Pancreatic Islets Accumulate Cadmium in a Rodent Model of Cadmium-Induced Hyperglycemia
Abstract
1. Introduction
2. Results
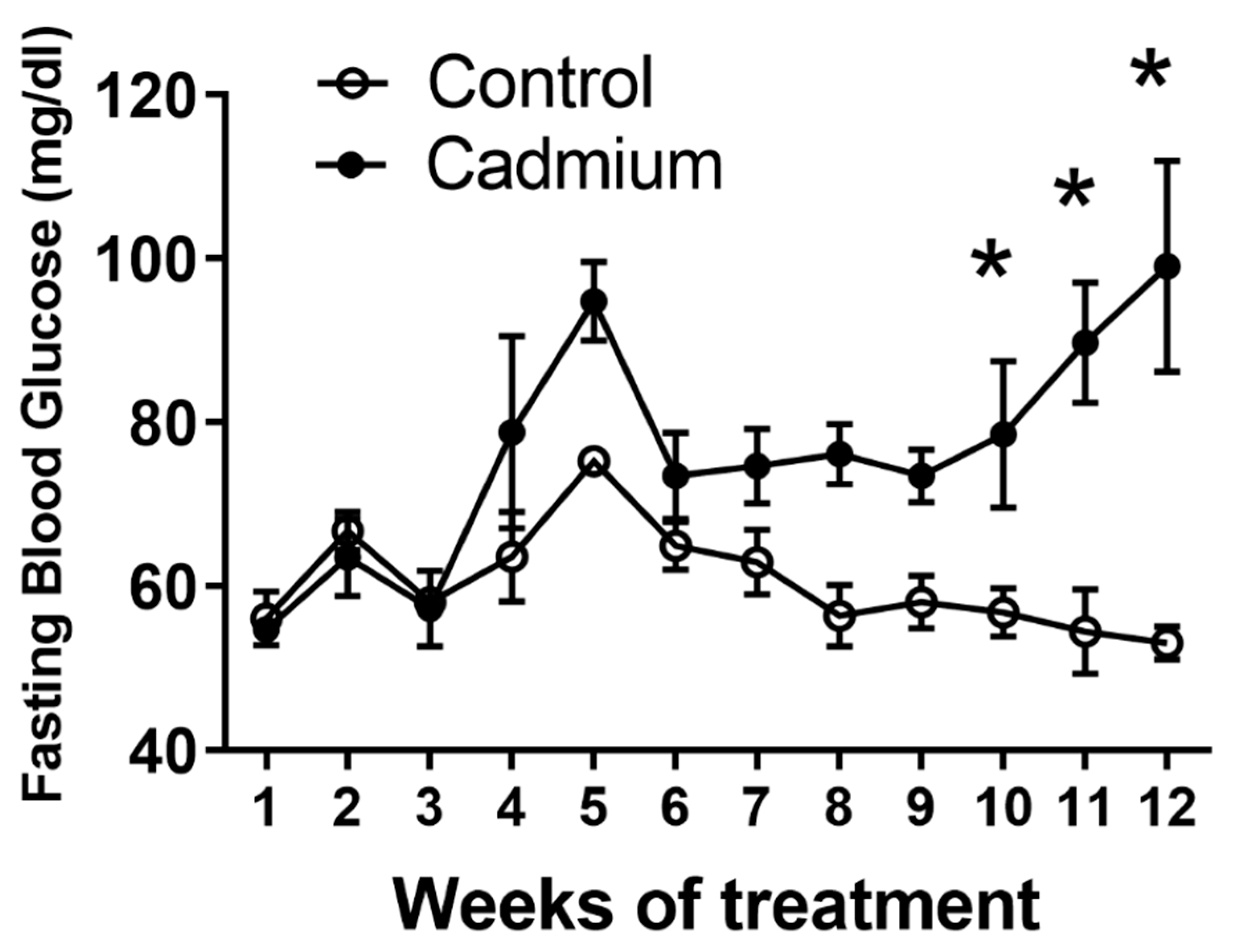
2.1. Long Term Cd Exposure Resulted in Time-Dependent Increase in Fasting Blood Glucose
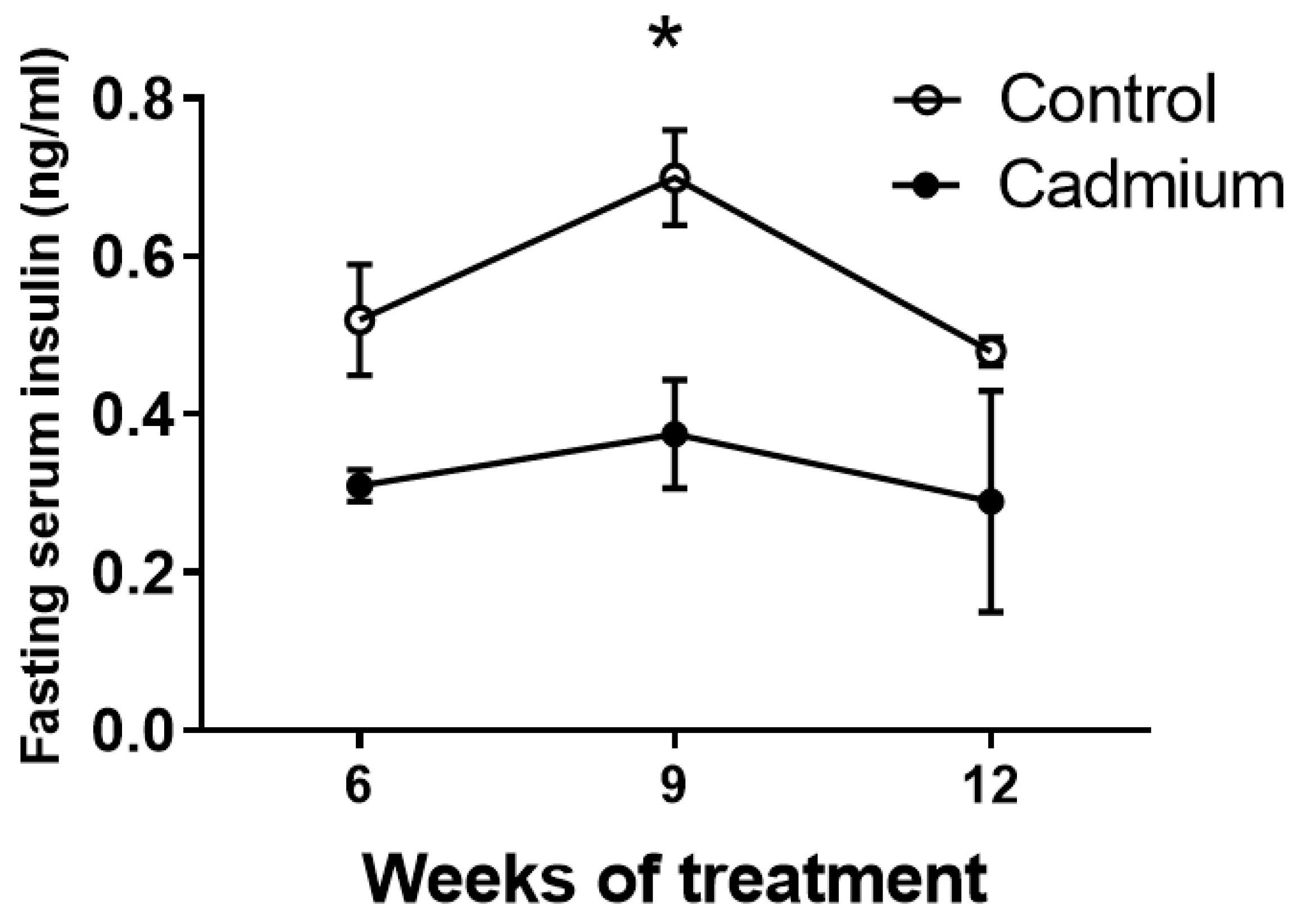
2.2. Fasting Serum Insulin is Reduced During Cd Exposure
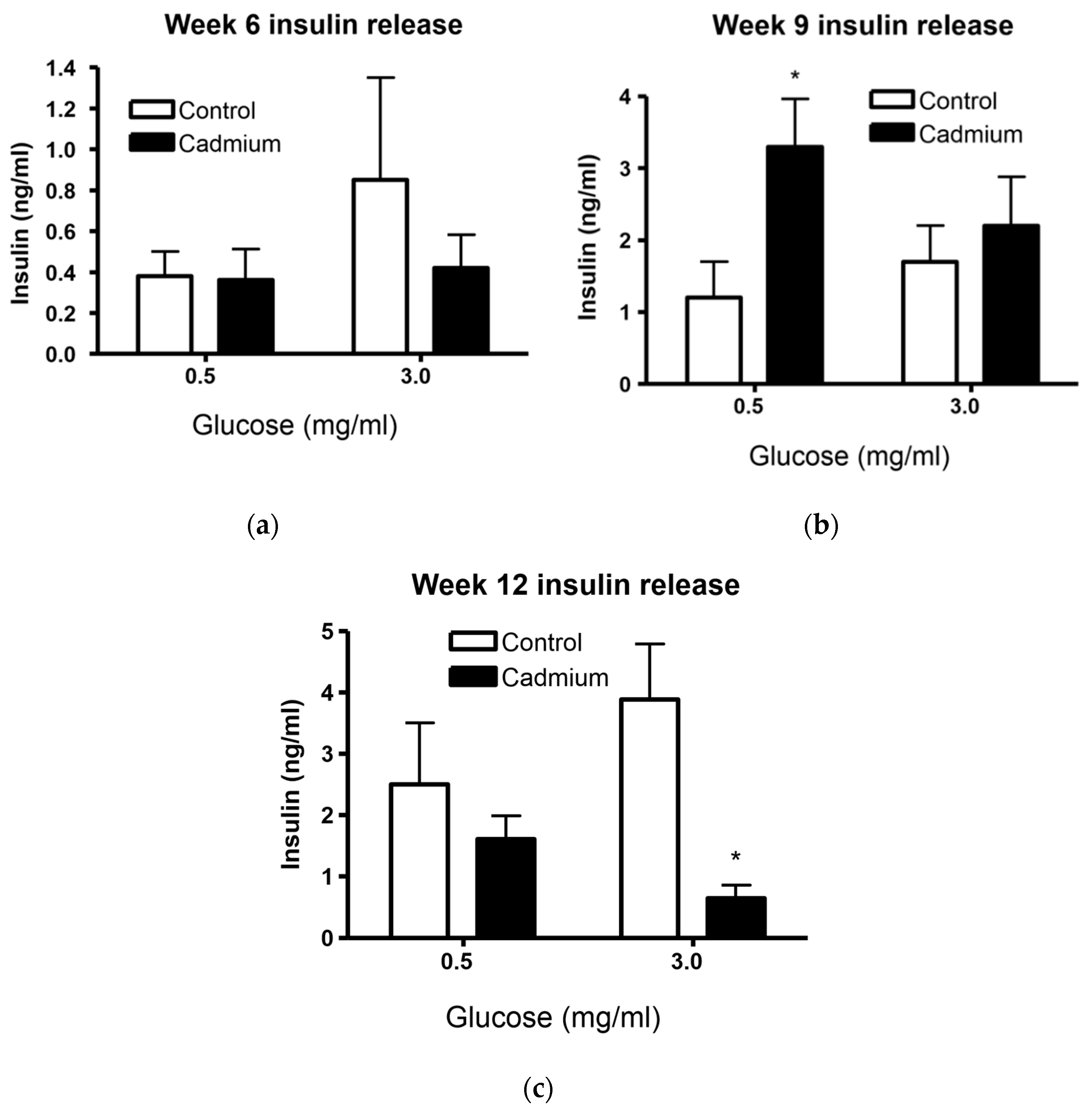
2.3. Islets From Cd-Treated Rats Have Altered Insulin Release
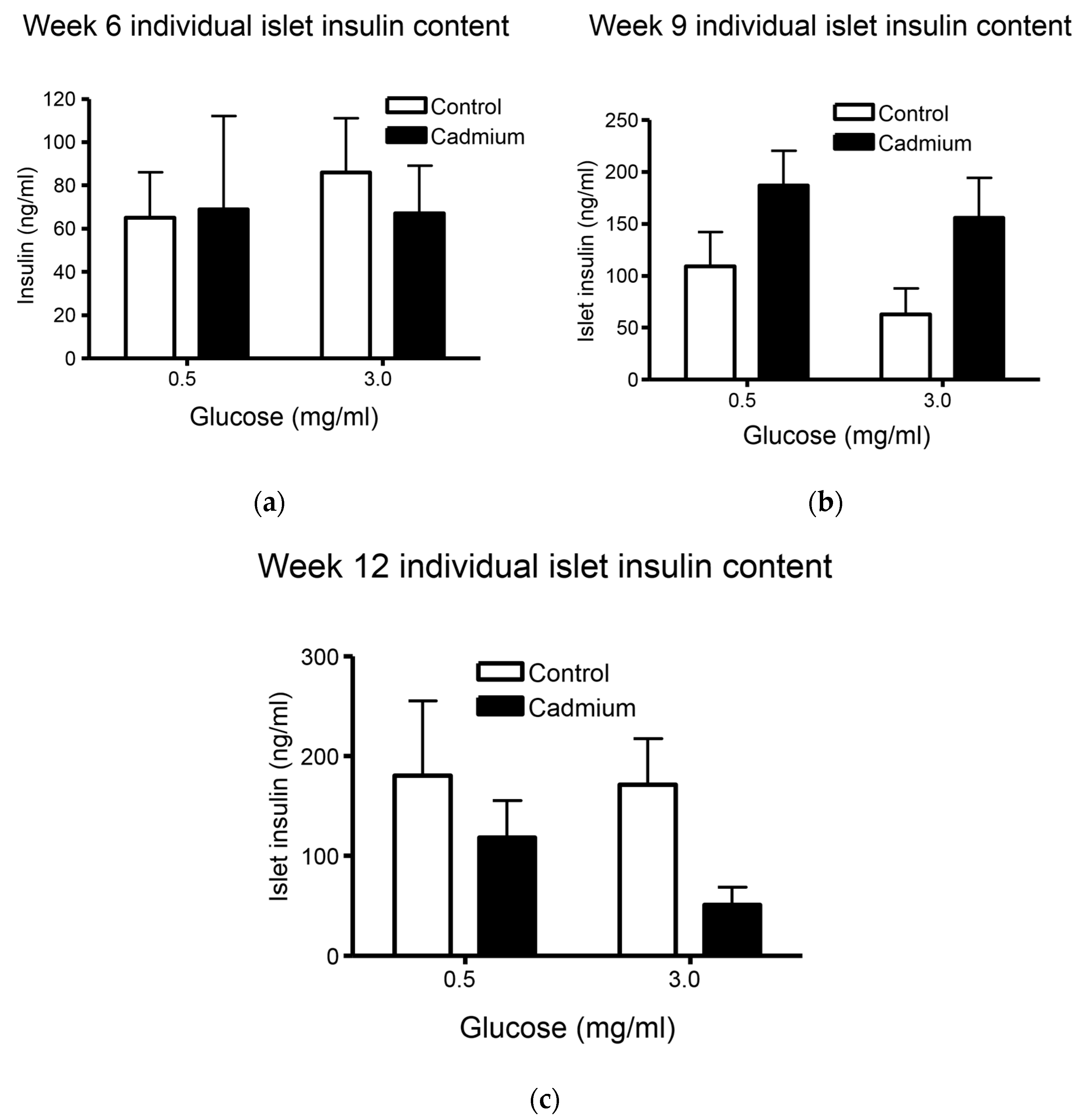
2.4. Total Insulin Content from Islets Isolated from Week 9 Cd-Treated Animals Was Significantly Increased
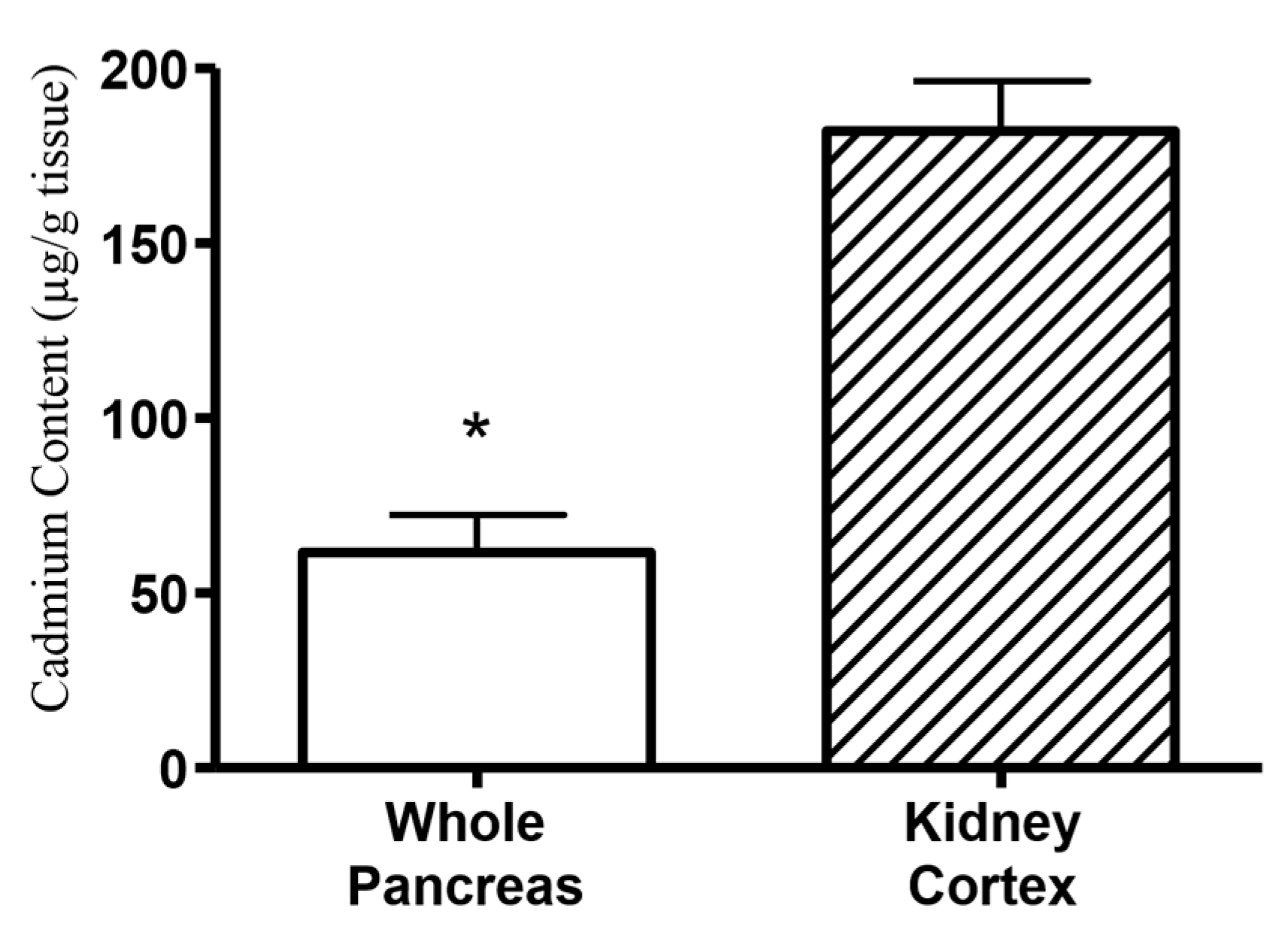
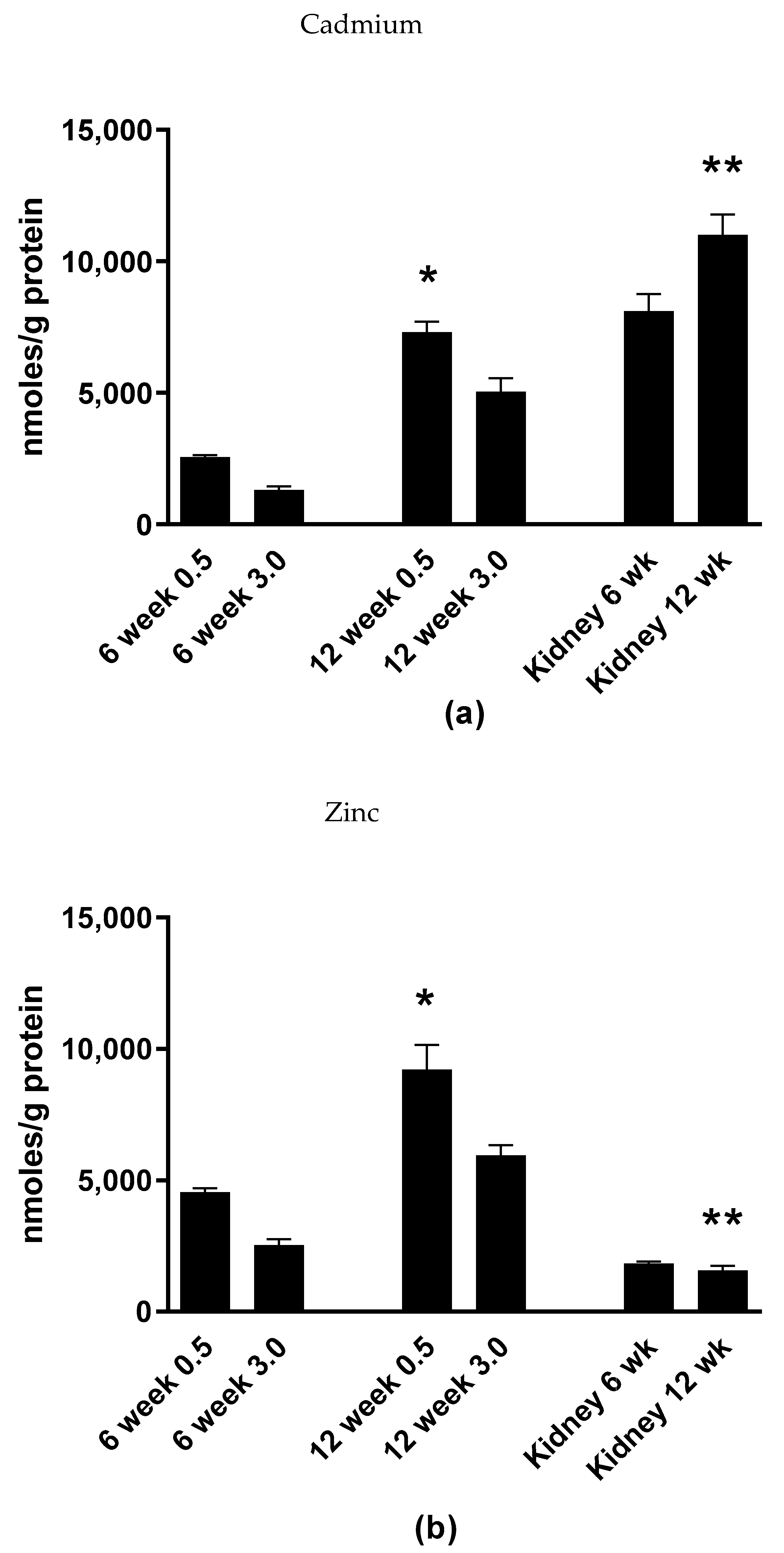
2.5. Cadmium Selectively Accumulates within the Islets of Langerhans within Whole Pancreatic Tissue
2.6. Cadmium Alters the Histopathology and Morphology of Islets of Langerhans
2.7. Pancreatic Islet Density Was Less in Cd-Treated Animals
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Islet Isolation and Treatment
4.3. Measurement of Insulin from Serum and Isolated Islets
4.4. Measurement of Cd and Zn Content in Freshly Isolated Pancreatic Islets, Whole Pancreata and Renal Cortex
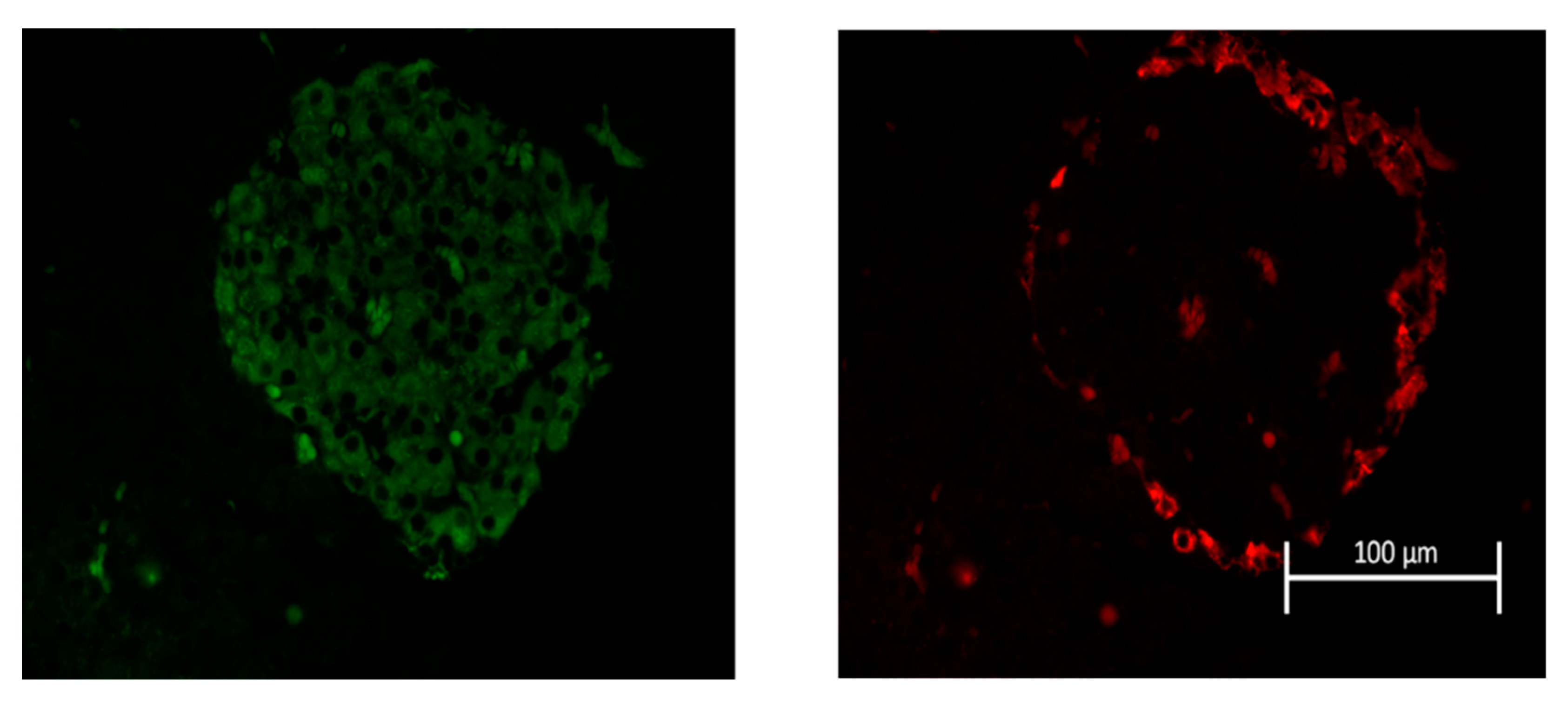
4.5. Insulin and Glucagon Immunolabelling of Pancreatic Islets
4.6. Islet Morphometric Analyses
4.7. Histopathological Analyses of Islets of Langerhans
4.8. Quantification of Islet Density in Pancreatic Tissue
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type-2 diabetes mellitus |
| ICP-MS | Inductively-coupled plasma mass spectrometry |
| Cd | Cadmium |
| Zn | Zinc |
References
- Jarup, L.; Berglund, M.; Elinder, C.G.; Nordberg, G.; Vahter, M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scand. J. Work Environ. Health 1998, 24 (Suppl. 1), 1–51. [Google Scholar] [PubMed]
- Lei, L.J.; Jin, T.Y.; Zhou, Y.F. Insulin expression in rats exposed to cadmium. Biomed. Environ. Sci. 2007, 20, 295–301. [Google Scholar] [PubMed]
