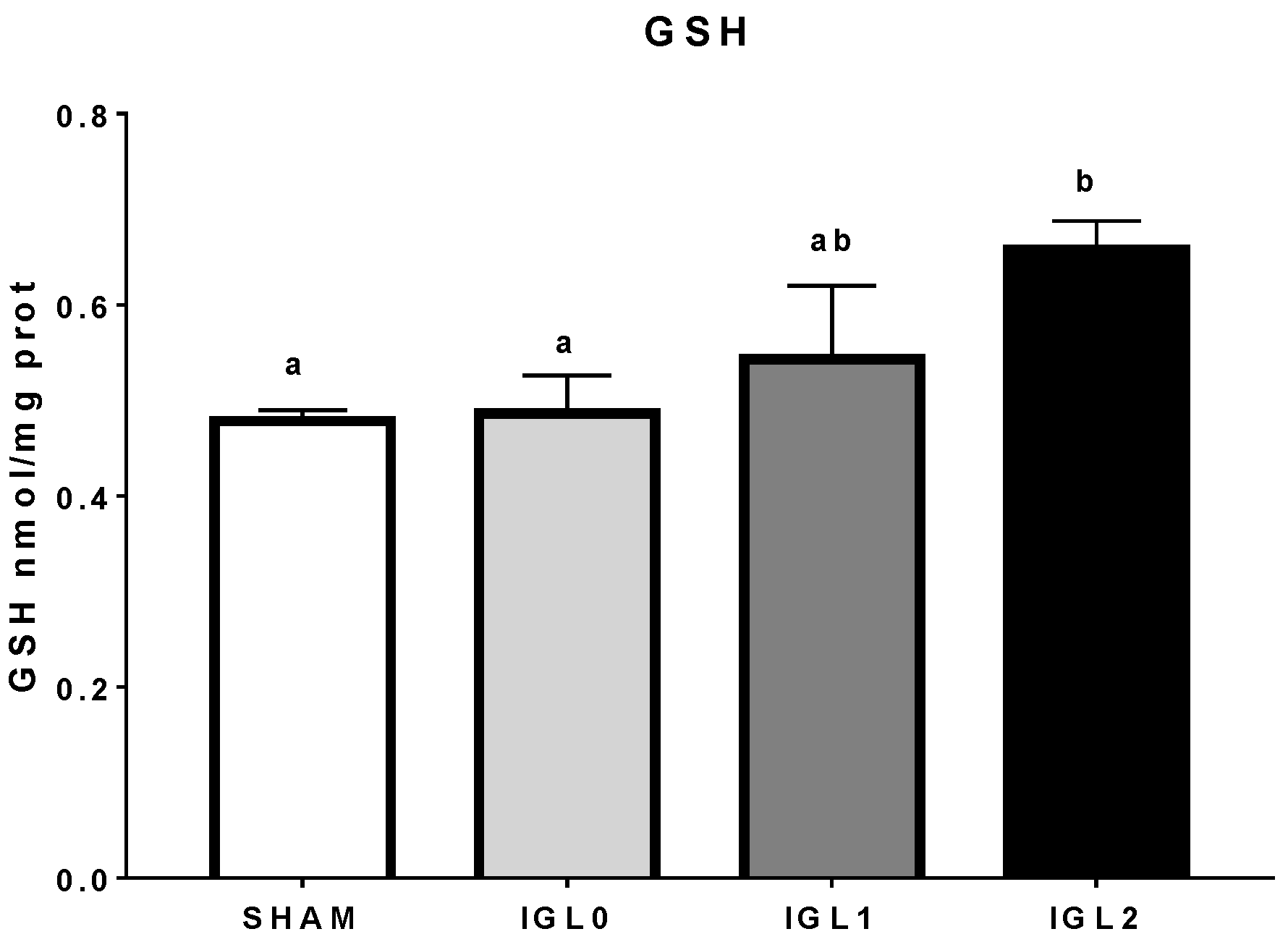
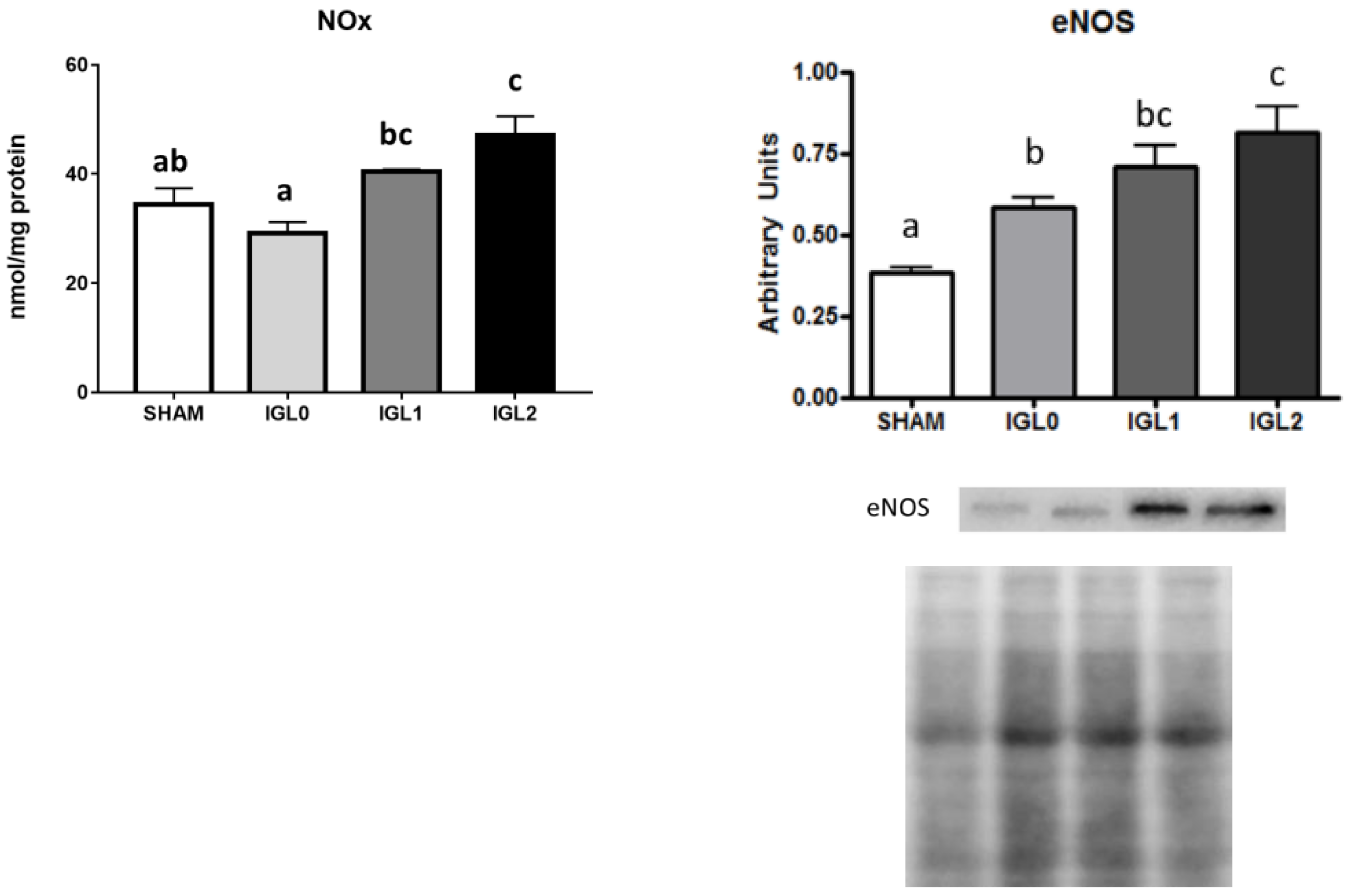
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Groups
- Group 1 (SHAM): Obese Zücker rats underwent transverse laparotomy, and silk ligatures of right suprarenal and diaphragmatic veins and hepatic artery were performed before retrieving the liver.
- Group 2 (IGL-0 solution): After organ recovery, fatty livers were flushed with 40 mL of IGL-0 preservation solution and were then stored in IGL-0 at 4 °C for 24 h.
- Group 3 (IGL-1 solution): After organ recovery, fatty livers were flushed with 40 mL of IGL-1 preservation solution and were then stored in IGL-1 at 4 °C for 24 h.
- Group 4 (IGL-2 solution): After organ recovery, fatty livers were flushed with 40 mL of IGL-1 preservation solution and were then stored in IGL-2 at 4 °C for 24 h.
