An Insight into the Role of Non-Porphyrinoid Photosensitizers for Skin Wound Healing
Abstract
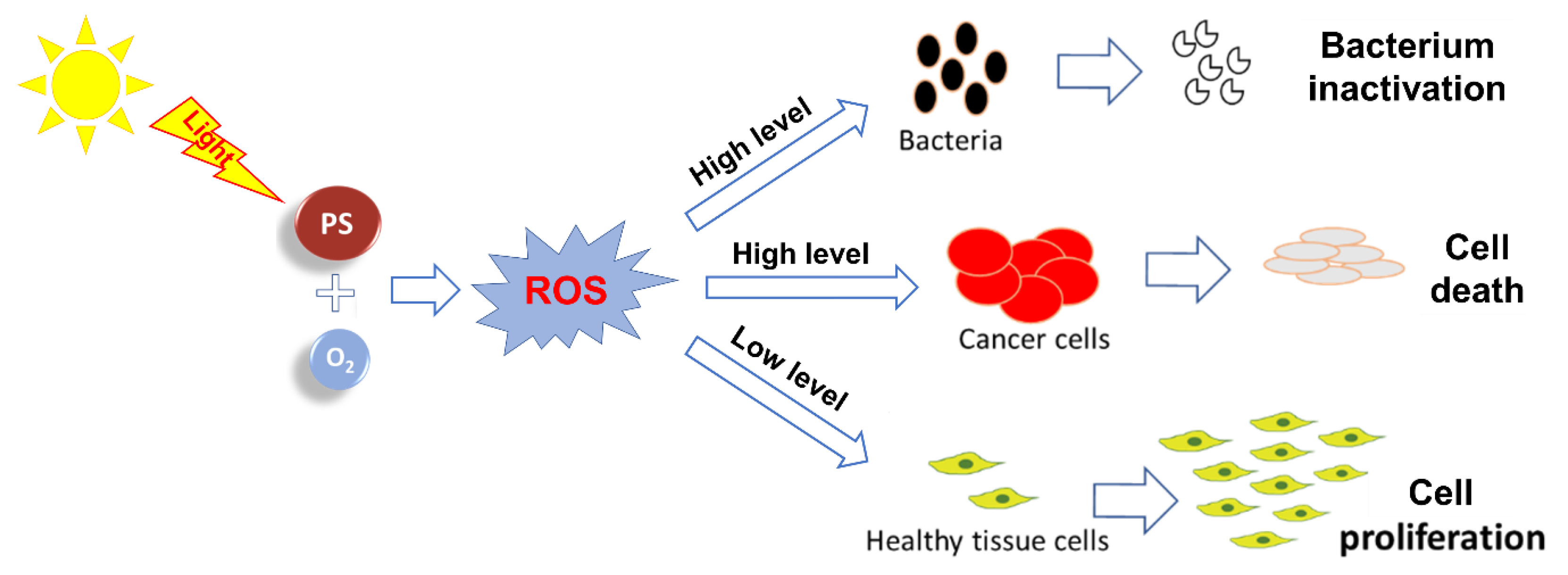
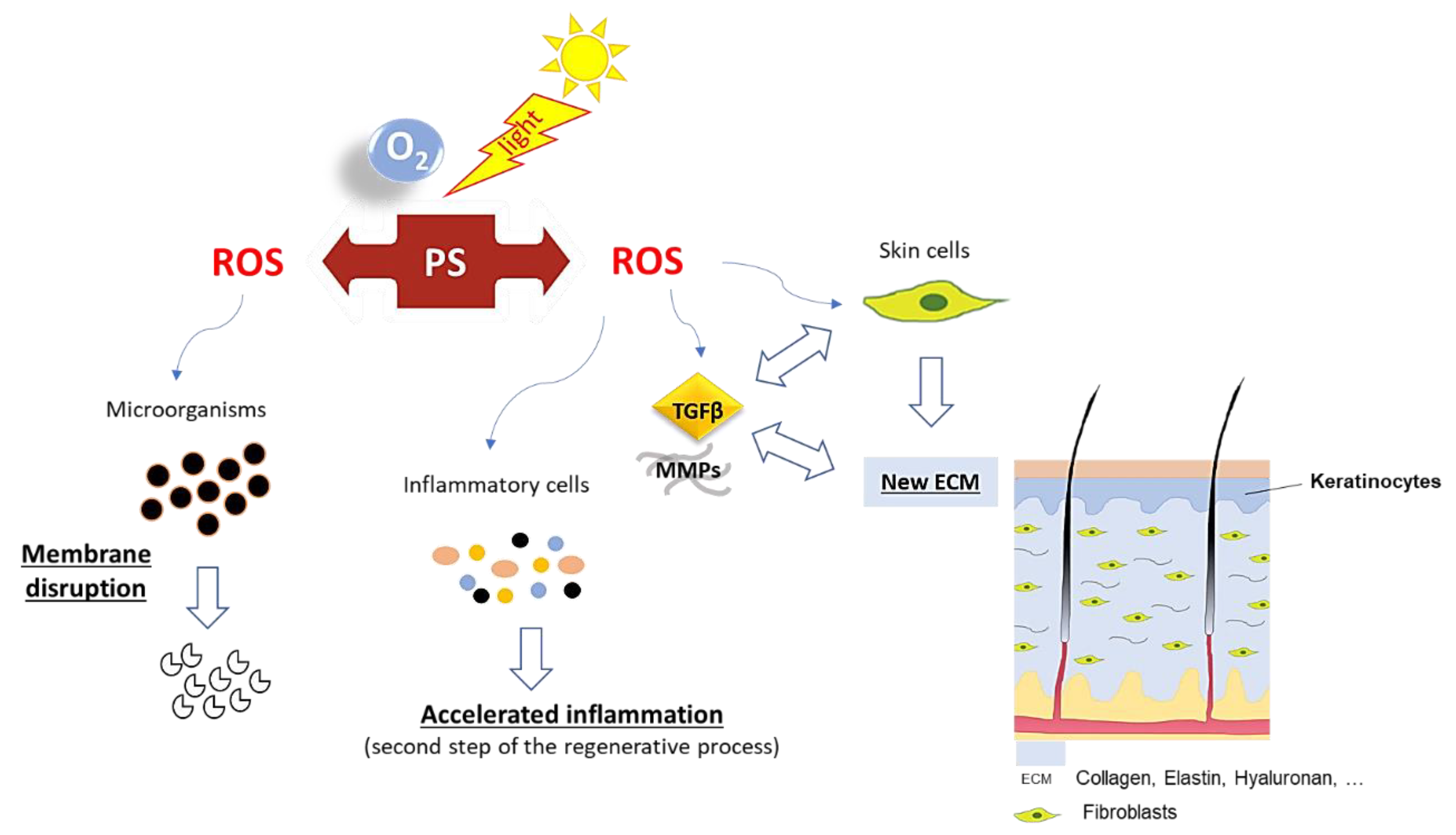
1. Introduction
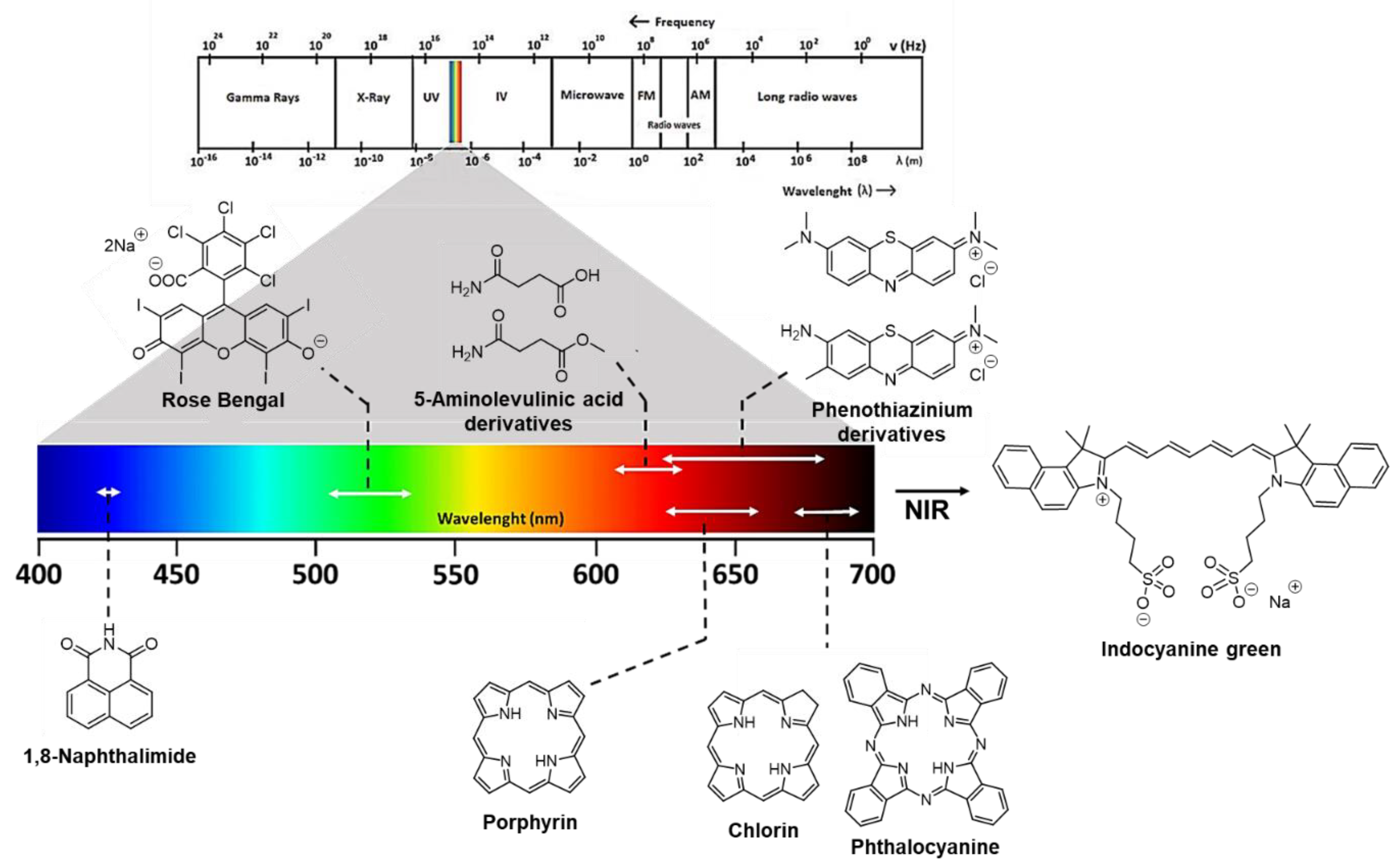
2. Photosensitizing Dyes
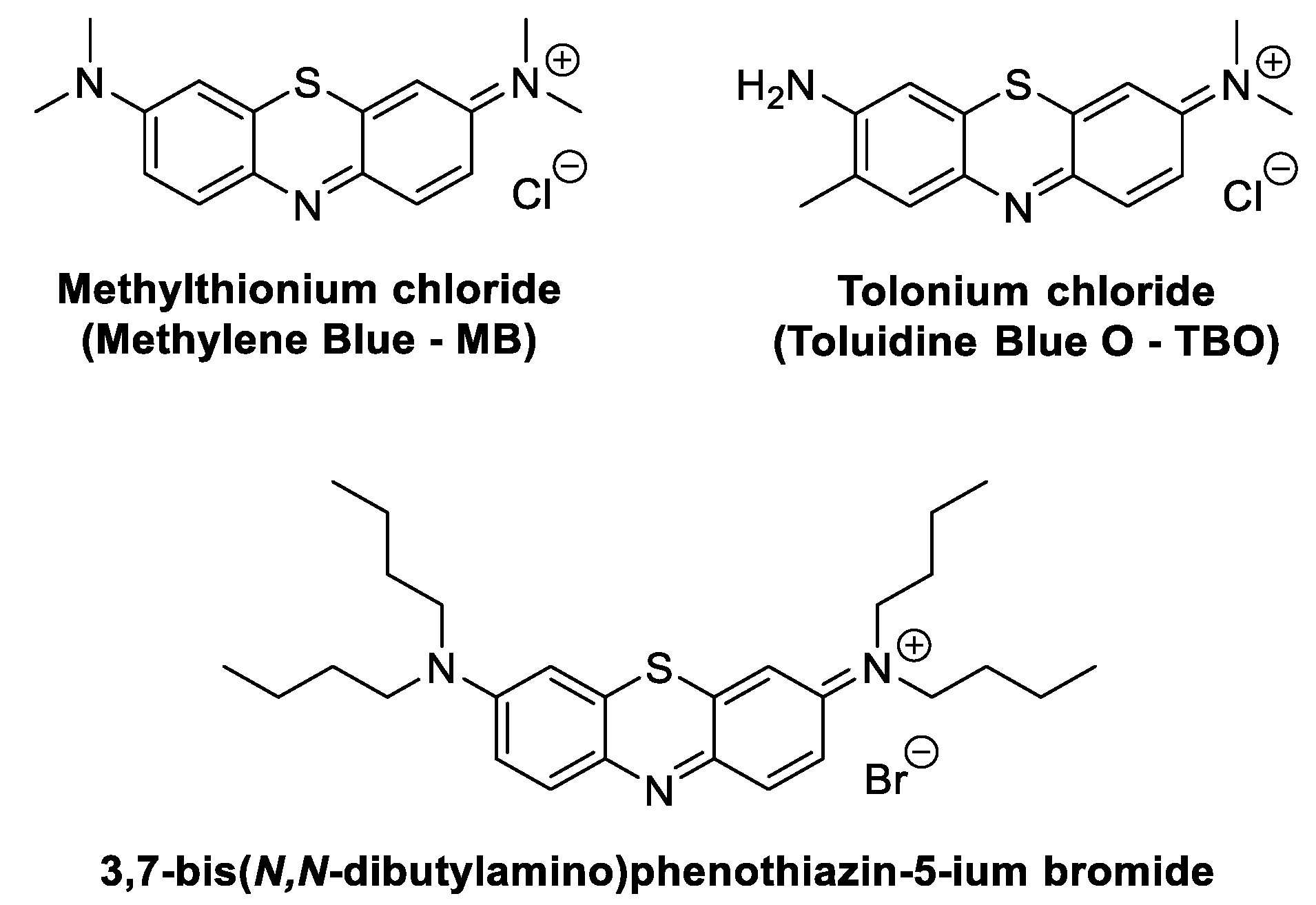
2.1. Phenothiazinium Derivatives
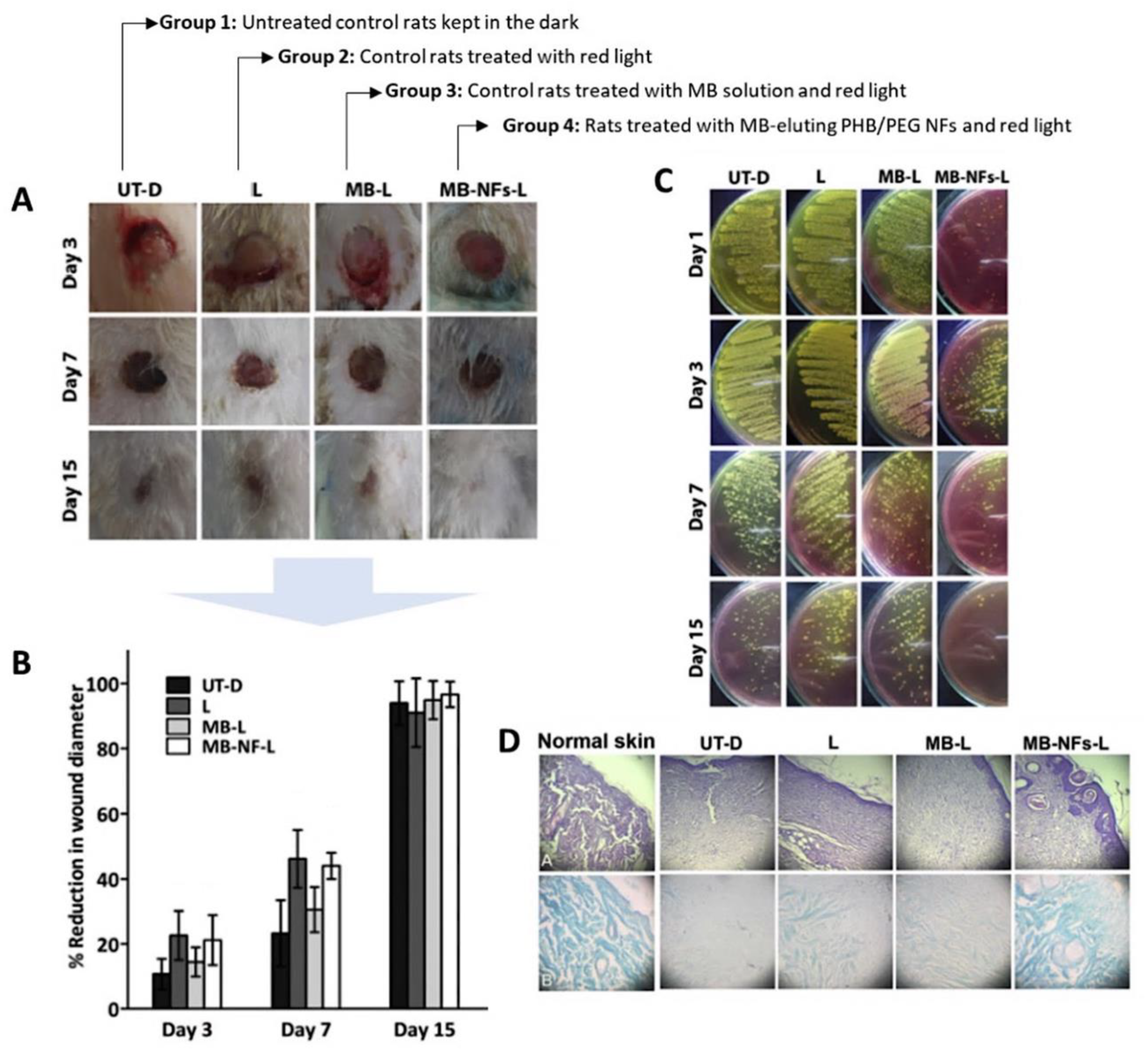
2.1.1. Methylene Blue
2.1.2. Toluidine O Blue Dye
2.1.3. 3,7-Bis(N,N-Dibutylamino)phenothiazine-5-ium Bromide
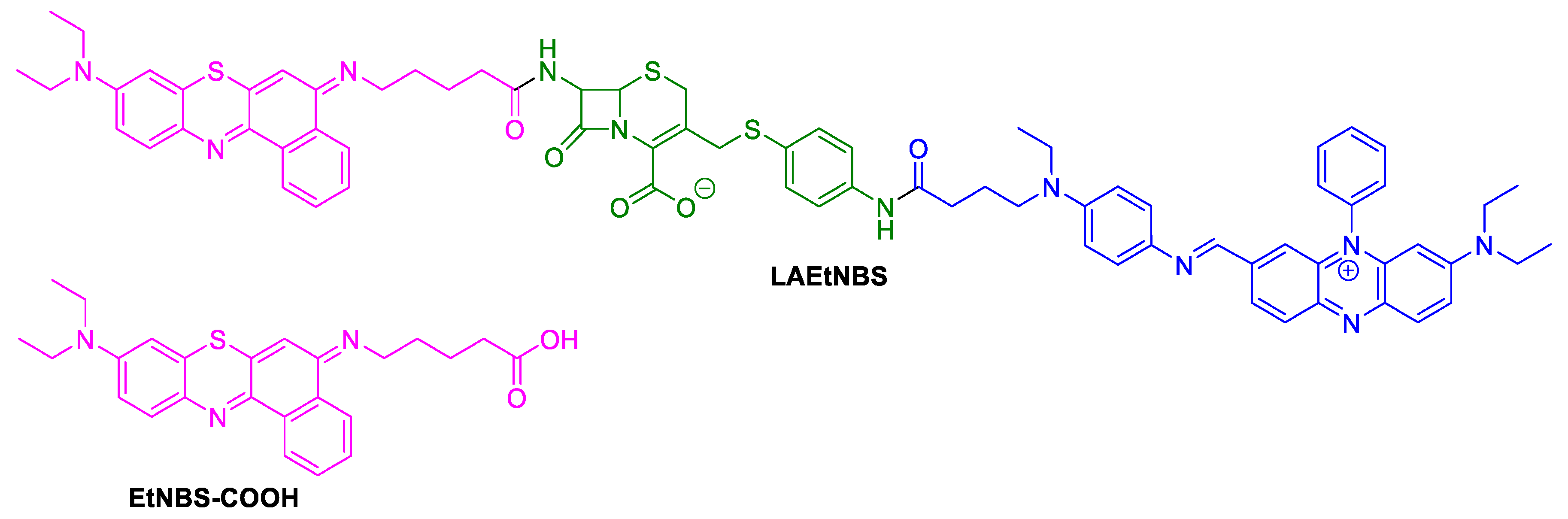
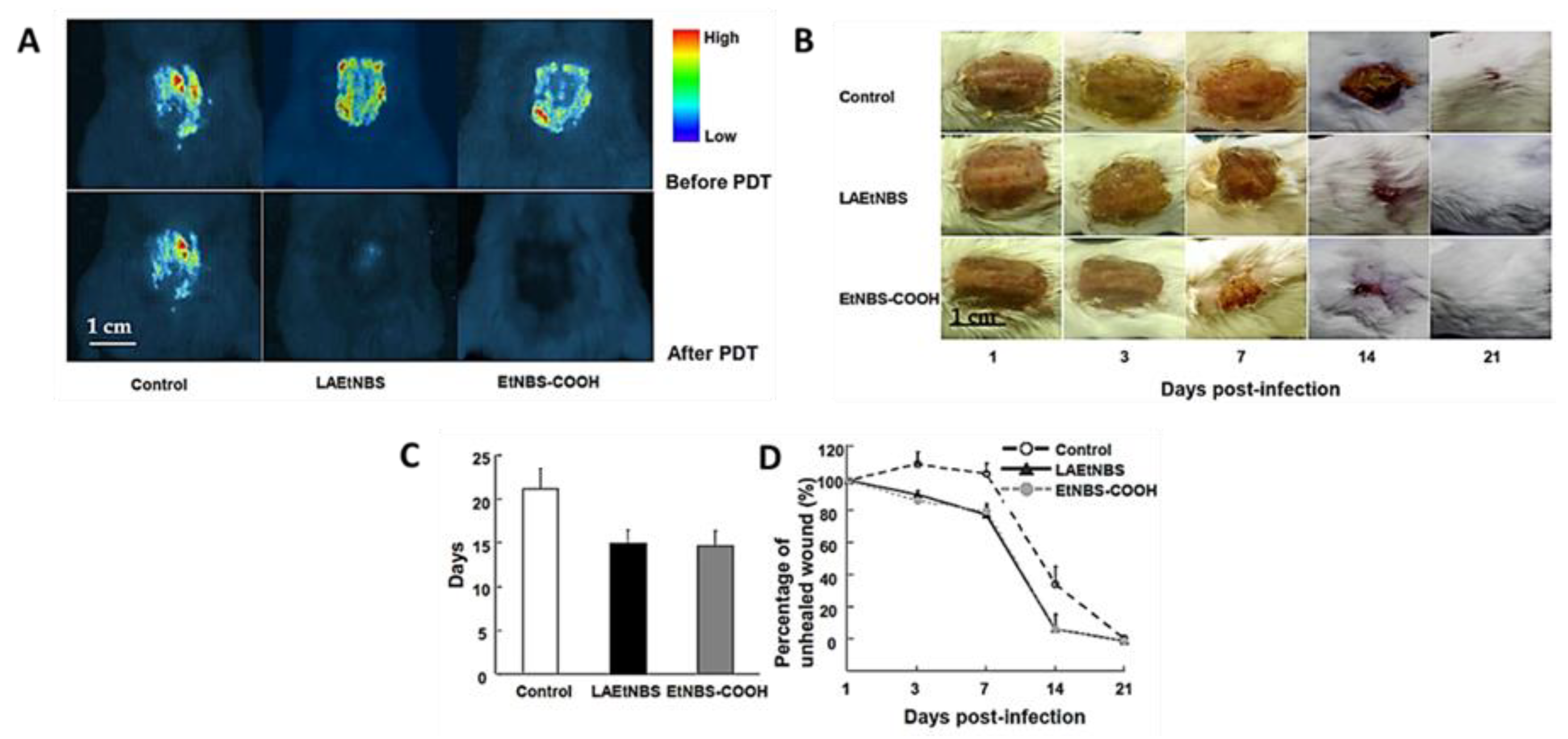
2.1.4. Benzophenothiazinium Dyes (EtNBS)
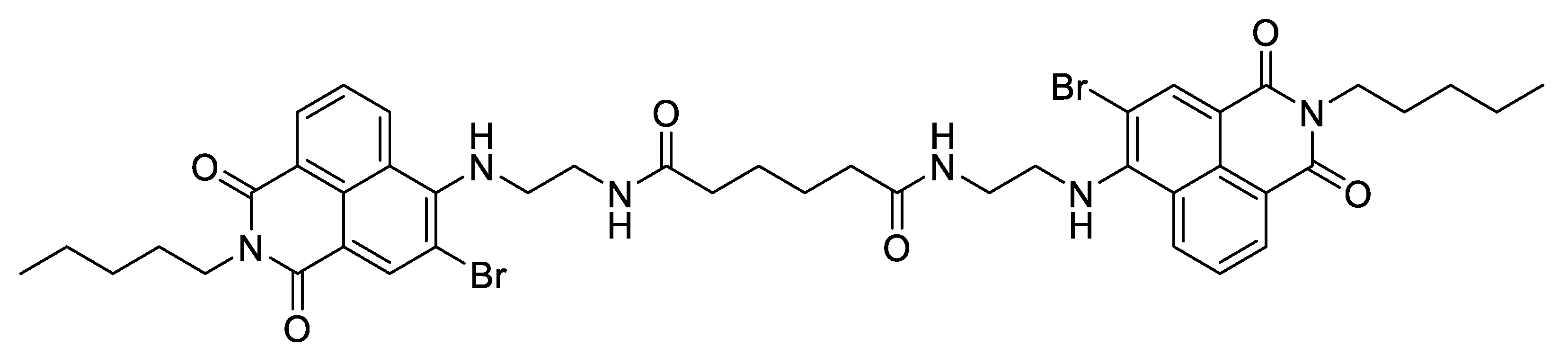
2.2. Naphthalimide Dye
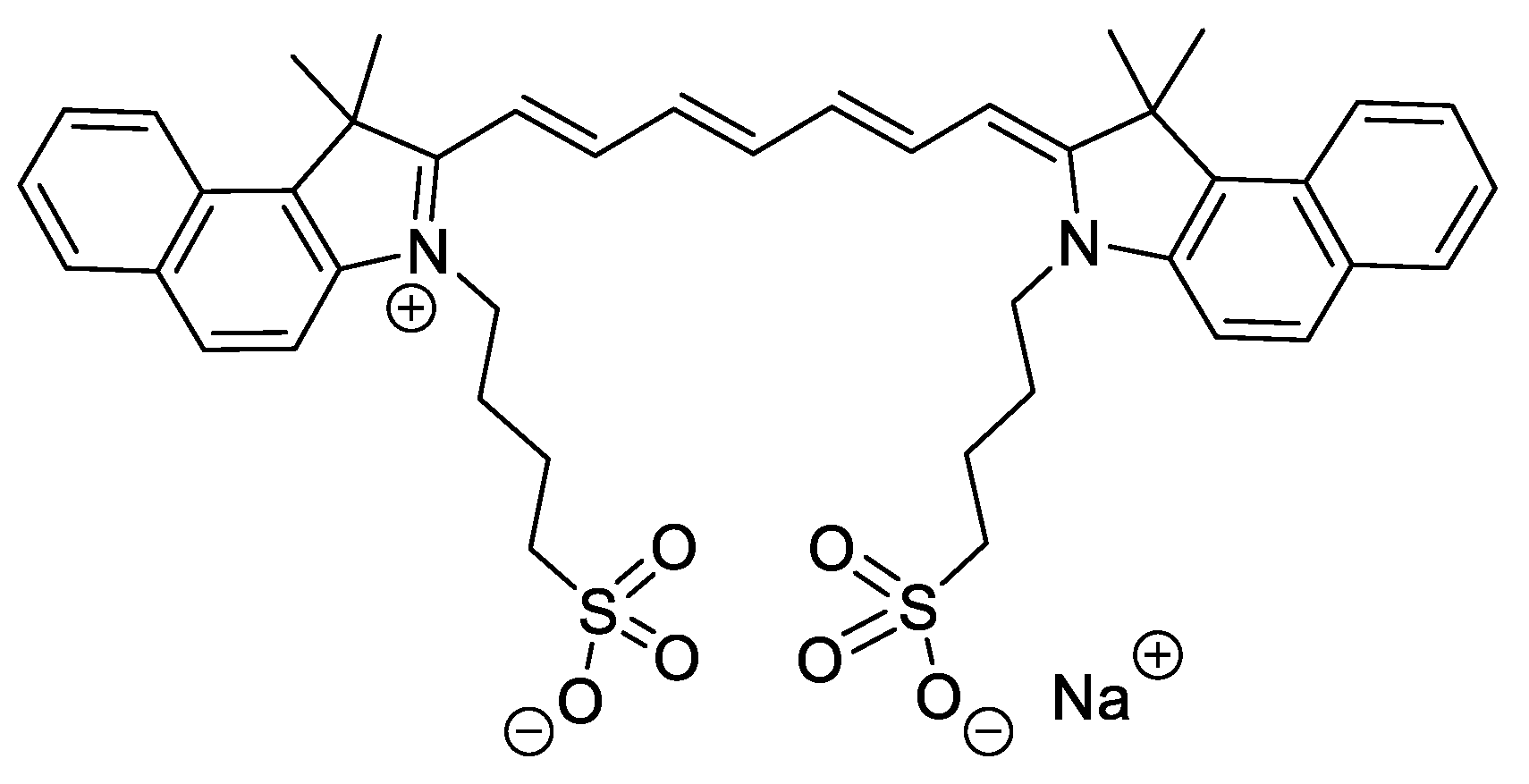
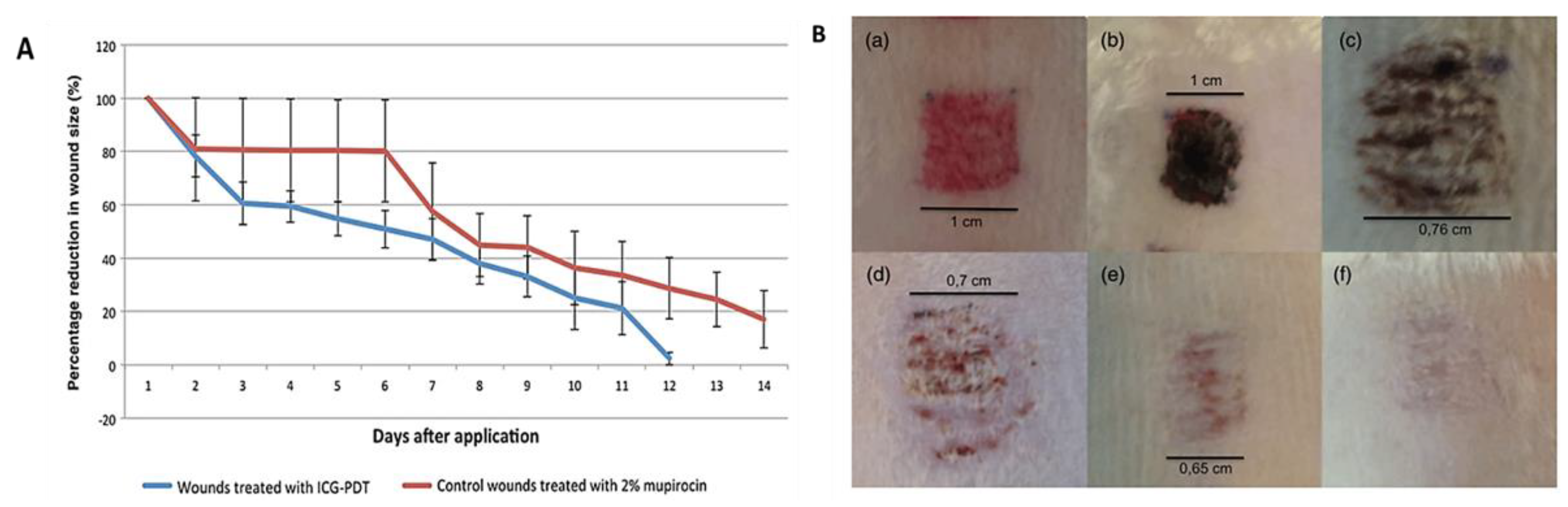
2.3. Indocyanine Green
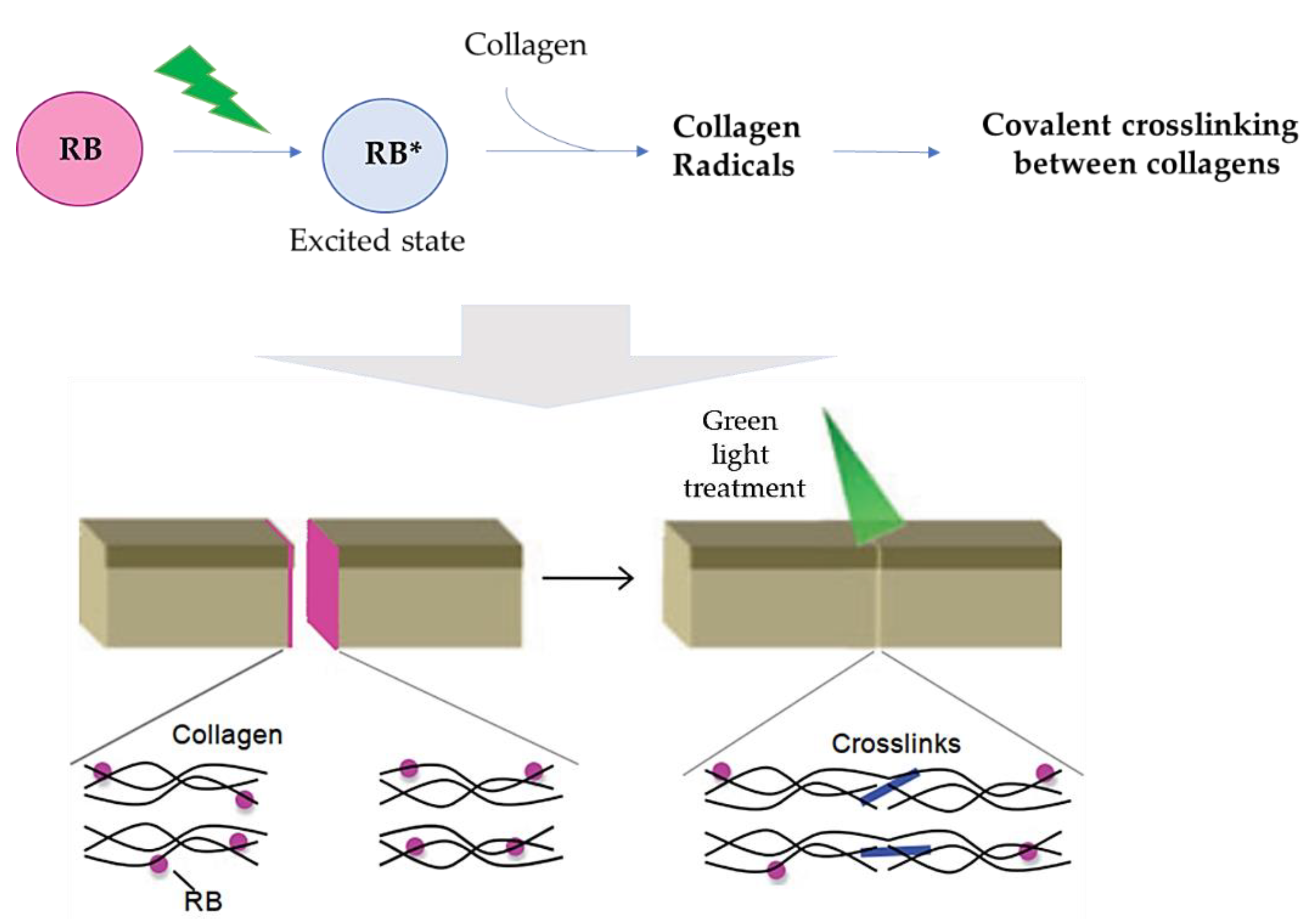
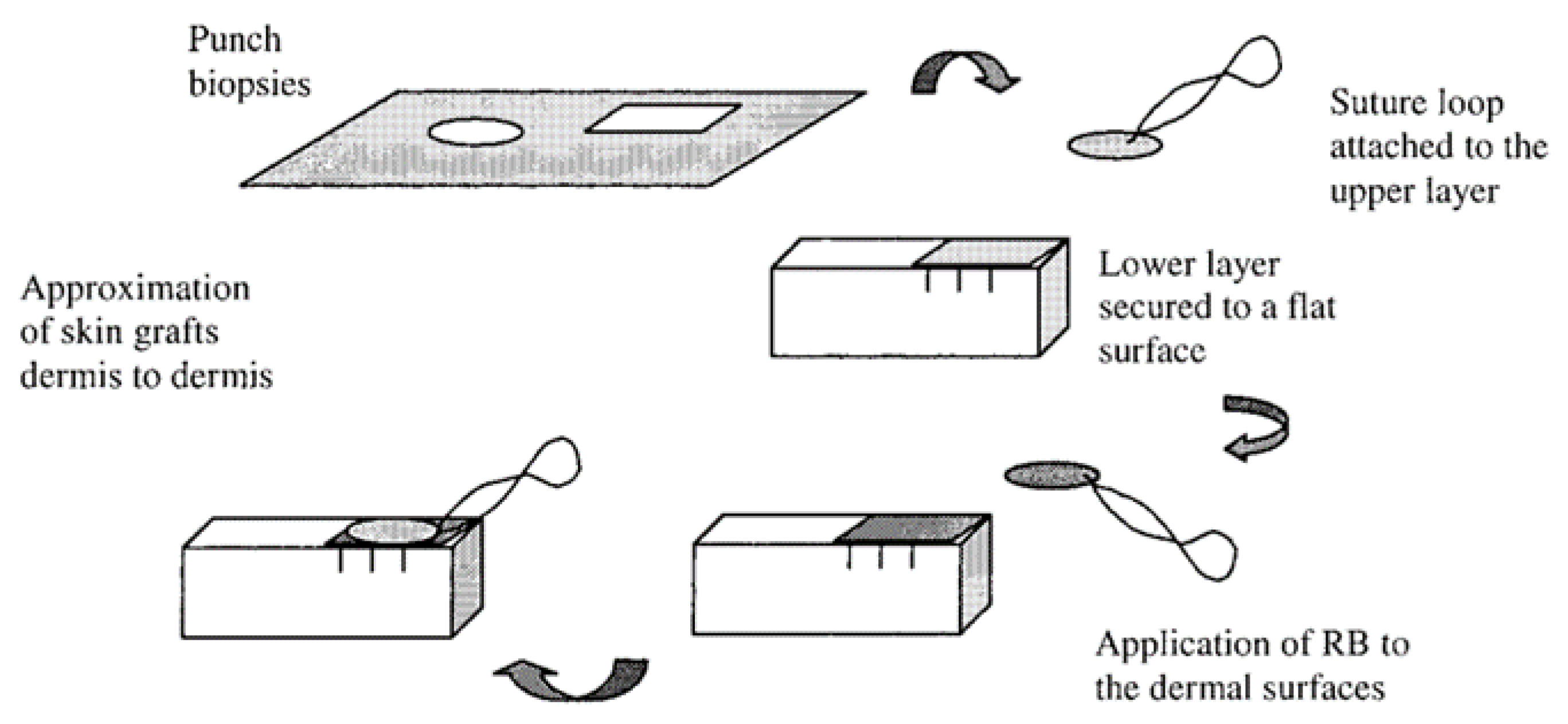
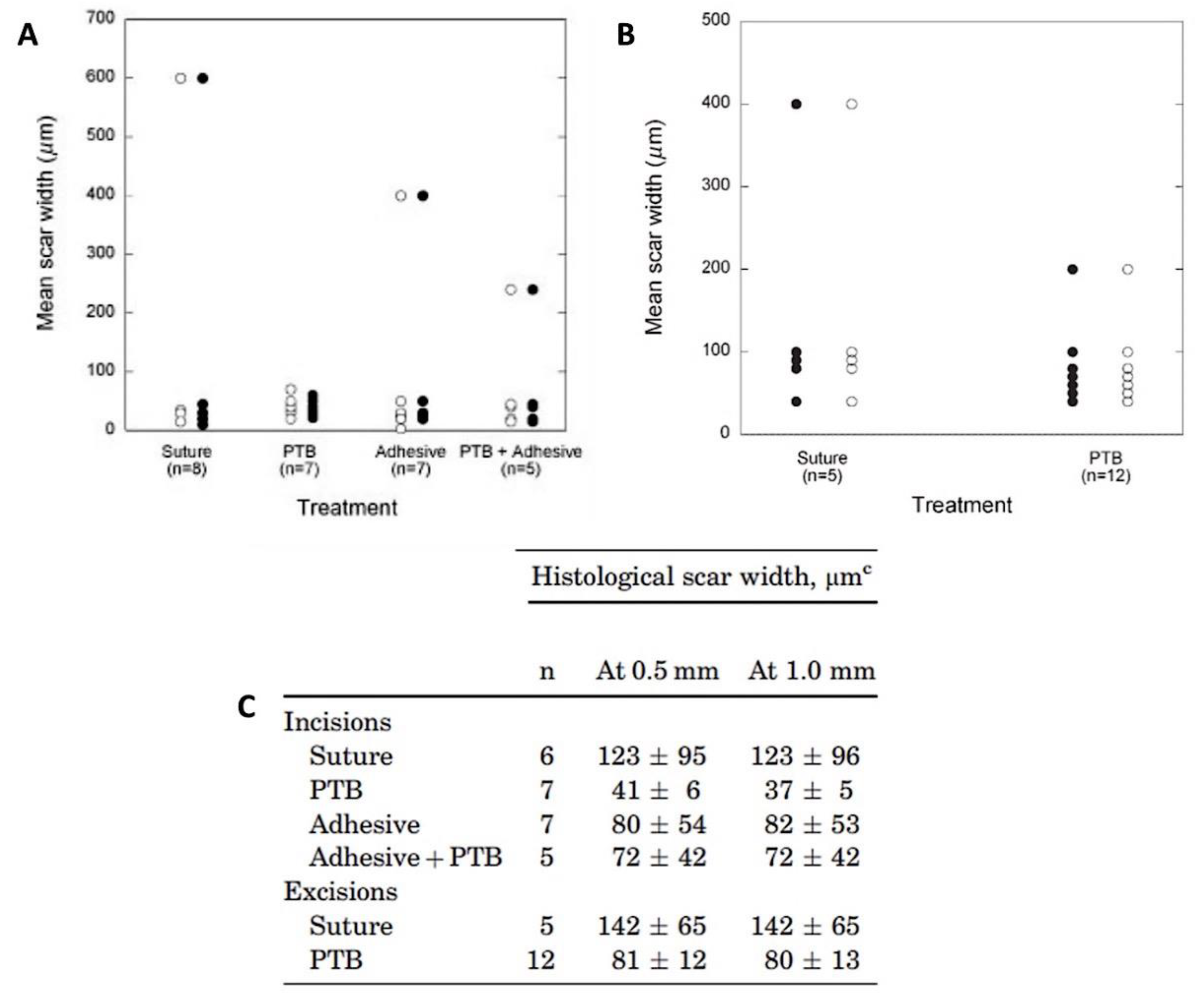
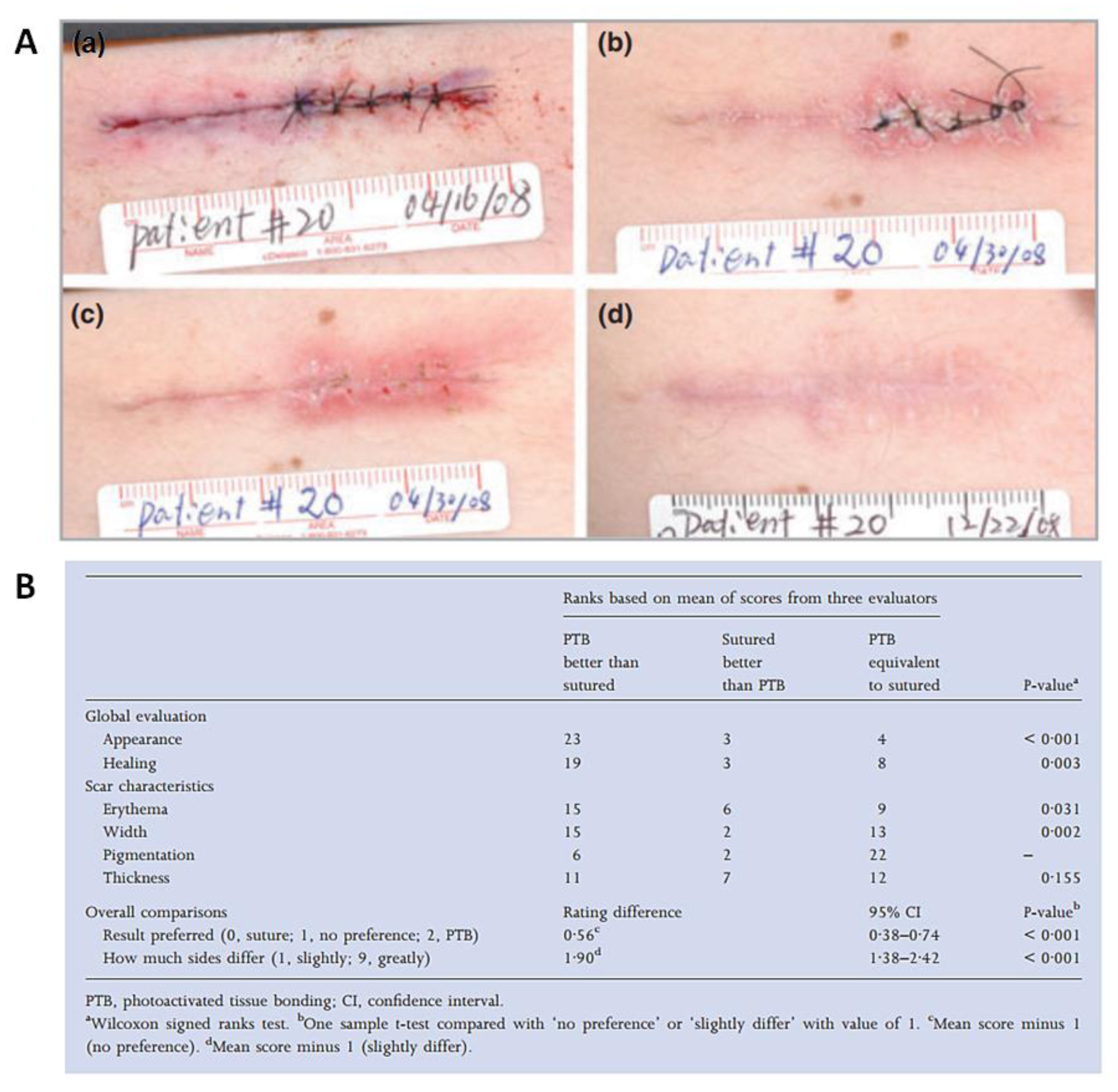
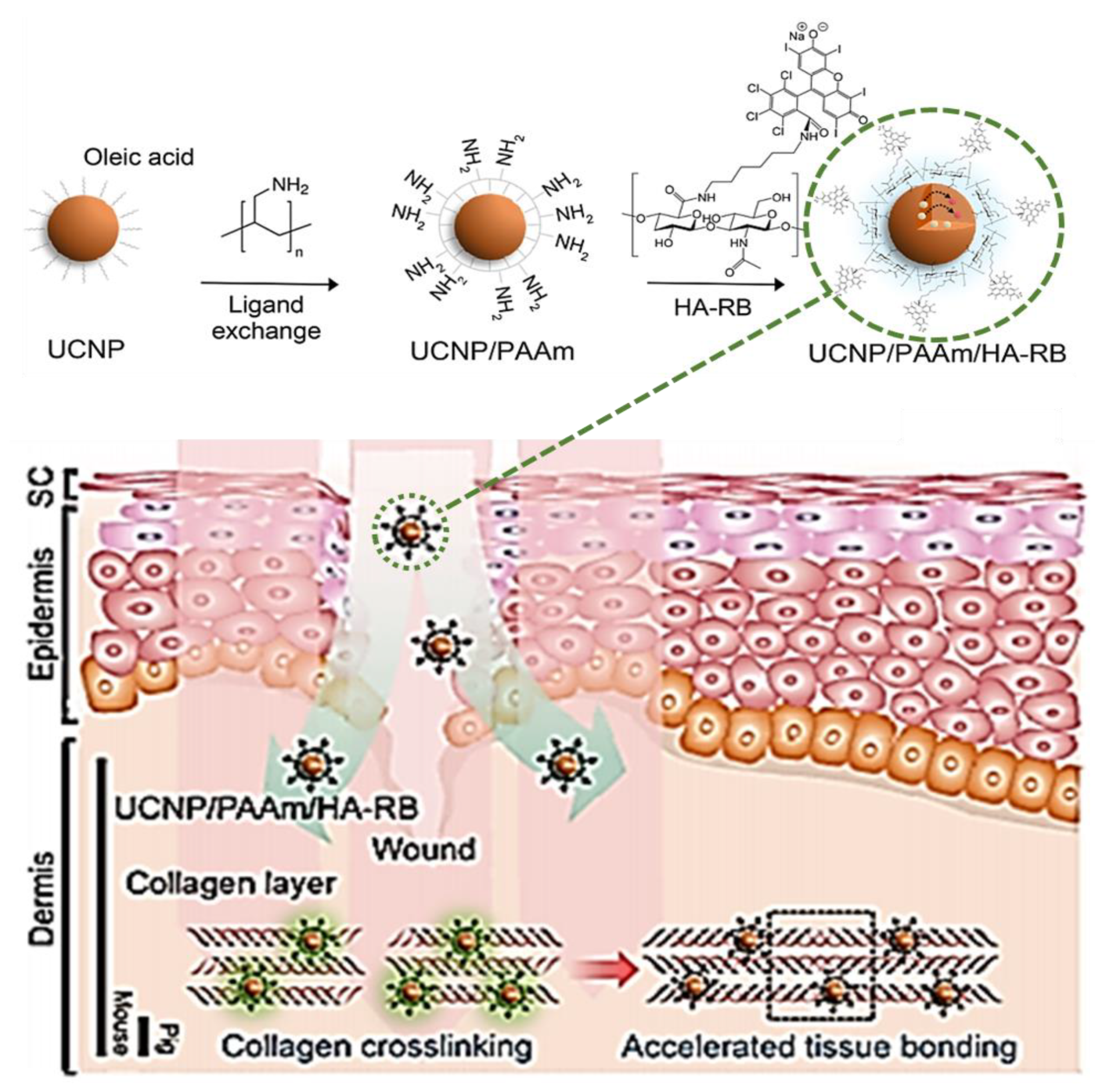
