Development of New Antiproliferative Compound against Human Tumor Cells from the Marine Microalgae Nannochloropsis gaditana by Applied Proteomics
Abstract
1. Introduction
2. Results
2.1. Identified Protein Analysis
2.2. Heterologous Expression and Purification of Recombinant UCA01
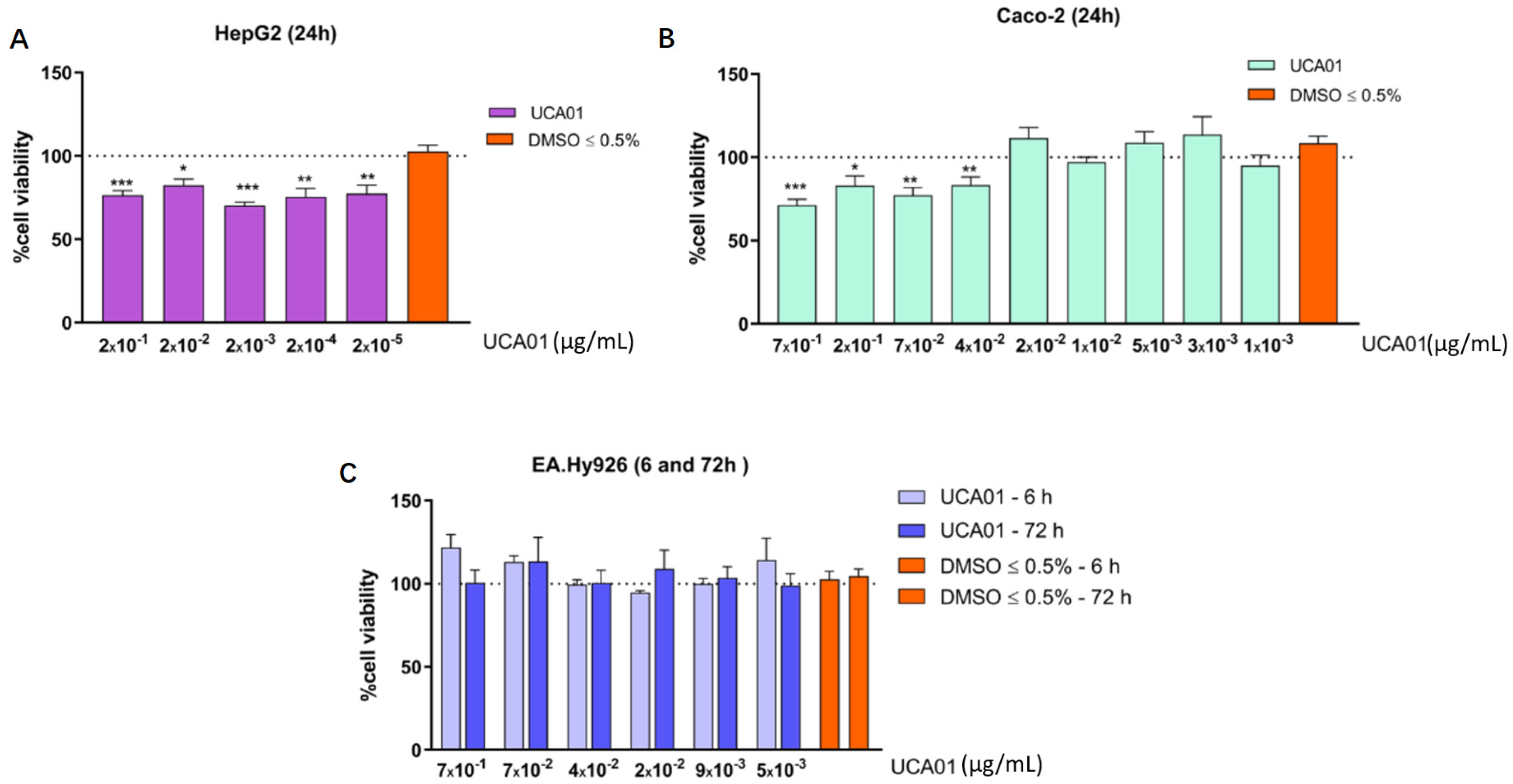
2.3. Antiproliferative Activity against Human Tumor Cells of N. gaditana Recombinant UCA01
3. Discussion
4. Materials and Methods
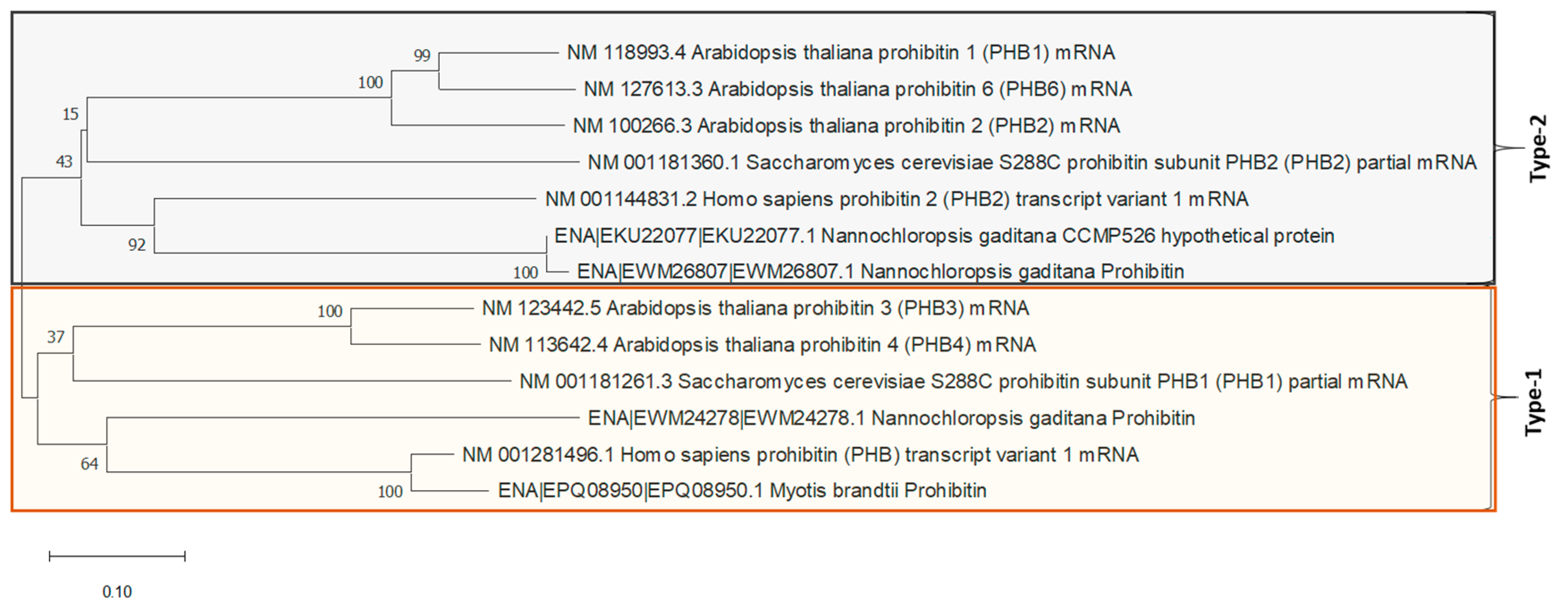
4.1. Construction and Phylogenetics Analysis
4.2. Heterologous Expression and Purification of Recombinant UCA01
4.2.1. RNA Extraction, cDNA Synthesis and PCR Amplification
4.2.2. Amplified Fragment Purification
4.2.3. Digestion with Restriction Enzymes
4.2.4. Plasmid (pET28a) Plus Amplification Product Construction
4.2.5. Competent E. coli Cells Transformed with PET28a-UCA01 Construction
4.2.6. UCA01 Transform Isolation
4.2.7. Induction of UCA01 Protein Expression
4.2.8. UCA01 Recombinant Protein Purification
- Human colorectal adenocarcinoma epithelial cell line: Caco-2 (ATCC® HTB-5 37);
- Human hepatocellular carcinoma cell line: HepG2 (ATCC®-HB-8065);
- Human endothelial cell line EA.hy926 (ATCC® CRL-2922 ™).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yates, J.R., 3rd. Recent technical advances in proteomics. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Rolain, J.M. Use of MALDI-TOF mass spectrometry for identification of bacteria that are difficult to culture. J. Microbiol. Methods 2013, 92, 14–24. [Google Scholar] [PubMed]
- Trauger, S.A.; Junker, T.; Siuzdak, G. Investigating Viral Proteins and Intact Viruses with Mass Spectrometry. In Modern Mass Spectrometry; Schalley, C.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 265–282. [Google Scholar]
- Novakova, K.; Sedo, O.; Zdrahal, Z. Mass spectrometry characterization of plant phosphoproteins. Curr. Protein Pept. Sci. 2011, 12, 112–125. [Google Scholar] [CrossRef]
- Liñeiro, E.; Cantoral, J.M.; Fernández-Acero, F.J. Contribution of Proteomics Research to Understanding Botrytis Biology and Pathogenicity. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 315–333. [Google Scholar]
- Fu, W.; Nelson, D.R.; Mystikou, A.; Daakour, S.; Salehi-Ashtiani, K. Advances in microalgal research and engineering development. Curr. Opin. Biotechnol. 2019, 59, 157–164. [Google Scholar]
- Wilken, S.E.; Swift, C.L.; Podolsky, I.A.; Lankiewicz, T.S.; Seppälä, S.; O’Malley, M.A. Linking ‘omics’ to function unlocks the biotech potential of non-model fungi. Curr. Opin. Syst. Biol. 2019, 14, 9–17. [Google Scholar] [CrossRef]
