What Can We Learn from FGF-2 Isoform-Specific Mouse Mutants? Differential Insights into FGF-2 Physiology In Vivo
Abstract
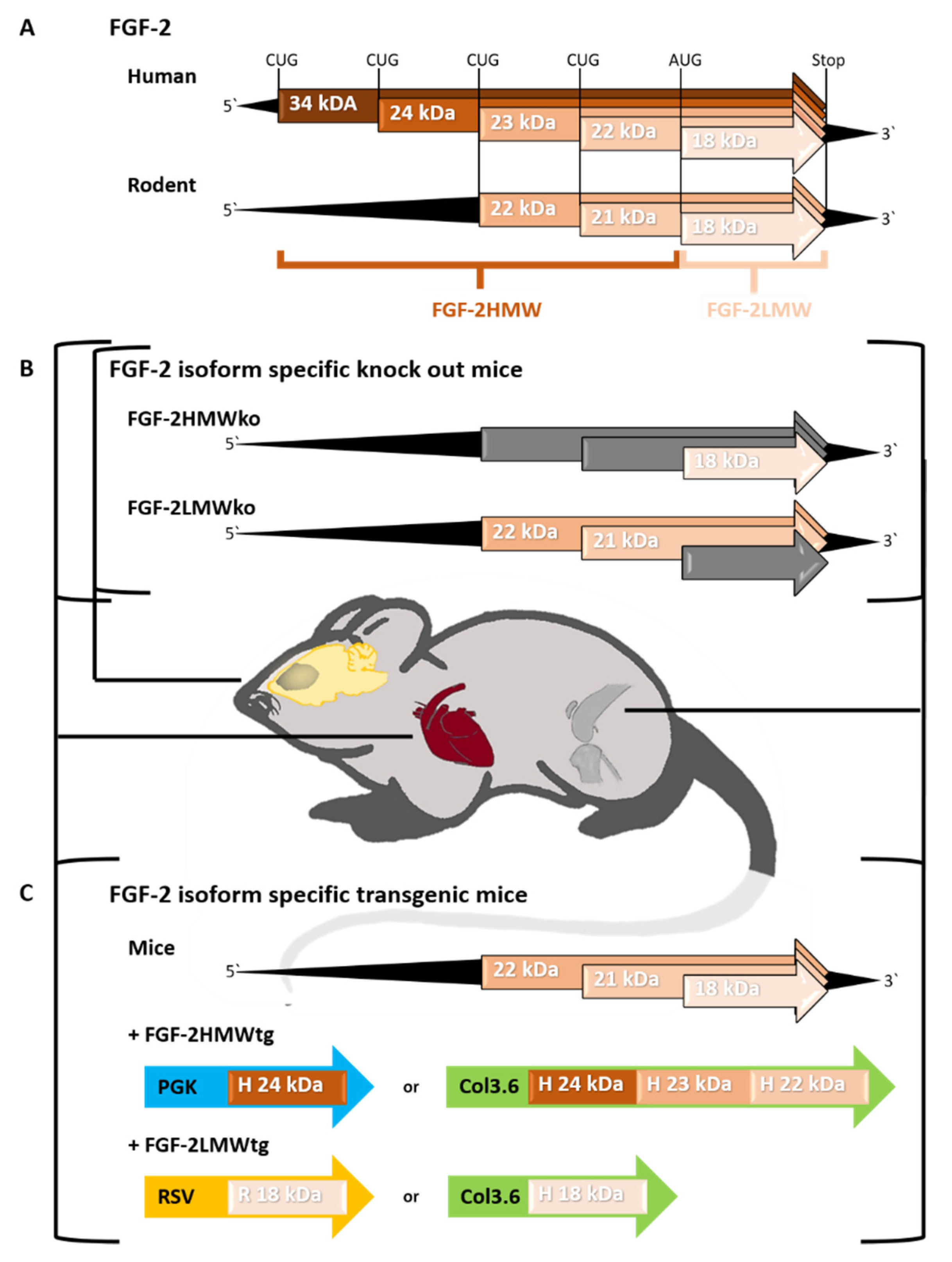
1. Introduction
2. FGF-2 Isoforms in the Cardiovascular System
| Strain | FGF-2 LMWko (FGF-2tm2Doe) | FGF-2 HMWko (FGF-2tm3Doe) | FGF-2HMWtg Overexpressed Human 24 kDa Driven by PGK Promoter | FGF-2LMWtg Overexpressed Rat 18 kDa Driven by RSV Promoter |
|---|---|---|---|---|
| Phenotype | ♂ ↑ left atrial dimensions ↓ E/A ratio ↓ decline in left ventricular pressure ↑ isovolumic relaxation time (impaired elaxation filling patterns) (Nusayr and Doetschman 2013) [50] ↑ α-SMA expression (Nusayr, Sadideen et al., 2013) [51] ♀ ↑ body weight ↑ heart hypoplasia ↓ LV posterior mass and wall thickness in iastole and systole ↑ LV volume in systole and diastole ↑ LV internal dimension in diastole ↑ E/A ratio ↑ myocardial stiffnessrestrictive diastolic filling patterns (Nusayr and Doetschman 2013) [50] ♀ ↑ ANF expression (Nusayr, Sadideen et al., 2013) [51] ♀ ↑ angiogenesis mediated by 17β-estradiol § (Garmy-Susini, Delmas et al., 2004) [42] | ♂/♀ ↑ FGF-2LMW protein levels in heart tissue, capillaries and vessels (Azhar, Yin et al., 2009) [43], (Liao, Bodmer et al., 2010) [44] ♂ ↓ LV volume in systole ↓ LV internal dimensions in systole ↑ systolic function indicated by greater cardiac output, stroke volume and fractional shortening ↑ flow velocity in the pulmonary artery ↑ mitral valve flow mean velocity and mitral valve flow mean pressure gradient (Nusayr and Doetschman 2013) [50] ♀ ↑ ANF expression (Nusayr, Sadideen et al., 2013) [51] ♀ no alterations compared to wt littermates (Nusayr and Doetschman 2013) [50] (Nusayr, Sadideen et al., 2013) [51] | ♂/♀ no alteration regarding heart growth, vasculogenesis/ angiogenesis or endogenous FGF-2 protein expression (Liao, Bodmer et al., 2010) [44] | ♂/♀ ↑ capillary density ↑ levels of phosphorylated c-Jun ↑ levels of phosphorylated p38MAPK ↑ levels of phosphorylated membrane associated PKCα and cytosolic PKCε (Sheikh, Sontag et al., 2001) [48] |
| Atherosclerosis | FGF-2tm2Doe x ApoEko ♂ ↓ atherosclerotic lesions in the aorta ↓ macrophage infiltration ↓ MCP-1, VCAM-1, Nox4 and p47phox expression (Liang, Wang et al., 2018) [60] |
3. FGF-2 Isoforms in Bone Physiology
| (A) | ||||
|---|---|---|---|---|
| Strain | FGF-2LMWko (FGF-2tm2Doe) | FGF-2HMWko (FGF-2tm3Doe) | FGF-2HMWtg Overexpressed Human 22, 23, 24 kDa Driven by Col3.6 Promoter | FGF-2LMWtg Overexpressed Human 18 kDa Driven by Col3.6 Promoter |
| Phenotype | ♂ ↓ vertebral bone mineral density and content ↑ sFRP1 protein levels in trabecular bones (Xiao, Liu et al., 2009) [47] | ♂ ↑ whole body bone mineral density and content ↑ vertebral, femoral bone mineral density and content ↑ femoral bone volume, trabecular thickness, number (cortical bone area, thickness, cortical mask) ↓ femoral trabecular spacing ↑ connective tissue density ↓ cortical porosity, bone resorption (↓ osteoclast surface, number) ↑ bone formation in cortical periosteum, trabecular bone (↑ osteoblast surface, inter-label thickness, mineral apposition rate) ↑ tibial Col1a1, Runx2, osterix, oc, op, Dmp1 gene expression ↓ femoral Sost gene expression ↓ serum sclerostin, protein levels ↓ tibial Fgf-2, Fgf-23 gene expression (Homer-Bouthiette, Doetschman et al., 2014) [78] | ♂ dwarfism, osteomalacia ↓ body weight ↓ whole body bone mineral density and content ↓ femoral bone length ↓ vertebral volume, bone mineral density and content ↓ femoral bone volume, trabecular number, thickness ↑ femoral trabecular spacing ↑ bone resorption (↑ osteoclast surface, number) ↓ bone formation (↓ osteoblast, mineralization surface, bone formation rate) ↓ tibial Col1a1, Oc gene expression ↑ tibial Op, Mgp gene expression↓ serum phosphate ↑ serum PTH, CTX, FGF-23 ↑ tibial, femoral Fgf-23, Phex gene expression, protein levels ↑ renal Fgfr-1c, Fgfr-3c, Klotho gene expression ♂ with continuous phosphate diet ↑ body weight, bone mineral content and density ↑ serum phosphate to a normal level ↑ serum FGF-23 (Xiao, Naganawa et al., 2010) [45] ♂ ↓ tibia bone mineral density and content ↑ renal FGFR-1, FGFR-3, Klotho, C-Fos, activated ERK protein levels ↑ renal C-fos, Egr1 gene expression ↓ renal Npt2 gene expression ↑ renal Cyp24, Cyp27b1 gene expression ↓ renal Npt2 protein levels (Du, Xiao et al., 2017) [83] ♂ ↓ tail length ↓ femoral length ↑ urinary phosphate level ↑ cortical porosity, trabecular spacing, osteoid volume ↓ cortical thickness, tissue ↓ endosteal/periosteal perimeter, subendosteal area ↓ mineralization of cortical bone area, metaphyseal cancellous bone volume, trabecular number ↑ femoral Fgfr-3c, Pthr1, Op, Mgp gene expression (Xiao, Du et al., 2017) [84] | ♂ ↑ vertebral, tibial, femoral bone mineral density and content ↑ femoral bone volume, trabecular thickness, cortical bone area, thickness ↓ sFrp1 gene expression, protein levels in trabecular bones ↑ β-catenin gene expression, protein levels (Xiao, Liu et al., 2009) [47] ♂ ↑ Fgfr-1, Fgfr2, oc, β-catenin gene expression in calvaria bone ↓ sFrp1 gene expression in calvaria bone ↑ calvarial inter-label thickness, mineral apposition rate (Xiao, Ueno et al., 2014) [77] |