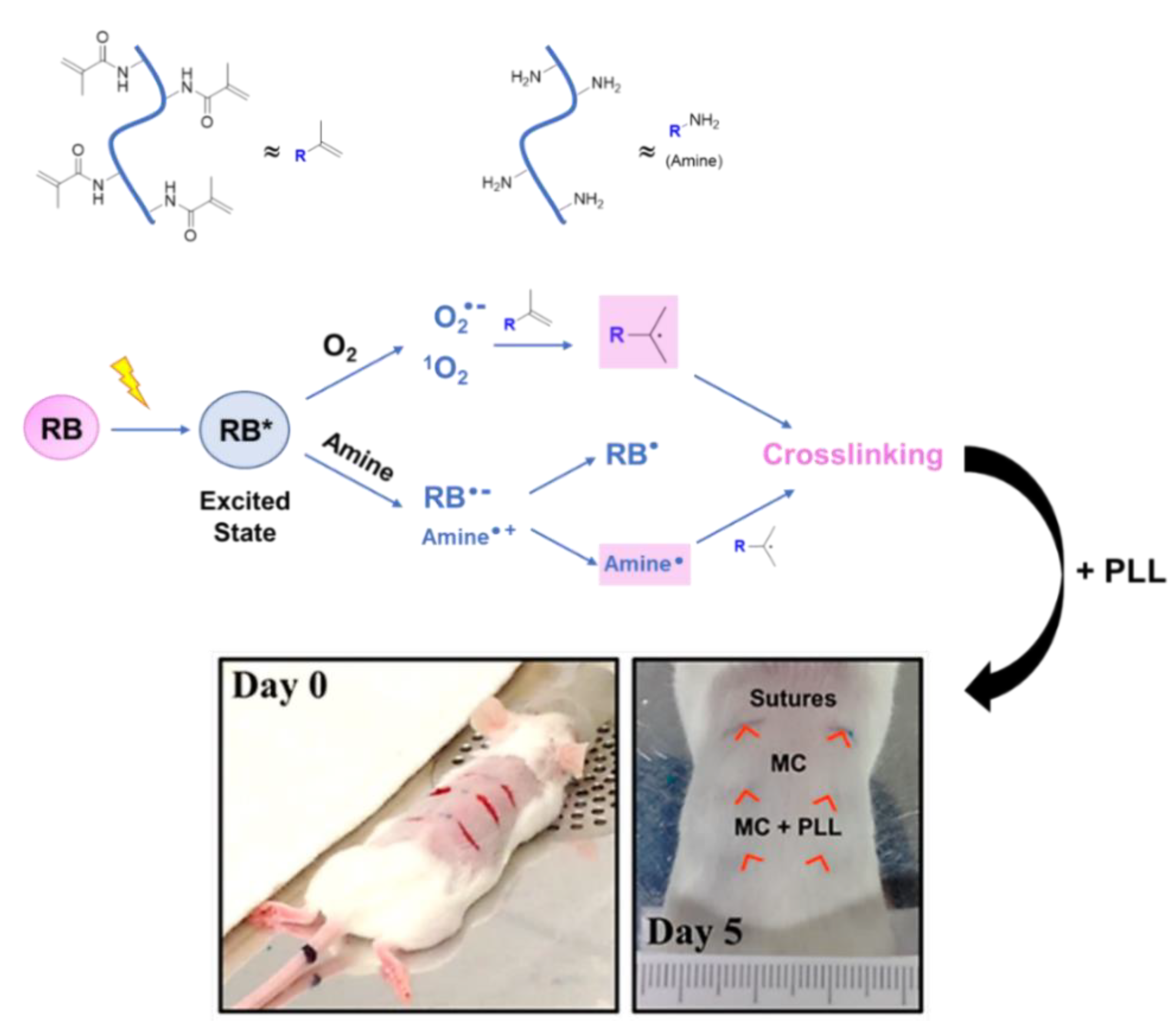
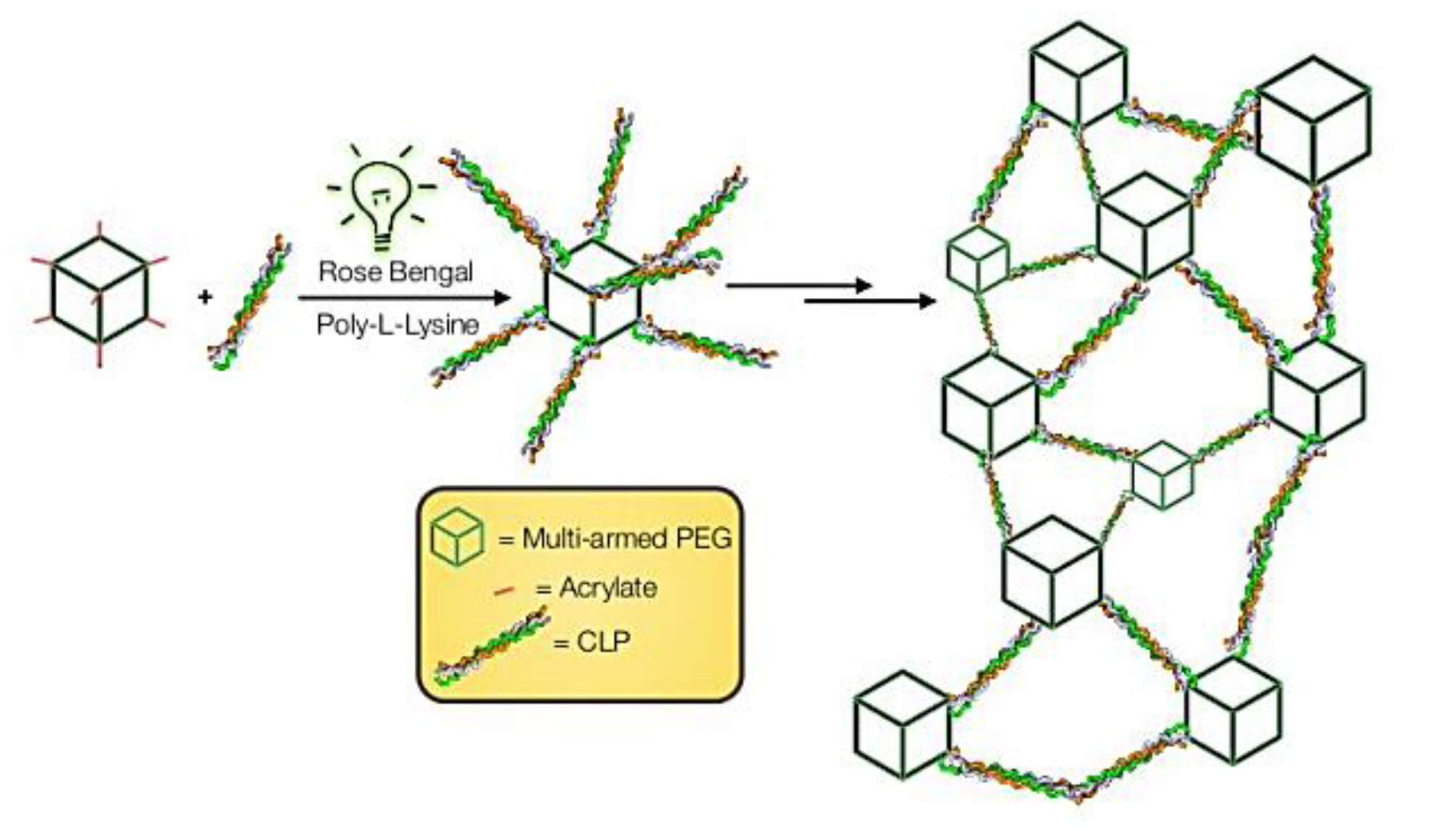
2.4. Rose Bengal Dye and the Concept of Photobonding
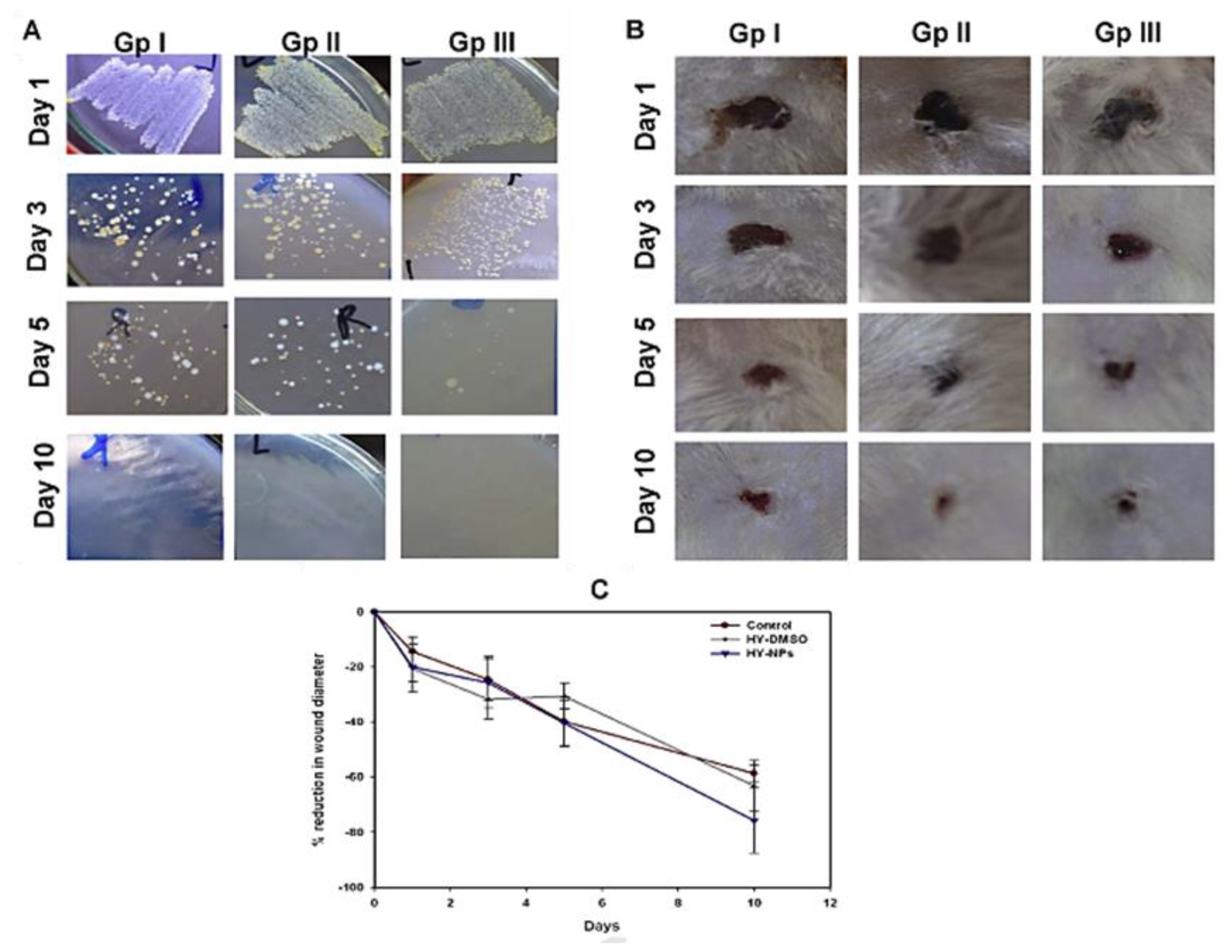
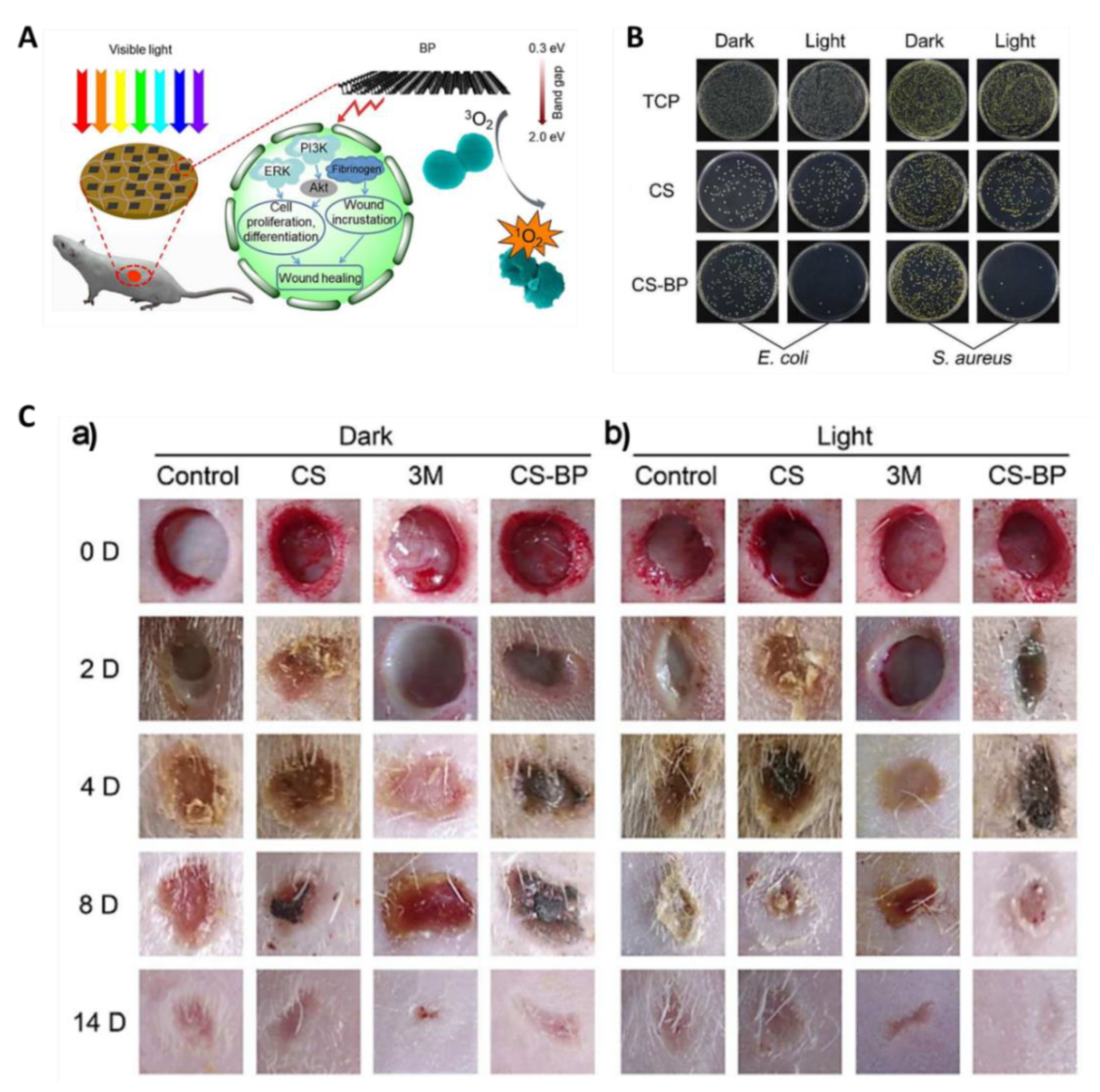
2.5. Other Photoactive Platforms
3. Final Remarks
Funding
Conflicts of Interest
Abbreviations
| AAm | Acrylamide |
| aPDT | Antimicrobial photodynamic therapy |
| BP | Black phosphorus |
| CFTR | Cystic fibrosis transmembrane conductance regulator R |
| CLP | Collagen-like peptides |
| CMC | Carboxymethyl cellulose |
| CS | Chitosan hydrogel |
| DA | Dopamine |
| DFT | Directional freezing–thawing |
| ECM | Extracellular matrix |
| FA | Folic acid |
| FAK | Focal adhesion kinase |
| FDA | Food and Drug Administration |
| Fmoc-FF | N-fluorenylmethoxycarbonyl diphenylalanine |
| g-C3N4 | Graphitic carbon nitride |
| GFs | Growth factors |
| HÁ | Hyaluronate |
| HIF-1α | Hypoxia inducible factor-1 |
| HY | Hypericin |
| ICG | Indocyanine green |
| LED | Light-emitting diode |