- Armengaud, J.; Trapp, J.; Pible, O.; Geffard, O.; Chaumot, A.; Hartmann, E.M. Non-model organisms, a species endangered by proteogenomics. J. Proteom. 2014, 105, 5–18. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, Y.; Chen, X.; Lee, E.J.; Barber, C.J.; Chakrabarty, R.; Desgagne-Penix, I.; Haslam, T.M.; Kim, Y.B.; Liu, E.; et al. Transcriptome analysis based on next-generation sequencing of non-model plants producing specialized metabolites of biotechnological interest. J. Biotechnol. 2013, 166, 122–134. [Google Scholar]
- Lauritano, C.; Ferrante, M.I.; Rogato, A. Marine Natural Products from Microalgae: An -Omics Overview. Mar. Drugs 2019, 17, 269. [Google Scholar] [CrossRef]
- Talero, E.; Garcia-Maurino, S.; Avila-Roman, J.; Rodriguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer. Mar. Drugs 2015, 13, 6152–6209. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-H.; Wang, Y.-J.; Sheng, J.; Wang, F.; Zheng, Y.; Lin, X.-K.; Sun, M. Antitumor peptides from marine organisms. Mar. Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X. Separation, antitumor activities, and encapsulation of polypeptide from Chlorella pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Tejano, L.A.; Peralta, J.P.; Yap, E.E.S.; Panjaitan, F.C.A.; Chang, Y.W. Prediction of Bioactive Peptides from Chlorella Sorokiniana Proteins Using Proteomic Techniques in Combination with Bioinformatics Analyses. Int. J. Mol. Sci. 2019, 20, 1786. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.P.; Williams, E.; Wang, D.Z.; Xie, Z.X.; Hsia, R.C.; Jenck, A.; Halden, R.; Li, J.; Chen, F.; Place, A.R. Responses of Nannochloropsis oceanica IMET1 to Long-Term Nitrogen Starvation and Recovery. Plant Physiol. 2013, 162, 1110–1126. [Google Scholar] [CrossRef]
- Tran, N.-A.T.; Padula, M.P.; Evenhuis, C.R.; Commault, A.S.; Ralph, P.J.; Tamburic, B. Proteomic and biophysical analyses reveal a metabolic shift in nitrogen deprived Nannochloropsis oculata. Algal Res. 2016, 19, 1–11. [Google Scholar] [CrossRef]
- Anand, V.; Singh, P.K.; Banerjee, C.; Shukla, P. Proteomic approaches in microalgae: Perspectives and applications. 3 Biotech 2017, 7, 197. [Google Scholar] [CrossRef]
- Rai, V.; Muthuraj, M.; Gandhi, M.N.; Das, D.; Srivastava, S. Real-time iTRAQ-based proteome profiling revealed the central metabolism involved in nitrogen starvation induced lipid accumulation in microalgae. Sci. Rep. 2017, 7, 45732. [Google Scholar] [CrossRef]
- Fernandez-Acero, F.J.; Amil-Ruiz, F.; Duran-Pena, M.J.; Carrasco, R.; Fajardo, C.; Guarnizo, P.; Fuentes-Almagro, C.; Vallejo, R.A. Valorisation of the microalgae Nannochloropsis gaditana biomass by proteomic approach in the context of circular economy. J. Proteom. 2019, 193, 239–242. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yoo, W.I.; Lee, S.H.; Lee, M.Y. Proteomic Analysis of Cadmium-Induced Protein Profile Alterations from Marine Alga Nannochloropsis oculata. Ecotoxicology 2005, 14, 589–596. [Google Scholar] [CrossRef]