| Phenotype | ♀ ↓ body weight ↓ femoral, tibial, vertebral bone mineral density and content ↓ serum phosphate ↑ serum FGF-23, 1,25D ↑ urinary phosphate level ↑ renal FGFR-1, En-1, klotho protein levels ↑ renal Klotho, Sostdc-1, En-1, Cyp24 gene expression ↑ activated renal ERK, Gsk-3β (Tr216) protein levels ↓ renal Npt2, Akt gene expression ↓ activated renal Gsk-3β (Ser9), active β-catenin and Akt protein levels (Du, Xiao et al., 2016) [82] ♀ ↓ femur length, cortical density, mineral apposition rate ↑ cortical porosity ↓ femoral bone volume, trabecular number ↑ femoral trabecular spacing ↑ osteoid volume ↑ serum ALP ↓ serum TNAP ↓ TNAP activity in osteocytes ↑ renal Fgfr-1c, Fgfr-3 gene expression ↑ tibia Fgf-2, Fgfr-1c, Col1a1, Mgp, Dmp4, Phex, Mepe, Enpp1, SLc20a1 gene expression ↓ tibia Dmp1, Rankl, Oc gene expression ↑ femur cortical ERK, FGFR-1 protein levels (Xiao, Homer-Bouthiette et al., 2018) [79] | |||
| (B) | ||||
| Strain | FGF-2 LMWko (FGF-2tm2Doe) | FGF-2 HMWko (FGF-2tm3Doe) | FGF-2HMWtg Overexpressed Human 22, 23, 24 kDa Driven by Col3.6 Promoter | FGF-2LMWtg Overexpressed Human 18 kDa Driven by Col3.6 Promoter |
| Aging/Osteo-arthritis | ♂/♀ ↑ OA in knee joints (flattening of tibial plateau, osteophyte formation) ♂ ↓ femoral, tibial bone volume, trabecular number, thickness ↑ femoral, tibial trabecular spacing ↓ proteoglycan content, cartilage thickness in knee joint ↑ tendonitis, arthritis ↑ MMP-13, ADAMTS-5, FGF-2, FGF-23, FGFR-1 protein levels in articular cartilages ↑ Igf1, IL-1β, Bmp4, Hif1α, Bax, Fgf-2, Fgf-23, Fgfr-3 Vegf, Col10 gene expression in knee joints ↑ activated ERK protein levels in articular cartilage ↓ activated FGFR-3 in articular cartilage ↑ signs of OA following tibial loading (loss of proteoglycan content, thinning of subchondral bone) (Burt, Xiao et al., 2019) [71] | ♂/♀ no radiographical signs of OA in knee joints ♂ ↑ activated FGFR-3 protein levels in knees ↓ FGF-2 protein levels in articular cartilage (Burt, Xiao et al., 2019) [71] | ♂ ↑ OA in knee joints (flattening of tibial plateau, osteophyte formation, femoral subchondral bone thinning, sclerotic bone development, narrowing of the patellofemoral space, loss of trabeculae, sclerosis of femur) ↓ epiphyseal bone volume density, trabecular thickness, number in femur, tibiae ↓ proteoglycan content, cartilage thickness in knee joint ↑ Mmp13, Col10, ADAMTS-5 gene expression in articular cartilages ↑ Igf1, IL-1β, Bmp2, Bmp4, Hif1α, Bax, Sox9, Vegf gene expression in knee joints ↑ FGF-23, FGFR-1 protein levels in knee joints↓ mineralization of hypertrophic chondrocytes (Meo Burt, Xiao et al., 2016) [89] ♂ ↓ Sost, Dkk1, Lrp6 gene expression in knee joints ↑ Wnt5a, Axin2, Lef1 gene expression in knee joints ↓ Sost, Lrp6 protein levels in knee joints ↑ Wnt7b, Wnt5a, Lrp5, Axin2, Gsk-3β, Lef1, nuclear β-catenin protein levels in knee joints ↑ Mmp9 gene expression in femoral cartilage (Meo Burt, Xiao et al., 2018) [87] ♂/♀ ↑ signs of OA in knee joints (flattening of tibial plateau, osteophyte formation, sclerosis) ↓ femoral, tibial bone volume, trabecular number, thickness ↓ proteoglycane content, cartilage thickness in knee joints ↑ cartilage calcification in knee cartilage ♂ ↑ Fgfr-1c, Fgf-18 gene expression in knee joints ↓ Fgfr-3c gene expression in knee joints ↑ FGF-2 protein level in subchondral bone ↑ MMP-13, SOX9, ADAMTS-5 protein level in articular cartilages ↓ Dkk1 Lrp6, Sost protein levels in articular cartilage (Xiao, Williams et al., 2020) [92] | ♂ no radiographical signs of OA in knee joints (Meo Burt, Xiao et al., 2016) [89] |
4. FGF-2 Isoforms in the Central Nervous System
| Strain | FGF-2 LMWko (FGF-2tm2Doe) | FGF-2 HMWko (FGF-2tm3Doe) |
|---|---|---|
| Phenotype | ♂/♀ no FGF-2HMW protein expression can be found in the embryonic nigrostriatal system ↑ number of DA precursor cells in the rostral subventricular zone at E14.5 ↑ signs of regulatory apoptosis in DA neurons of the ventral midbrain at P0 ↑ number of DA neurons in the adult female SNpc ↓ explorative behavior of adult male mice (von Hövel, Leiter et al., 2019) [22] | ♂/♀ ↑ FGF-2LMW protein in brain tissue (Azhar, Yin et al., 2009) [43] ♂/♀ ↑ FGF-2LMW protein expression in the nigrostriatal pathway from P0 through development ↑ number of DA precursor cells in the rostral subventricular zone at E14.5 ↑ signs of apoptosis in DA neurons of the ventral midbrain at P0 ↑ number of DA neurons in the adult female SNpc ↑ explorative behavior of adult male mice (von Hövel, Leiter et al., 2019) [22] |
| ALS mouse model | ♂/♀ SOD1G93A × FGF-2LMWko ↓ body weight, general condition score, and survival rate from 17 weeks onwards ↓ performance in the rotarod test in weeks 14–17 ↓ runtime in the footprint track with 21 weeks ↓ EGF mRNA expression in the gastrocnemius muscle at 21 weeks (Kefalakes, Sarikidi et al., 2019) [113] | ♂/♀ SOD1G93A × FGF-2HMWko ↑ performance in the rotarod test in weeks 17, 18, 19, 20 and 21 (Kefalakes, Sarikidi et al., 2019) [113] |
