Adenosine Signaling in Mast Cells and Allergic Diseases
Abstract
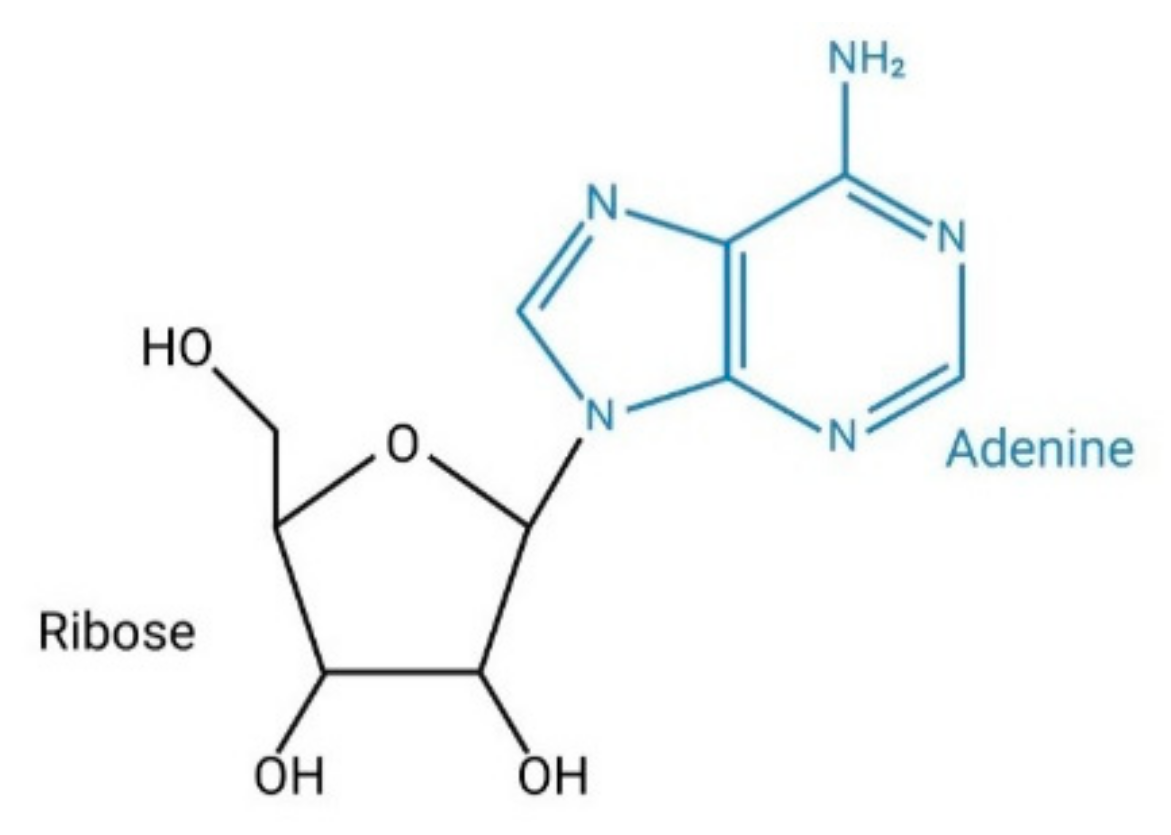
1. Introduction
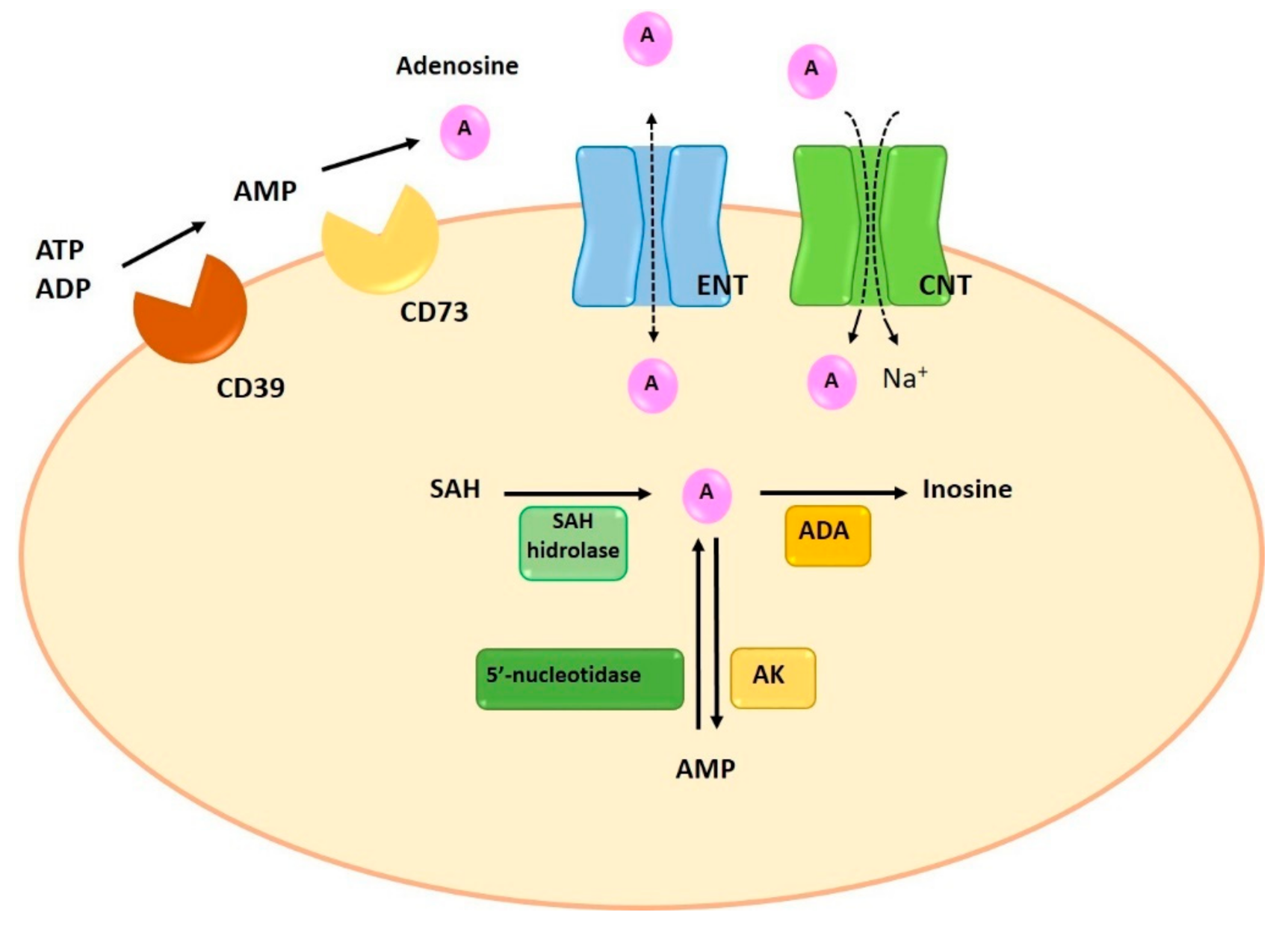
1.1. Synthesis and Degradation of Adenosine
1.2. Adenosine Transporters
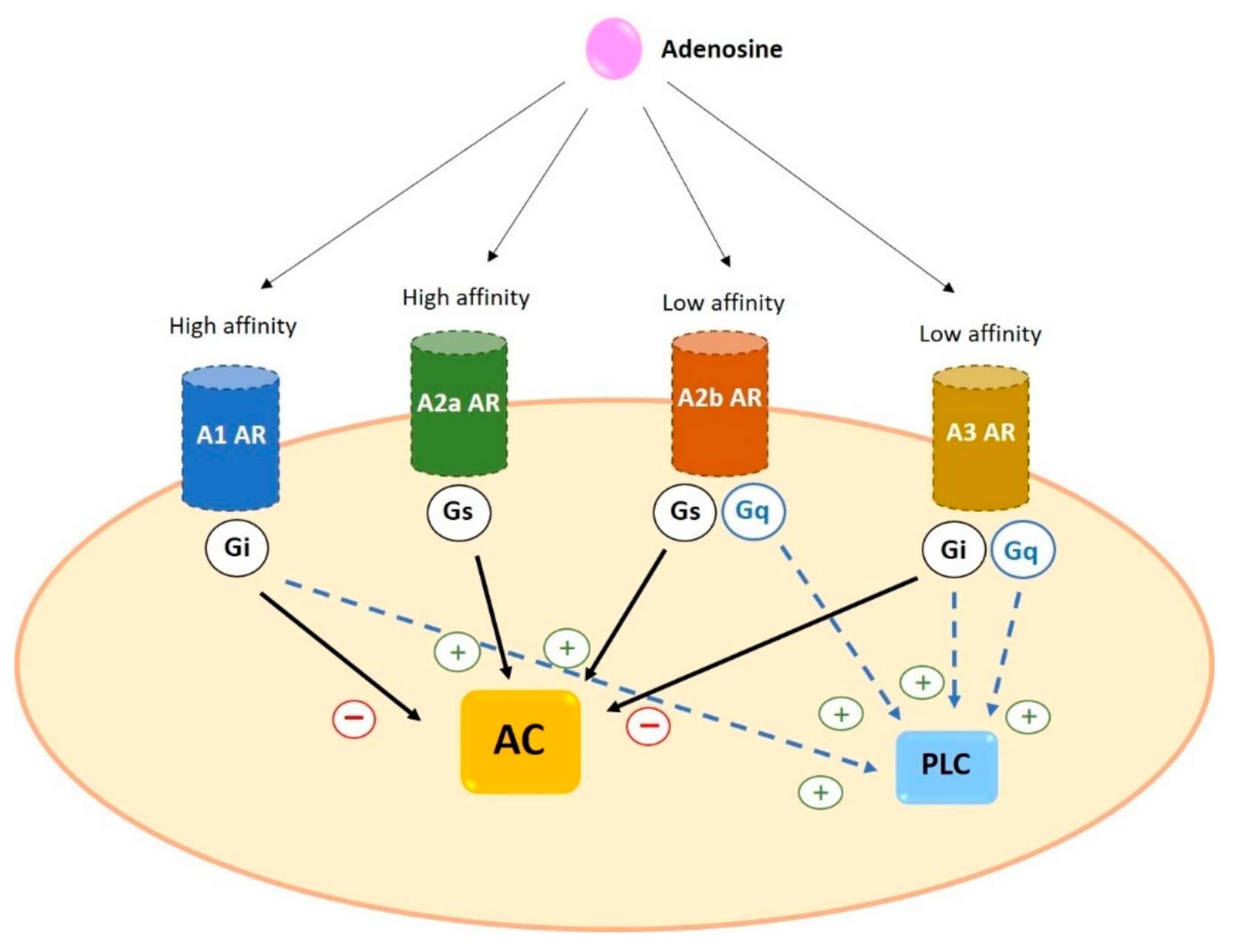
2. Adenosine Receptors
2.1. Expression of Adenosine Receptors in Human Cells
Expression of Adenosine Receptors in Human Basophils and Mast Cells
2.2. Signal Transduction of Adenosine Receptors in Human Cells
2.2.1. A1
2.2.2. A2a
2.2.3. A2b
2.2.4. A3
2.3. Desensitization of Adenosine Receptors
3. Adenosine in Allergic Diseases
3.1. Role of Specific Receptor Stimulation in Allergic Diseases
3.1.1. A1
3.1.2. A2a
3.1.3. A2b
3.1.4. A3
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sachdeva, S.; Gupta, M. Adenosine and its receptors as therapeutic targets: An overview. Saudi Pharm. J. 2013, 21, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-T.T.; Berg, N.K.; Harting, M.T.; Li, X.; Eltzschig, H.K.; Yuan, X. Purinergic Signaling in Pulmonary Inflammation. Front. Immunol. 2019, 10, 1633. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, P. Adenosine A2B Receptor: From Cell Biology to Human Diseases. Front. Chem. 2016, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharmacol. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef]
- Borea, P.A.; Varani, K.; Vincenzi, F.; Baraldi, P.G.; Tabrizi, M.A.; Merighi, S.; Gessi, S. The A3Adenosine Receptor: History and Perspectives. Pharmacol. Rev. 2015, 67, 74–102. [Google Scholar] [CrossRef]
- Antonioli, L.; Fornai, M.; Blandizzi, C.; Pacher, P.; Haskó, G. Adenosine signaling and the immune system: When a lot could be too much. Immunol. Lett. 2019, 205, 9–15. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol. Lett. 2019, 205, 16–24. [Google Scholar] [CrossRef]
- Van Linden, A.; Eltzschig, H.K. Role of pulmonary adenosine during hypoxia: Extracellular generation, signaling and metabolism by surface adenosine deaminase/CD26. Expert Opin. Biol. Ther. 2007, 7, 1437–1447. [Google Scholar] [CrossRef]