- Siegler, H.; Valerius, O.; Ischebeck, T.; Popko, J.; Tourasse, N.J.; Vallon, O.; Khozin-Goldberg, I.; Braus, G.H.; Feussner, I. Analysis of the lipid body proteome of the oleaginous alga Lobosphaera incisa. BMC Plant Biol. 2017, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Li, H.; Zhao, P.; Yu, R.; Pan, G.; Gao, S.; Xie, X.; Huang, A.; He, L.; Wang, G. Quantitative proteomic analysis of thylakoid from two microalgae (Haematococcus pluvialis and Dunaliella salina) reveals two different high light-responsive strategies. Sci. Rep. 2014, 4, 6661. [Google Scholar] [CrossRef] [PubMed]
- Garibay-Hernández, A.; Barkla, B.J.; Vera-Estrella, R.; Martinez, A.; Pantoja, O. Membrane Proteomic Insights into the Physiology and Taxonomy of an Oleaginous Green Microalga. Plant Physiol. 2017, 173, 390–416. [Google Scholar] [CrossRef] [PubMed]
- Ben Amor, F.; Elleuch, F.; Ben Hlima, H.; Garnier, M.; Saint-Jean, B.; Barkallah, M.; Pichon, C.; Abdelkafi, S.; Fendri, I. Proteomic Analysis of the Chlorophyta Dunaliella New Strain AL-1 Revealed Global Changes of Metabolism during High Carotenoid Production. Mar. Drugs 2017, 15, 293. [Google Scholar] [CrossRef]
- Guarnieri, M.T.; Nag, A.; Smolinski, S.L.; Darzins, A.; Seibert, M.; Pienkos, P.T. Examination of Triacylglycerol Biosynthetic Pathways via De Novo Transcriptomic and Proteomic Analyses in an Unsequenced Microalga. PLoS ONE 2011, 6, e25851. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, J.; Lu, S.; Lv, Y.; Yuan, Y. Quantitative proteomic profiling reveals photosynthesis responsible for inoculum size dependent variation in Chlorella sorokiniana. Biotechnol. Bioeng. 2013, 110, 773–784. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Shen, Y.; Yan, D.; He, X.; Dai, J.; Wu, Q. Oil accumulation mechanisms of the oleaginous microalga Chlorella protothecoides revealed through its genome, transcriptomes, and proteomes. BMC Genom. 2014, 15, 582. [Google Scholar] [CrossRef]
- Roustan, V.; Bakhtiari, S.; Roustan, P.-J.; Weckwerth, W. Quantitative in vivo phosphoproteomics reveals reversible signaling processes during nitrogen starvation and recovery in the biofuel model organism Chlamydomonas reinhardtii. Biotechnol. Biofuels 2017, 10, 280. [Google Scholar] [CrossRef]
- Krohn-Molt, I.; Alawi, M.; Förstner, K.U.; Wiegandt, A.; Burkhardt, L.; Indenbirken, D.; Thieß, M.; Grundhoff, A.; Kehr, J.; Tholey, A.; et al. Insights into Microalga and Bacteria Interactions of Selected Phycosphere Biofilms Using Metagenomic, Transcriptomic, and Proteomic Approaches. Front. Microbiol. 2017, 8, 1941. [Google Scholar] [CrossRef]