- Uetani, M.; Kobayashi, E.; Suwazono, Y.; Honda, R.; Nishijo, M.; Nakagawa, H.; Kido, T.; Nogawa, K. Tissue cadmium (Cd) concentrations of people living in a Cd polluted area, Japan. Biometals 2006, 19, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L. Cadmium and the kidney. Environ. Health Perspect. 1984, 54, 1–11. [Google Scholar] [CrossRef]
- Sabolic, I.; Breljak, D.; Skarica, M.; Herak-Kramberger, C.M. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals 2010, 23, 897–926. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Kazi, N.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Baig, J.A.; Sarfraz, R.A. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res. Clin. Pract. 2008, 80, 280–288. [Google Scholar] [CrossRef]
- Kolachi, N.F.; Kazi, T.G.; Afridi, H.I.; Kazi, N.; Khan, S.; Kandhro, G.A.; Shah, A.Q.; Baig, J.A.; Wadhwa, S.K.; Shah, F.; et al. Status of Toxic Metals in Biological Samples of Diabetic Mothers and Their Neonates. Biol. Trace Elem. Res. 2010, 143, 196–212. [Google Scholar] [CrossRef]
- Chen, L.; Lei, L.; Jin, T.; Nordberg, M.; Nordberg, G.F. Plasma metallothionein antibody, urinary cadmium, and renal dysfunction in a Chinese type 2 diabetic population. Diabetes Care 2006, 29, 2682–2687. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Il’yasova, D.; Ivanova, A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 2003, 26, 468–470. [Google Scholar] [CrossRef]
- Wallia, A.; Allen, N.B.; Badon, S.; El Muayed, M. Association between urinary cadmium levels and prediabetes in the NHANES 2005-2010 population. Int. J. Hyg. Environ. Health 2014, 217, 854–860. [Google Scholar] [CrossRef]