4.3. Biochemical Analyses
Transaminase Assay
4.4. Glutamate Dehydrogenase (GLDH) Activity
4.5. Energy Metabolism (ATP Breakdown)
4.6. 4-Hydroxynonenal Protein Adducts Assay
4.7. Advanced Oxidation Protein Products (AOPP)
4.8. Glutathione Analysis
4.9. Nitrite/Nitrate Analysis
4.10. Western Blot Analysis
ALDH2, Beclin-1, and LC3B
4.11. 4-HNE Protein Adducts and eNOS
4.12. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Peralta, C.; Roselló-Catafau, J. The future of fatty livers. J. Hepatol. 2004, 41, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Busuttil, R.W.; Tanaka, K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003, 9, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Selzner, M.; Clavien, P.A. Fatty liver in transplantation and surgery. Semin. Liver Dis. 2001, 21, 1103–1115. [Google Scholar] [CrossRef]
- Said, A. Non-alcoholic fatty liver disease and liver transplantation: Outcomes and advances. World J. Gastroenterol. 2013, 19, 9146–9155. [Google Scholar] [CrossRef]
- Tashiro, H.; Kuroda, S.; Mikuriya, Y.; Ohdan, H. Ischemia-reperfuson injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. Surg. Today 2014, 44, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ Preservation: Current Con-cepts and New Strategies for the Next Decade. Transfus. Med. Hemother. 2011, 38, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, A.V.Y.; Carnevale, M.; Somov, A.Y.; Osorio, J.; Rodriguez, J.V.; Guibert, R.; Fuller, B.J.; Froghi, F. Organ Preservation into the 2020s: The era of dynamic preservation. Transfus. Med. Hemother. 2019, 46, 151–172. [Google Scholar] [CrossRef]
- Stewart, Z.A.; Cameron, A.M.; Singer, A.L.; Montgomery, R.A.; Segev, D.L. Histidine-tryptophan-ketoglutarate (HTK) is as-sociated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am. J. Transplant. 2009, 9, 286–293. [Google Scholar] [CrossRef]
- Adam, R.; Delvart, V.; Karam, V.; Ducerf, C.; Navarro, F.; Letoublon, C.; Belghiti, J.; Pezet, D.; Castaing, D.; Le Treut, Y.P.; et al. Compared Efficacy of Preservation Solutions in Liver Transplantation: A Long-Term Graft Outcome Study From the European Liver Transplant Registry. Arab. Archaeol. Epigr. 2015, 15, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Ben Abdennebi, H.; Padrissa-Altés, S.; Mahfoudh-Boussaid, A.; Roselló-Catafau, J. Pharmacological strate-gies against cold ischemia reperfusion injury. Expert Opin Pharmacother. 2010, 11, 537–555. [Google Scholar] [CrossRef]
- Ben Mosbah, I.; Roselló-Catafau, J.; Franco-Gou, R.; Ben Abdennebi, H.; Saidane, D.; Ramella-Virieux, S.; Boillot, O.; Peralta, C. Preservation of steatotic livers in IGL-1 solution. Liver Transplant. 2006, 12, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Mosbah, I.; Franco-Gou, R.; Abdennebi, H.; Hernandez, R.; Escolar, G.; Saidane, D.; Rosello-Catafau, J.; Peralta, C. Effects of Polyethylene Glycol and Hydroxyethyl Starch in University of Wisconsin Preservation Solution on Human Red Blood Cell Aggregation and Viscosity. Transplant. Proc. 2006, 38, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, J.; Achtergaele, J.; Fieuws, S.; Jochmans, I.; Sainz-Barriga, M.; Diethard Monbaliu, D.; Pirenne, J.; Gilbo, N. The effect of organ preservation solutions on short-term outcomes after liver transplantation: Single-center retrospec-tive study. Trasplant. Int. 2021, 34, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Bejaoui, M.; Calvo, M.; Folch-Puy, E.; Pantazi, E.; Pasut, G.; Rimola, A.; Ben Abdennebi, H.; Adam, R.; Roselló-Catafau, J. Polyethylene glycol rinse solution: An effective way to prevent ischemia-reperfusion injury. World J. Gastroenterol. 2014, 20, 16203–16214. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amara, M.; Yang, S.Y.; Seifalian, A.; Davidson, B.; Fuller, B. The nitric oxide pathway-evidence and mechanisms for protection against liver ischaemia reperfusion injury. Liver Int. 2012, 32, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdennebi, H.; Zaouali, M.A.; Alfany-Fernandez, I.; Tabka, D.; Roselló-Catafau, J. How to protect liver graft with nitric oxide. World J. Gastroenterol. 2011, 17, 2879–2889. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol. Physiol. 2017, 312, C254–C262. [Google Scholar] [CrossRef]