- Longworth, J.; Wu, D.; Huete-Ortega, M.; Wright, P.C.; Vaidyanathan, S. Proteome response of Phaeodactylum tricornutum, during lipid accumulation induced by nitrogen depletion. Algal Res. 2016, 18, 213–224. [Google Scholar] [CrossRef]
- Beisser, D.; Kaschani, F.; Graupner, N.; Grossmann, L.; Jensen, M.; Ninck, S.; Schulz, F.; Rahmann, S.; Boenigk, J.; Kaiser, M. Quantitative Proteomics Reveals Ecophysiological Effects of Light and Silver Stress on the Mixotrophic Protist Poterioochromonas malhamensis. PLoS ONE 2017, 12, e0168183. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Eravci, M.; Weise, C.; Roesler, U. Mass spectrometry data from label-free quantitative proteomic analysis of harmless and pathogenic strains of infectious microalgae, Prototheca spp. Data Brief 2017, 12, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Garnier, M.; Carrier, G.; Rogniaux, H.; Nicolau, E.; Bougaran, G.; Saint-Jean, B.; Cadoret, J.P. Comparative proteomics reveals proteins impacted by nitrogen deprivation in wild-type and high lipid-accumulating mutant strains of Tisochrysis lutea. J. Proteom. 2014, 105, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Read, B.A.; Kegel, J.; Klute, M.J.; Kuo, A.; Lefebvre, S.C.; Maumus, F.; Mayer, C.; Miller, J.; Monier, A.; Salamov, A.; et al. Pan genome of the phytoplankton Emiliania underpins its global distribution. Nature 2013, 499, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, R.; Fajardo, C.; Guarnizo, P.; Vallejo, R.A.; Fernandez-Acero, F.J. Biotechnology Applications of Microalgae in the Context of EU “Blue Growth” Initiatives. J. Microbiol. Genet. 2018. [Google Scholar] [CrossRef]
- Bendaoud, A.; Ahmed, F.-Z.; Merzouk, H.; Bouanane, S.; Bendimerad, S. Effects of Dietary Microalgae Nannochloropsis Gaditana on Serum and Redox Status in Obese Rats Subjected to a High Fat Diet. Phytothérapie 2018, 17, 177–187. [Google Scholar] [CrossRef]
- Peña, E.H.; Medina, A.R.; Callejón, M.J.J.; Sánchez, M.D.M.; Cerdán, L.E.; Moreno, P.A.G.; Grima, E.M. Extraction of free fatty acids from wet Nannochloropsis gaditana biomass for biodiesel production. Renew. Energy 2015, 75, 366–373. [Google Scholar] [CrossRef]
- Zou, N.; Zhang, C.; Cohen, Z.; Richmond, A. Production of cell mass and eicosapentaenoic acid (EPA) in ultrahigh cell density cultures of Nannochloropsis sp. (Eustigmatophyceae). Eur. J. Phycol. 2000, 35, 127–133. [Google Scholar] [CrossRef]
- Letsiou, S.; Kalliampakou, K.; Gardikis, K.; Mantecon, L.; Infante, C.; Chatzikonstantinou, M.; Labrou, N.E.; Flemetakis, E. Skin protective effects of Nannochloropsis gaditana extract on H2O2-stressed human dermal fibroblasts. Front. Mar. Sci. 2017, 4, 221. [Google Scholar] [CrossRef]