| In vitro model | ♂/♀ ↓ RIG-1 protein level in MEF independent of IAV infection ↑ INF-α, INF-β, IL-6, and TNF-α expression in macrophages and MEFs following polyIC or SeV stimulation ↑ phosphorylation of IRF3 following SeV infection (Liu, Luo et al., 2015) [122] |
5. Outlook
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, REVIEWS3005. [Google Scholar] [CrossRef]
- Gospodarowicz, D. Purification of a fibroblast growth factor from bovine pituitary. J. Biol. Chem. 1975, 250, 2515–2520. [Google Scholar] [CrossRef]
- Gospodarowicz, D.; Jones, K.L.; Sato, G. Purification of a growth factor for ovarian cells from bovine pituitary glands. Proc. Natl. Acad. Sci. USA 1974, 71, 2295–2299. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, P.; Esch, F.; Baird, A.; Gospodarowicz, D. Acidic fibroblast growth factor (FGF) from bovine brain: Amino-terminal sequence and comparison with basic FGF. EMBO J. 1985, 4, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Petryszak, R.; Keays, M.; Tang, Y.A.; Fonseca, N.A.; Barrera, E.; Burdett, T.; Fullgrabe, A.; Fuentes, A.M.; Jupp, S.; Koskinen, S.; et al. Expression Atlas update—An integrated database of gene and protein expression in humans, animals and plants. Nucleic Acids Res. 2016, 44, D746–D752. [Google Scholar] [CrossRef]
- Amit, M.; Carpenter, M.K.; Inokuma, M.S.; Chiu, C.P.; Harris, C.P.; Waknitz, M.A.; Itskovitz-Eldor, J.; Thomson, J.A. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000, 227, 271–278. [Google Scholar] [CrossRef]
- Kawai, T.; Takahashi, T.; Esaki, M.; Ushikoshi, H.; Nagano, S.; Fujiwara, H.; Kosai, K. Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circ. J. 2004, 68, 691–702. [Google Scholar] [CrossRef][Green Version]
- Kizhner, T.; Ben-David, D.; Rom, E.; Yayon, A.; Livne, E. Effects of FGF2 and FGF9 on osteogenic differentiation of bone marrow-derived progenitors. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 294–301. [Google Scholar] [CrossRef]
- Lotz, S.; Goderie, S.; Tokas, N.; Hirsch, S.E.; Ahmad, F.; Corneo, B.; Le, S.; Banerjee, A.; Kane, R.S.; Stern, J.H.; et al. Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS ONE 2013, 8, e56289. [Google Scholar] [CrossRef]
- Qian, X.; Davis, A.A.; Goderie, S.K.; Temple, S. FGF2 concentration regulates the generation of neurons and glia from multipotent cortical stem cells. Neuron 1997, 18, 81–93. [Google Scholar] [CrossRef]
- Mimura, S.; Suga, M.; Liu, Y.; Kinehara, M.; Yanagihara, K.; Ohnuma, K.; Nikawa, H.; Furue, M.K. Synergistic effects of FGF-2 and Activin A on early neural differentiation of human pluripotent stem cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Florkiewicz, R.Z.; Sommer, A. Human basic fibroblast growth factor gene encodes four polypeptides: Three initiate translation from non-AUG codons. Proc. Natl. Acad. Sci. USA 1989, 86, 3978–3981. [Google Scholar] [CrossRef] [PubMed]
- Prats, H.; Kaghad, M.; Prats, A.C.; Klagsbrun, M.; Lelias, J.M.; Liauzun, P.; Chalon, P.; Tauber, J.P.; Amalric, F.; Smith, J.A.; et al. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc. Natl. Acad. Sci. USA 1989, 86, 1836–1840. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, E.; Touriol, C.; Boutonnet, C.; Gensac, M.C.; Vagner, S.; Prats, H.; Prats, A.C. A new 34-kilodalton isoform of human fibroblast growth factor 2 is cap dependently synthesized by using a non-AUG start codon and behaves as a survival factor. Mol. Cell. Biol. 1999, 19, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Powell, P.P.; Klagsbrun, M. Three forms of rat basic fibroblast growth factor are made from a single mRNA and localize to the nucleus. J. Cell. Physiol. 1991, 148, 202–210. [Google Scholar] [CrossRef]
- Zhou, M.; Sutliff, R.L.; Paul, R.J.; Lorenz, J.N.; Hoying, J.B.; Haudenschild, C.C.; Yin, M.; Coffin, J.D.; Kong, L.; Kranias, E.G.; et al. Fibroblast growth factor 2 control of vascular tone. Nat. Med. 1998, 4, 201–207. [Google Scholar] [CrossRef]
- Dono, R.; Texido, G.; Dussel, R.; Ehmke, H.; Zeller, R. Impaired cerebral cortex development and blood pressure regulation in FGF-2-deficient mice. EMBO J. 1998, 17, 4213–4225. [Google Scholar] [CrossRef]
- Ortega, S.; Ittmann, M.; Tsang, S.H.; Ehrlich, M.; Basilico, C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc. Natl. Acad. Sci. USA 1998, 95, 5672–5677. [Google Scholar] [CrossRef]
- Coffin, J.D.; Florkiewicz, R.Z.; Neumann, J.; Mort-Hopkins, T.; Dorn, G.W., 2nd; Lightfoot, P.; German, R.; Howles, P.N.; Kier, A.; O’Toole, B.A.; et al. Abnormal bone growth and selective translational regulation in basic fibroblast growth factor (FGF-2) transgenic mice. Mol. Biol. Cell 1995, 6, 1861–1873. [Google Scholar] [CrossRef]
- Rumpel, R.; Baron, O.; Ratzka, A.; Schroder, M.L.; Hohmann, M.; Effenberg, A.; Claus, P.; Grothe, C. Increased innervation of forebrain targets by midbrain dopaminergic neurons in the absence of FGF-2. Neuroscience 2016, 314, 134–144. [Google Scholar] [CrossRef] [PubMed]