- Haswell-Elkins, M.; Satarug, S.; O’Rourke, P.; Moore, M.; Ng, J.; McGrath, V.; Walmby, M. Striking association between urinary cadmium level and albuminuria among Torres Strait Islander people with diabetes. Environ. Res. 2008, 106, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.M.; Ricardo, A.C.; Persky, V.; Turyk, M. Associations between blood cadmium concentration and kidney function in the U.S. population: Impact of sex, diabetes and hypertension. Environ. Res. 2019, 169, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Guallar, E.; Cowie, C.C. Metals in Urine and Diabetes in US Adults. Diabetes 2016, 65, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.P.; Wallia, A.; Edwards, J.R.; El Muayed, M. Comment on Menke et al. Metals in Urine and Diabetes in US Adults. Diabetes 2016; 65: 164–171. Diabetes 2016, 65, e31. [Google Scholar] [CrossRef]
- Edwards, J.R.; Prozialeck, W.C. Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 2009, 238, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.R.; Early, J.L.; Nonavinakere, V.K.; Mallory, Z. Effect of cadmium on blood glucose level in the rat. Toxicol. Lett. 1990, 54, 199–205. [Google Scholar] [CrossRef]
- Han, J.C.; Park, S.Y.; Hah, B.G.; Choi, G.H.; Kim, Y.K.; Kwon, T.H.; Kim, E.K.; Lachaal, M.; Jung, C.Y.; Lee, W. Cadmium induces impaired glucose tolerance in rat by down-regulating GLUT4 expression in adipocytes. Arch. Biochem. Biophys. 2003, 413, 213–220. [Google Scholar] [CrossRef]
- Merali, Z.; Singhal, R.L. Diabetogenic effects of chronic oral cadmium adminstration to neonatal rats. Br. J. Pharmacol. 1980, 69, 151–157. [Google Scholar] [CrossRef]
- Merali, Z.; Singhal, R.L. Protective effect of selenium on certain hepatotoxic and pancreotoxic manifestations of subacute cadmium administration. J. Pharmacol. Exp. Ther. 1975, 195, 58–66. [Google Scholar]
- Kurata, Y.; Katsuta, O.; Doi, T.; Kawasuso, T.; Hiratsuka, H.; Tsuchitani, M.; Umemura, T. Chronic cadmium treatment induces islet B cell injury in ovariectomized cynomolgus monkeys. Jpn. J. Vet. Res. 2003, 50, 175–183. [Google Scholar] [PubMed]