- Menegol, T.; Romero-Villegas, G.I.; López-Rodríguez, M.; Navarro-López, E.; López-Rosales, L.; Chisti, Y.; Cerón-García, M.C.; Molina-Grima, E. Mixotrophic production of polyunsaturated fatty acids and carotenoids by the microalga Nannochloropsis gaditana. J. Appl. Phycol. 2019, 31, 2823–2832. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Fuerstenberg, S.I.; Tae, H.; Settlage, R.E.; Boore, J.L.; Posewitz, M.C. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat. Commun. 2012, 3, 686. [Google Scholar] [CrossRef]
- Alboresi, A.; Le Quiniou, C.; Yadav, S.K.; Scholz, M.; Meneghesso, A.; Gerotto, C.; Simionato, D.; Hippler, M.; Boekema, E.J.; Croce, R.; et al. Conservation of core complex subunits shaped the structure and function of photosystem I in the secondary endosymbiont alga Nannochloropsis gaditana. New Phytol. 2017, 213, 714–726. [Google Scholar] [CrossRef]
- Weiner, A.K.; Sidoli, S.; Diskin, S.J.; Garcia, B.A. Graphical Interpretation and Analysis of Proteins and Their Ontologies (GiaPronto): A One-Click Graph Visualization Software for Proteomics Data Sets. Mol. Cell. Proteom. 2018, 17, 1426–1431. [Google Scholar] [CrossRef]
- Sinitcyn, P.; Rudolph, J.D.; Cox, J. Computational Methods for Understanding Mass Spectrometry–Based Shotgun Proteomics Data. Annu. Rev. Biomed. Data Sci. 2018, 1, 207–234. [Google Scholar] [CrossRef]
- Laukens, K.; Naulaerts, S.; Berghe, W.V. Bioinformatics approaches for the functional interpretation of protein lists: From ontology term enrichment to network analysis. Proteomics 2015, 15, 981–996. [Google Scholar] [CrossRef]
- Li, W.; McWilliam, H.; de la Torre, A.R.; Grodowski, A.; Benediktovich, I.; Goujon, M.; Nauche, S.; Lopez, R. Non-redundant patent sequence databases with value-added annotations at two levels. Nucleic Acids Res. 2009, 38 (Suppl. 1), D52–D56. [Google Scholar] [CrossRef]
- Carpinelli, E.C.; Telatin, A.; Vitulo, N.; Forcato, C.; D’Angelo, M.; Schiavon, R.; Vezzi, A.; Giacometti, G.M.; Morosinotto, T.; Valle, G. Chromosome scale genome assembly and transcriptome profiling of Nannochloropsis gaditana in nitrogen depletion. Mol. Plant 2014, 7, 323–335. [Google Scholar] [CrossRef]
- Jean Beltran, P.M.; Federspiel, J.D.; Sheng, X.; Cristea, I.M. Proteomics and integrative omic approaches for understanding host-pathogen interactions and infectious diseases. Mol. Syst. Biol. 2017, 13, 922. [Google Scholar] [CrossRef]
- Peri, S.; Navarro, J.D.; Kristiansen, T.Z.; Amanchy, R.; Surendranath, V.; Muthusamy, B.; Gandhi, T.K.; Chandrika, K.N.; Deshpande, N.; Suresh, S.; et al. Human protein reference database as a discovery resource for proteomics. Nucleic Acids Res. 2004, 32, D497–D501. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evol. Int. J. Org. Evol. 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Lineiro, E.; Chiva, C.; Cantoral, J.M.; Sabido, E.; Fernandez-Acero, F.J. Phosphoproteome analysis of B. cinerea in response to different plant-based elicitors. J. Proteom. 2016, 139, 84–94. [Google Scholar] [CrossRef]