- von Hövel, F.F.; Leiter, I.; Rumpel, R.; Langenhagen, A.; Wedekind, D.; Hager, C.; Bleich, A.; Palme, R.; Grothe, C. FGF-2 isoforms influence the development of dopaminergic neurons in the murine substantia nigra, but not anxiety-like behavior, stress susceptibility, or locomotor behavior. Behav. Brain Res. 2019, 374, 112113. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Sherman, L.; Lyman, W.; Morrison, R. Multiple molecular weight forms of basic fibroblast growth factor are developmentally regulated in the central nervous system. Dev. Biol. 1992, 152, 293–303. [Google Scholar] [CrossRef]
- Ebert, A.D.; Laussmann, M.; Wegehingel, S.; Kaderali, L.; Erfle, H.; Reichert, J.; Lechner, J.; Beer, H.D.; Pepperkok, R.; Nickel, W. Tec-kinase-mediated phosphorylation of fibroblast growth factor 2 is essential for unconventional secretion. Traffic 2010, 11, 813–826. [Google Scholar] [CrossRef]
- Florkiewicz, R.Z.; Baird, A.; Gonzalez, A.M. Multiple forms of bFGF: Differential nuclear and cell surface localization. Growth Factors 1991, 4, 265–275. [Google Scholar] [CrossRef]
- Taverna, S.; Ghersi, G.; Ginestra, A.; Rigogliuso, S.; Pecorella, S.; Alaimo, G.; Saladino, F.; Dolo, V.; Dell’Era, P.; Pavan, A.; et al. Shedding of membrane vesicles mediates fibroblast growth factor-2 release from cells. J. Biol. Chem. 2003, 278, 51911–51919. [Google Scholar] [CrossRef]
- Backhaus, R.; Zehe, C.; Wegehingel, S.; Kehlenbach, A.; Schwappach, B.; Nickel, W. Unconventional protein secretion: Membrane translocation of FGF-2 does not require protein unfolding. J. Cell Sci. 2004, 117, 1727–1736. [Google Scholar] [CrossRef]
- Sleeman, M.; Fraser, J.; McDonald, M.; Yuan, S.; White, D.; Grandison, P.; Kumble, K.; Watson, J.D.; Murison, J.G. Identification of a new fibroblast growth factor receptor, FGFR5. Gene 2001, 271, 171–182. [Google Scholar] [CrossRef]
- Grothe, C.; Timmer, M. The physiological and pharmacological role of basic fibroblast growth factor in the dopaminergic nigrostriatal system. Brain Res. Rev. 2007, 54, 80–91. [Google Scholar] [CrossRef]
- Mason, I. Initiation to end point: The multiple roles of fibroblast growth factors in neural development. Nat. Rev. Neurosci. 2007, 8, 583–596. [Google Scholar] [CrossRef]
- Quarto, N.; Finger, F.P.; Rifkin, D.B. The NH2-terminal extension of high molecular weight bFGF is a nuclear targeting signal. J. Cell. Physiol. 1991, 147, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Claus, P.; Doring, F.; Gringel, S.; Muller-Ostermeyer, F.; Fuhlrott, J.; Kraft, T.; Grothe, C. Differential intranuclear localization of fibroblast growth factor-2 isoforms and specific interaction with the survival of motoneuron protein. J. Biol. Chem. 2003, 278, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bugler, B.; Amalric, F.; Prats, H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol. Cell. Biol. 1991, 11, 573–577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Renko, M.; Quarto, N.; Morimoto, T.; Rifkin, D.B. Nuclear and cytoplasmic localization of different basic fibroblast growth factor species. J. Cell. Physiol. 1990, 144, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, V.; Nilsen, T.; Wiedlocha, A. Functional diversity of FGF-2 isoforms by intracellular sorting. Bioessays 2006, 28, 504–514. [Google Scholar] [CrossRef]
- Dunham-Ems, S.M.; Lee, Y.W.; Stachowiak, E.K.; Pudavar, H.; Claus, P.; Prasad, P.N.; Stachowiak, M.K. Fibroblast growth factor receptor-1 (FGFR1) nuclear dynamics reveal a novel mechanism in transcription control. Mol. Biol. Cell 2009, 20, 2401–2412. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Moffett, J.; Myers, J.; Fang, X.; Stachowiak, E.K.; Maher, P.; Kratz, E.; Hines, J.; Fluharty, S.J.; Mizukoshi, E.; et al. Novel nuclear signaling pathway mediates activation of fibroblast growth factor-2 gene by type 1 and type 2 angiotensin II receptors. Mol. Biol. Cell 2001, 12, 449–462. [Google Scholar] [CrossRef]
- Foletti, A.; Ackermann, J.; Schmidt, A.; Hummler, E.; Beermann, F. Absence of fibroblast growth factor 2 does not prevent tumor formation originating from the RPE. Oncogene 2002, 21, 1841–1847. [Google Scholar] [CrossRef][Green Version]
- Eppig, J.T.; Strivens, M. Finding a mouse: The International Mouse Strain Resource (IMSR). Trends Genet. 1999, 15, 81–82. [Google Scholar] [CrossRef]
- Strivens, M.; Eppig, J.T. Visualizing the laboratory mouse: Capturing phenotype information. Genetica 2004, 122, 89–97. [Google Scholar] [CrossRef]
- Eppig, J.T.; Motenko, H.; Richardson, J.E.; Richards-Smith, B.; Smith, C.L. The International Mouse Strain Resource (IMSR): Cataloging worldwide mouse and ES cell line resources. Mamm. Genome 2015, 26, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Garmy-Susini, B.; Delmas, E.; Gourdy, P.; Zhou, M.; Bossard, C.; Bugler, B.; Bayard, F.; Krust, A.; Prats, A.C.; Doetschman, T.; et al. Role of fibroblast growth factor-2 isoforms in the effect of estradiol on endothelial cell migration and proliferation. Circ. Res. 2004, 94, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.; Yin, M.; Zhou, M.; Li, H.; Mustafa, M.; Nusayr, E.; Keenan, J.B.; Chen, H.; Pawlosky, S.; Gard, C.; et al. Gene targeted ablation of high molecular weight fibroblast growth factor-2. Dev. Dyn. 2009, 238, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Bodmer, J.R.; Azhar, M.; Newman, G.; Coffin, J.D.; Doetschman, T.; Schultz Jel, J. The influence of FGF2 high molecular weight (HMW) isoforms in the development of cardiac ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2010, 48, 1245–1254. [Google Scholar] [CrossRef]