- Haskó, G.; Antonioli, L.; Cronstein, B.N. Adenosine metabolism, immunity and joint health. Biochem. Pharmacol. 2018, 151, 307–313. [Google Scholar] [CrossRef]
- Zhou, Y.; Murthy, J.N.; Zeng, D.; Belardinelli, L.; Blackburn, M.R. Alterations in Adenosine Metabolism and Signaling in Patients with Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. PLoS ONE 2010, 5, e9224. [Google Scholar] [CrossRef]
- Idzko, M.; Ayata, C.K.; Müller, T.; Dürk, T.; Grimm, M.; Baudiß, K.; Vieira, R.P.; Cicko, S.; Boehlke, C.; Zech, A.; et al. Attenuated allergic airway inflammation inCd39null mice. Allergy 2013, 68, 472–480. [Google Scholar] [CrossRef]
- Moreno, E.; Canet, J.; Gracia, E.; Lluis, C.; Mallol, J.; Canela, E.I.; Cortés, A.; Casadó, V. Molecular Evidence of Adenosine Deaminase Linking Adenosine A2A Receptor and CD26 Proteins. Front. Pharmacol. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Boison, D. Regulation of Extracellular Adenosine. Nicotinic Recept. 2018, 34, 13–32. [Google Scholar] [CrossRef]
- Zhong, H.; Chunn, J.L.; Volmer, J.B.; Fozard, J.R.; Blackburn, M.R. Adenosine-mediated mast cell degranulation in adenosine deaminase-deficient mice. J. Pharmacol. Exp. Ther. 2001, 298, 433. [Google Scholar]
- Jin, X.; Shepherd, R.K.; Duling, B.R.; Lindén, J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J. Clin. Investig. 1997, 100, 2849–2857. [Google Scholar] [CrossRef]
- Yu, M.; Cui, F.-X.; Jia, H.-M.; Zhou, C.; Yang, Y.; Zhang, H.-W.; Ding, G.; Zou, Z.-M. Aberrant purine metabolism in allergic asthma revealed by plasma metabolomics. J. Pharm. Biomed. Anal. 2016, 120, 181–189. [Google Scholar] [CrossRef]
- Young, J.D. The SLC28 (CNT) and SLC29 (ENT) nucleoside transporter families: A 30-year collaborative odyssey. Biochem. Soc. Trans. 2016, 44, 869–876. [Google Scholar] [CrossRef]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef]
- Sheth, S.; Brito, R.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Adenosine Receptors: Expression, Function and Regulation. Int. J. Mol. Sci. 2014, 15, 2024–2052. [Google Scholar] [CrossRef]