- Rosello, A.P.; Da Silva, R.T.; Castro, C.; Bardallo, R.G.; Calvo, M.; Folch-Puy, E.; Carbonell, T.; Palmeira, C.; Catafau, J.R.; Adam, R. Polyethylene Glycol 35 as a Perfusate Additive for Mitochondrial and Glycocalyx Protection in HOPE Liver Preservation. Int. J. Mol. Sci. 2020, 21, 5703. [Google Scholar] [CrossRef]
- Panisello-Rosello, A.; Roselló-Catafau, J. HOPE (hypothermic oxygenated perfusion) strategies in the era of dynamic liver graft preservation. EBioMedicine 2020, 61, 103071. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.-A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2018, 68, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; Lopez, A.; Folch-Puy, E.; Carbonell, T.; Rolo, A.; Palmeira, C.; Adam, R.; Net, M.; Roselló-Catafau, J. Role of aldehyde dehydrogenase 2 in ischemia reperfusion injury: An update. World J. Gastroenterol. 2018, 24, 2984–2994. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; Alva, N.; Flores, M.; Lopez, A.; Benítez, C.C.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Adam, R.; Carbonell, T.; et al. Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2479. [Google Scholar] [CrossRef]
- Panisello-Roselló, A.; Verde, E.; Lopez, A.; Flores, M.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Hotter, G.; Carbonel, T.; Adam, R.; et al. Cytoprotective Mechanisms in Fatty Liver Preservation against Cold Ischemia Injury: A Comparison between IGL-1 and HTK. Int. J. Mol. Sci. 2018, 19, 348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Q.; Ye, F.; Huang, C.Y.; Chen, W.M.; Huang, W.Q. Alda-1, an ALDH2 activator, protects against hepatic/ischemia reperfusion injury in rats via inhibition of oxidative stress. Free Radic. Res. 2018, 52, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, M.; Li, J.; Chen, L.; Xu, D.; Tong, Y.; Zhang, J.; Wu, H.; Kong, X.; Xia, Q. Alda-1 ameliorates Liver ische-mia-reperfusion injury by activating aldehyde dehydrogenase 2 and enhancing autophagy in mice. J. Immunol. Res. 2018, 2018, 9807139. [Google Scholar] [CrossRef]
- Van Erp, A.C.; Hoeksma, D.; Rebolledo, R.A.; Ottens, P.J.; Jochmans, I.; Monbaliu, D.; Pirenne, J.; Leuvenink, H.G.D.; Decuypere, J.-P. The Crosstalk between ROS and Autophagy in the Field of Transplantation Medicine. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Van Breussegem, A.; Van Pelt, J.; Wylin, T.; Heedfeld, V.; Zeegers, M.; Monbaliu, D.; Pirenne, J.; Vekemans, K. Presumed and Actual Concentrations of Reduced Glutathione in Preservation Solutions. Transplant. Proc. 2011, 43, 3451–3454. [Google Scholar] [CrossRef]
- Yin, X.-M.; Ding, W.-X.; Gao, W. Autophagy in the liver. Hepatology 2008, 47, 1773–1785. [Google Scholar] [CrossRef]
- Pasut, G.F.; Panisello-Roselló, A.; Folch-Puy, E.; Lopez, A.; Castro-Benítez, C.; Calvo, M.; Carbonell, T.; Garcia-Gil, A.; Adam, R.; Roselló-Catafau, J. Polyehtylene glycols: An effective strategy for limiting liver ischemia reperfusion injury. World J. Gastroenterol. 2016, 22, 6501–6508. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.-A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. EBioMedicine 2020, 60. [Google Scholar] [CrossRef]