- Blackstock, W.P.; Weir, M.P. Proteomics: Quantitative and physical mapping of cellular proteins. Trends Biotechnol. 1999, 17, 121–127. [Google Scholar] [CrossRef]
- European Commission: Blue Growth. Available online: https://ec.europa.eu/maritimeaffairs/policy/blue_growth_en (accessed on 23 December 2020).
- Mishra, S.; Murphy, L.; Nyomba, G.; Murphy, L. Prohibitin: A potential target for new therapeutics. Trends Mol. Med. 2005, 11, 192–197. [Google Scholar] [CrossRef]
- Sato, T.; Sakamoto, T.; Takita, K.; Saito, H.; Okui, K.; Nakamura, Y. The human prohibitin (PHB) gene family and its somatic mutations in human tumors. Genomics 1993, 17, 762–764. [Google Scholar] [CrossRef]
- Mishra, S.; Murphy, L.C.; Murphy, L.J. The Prohibitins: Emerging roles in diverse functions. J. Cell. Mol. Med. 2006, 10, 353–363. [Google Scholar] [CrossRef]
- Van Aken, O.; Pecenkova, T.; van de Cotte, B.; De Rycke, R.; Eeckhout, D.; Fromm, H.; De Jaeger, G.; Witters, E.; Beemster, G.T.; Inze, D.; et al. Mitochondrial type-I prohibitins of Arabidopsis thaliana are required for supporting proficient meristem development. Plant J. Cell Mol. Biol. 2007, 52, 850–864. [Google Scholar] [CrossRef]
- Signorile, A.; Sgaramella, G.; Bellomo, F.; De Rasmo, D. Prohibitins: A Critical Role in Mitochondrial Functions and Implication in Diseases. Cells 2019, 8, 71. [Google Scholar] [CrossRef]
- Yang, J.; Li, B.; He, Q.Y. Significance of prohibitin domain family in tumorigenesis and its implication in cancer diagnosis and treatment. Cell Death Dis. 2018, 9, 580. [Google Scholar] [CrossRef]
- McClung, J.K.; Danner, D.B.; Stewart, D.A.; Smith, J.R.; Schneider, E.L.; Lumpkin, C.K.; Dell’Orco, R.T.; Nuell, M.J. Isolation of a cDNA that hybrid selects antiproliferative mRNA from rat liver. Biochem. Biophys. Res. Commun. 1989, 164, 1316–1322. [Google Scholar] [CrossRef]
- Nuell, M.J.; Stewart, D.A.; Walker, L.; Friedman, V.; Wood, C.M.; Owens, G.A.; Smith, J.R.; Schneider, E.L.; Dell’Orco, R.; Lumpkin, C.K. Prohibitin, an evolutionarily conserved intracellular protein that blocks DNA synthesis in normal fibroblasts and HeLa cells. Mol. Cell. Biol. 1991, 11, 1372–1381. [Google Scholar] [CrossRef]
- Mishra, S.; Ande, S.R.; Nyomba, B.L.G. The role of prohibitin in cell signaling. FEBS J. 2010, 277, 3937–3946. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Sharma, J.R.; Sibuyi, N.R.S.; Tincho, M.B.; Boyom, F.F.; Meyer, M. Synthesis of Biogenic Gold Nanoparticles from Terminalia mantaly Extracts and the Evaluation of Their In Vitro Cytotoxic Effects in Cancer Cells. Molecules 2020, 25, 4469. [Google Scholar] [CrossRef]