- Merighi, S.; Gessi, S.; Borea, P.A. Adenosine Receptors: Structure, Distribution, and Signal Transduction. Nicotinic Recept. 2018, 34, 33–57. [Google Scholar] [CrossRef]
- Alnouri, M.W.; Jepards, S.; Casari, A.; Schiedel, A.C.; Hinz, S.; Müller, C.E. Selectivity is species-dependent: Characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal. 2015, 11, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Peleli, M.; Fredholm, B.B.; Sobrevia, L.; Carlström, M. Pharmacological targeting of adenosine receptor signaling. Mol. Asp. Med. 2017, 55, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets—What are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef]
- Gao, Z.-G.; Tosh, D.K.; Jain, S.; Yu, J.; Suresh, R.R.; Jacobson, K.A. A1 Adenosine Receptor Agonists, Antagonists, and Allosteric Modulators. Nicotinic Recept. 2018, 34, 59–89. [Google Scholar] [CrossRef]
- Ciancetta, A.; Jacobson, K.A. Structural Probing and Molecular Modeling of the A3 Adenosine Receptor: A Focus on Agonist Binding. Molecules 2017, 22, 449. [Google Scholar] [CrossRef]
- Méndez-Enríquez, E.; Hallgren, J. Mast Cells and Their Progenitors in Allergic Asthma. Front. Immunol. 2019, 10, 821. [Google Scholar] [CrossRef]
- Matsuo, Y.; Yanase, Y.; Irifuku, R.; Ishii, K.; Kawaguchi, T.; Takahagi, S.; Hide, I.; Hide, M. The role of adenosine for IgE receptor-dependent degranulation of human peripheral basophils and skin mast cells. Allergol. Int. 2018, 67, 524–526. [Google Scholar] [CrossRef]
- Gao, Z.-G.; Jacobson, K.A. Purinergic Signaling in Mast Cell Degranulation and Asthma. Front. Pharmacol. 2017, 8, 947. [Google Scholar] [CrossRef]
- Rudich, N.; Dekel, O.; Sagi-Eisenberg, R. Down-regulation of the A3 adenosine receptor in human mast cells upregulates mediators of angiogenesis and remodeling. Mol. Immunol. 2015, 65, 25–33. [Google Scholar] [CrossRef]
- Gomez, G.; Zhao, W.; Schwartz, L.B. Disparity in FcεRI-Induced Degranulation of Primary Human Lung and Skin Mast Cells Exposed to Adenosine. J. Clin. Immunol. 2011, 31, 479–487. [Google Scholar] [CrossRef]