- Xiao, L.; Naganawa, T.; Lorenzo, J.; Carpenter, T.O.; Coffin, J.D.; Hurley, M.M. Nuclear isoforms of fibroblast growth factor 2 are novel inducers of hypophosphatemia via modulation of FGF23 and KLOTHO. J. Biol. Chem. 2010, 285, 2834–2846. [Google Scholar] [CrossRef]
- Davis, M.G.; Zhou, M.; Ali, S.; Coffin, J.D.; Doetschman, T.; Dorn, G.W., 2nd. Intracrine and autocrine effects of basic fibroblast growth factor in vascular smooth muscle cells. J. Mol. Cell. Cardiol. 1997, 29, 1061–1072. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, P.; Li, X.; Doetschman, T.; Coffin, J.D.; Drissi, H.; Hurley, M.M. Exported 18-kDa isoform of fibroblast growth factor-2 is a critical determinant of bone mass in mice. J. Biol. Chem. 2009, 284, 3170–3182. [Google Scholar] [CrossRef]
- Sheikh, F.; Sontag, D.P.; Fandrich, R.R.; Kardami, E.; Cattini, P.A. Overexpression of FGF-2 increases cardiac myocyte viability after injury in isolated mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1039–H1050. [Google Scholar] [CrossRef]
- Liao, S.; Bodmer, J.; Pietras, D.; Azhar, M.; Doetschman, T.; Schultz Jel, J. Biological functions of the low and high molecular weight protein isoforms of fibroblast growth factor-2 in cardiovascular development and disease. Dev. Dyn. 2009, 238, 249–264. [Google Scholar] [CrossRef]
- Nusayr, E.; Doetschman, T. Cardiac development and physiology are modulated by FGF2 in an isoform- and sex-specific manner. Physiol. Rep. 2013, 1. [Google Scholar] [CrossRef]
- Nusayr, E.; Sadideen, D.T.; Doetschman, T. FGF2 modulates cardiac remodeling in an isoform- and sex-specific manner. Physiol. Rep. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Pasumarthi, K.B.; Jin, Y.; Bock, M.E.; Lytras, A.; Kardami, E.; Cattini, P.A. Characterization of fibroblast growth factor receptor 1 RNA expression in the embryonic mouse heart. Ann. N. Y. Acad. Sci. 1995, 752, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.; Fandrich, R.R.; Kardami, E.; Cattini, P.A. Overexpression of long or short FGFR-1 results in FGF-2-mediated proliferation in neonatal cardiac myocyte cultures. Cardiovasc. Res. 1999, 42, 696–705. [Google Scholar] [CrossRef]
- Hayek, A.; Culler, F.L.; Beattie, G.M.; Lopez, A.D.; Cuevas, P.; Baird, A. An in vivo model for study of the angiogenic effects of basic fibroblast growth factor. Biochem. Biophys. Res. Commun. 1987, 147, 876–880. [Google Scholar] [CrossRef]
- Liao, S.; Porter, D.; Scott, A.; Newman, G.; Doetschman, T.; Schultz Jel, J. The cardioprotective effect of the low molecular weight isoform of fibroblast growth factor-2: The role of JNK signaling. J. Mol. Cell. Cardiol. 2007, 42, 106–120. [Google Scholar] [CrossRef]
- Piotrowicz, R.S.; Martin, J.L.; Dillman, W.H.; Levin, E.G. The 27-kDa heat shock protein facilitates basic fibroblast growth factor release from endothelial cells. J. Biol. Chem. 1997, 272, 7042–7047. [Google Scholar] [CrossRef]
- Piotrowicz, R.S.; Maher, P.A.; Levin, E.G. Dual activities of 22-24 kDA basic fibroblast growth factor: Inhibition of migration and stimulation of proliferation. J. Cell. Physiol. 1999, 178, 144–153. [Google Scholar] [CrossRef]
- Gonzalez-Herrera, I.G.; Prado-Lourenco, L.; Pileur, F.; Conte, C.; Morin, A.; Cabon, F.; Prats, H.; Vagner, S.; Bayard, F.; Audigier, S.; et al. Testosterone regulates FGF-2 expression during testis maturation by an IRES-dependent translational mechanism. FASEB J. 2006, 20, 476–478. [Google Scholar] [CrossRef]
- Hodgin, J.B.; Maeda, N. Minireview: Estrogen and mouse models of atherosclerosis. Endocrinology 2002, 143, 4495–4501. [Google Scholar] [CrossRef]
- Liang, W.; Wang, Q.; Ma, H.; Yan, W.; Yang, J. Knockout of Low Molecular Weight FGF2 Attenuates Atherosclerosis by Reducing Macrophage Infiltration and Oxidative Stress in Mice. Cell. Physiol. Biochem. 2018, 45, 1434–1443. [Google Scholar] [CrossRef]
- Hodgin, J.B.; Knowles, J.W.; Kim, H.S.; Smithies, O.; Maeda, N. Interactions between endothelial nitric oxide synthase and sex hormones in vascular protection in mice. J. Clin. Investig. 2002, 109, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Delrieu, I.; Chinestra, P.; Delassus, F.; Bayard, F.; Prats, H.; Faye, J.C. IL-6 promoter is modulated by the 24 kDa FGF-2 isoform fused to the hormone binding domain of the oestrogen receptor. Cytokine 2000, 12, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Koleini, N.; Santiago, J.J.; Nickel, B.E.; Sequiera, G.L.; Wang, J.; Fandrich, R.R.; Jassal, D.S.; Dhingra, S.; Kirshenbaum, L.A.; Cattini, P.A.; et al. Elimination or neutralization of endogenous high-molecular-weight FGF2 mitigates doxorubicin-induced cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H279–H288. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.R.; Perkins, S.O.; Sinclair, E.A.; Gao, X.; Zhang, Y.; Newman, G.; Pyle, W.G.; Schultz Jel, J. Low molecular weight fibroblast growth factor-2 signals via protein kinase C and myofibrillar proteins to protect against postischemic cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1382–H1396. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Okigaki, M.; Takeda, M.; Matsui, A.; Honsho, S.; Katsume, A.; Kishita, E.; Che, J.; Kurihara, T.; Adachi, Y.; et al. Endothelium-targeted overexpression of constitutively active FGF receptor induces cardioprotection in mice myocardial infarction. J. Mol. Cell. Cardiol. 2009, 46, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Galzie, Z.; Kinsella, A.R.; Smith, J.A. Fibroblast growth factors and their receptors. Biochem. Cell Biol. 1997, 75, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Hoerstrup, S.P.; Zund, G.; Schnell, A.M.; Kolb, S.A.; Visjager, J.F.; Schoeberlein, A.; Turina, M. Optimized growth conditions for tissue engineering of human cardiovascular structures. Int. J. Artif. Organs 2000, 23, 817–823. [Google Scholar] [CrossRef]
- Parker, T.G.; Packer, S.E.; Schneider, M.D. Peptide growth factors can provoke “fetal” contractile protein gene expression in rat cardiac myocytes. J. Clin. Investig. 1990, 85, 507–514. [Google Scholar] [CrossRef]
- Padua, R.R.; Kardami, E. Increased basic fibroblast growth factor (bFGF) accumulation and distinct patterns of localization in isoproterenol-induced cardiomyocyte injury. Growth Factors 1993, 8, 291–306. [Google Scholar] [CrossRef]
- Sabbieti, M.G.; Marchetti, L.; Abreu, C.; Montero, A.; Hand, A.R.; Raisz, L.G.; Hurley, M.M. Prostaglandins regulate the expression of fibroblast growth factor-2 in bone. Endocrinology 1999, 140, 434–444. [Google Scholar] [CrossRef][Green Version]
- Burt, P.M.; Xiao, L.; Doetschman, T.; Hurley, M.M. Ablation of low-molecular-weight FGF2 isoform accelerates murine osteoarthritis while loss of high-molecular-weight FGF2 isoforms offers protection. J. Cell. Physiol. 2019, 234, 4418–4431. [Google Scholar] [CrossRef]
- Montero, A.; Okada, Y.; Tomita, M.; Ito, M.; Tsurukami, H.; Nakamura, T.; Doetschman, T.; Coffin, J.D.; Hurley, M.M. Disruption of the fibroblast growth factor-2 gene results in decreased bone mass and bone formation. J. Clin. Investig. 2000, 105, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Sobue, T.; Naganawa, T.; Xiao, L.; Okada, Y.; Tanaka, Y.; Ito, M.; Okimoto, N.; Nakamura, T.; Coffin, J.D.; Hurley, M.M. Over-expression of fibroblast growth factor-2 causes defective bone mineralization and osteopenia in transgenic mice. J. Cell. Biochem. 2005, 95, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Dacic, S.; Kalajzic, I.; Visnjic, D.; Lichtler, A.C.; Rowe, D.W. Col1a1-driven transgenic markers of osteoblast lineage progression. J. Bone Miner. Res. 2001, 16, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Boban, I.; Jacquin, C.; Prior, K.; Barisic-Dujmovic, T.; Maye, P.; Clark, S.H.; Aguila, H.L. The 3.6 kb DNA fragment from the rat Col1a1 gene promoter drives the expression of genes in both osteoblast and osteoclast lineage cells. Bone 2006, 39, 1302–1312. [Google Scholar] [CrossRef]
- Coffin, J.D.; Homer-Bouthiette, C.; Hurley, M.M. Fibroblast Growth Factor 2 and Its Receptors in Bone Biology and Disease. J. Endocr. Soc. 2018, 2, 657–671. [Google Scholar] [CrossRef]
- Xiao, L.; Ueno, D.; Catros, S.; Homer-Bouthiette, C.; Charles, L.; Kuhn, L.; Hurley, M.M. Fibroblast growth factor-2 isoform (low molecular weight/18 kDa) overexpression in preosteoblast cells promotes bone regeneration in critical size calvarial defects in male mice. Endocrinology 2014, 155, 965–974. [Google Scholar] [CrossRef]
- Homer-Bouthiette, C.; Doetschman, T.; Xiao, L.; Hurley, M.M. Knockout of nuclear high molecular weight FGF2 isoforms in mice modulates bone and phosphate homeostasis. J. Biol. Chem. 2014, 289, 36303–36314. [Google Scholar] [CrossRef]
- Xiao, L.; Homer-Bouthiette, C.; Hurley, M.M. FGF23 Neutralizing Antibody Partially Improves Bone Mineralization Defect of HMWFGF2 Isoforms in Transgenic Female Mice. J. Bone Miner. Res. 2018, 33, 1347–1361. [Google Scholar] [CrossRef]
- Martin, A.; Liu, S.; David, V.; Li, H.; Karydis, A.; Feng, J.Q.; Quarles, L.D. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011, 25, 2551–2562. [Google Scholar] [CrossRef]
- Eicher, E.M.; Southard, J.L.; Scriver, C.R.; Glorieux, F.H. Hypophosphatemia: Mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc. Natl. Acad. Sci. USA 1976, 73, 4667–4671. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Xiao, L.; Hurley, M.M. FGFR Inhibitor Ameliorates Hypophosphatemia and Impaired Engrailed-1/Wnt Signaling in FGF2 High Molecular Weight Isoform Transgenic Mice. J. Cell. Biochem. 2016, 117, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Xiao, L.; Hurley, M.M. FGF23 Neutralizing Antibody Ameliorates Hypophosphatemia and Impaired FGF Receptor Signaling in Kidneys of HMWFGF2 Transgenic Mice. J. Cell. Physiol. 2017, 232, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Du, E.; Homer-Bouthiette, C.; Hurley, M.M. Inhibition of FGFR Signaling Partially Rescues Hypophosphatemic Rickets in HMWFGF2 Tg Male Mice. Endocrinology 2017, 158, 3629–3646. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M. Chapter 11—Minerals and Trace Elements. In Nutrient Metabolism, 2nd ed.; Kohlmeier, M., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 673–807. [Google Scholar] [CrossRef]