| LLLT | Low-level laser therapy |
| MB | Methylene blue |
| min | Minute |
| MMPS | Metalloproteinases |
| m-PEG | Poly(ethylene glycol)monomethyl ether |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| NF | Nanofiber |
| NIPAAM | N-isopropylacrylamide |
| NIR | Near-infrared |
| NP | Nanoparticle |
| PAAm | Poly(allylamine) |
| PB | Photobonding |
| PCL-PEG | Polycaprolactone-poly(ethylene glycol) |
| PDT | Photodynamic therapy |
| PEG-Ac | Polyethylene glycol-acrylate |
| PLL | Poly-L-lysine |
| PS | Photosensitizer |
| PTB | Photoactivated tissue bonding |
| PVA | Poly(vinyl alcohol) |
| PTT | Photothermal therapy |
| RB | Rose Bengal |
| ROS | Reactive oxygen species |
| SDBS | Sodium dodecylbenzenesulfonate |
| TBO | Toluidine O blue |
| TCP | Tissue culture plate |
| UPNP | Upconversion nanoparticles |
| VEGF | Vascular endothelial growth factor |
References
- Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Cancer, Photodynamic Therapy and Porphyrin-Type Derivatives. An. Acad. Bras. Ciências 2018, 90, 993–1026. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Dias, C.J.; Gamelas, S.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. An insight on the role of photosensitizer nanocarriers for Photodynamic Therapy. An. Acad. Bras. Ciências 2018, 90, 1101–1130. [Google Scholar] [CrossRef] [PubMed]
- Scherer, K.M.; Bisby, R.H.; Botchway, S.W.; Parker, A.W. New Approaches to Photodynamic Therapy from Types I, II and III to Type IV Using One or More Photons. Anti-Cancer Agents Med. Chem. 2017, 17, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Su, Y.X.; Wu, J.W.; Gu, Y. Photodynamic therapy in combination with ranibizumab versus ranibizumab monotherapy for wet age-related macular degeneration: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2018, 22, 263–273. [Google Scholar] [CrossRef]