- Muñoz-Cano, R.; Pascal, M.; Bartra, J.; Picado, C.; Valero, A.; Kim, D.-K.; Brooks, S.; Ombrello, M.; Metcalfe, D.D.; Rivera, J.; et al. Distinct transcriptome profiles differentiate nonsteroidal anti-inflammatory drug–dependent from nonsteroidal anti-inflammatory drug–independent food-induced anaphylaxis. J. Allergy Clin. Immunol. 2016, 137, 137–146. [Google Scholar] [CrossRef]
- Kim, S.-H.; Nam, E.-J.; Kim, Y.-K.; Ye, Y.-M.; Park, H.-S. Functional variability of the adenosine A3 receptor (ADORA3) gene polymorphism in aspirin-induced urticaria. Br. J. Dermatol. 2010, 163, 977–985. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, Y.-K.; Park, H.-W.; Kim, S.-H.; Ye, Y.-M.; Min, K.-U.; Park, H.-S. Adenosine deaminase and adenosine receptor polymorphisms in aspirin-intolerant asthma. Respir. Med. 2009, 103, 356–363. [Google Scholar] [CrossRef]
- Liu, Y.; Saccucci, P.; Qi, H.; Wu, H.C.; Zhao, F.; Dai, Y.; Bottini, N.; Gloria-Bottini, F. ADA Polymorphisms and Asthma: A Study in the Chinese Han Population. J. Asthma 2006, 43, 203–206. [Google Scholar] [CrossRef]
- Marone, G.; Findlay, S.R.; Lichtenstein, L.M. Adenosine receptor on human basophils: Modulation of histamine release. J. Immunol. 1979, 123, 1473. [Google Scholar]
- Jacobson, K.A.; Gao, Z.-G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef]
- Guerrero, Á. A2A Adenosine Receptor Agonists and their Potential Therapeutic Applications. An Update. Curr. Med. Chem. 2018, 25, 3597–3612. [Google Scholar] [CrossRef]
- Merighi, S.; Bencivenni, S.; Vincenzi, F.; Varani, K.; Borea, P.A.; Gessi, S. A 2B adenosine receptors stimulate IL-6 production in primary murine microglia through p38 MAPK kinase pathway. Pharmacol. Res. 2017, 117, 9–19. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Merighi, S.; Varani, K.; Borea, P.A.; Baraldi, S.; Tabrizi, M.A.; Romagnoli, R.; Baraldi, P.G.; Ciancetta, A.; Tosh, D.K.; et al. A3Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy. Med. Res. Rev. 2018, 38, 1031–1072. [Google Scholar] [CrossRef]
- Klaasse, E.C.; Ijzerman, A.P.; De Grip, W.J.; Beukers, M.W. Internalization and desensitization of adenosine receptors. Purinergic Signal. 2007, 4, 21–37. [Google Scholar] [CrossRef]