- El Muayed, M.; Raja, M.R.; Zhang, X.; MacRenaris, K.W.; Bhatt, S.; Chen, X.; Urbanek, M.; O’Halloran, T.V.; Lowe, W.L., Jr. Accumulation of cadmium in insulin-producing beta cells. Islets 2012, 4, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.J.; Chen, L.; Jin, T.Y.; Nordberg, M.; Chang, X.L. Estimation of benchmark dose for pancreatic damage in cadmium-exposed smelters. Toxicol. Sci. 2007, 97, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Rajanna, B.; Hobson, M.; Reese, J.; Sample, E.; Chapatwala, K.D. Chronic hepatic and renal toxicity by cadmium in rats. Drug Chem. Toxicol. 1984, 7, 229–241. [Google Scholar] [CrossRef]
- Chapatwala, K.D.; Boykin, M.; Butts, A.; Rajanna, B. Effect of intraperitoneally injected cadmium on renal and hepatic gluconeogenic enzymes in rats. Drug Chem. Toxicol. 1982, 5, 305–317. [Google Scholar] [CrossRef]
- Edwards, J.; Ackerman, C. A Review of Diabetes Mellitus and Exposure to the Environmental Toxicant Cadmium with an Emphasis on Likely Mechanisms of Action. Curr. Diabetes Rev. 2016, 12, 252–258. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Vaidya, V.S.; Liu, J.; Waalkes, M.P.; Edwards, J.R.; Lamar, P.C.; Bernard, A.M.; Dumont, X.; Bonventre, J.V. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007, 72, 985–993. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R.; Lamar, P.C.; Liu, J.; Vaidya, V.S.; Bonventre, J.V. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol. Appl. Pharmacol. 2009, 238, 306–314. [Google Scholar] [CrossRef]
- Prozialeck, W.C.; Edwards, J.R.; Vaidya, V.S.; Bonventre, J.V. Preclinical Evaluation of Novel Urinary Biomarkers of Cadmium Nephrotoxicity. Toxicol. Appl. Pharmacol. 2009, 238, 301–305. [Google Scholar] [CrossRef]
- Dalton, T.P.; He, L.; Wang, B.; Miller, M.L.; Jin, L.; Stringer, K.F.; Chang, X.; Baxter, C.S.; Nebert, D.W. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc. Natl. Acad. Sci. USA 2005, 102, 3401–3406. [Google Scholar] [CrossRef]