- Dharmaratne, P.; Sapugahawatte, D.N.; Wang, B.Y.; Chan, C.L.; Lau, K.M.; Lau, C.; Fung, K.P.; Ng, D.K.P.; Margaret, I.P. Contemporary approaches and future perspectives of antibacterial photodynamic therapy (aPDT) against methicillin-resistant Staphylococcus aureus (MRSA): A systematic review. Eur. J. Med. Chem. 2020, 200, 27. [Google Scholar] [CrossRef]
- Jaber, G.; Dariush, R.; Shahin, A.; Alireza, T.; Abbas, B. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef]
- Esmatabadi, M.J.D.; Bozorgmehr, A.; Hajjari, S.N.; Sombolestani, A.S.; Malekshahi, Z.V.; Sadeghizadeh, M. Review of new insights into antimicrobial agents. Cell. Mol. Biol. 2017, 63, 40–48. [Google Scholar] [CrossRef]
- Oyama, J.; Fernandes Herculano Ramos-Milaré, Á.C.; Lopes Lera-Nonose, D.S.S.; Nesi-Reis, V.; Galhardo Demarchi, I.; Alessi Aristides, S.M.; Juarez Vieira Teixeira, J.; Gomes Verzignassi Silveira, T.; Campana Lonardoni, M.V. Photodynamic therapy in wound healing in vivo: A systematic review. Photodiagnosis Photodyn. Ther. 2020, 30, 101682. [Google Scholar] [CrossRef] [PubMed]