- Mundell, S.; Kelly, E. Adenosine receptor desensitization and trafficking. Biochim. Biophys. Acta BBA Biomembr. 2011, 1808, 1319–1328. [Google Scholar] [CrossRef]
- Stoddart, L.A.; Vernall, A.J.; Briddon, S.J.; Kellam, B.; Hill, S.J. Direct visualisation of internalization of the adenosine A3 receptor and localization with arrestin3 using a fluorescent agonist. Neuropharmacology 2015, 98, 68–77. [Google Scholar] [CrossRef]
- Soave, M.; Kellam, B.; Woolard, J.; Briddon, S.J.; Hill, S.J. NanoBiT Complementation to Monitor Agonist-Induced Adenosine A1 Receptor Internalization. SLAS Discov. Adv. Life Sci. R&D 2020, 25, 186–194. [Google Scholar] [CrossRef]
- Cox, C.A.; Boudewijn, I.M.; Vroegop, S.J.; Schokker, S.; Lexmond, A.J.; Frijlink, H.W.; Hagedoorn, P.; Vonk, J.M.; Farenhorst, M.P.; Hacken, N.H.T.T.; et al. Associations of AMP and adenosine induced dyspnea sensation to large and small airways dysfunction in asthma. BMC Pulm. Med. 2019, 19, 23. [Google Scholar] [CrossRef]
- Wilson, C.N. Adenosine receptors and asthma in humans. Br. J. Pharmacol. 2008, 155, 475–486. [Google Scholar] [CrossRef]
- Mao, M.; Liu, H.; Yan, S.; Yuan, Y.; Liu, R.; Wu, Y.; Peng, C.; Li, J.; Chen, X. Plasma adenosine is linked to disease activity and response to treatment in patients with chronic spontaneous urticaria. Allergy 2021, 76, 571–573. [Google Scholar] [CrossRef]
- Leung, C.T.; Li, A.; Banerjee, J.; Gao, Z.-G.; Kambayashi, T.; Jacobson, K.A.; Civan, M.M. The role of activated adenosine receptors in degranulation of human LAD2 mast cells. Purinergic Signal. 2014, 10, 465–475. [Google Scholar] [CrossRef]
- Schulman, E.S.; Glaum, M.C.; Post, T.; Wang, Y.; Raible, D.G.; Mohanty, J.; Butterfield, J.H.; Pelleg, A. ATP Modulates Anti-IgE–Induced Release of Histamine from Human Lung Mast Cells. Am. J. Respir. Cell Mol. Biol. 1999, 20, 530–537. [Google Scholar] [CrossRef]
- Caruso, M.; Alamo, A.; Crisafulli, E.; Raciti, C.; Fisichella, A.; Polosa, R. Adenosine signaling pathways as potential therapeutic targets in respiratory disease. Expert Opin. Ther. Targets 2013, 17, 761–772. [Google Scholar] [CrossRef]