- Medrano-Padial, C.; Puerto, M.; Merchan-Gragero, M.D.M.; Moreno, F.J.; Richard, T.; Cantos-Villar, E.; Pichardo, S. Cytotoxicity studies of a stilbene extract and its main components intended to be used as preservative in the wine industry. Food Res. Int. 2020, 137, 109738. [Google Scholar] [CrossRef]
- Sandate-Flores, L.; Romero-Esquivel, E.; Rodriguez-Rodriguez, J.; Rostro-Alanis, M.; Melchor-Martinez, E.M.; Castillo-Zacarias, C.; Ontiveros, P.R.; Celaya, M.F.M.; Chen, W.N.; Iqbal, H.M.N.; et al. Functional Attributes and Anticancer Potentialities of Chico (Pachycereus Weberi) and Jiotilla (Escontria Chiotilla) Fruits Extract. Plants 2020, 9, 1623. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). Available online: https://www.nih.gov/about-nih/what-we-do/nih-almanac/national-cancer-institute-nci (accessed on 23 December 2020).
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Reinado, R.; Escobar-Niño, A.; Fajardo, C.; Morano, I.M.; Amil-Ruiz, F.; Martinez-Rodríguez, G.; Fuentes-Almagro, C.; Capilla, V.; Tomás-Cobos, L.; Soriano-Romaní, L.; et al. Development of New Antiproliferative Compound against Human Tumor Cells from the Marine Microalgae Nannochloropsis gaditana by Applied Proteomics. Int. J. Mol. Sci. 2021, 22, 96. https://doi.org/10.3390/ijms22010096
Carrasco-Reinado R, Escobar-Niño A, Fajardo C, Morano IM, Amil-Ruiz F, Martinez-Rodríguez G, Fuentes-Almagro C, Capilla V, Tomás-Cobos L, Soriano-Romaní L, et al. Development of New Antiproliferative Compound against Human Tumor Cells from the Marine Microalgae Nannochloropsis gaditana by Applied Proteomics. International Journal of Molecular Sciences. 2021; 22(1):96. https://doi.org/10.3390/ijms22010096
Chicago/Turabian StyleCarrasco-Reinado, Rafael, Almudena Escobar-Niño, Carlos Fajardo, Ines M. Morano, Francisco Amil-Ruiz, Gonzalo Martinez-Rodríguez, Carlos Fuentes-Almagro, Victoria Capilla, Lidia Tomás-Cobos, Laura Soriano-Romaní, and et al. 2021. "Development of New Antiproliferative Compound against Human Tumor Cells from the Marine Microalgae Nannochloropsis gaditana by Applied Proteomics" International Journal of Molecular Sciences 22, no. 1: 96. https://doi.org/10.3390/ijms22010096
APA StyleCarrasco-Reinado, R., Escobar-Niño, A., Fajardo, C., Morano, I. M., Amil-Ruiz, F., Martinez-Rodríguez, G., Fuentes-Almagro, C., Capilla, V., Tomás-Cobos, L., Soriano-Romaní, L., Guarnizo, P., Vallejo, R. A., & Fernández-Acero, F. J. (2021). Development of New Antiproliferative Compound against Human Tumor Cells from the Marine Microalgae Nannochloropsis gaditana by Applied Proteomics. International Journal of Molecular Sciences, 22(1), 96. https://doi.org/10.3390/ijms22010096