- Wang, B.; Schneider, S.N.; Dragin, N.; Girijashanker, K.; Dalton, T.P.; He, L.; Miller, M.L.; Stringer, K.F.; Soleimani, M.; Richardson, D.D.; et al. Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am. J. Physiol. Cell Physiol. 2007, 292, C1523–C1535. [Google Scholar] [CrossRef] [PubMed]
- Ohana, E.; Sekler, I.; Kaisman, T.; Kahn, N.; Cove, J.; Silverman, W.F.; Amsterdam, A.; Hershfinkel, M. Silencing of ZnT-1 expression enhances heavy metal influx and toxicity. J. Mol. Med. 2006, 84, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Girijashanker, K.; He, L.; Soleimani, M.; Reed, J.M.; Li, H.; Liu, Z.; Wang, B.; Dalton, T.P.; Nebert, D.W. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: Similarities to the ZIP8 transporter. Mol. Pharmacol. 2008, 73, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Habeebu, S.S.; Liu, J.; Liu, Y.; Klaassen, C.D. Metallothionein-null mice are more sensitive than wild-type mice to liver injury induced by repeated exposure to cadmium. Toxicol. Sci. 2000, 55, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Zangger, K.; Oz, G.; Otvos, J.D.; Armitage, I.M. Three-dimensional solution structure of mouse [Cd7]-metallothionein-1 by homonuclear and heteronuclear NMR spectroscopy. Protein Sci. 1999, 8, 2630–2638. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.P.; Allen, N.B.; Meyers, M.S.; Link, E.O.; Zhang, X.; MacRenaris, K.W.; El Muayed, M. Exploring the Association Between Demographics, SLC30A8 Genotype, and Human Islet Content of Zinc, Cadmium, Copper, Iron, Manganese and Nickel. Sci. Rep. 2017, 7, 473. [Google Scholar] [CrossRef]
- Prentki, M.; Nolan, C.J. Islet beta cell failure in type 2 diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Pancreatic Islet Biology, Insulin Sensitivity, and Diabetes. Prev. Nutr. Food Sci. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Abu-Hayyeh, S.; Sian, M.; Jones, K.G.; Manuel, A.; Powell, J.T. Cadmium accumulation in aortas of smokers. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 863–867. [Google Scholar] [CrossRef]