- Fernandez, L.G.; Sharma, A.K.; LaPar, D.J.; Kron, I.L.; Laubach, V.E. Adenosine A1 receptor activation attenuates lung ischemia–reperfusion injury. J. Thorac. Cardiovasc. Surg. 2013, 145, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Narke, D.; Kurade, M.; Frey, K.M.; Rajalingam, S.; Siddiquee, A.; Mustafa, S.J.; Ledent, C.; Ponnoth, D.S. Limonene-induced activation of A2A adenosine receptors reduces airway inflammation and reactivity in a mouse model of asthma. Purinergic Signal. 2020, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nat. Cell Biol. 2001, 414, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zuo, Y.; Huang, Y.; Ge, J. Adenosine A2a receptor agonist CGS21680 treatment attenuates cardiopulmonary bypass-associated inflammatory lung injury in juvenile rats. Mol. Med. Rep. 2019, 20, 117–124. [Google Scholar] [CrossRef]
- Alfaro, T.M.; Rodrigues, D.I.; Tomé, Â.R.; Cunha, R.A.; Cordeiro, C.R. Adenosine A 2A receptors are up-regulated and control the activation of human alveolar macrophages. Pulm. Pharmacol. Ther. 2017, 45, 90–94. [Google Scholar] [CrossRef]
- Pei, H.; Linden, J. Adenosine influences myeloid cells to inhibit aeroallergen sensitization. Am. J. Physiol. Cell. Mol. Physiol. 2016, 310, L985–L992. [Google Scholar] [CrossRef]
- Yuryeva, K.; Saltykova, I.; Ogorodova, L.; Kirillova, N.; Kulikov, E.; Korotkaya, E.; Iakovleva, Y.; Feoktistov, I.; Sazonov, A.; Ryzhov, S. Expression of adenosine receptors in monocytes from patients with bronchial asthma. Biochem. Biophys. Res. Commun. 2015, 464, 1314–1320. [Google Scholar] [CrossRef]
- Duffy, S.M.; Cruse, G.; Brightling, C.E.; Bradding, P. Adenosine closes the K+ channel KCa3.1 in human lung mast cells and inhibits their migrationvia the adenosine A2A receptor. Eur. J. Immunol. 2007, 37, 1653–1662. [Google Scholar] [CrossRef]
- Suzuki, H.; Takei, M.; Nakahata, T.; Fukamachi, H. Inhibitory Effect of Adenosine on Degranulation of Human Cultured Mast Cells upon Cross-Linking of FceRI. Biochem. Biophys. Res. Commun. 1998, 242, 6. [Google Scholar]