- Xiao, L.; Esliger, A.; Hurley, M.M. Nuclear fibroblast growth factor 2 (FGF2) isoforms inhibit bone marrow stromal cell mineralization through FGF23/FGFR/MAPK in vitro. J. Bone Miner. Res. 2013, 28, 35–45. [Google Scholar] [CrossRef]
- Meo Burt, P.; Xiao, L.; Hurley, M.M. FGF23 Regulates Wnt/β-Catenin Signaling-Mediated Osteoarthritis in Mice Overexpressing High-Molecular-Weight FGF2. Endocrinology 2018, 159, 2386–2396. [Google Scholar] [CrossRef]
- Chia, S.L.; Sawaji, Y.; Burleigh, A.; McLean, C.; Inglis, J.; Saklatvala, J.; Vincent, T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009, 60, 2019–2027. [Google Scholar] [CrossRef]
- Meo Burt, P.; Xiao, L.; Dealy, C.; Fisher, M.C.; Hurley, M.M. FGF2 High Molecular Weight Isoforms Contribute to Osteoarthropathy in Male Mice. Endocrinology 2016, 157, 4602–4614. [Google Scholar] [CrossRef]
- Yan, D.; Chen, D.; Cool, S.M.; van Wijnen, A.J.; Mikecz, K.; Murphy, G.; Im, H.J. Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Res. Ther. 2011, 13, R130. [Google Scholar] [CrossRef]
- Guibert, M.; Gasser, A.; Kempf, H.; Bianchi, A. Fibroblast-growth factor 23 promotes terminal differentiation of ATDC5 cells. PLoS ONE 2017, 12, e0174969. [Google Scholar] [CrossRef]
- Xiao, L.; Williams, D.; Hurley, M.M. Inhibition of FGFR Signaling Partially Rescues Osteoarthritis in Mice Overexpressing High Molecular Weight FGF2 Isoforms. Endocrinology 2020, 161. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, J.; Cao, L.; Liang, Y.; Han, X.; Quarles, L.D. Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLoS ONE 2014, 9, e104154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hurley, M.M.; Adams, D.J.; Wang, L.; Jiang, X.; Burt, P.M.; Du, E.; Xiao, L. Accelerated fracture healing in transgenic mice overexpressing an anabolic isoform of fibroblast growth factor 2. J. Cell. Biochem. 2016, 117, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.C.; Yuan, Q. Fibroblast growth factor 23 and bone mineralisation. Int. J. Oral Sci. 2015, 7, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, D.; Shimazu, K.; Zhou, Y.X.; Lu, B.; Deng, C.X. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol. Psychiatry 2007, 62, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.P.; Black, I.B.; DiCicco-Bloom, E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J. Neurosci. 1999, 19, 6006–6016. [Google Scholar] [CrossRef]
- Raballo, R.; Rhee, J.; Lyn-Cook, R.; Leckman, J.F.; Schwartz, M.L.; Vaccarino, F.M. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J. Neurosci. 2000, 20, 5012–5023. [Google Scholar] [CrossRef]
- Ratzka, A.; Baron, O.; Stachowiak, M.K.; Grothe, C. Fibroblast growth factor 2 regulates dopaminergic neuron development in vivo. J. Neurochem. 2012, 122, 94–105. [Google Scholar] [CrossRef]
- Timmer, M.; Cesnulevicius, K.; Winkler, C.; Kolb, J.; Lipokatic-Takacs, E.; Jungnickel, J.; Grothe, C. Fibroblast growth factor (FGF)-2 and FGF receptor 3 are required for the development of the substantia nigra, and FGF-2 plays a crucial role for the rescue of dopaminergic neurons after 6-hydroxydopamine lesion. J. Neurosci. 2007, 27, 459–471. [Google Scholar] [CrossRef]
- Forthmann, B.; Grothe, C.; Claus, P. A nuclear odyssey: Fibroblast growth factor-2 (FGF-2) as a regulator of nuclear homeostasis in the nervous system. Cell. Mol. Life Sci. 2015, 72, 1651–1662. [Google Scholar] [CrossRef]
- Even-Chen, O.; Barak, S. The role of fibroblast growth factor 2 in drug addiction. Eur. J. Neurosci. 2019, 50, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Esnafoglu, E.; Ayyildiz, S.N. Decreased levels of serum fibroblast growth factor-2 in children with autism spectrum disorder. Psychiatry Res. 2017, 257, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Thau, N.; Jungnickel, J.; Knippenberg, S.; Ratzka, A.; Dengler, R.; Petri, S.; Grothe, C. Prolonged survival and milder impairment of motor function in the SOD1 ALS mouse model devoid of fibroblast growth factor 2. Neurobiol. Dis. 2012, 47, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Petri, S.; Krampfl, K.; Kuhlemann, K.; Dengler, R.; Grothe, C. Preserved expression of fibroblast growth factor (FGF)-2 and FGF receptor 1 in brain and spinal cord of amyotrophic lateral sclerosis patients. Histochem. Cell Biol. 2009, 131, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Hensel, N.; Ratzka, A.; Brinkmann, H.; Klimaschewski, L.; Grothe, C.; Claus, P. Analysis of the fibroblast growth factor system reveals alterations in a mouse model of spinal muscular atrophy. PLoS ONE 2012, 7, e31202. [Google Scholar] [CrossRef]
- Sarchielli, P.; Di Filippo, M.; Ercolani, M.V.; Chiasserini, D.; Mattioni, A.; Bonucci, M.; Tenaglia, S.; Eusebi, P.; Calabresi, P. Fibroblast growth factor-2 levels are elevated in the cerebrospinal fluid of multiple sclerosis patients. Neurosci. Lett. 2008, 435, 223–228. [Google Scholar] [CrossRef]
- Clemente, D.; Ortega, M.C.; Arenzana, F.J.; de Castro, F. FGF-2 and Anosmin-1 are selectively expressed in different types of multiple sclerosis lesions. J. Neurosci. 2011, 31, 14899–14909. [Google Scholar] [CrossRef]