- Thijssen, S.; Cuypers, A.; Maringwa, J.; Smeets, K.; Horemans, N.; Lambrichts, I.; Van Kerkhove, E. Low cadmium exposure triggers a biphasic oxidative stress response in mice kidneys. Toxicology 2007, 236, 29–41. [Google Scholar] [CrossRef]
- Chen, S.; Han, J.; Liu, Y. Dual Opposing Roles of Metallothionein Overexpression in C57BL/6J Mouse Pancreatic beta-Cells. PLoS ONE 2015, 10, e0137583. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Epstein, P.N. Metallothionein and catalase sensitize to diabetes in nonobese diabetic mice: Reactive oxygen species may have a protective role in pancreatic beta-cells. Diabetes 2006, 55, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.V. Zinc and insulin in pancreatic beta-cells. Endocrine 2014, 45, 178–189. [Google Scholar] [CrossRef]
- Flannick, J.; Thorleifsson, G.; Beer, N.L.; Jacobs, S.B.; Grarup, N.; Burtt, N.P.; Mahajan, A.; Fuchsberger, C.; Atzmon, G.; Benediktsson, R.; et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 2014, 46, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Malaiyandi, L.M.; Sharthiya, H.; Barakat, A.N.; Edwards, J.R.; Dineley, K.E. Using FluoZin-3 and fura-2 to monitor acute accumulation of free intracellular Cd(2+) in a pancreatic beta cell line. Biometals 2019, 32, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Kashiv, Y.; Austin, J.R., 2nd; Lai, B.; Rose, V.; Vogt, S.; El-Muayed, M. Imaging trace element distributions in single organelles and subcellular features. Sci. Rep. 2016, 6, 21437. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Montague, W.; Taylor, K.W. Pentitols and insulin release by isolated rat islets of Langerhans. Biochem. J. 1968, 109, 333–339. [Google Scholar] [CrossRef]