- Arizmendi, N.; Kulka, M. Adenosine activates Gαs proteins and inhibits C3a-induced activation of human mast cells. Biochem. Pharmacol. 2018, 156, 157–167. [Google Scholar] [CrossRef]
- Hua, X.; Kovarova, M.; Chason, K.D.; Nguyen, M.; Koller, B.H.; Tilley, S.L. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J. Exp. Med. 2007, 204, 117–128. [Google Scholar] [CrossRef]
- Schingnitz, U.; Hartmann, K.; MacManus, C.F.; Eckle, T.; Zug, S.; Colgan, S.P.; Eltzschig, H.K. Signaling through the A2B Adenosine Receptor Dampens Endotoxin-Induced Acute Lung Injury. J. Immunol. 2010, 184, 5271–5279. [Google Scholar] [CrossRef]
- Cohen, H.B.; Ward, A.; Hamidzadeh, K.; Ravid, K.; Mosser, D.M. IFN-γ Prevents Adenosine Receptor (A2bR) Upregulation to Sustain the Macrophage Activation Response. J. Immunol. 2015, 195, 3828–3837. [Google Scholar] [CrossRef]
- Hoegl, S.; Brodsky, K.S.; Blackburn, M.R.; Karmouty-Quintana, H.; Zwissler, B.; Eltzschig, H.K. Alveolar Epithelial A2B Adenosine Receptors in Pulmonary Protection during Acute Lung Injury. J. Immunol. 2015, 195, 1815–1824. [Google Scholar] [CrossRef]
- Greer, S.; Page, C.W.; Joshi, T.; Yan, N.; Newton, R.; Giembycz, M.A. Concurrent Agonism of Adenosine A2B and Glucocorticoid Receptors in Human Airway Epithelial Cells Cooperatively Induces Genes with Anti-Inflammatory Potential: A Novel Approach to Treat Chronic Obstructive Pulmonary Disease. J. Pharmacol. Exp. Ther. 2013, 346, 473–485. [Google Scholar] [CrossRef]
- Liu, B.; Bing, Q.; Li, S.; Han, B.; Lu, J.; Baiyun, R.; Zhang, X.; Lv, Y.; Wu, H.; Zhang, Z. Role of A2B adenosine receptor-dependent adenosine signaling in multi-walled carbon nanotube-triggered lung fibrosis in mice. J. Nanobiotechnol. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Huerter, M.E.; Sharma, A.K.; Zhao, Y.; Charles, E.J.; Kron, I.L.; Laubach, V.E. Attenuation of Pulmonary Ischemia-Reperfusion Injury by Adenosine A 2B Receptor Antagonism. Ann. Thorac. Surg. 2016, 102, 385–393. [Google Scholar] [CrossRef][Green Version]
- Jamwal, S.; Mittal, A.; Kumar, P.; Alhayani, D.M.; Al-Aboudi, A. Therapeutic Potential of Agonists and Antagonists of A1, A2a, A2b and A3 Adenosine Receptors. Curr. Pharm. Des. 2019, 25, 2892–2905. [Google Scholar] [CrossRef]