- Horváth, T.; Jász, D.K.; Baráth, B.; Poles, M.Z.; Boros, M.; Hartmann, P. Mitochondrial consequences of organ preserva-tion techniques during liver transplantation. Int. J. Mol. Sci. 2021, 22, 2816. [Google Scholar] [CrossRef]
- Chen, C.H.; Ferreira, J.C.; Gross, E.R.; Mochly-Rosen, D. Targeting aldehyde dehydrogenase 2: New therapeutic opportuni-ties. Physiol. Rev. 2014, 94, 1–34. [Google Scholar] [CrossRef]
- Solito, F.; Corti, C. Mitochondrial aldehyde dehydrogenase-2 activation prevents β-amyloid-induced en-dothelial cell dysfunction and restores angiogenesis. J. Cell Sci. 2013, 126, 1952–1961. [Google Scholar] [PubMed]
- Nannelli, E.; Terzuoli, V.; Giorgio, S.; Donnini, P.; Lupetti, A.; Giachetti, P.; Bernardi, M. ALDH2 Activity Re-duces Mitochondrial Oxygen Reserve Capacity in Endothelial Cells and Induces Senescence Properties. Oxidative Med. Cell. Longev. 2018, 2018, 9765027. [Google Scholar] [CrossRef] [PubMed]
- Nannelli, G.; Ziche, M.; Donnini, S.; Morbidelli, L. Endothelial Aldehyde Dehydrogenase 2 as a Target to Maintain Vascular Wellness and Function in Ageing. Biomediences 2020, 8, 4. [Google Scholar] [CrossRef]
- Ramalho, F.S.; Fernandez-Monteiro, I.; Rosello-Catafau, J.; Peralta, C. Hepatic microcirculatory failure. Acta Cir. Bras. 2006, 21, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Gausson, V.; Nguyen, A.T.; Touam, M.; Drüeke, T.; Santangelo, F.; Descamps-Latscha, B. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: A potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003, 64, 82–91. [Google Scholar] [CrossRef] [PubMed]
| Preservation Solution | IGL-1 | IGL-2 |
|---|---|---|
| Electrolytes (mmol/L) | ||
| K+ | 25 | 25 |
| Na+ | 125 | 125 |
| Mg2+ | 5.5 | |
| SO4 | 5.5 | |
| Zn2+ | 0.091 | |
| Buffers (mmol/L) | ||
| Phosphate | 25 | 25 |
| Histidine | 30 | |
| Impermeants (mmol/L) | ||
| Mannitol | 60 | 60 |
| Lactobionic acid | 80 | |
| Colloids (g/L) | ||
| Polyethylene glycol- 35 | 1 | 5 |
| Antioxydants (mmol/L) | ||
| Glutathione | 3 | 9 |
| Metabolic precursors (mmol/L) | ||
| Adenosine | 5 | 5 |
| NaNO2 (nmol/L) | 50 | |
| pH | 7.4 | 7.4 |
| Osmolarity (mosmol/L) | 320 | 320 |
| Viscosity (cP) | 1.2 | 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardallo, R.G.; da Silva, R.T.; Carbonell, T.; Folch-Puy, E.; Palmeira, C.; Roselló-Catafau, J.; Pirenne, J.; Adam, R.; Panisello-Roselló, A. Role of PEG35, Mitochondrial ALDH2, and Glutathione in Cold Fatty Liver Graft Preservation: An IGL-2 Approach. Int. J. Mol. Sci. 2021, 22, 5332. https://doi.org/10.3390/ijms22105332
Bardallo RG, da Silva RT, Carbonell T, Folch-Puy E, Palmeira C, Roselló-Catafau J, Pirenne J, Adam R, Panisello-Roselló A. Role of PEG35, Mitochondrial ALDH2, and Glutathione in Cold Fatty Liver Graft Preservation: An IGL-2 Approach. International Journal of Molecular Sciences. 2021; 22(10):5332. https://doi.org/10.3390/ijms22105332
Chicago/Turabian StyleBardallo, Raquel G., Rui Teixeira da Silva, Teresa Carbonell, Emma Folch-Puy, Carlos Palmeira, Joan Roselló-Catafau, Jacques Pirenne, René Adam, and Arnau Panisello-Roselló. 2021. "Role of PEG35, Mitochondrial ALDH2, and Glutathione in Cold Fatty Liver Graft Preservation: An IGL-2 Approach" International Journal of Molecular Sciences 22, no. 10: 5332. https://doi.org/10.3390/ijms22105332
APA StyleBardallo, R. G., da Silva, R. T., Carbonell, T., Folch-Puy, E., Palmeira, C., Roselló-Catafau, J., Pirenne, J., Adam, R., & Panisello-Roselló, A. (2021). Role of PEG35, Mitochondrial ALDH2, and Glutathione in Cold Fatty Liver Graft Preservation: An IGL-2 Approach. International Journal of Molecular Sciences, 22(10), 5332. https://doi.org/10.3390/ijms22105332