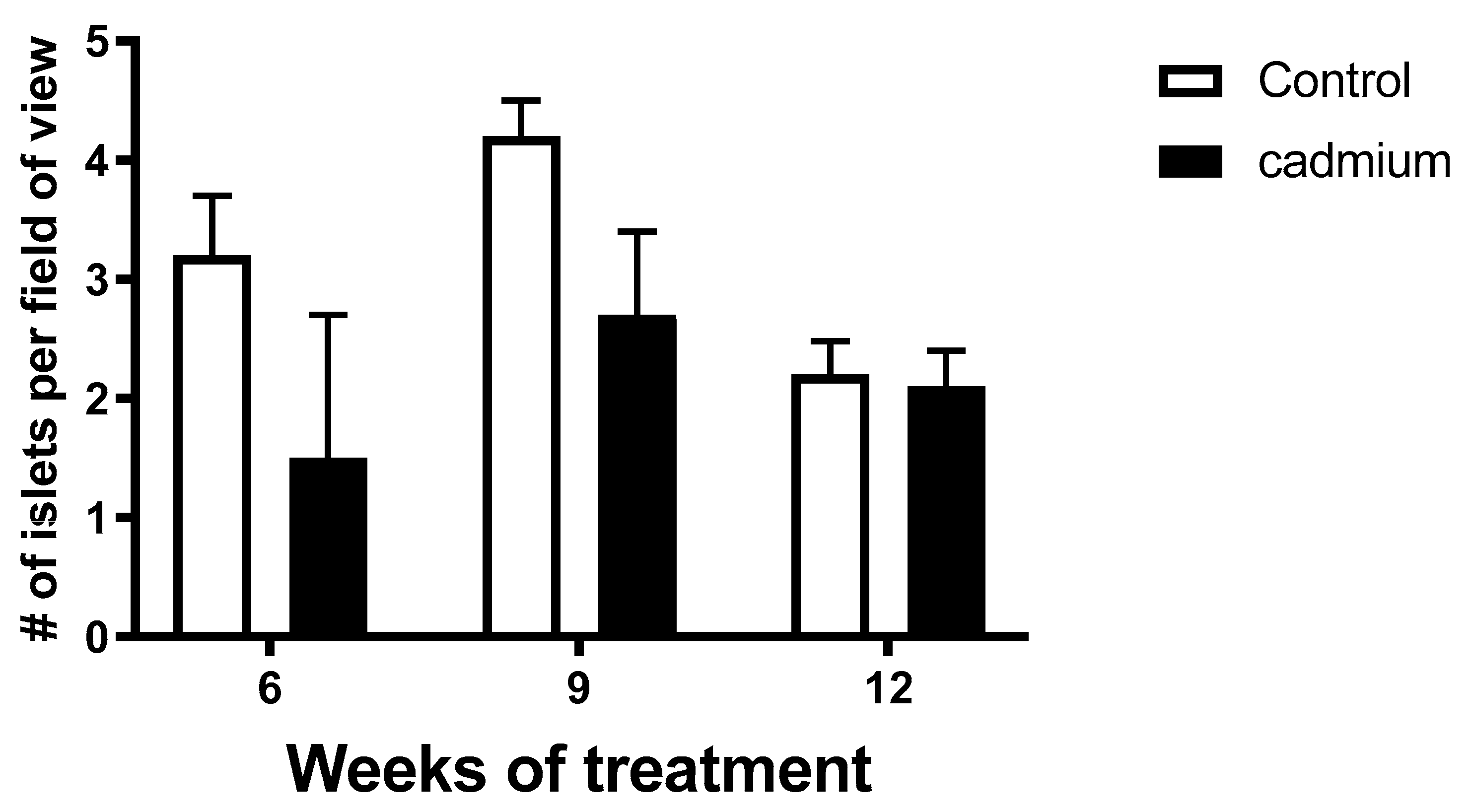
| Apoptosis | Pyknosis | Vacuolation | Dissociation | |
|---|---|---|---|---|
| Control Week 9 | 0 ± 0 | 1 ± 0 | 1.7 ± 0.3 | 0 ± 0 |
| Cadmium Week 9 | 0.8 ± 0.6 | 1.7 ± 0.7 | 2.3 ± 0.3 | 1.2 ± 0.4 |
| Control Week 12 | 0 ± 0 | 1 ± 0 | 2.3 ± 0.3 | 0 ± 0 |
| Cadmium Week 12 | 0.7 ± 0.3 | 2 ± 0 | 2.6 ± 0.3 | 0.8 ± 0.2 |
| Characteristics | Week 6 | Week 9 | Week 12 | ||||
|---|---|---|---|---|---|---|---|
| Control | Cd | Control | Cd | Control | Cd | p-Value | |
| Cells/Surface Area | * 0.12 ± 0.001 | 0.09 ± 0.001 | * 0.125 ± 0.001 | 0.1 ± 0.001 | * 0.12 ± 0.001 | 0.11 ± 0.001 | 0.04 |
| Dia Mean (µm) | 118 ± 9 (38–236) | 108 ± 20 (39–219) | 77 ± 7 (36–249) | 123 ± 9 (46–243) | 134 ± 9 (41–289) | 127 ± 9 (40–282) | 0.062 |
| Dia Min (µm) | 100 ± 4 (32–254) | 88 ± 17 (29–135) | * 63 ± 6 (12–186) | 102 ± 7 (33–243) | 111 ± 8 (43–245) | 105 ± 7 (29–256) | 0.046 |
| Dia Max (µm) | 141 ± 11 (43–327) | 131 ± 25 (49–269) | 93 ± 9 (43–311) | 130 ± 11 (41–369) | 165 ± 11 (42–366) | 157 ± 12 (46–368) | 0.194 |
| Roundness | 1.20 ± 0.014 (1.05–1.55) | 1.12 ± 0.031 (1.12–1.45) | 1.21 ± 0.016 (1.09–1.54) | 1.19 ± 0.019 (1.08–1.66) | 1.22 ± 0.017 (1.08–1.65) | 1.23 ± 0.025 (0.06–1.64) | 1.000 |
| Surface Area (µm2) | 13,918 ± 2057 (1172–16,2802) | 14,256 ± 4012 (1270–38,503) | 6209 ± 1484 (993–49,836) | 15,112 ± 2197 (1111–35,201) | 18,245 ± 2234 (1105–66,452) | 16,544 ± 2296 (1470–58,057) | 0.151 |
| Perimeter (µm) | 205 ± 16 (65–491) | 192 ± 36 (72–382) | 135 ± 14 (61–471) | 216 ± 18 (79–569) | 237 ± 17 (62–489) | 224 ± 16 (74–530) | 0.051 |
| Number of Nuclei | 86 ± 12 (5–338) | 68 ± 20 (6–178) | 43 ± 10 (4–348) | 81 ± 11 (7–273) | 115 ± 14 (10–379) | 96 ± 13 (6–382) | 0.136 |
| Characteristics | Week 9 | Week 12 | |||
|---|---|---|---|---|---|
| Control | Cd | Control | Cd | p-Value | |