- Tooyama, I.; Kremer, H.P.; Hayden, M.R.; Kimura, H.; McGeer, E.G.; McGeer, P.L. Acidic and basic fibroblast growth factor-like immunoreactivity in the striatum and midbrain in Huntington’s disease. Brain Res. 1993, 610, 1–7. [Google Scholar] [CrossRef]
- Zucchini, S.; Barbieri, M.; Simonato, M. Alterations in seizure susceptibility and in seizure-induced plasticity after pharmacologic and genetic manipulation of the fibroblast growth factor-2 system. Epilepsia 2005, 46 (Suppl. 5), 52–58. [Google Scholar] [CrossRef]
- Graham, B.M. Fibroblast Growth Factor-2: A Promising Biomarker for Anxiety and Trauma Disorders. J. Exp. Neurosci. 2017, 11, 1179069517749589. [Google Scholar] [CrossRef]
- Gaughran, F.; Payne, J.; Sedgwick, P.M.; Cotter, D.; Berry, M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res. Bull. 2006, 70, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kefalakes, E.; Sarikidi, A.; Bursch, F.; Ettcheto, M.; Schmuck, M.; Rumpel, R.; Grothe, C.; Petri, S. Isoform-selective as opposed to complete depletion of fibroblast growth factor 2 (FGF-2) has no major impact on survival and gene expression in SOD1(G93A) amyotrophic lateral sclerosis mice. Eur. J. Neurosci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ratzka, A.; Baron, O.; Grothe, C. FGF-2 deficiency does not influence FGF ligand and receptor expression during development of the nigrostriatal system. PLoS ONE 2011, 6, e23564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tooyama, I.; Kawamata, T.; Walker, D.; Yamada, T.; Hanai, K.; Kimura, H.; Iwane, M.; Igarashi, K.; McGeer, E.G.; McGeer, P.L. Loss of basic fibroblast growth factor in substantia nigra neurons in Parkinson’s disease. Neurology 1993, 43, 372–376. [Google Scholar] [CrossRef]
- Grothe, C.; Schulze, A.; Semkova, I.; Muller-Ostermeyer, F.; Rege, A.; Wewetzer, K. The high molecular weight fibroblast growth factor-2 isoforms (21,000 mol. wt and 23,000 mol. wt) mediate neurotrophic activity on rat embryonic mesencephalic dopaminergic neurons in vitro. Neuroscience 2000, 100, 73–86. [Google Scholar] [CrossRef]
- Fadda, P.; Bedogni, F.; Fresu, A.; Collu, M.; Racagni, G.; Riva, M.A. Reduction of corticostriatal glutamatergic fibers in basic fibroblast growth factor deficient mice is associated with hyperactivity and enhanced dopaminergic transmission. Biol. Psychiatry 2007, 62, 235–242. [Google Scholar] [CrossRef]
- Salmaso, N.; Stevens, H.E.; McNeill, J.; ElSayed, M.; Ren, Q.; Maragnoli, M.E.; Schwartz, M.L.; Tomasi, S.; Sapolsky, R.M.; Duman, R.; et al. Fibroblast Growth Factor 2 Modulates Hypothalamic Pituitary Axis Activity and Anxiety Behavior Through Glucocorticoid Receptors. Biol. Psychiatry 2016, 80, 479–489. [Google Scholar] [CrossRef]
- Ferraiuolo, L.; Higginbottom, A.; Heath, P.R.; Barber, S.; Greenald, D.; Kirby, J.; Shaw, P.J. Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain 2011, 134, 2627–2641. [Google Scholar] [CrossRef]
- Kefalakes, E.; Boselt, S.; Sarikidi, A.; Ettcheto, M.; Bursch, F.; Naujock, M.; Stanslowsky, N.; Schmuck, M.; Barenys, M.; Wegner, F.; et al. Characterizing the multiple roles of FGF-2 in SOD1(G93A) ALS mice in vivo and in vitro. J. Cell. Physiol. 2019, 234, 7395–7410. [Google Scholar] [CrossRef]
- Labzin, L.I.; Heneka, M.T.; Latz, E. Innate Immunity and Neurodegeneration. Annu. Rev. Med. 2018, 69, 437–449. [Google Scholar] [CrossRef]
- Liu, X.; Luo, D.; Yang, N. Cytosolic Low Molecular Weight FGF2 Orchestrates RIG-I-Mediated Innate Immune Response. J. Immunol. 2015, 195, 4943–4952. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lai, C.; Gu, H.; Zhao, L.; Xia, M.; Yang, P.; Wang, X. miR-194 Inhibits Innate Antiviral Immunity by Targeting FGF2 in Influenza H1N1 Virus Infection. Front. Microbiol. 2017, 8, 2187. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Ornitz, D.M. Fibroblast growth factors: From molecular evolution to roles in development, metabolism and disease. J. Biochem. 2011, 149, 121–130. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freiin von Hövel, F.; Kefalakes, E.; Grothe, C. What Can We Learn from FGF-2 Isoform-Specific Mouse Mutants? Differential Insights into FGF-2 Physiology In Vivo. Int. J. Mol. Sci. 2021, 22, 390. https://doi.org/10.3390/ijms22010390
Freiin von Hövel F, Kefalakes E, Grothe C. What Can We Learn from FGF-2 Isoform-Specific Mouse Mutants? Differential Insights into FGF-2 Physiology In Vivo. International Journal of Molecular Sciences. 2021; 22(1):390. https://doi.org/10.3390/ijms22010390
Chicago/Turabian StyleFreiin von Hövel, Friederike, Ekaterini Kefalakes, and Claudia Grothe. 2021. "What Can We Learn from FGF-2 Isoform-Specific Mouse Mutants? Differential Insights into FGF-2 Physiology In Vivo" International Journal of Molecular Sciences 22, no. 1: 390. https://doi.org/10.3390/ijms22010390
APA StyleFreiin von Hövel, F., Kefalakes, E., & Grothe, C. (2021). What Can We Learn from FGF-2 Isoform-Specific Mouse Mutants? Differential Insights into FGF-2 Physiology In Vivo. International Journal of Molecular Sciences, 22(1), 390. https://doi.org/10.3390/ijms22010390