- Yoshida, K.; Ito, M.; Hoshino, Y.; Matsuoka, I. Effects of dexamethasone on purinergic signaling in murine mast cells: Selective suppression of P2X7 receptor expression. Biochem. Biophys. Res. Commun. 2017, 493, 1587–1593. [Google Scholar] [CrossRef]
- Yoshida, K.; Ito, M.-A.; Sato, N.; Obayashi, K.; Yamamoto, K.; Koizumi, S.; Tanaka, S.; Furuta, K.; Matsuoka, I. Extracellular ATP Augments Antigen-Induced Murine Mast Cell Degranulation and Allergic Responses via P2X4 Receptor Activation. J. Immunol. 2020, 204, 3077–3085. [Google Scholar] [CrossRef]
- Müller, C.E.; Jacobson, K.A. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta BBA Biomembr. 2011, 1808, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Åstrand, A.B.M.; Bergström, E.L.; Zhang, H.; Börjesson, L.; Söderdahl, T.; Wingren, C.; Jansson, A.; Smailagic, A.; Johansson, C.; Bladh, H.; et al. The discovery of a selective and potent A 2a agonist with extended lung retention. Pharmacol. Res. Perspect. 2015, 3, e00134. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Garcia, L.; Olle, L.; Martin, M.; Roca-Ferrer, J.; Muñoz-Cano, R. Adenosine Signaling in Mast Cells and Allergic Diseases. Int. J. Mol. Sci. 2021, 22, 5203. https://doi.org/10.3390/ijms22105203
Garcia-Garcia L, Olle L, Martin M, Roca-Ferrer J, Muñoz-Cano R. Adenosine Signaling in Mast Cells and Allergic Diseases. International Journal of Molecular Sciences. 2021; 22(10):5203. https://doi.org/10.3390/ijms22105203
Chicago/Turabian StyleGarcia-Garcia, Lucia, Laia Olle, Margarita Martin, Jordi Roca-Ferrer, and Rosa Muñoz-Cano. 2021. "Adenosine Signaling in Mast Cells and Allergic Diseases" International Journal of Molecular Sciences 22, no. 10: 5203. https://doi.org/10.3390/ijms22105203
APA StyleGarcia-Garcia, L., Olle, L., Martin, M., Roca-Ferrer, J., & Muñoz-Cano, R. (2021). Adenosine Signaling in Mast Cells and Allergic Diseases. International Journal of Molecular Sciences, 22(10), 5203. https://doi.org/10.3390/ijms22105203