| Insulin & Glucagon Ratio | 3.15 ± 0.81 (1.33–6.0) | 9.08 ± 3.1 (2.95–17) | 4.02 ± 1.6 (2.04–10) | 3.32 ± 0.51 (2.75–5.8) | 0.088 |
| Insulin/SA (#/µm2) | 3.81 × 10−3 ± 4.1 × 10−4 (2.70 × 10−3–5.22 × 10−3) | 4.12 × 10−3 ± 3.24 × 10−5 (3.20 × 10−3–4.61 × 10−3) | 4.18 × 10−3 ± 3.80 × 10−4 (3.4 × 10−3–5.53 × 10−3) | 3.42 × 10−3 ± 6.63 × 10−5 (1.97 × 10−3–5.54 × 10−3) | 0.266 |
| Glucagon/SA (#/µm2) | 1.63 × 10−3 ± 6.00 × 10−4 (6.97× 10−5–3.04 × 10−3) | 7.00 × 10−4 ± 2.00 × 10−4 (9.91× 10−5–7.94 × 10−3) | 1.41 × 10−3 ± 2.00 × 10−4 (5.53 × 10−5–1.94 × 10−3) | 1.14 × 10−3 ± 3.24 × 10−4 (7.96 × 10−5–2.47 × 10−3) | 0.380 |
| # of Gluc Nuc/5000 µm (#/µm) | 6 ± 2 (3–14) | 3 ± 1 (1–4) | 5 ± 1 (2–7) | 4 ± 1 (2–9) | 0.388 |
| # of Ins Nuc/5000 µm(#/µm) | 14 ± 2 (10–19) | 15 ± 1 (12–19) | 15 ± 1 (13–20) | 13 ± 3 (7–20) | 0.273 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzgerald, R.; Olsen, A.; Nguyen, J.; Wong, W.; El Muayed, M.; Edwards, J. Pancreatic Islets Accumulate Cadmium in a Rodent Model of Cadmium-Induced Hyperglycemia. Int. J. Mol. Sci. 2021, 22, 360. https://doi.org/10.3390/ijms22010360
Fitzgerald R, Olsen A, Nguyen J, Wong W, El Muayed M, Edwards J. Pancreatic Islets Accumulate Cadmium in a Rodent Model of Cadmium-Induced Hyperglycemia. International Journal of Molecular Sciences. 2021; 22(1):360. https://doi.org/10.3390/ijms22010360
Chicago/Turabian StyleFitzgerald, Ryan, Andrew Olsen, Jessica Nguyen, Winifred Wong, Malek El Muayed, and Joshua Edwards. 2021. "Pancreatic Islets Accumulate Cadmium in a Rodent Model of Cadmium-Induced Hyperglycemia" International Journal of Molecular Sciences 22, no. 1: 360. https://doi.org/10.3390/ijms22010360
APA StyleFitzgerald, R., Olsen, A., Nguyen, J., Wong, W., El Muayed, M., & Edwards, J. (2021). Pancreatic Islets Accumulate Cadmium in a Rodent Model of Cadmium-Induced Hyperglycemia. International Journal of Molecular Sciences, 22(1), 360. https://doi.org/10.3390/ijms22010360