- El-Khordagui, L.; El-Sayed, N.; Galal, S.; El-Gowelli, H.; Omar, H.; Mohamed, M. Photosensitizer-eluting nanofibers for enhanced photodynamic therapy of wounds: A preclinical study in immunocompromized rats. Int. J. Pharm. 2017, 520, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Nesi-Reis, V.; Lera-Nonose, D.S.S.L.; Oyama, J.; Silva-Lalucci, M.P.P.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Contribution of photodynamic therapy in wound healing: A systematic review. Photodiagnosis Photodyn. Ther. 2018, 21, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, A.; Jesus, P. Low Level Energy Photodynamic Therapy for Skin Processes and Regeneration. In Photomedicine—Advances in Clinical Practice; Tanaka, Y., Ed.; IntechOpen: London, UK, 2017; Volume 5, pp. 75–94. [Google Scholar]
- Peplow, P.V.; Chung, T.-Y.; Baxter, G.D. Photodynamic Modulation of Wound Healing: A Review of Human and Animal Studies. Photomed. Laser Surg. 2012, 30, 118–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Dias, C.J.; Sardo, I.; Moura, N.M.M.; Felgueiras, J.; Neves, M.G.P.M.S.; Fardilha, M.; Faustino, M.A.F. An efficient synthetic access to new uracil-alditols bearing a porphyrin unit and biological assessment in prostate cancer cells. Dye. Pigment. 2020, 173, 107996. [Google Scholar] [CrossRef]
- Gomes, A.T.P.C.; Faustino, M.A.F.; Neves, M.G.P.S.N.; Ferreira, V.F.; Juarranz, A.; Cavaleiro, J.A.S.; Sanz-Rodriguez, F. Photodynamic effect of glycochlorin conjugates in human cancer epithelial cells. RSC Adv. 2015, 5, 33496–33502. [Google Scholar] [CrossRef]
- Gupta, A.; Keshri, G.K.; Yadav, A. Non-thermal Therapeutic Applications of Light. Natl. Acad. Sci. India Sect. A Phys. Sci. 2018, 88, 473–478. [Google Scholar] [CrossRef]
- Moreira, X.; Santos, P.; Faustino, M.A.F.; Raposo, M.M.M.; Costa, S.P.G.; Moura, N.M.M.; Gomes, A.T.P.C.; Almeida, A.; Neves, M.G.P.M.S. An insight into the synthesis of cationic porphyrin-imidazole derivatives and their photodynamic inactivation efficiency against Escherichia coli. Dye. Pigment. 2020, 178, 108330. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Moura, N.M.M.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Faustino, M.d.A.F.; Cunha, Â.; Almeida Paz, F.A.; Mendes, R.F.; Almeida, A.; Freire, C.S.R.; et al. Synthesis and characterization of photoactive porphyrin and poly(2-hydroxyethyl methacrylate) based materials with bactericidal properties. Appl. Mater. Today 2019, 16, 332–341. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Moura, N.M.M.; Figueira, F.; Ferreira, R.I.; Simões, M.M.Q.; Cavaleiro, J.A.S.; Faustino, M.A.F.; Silvestre, A.J.D.; Freire, C.S.R.; Tomé, J.P.C.; et al. ew Materials Based on Cationic Porphyrins Conjugated to Chitosan or Titanium Dioxide: Synthesis, Characterization and Antimicrobial Efficacy. Int. J. Mol. Sci. 2019, 20, 2522. [Google Scholar] [CrossRef] [PubMed]
- Deyhimi, P.; Khademi, H.; Birang, R.; Akhoondzadeh, M. Histological Evaluation of Wound Healing Process after Photodynamic Therapy of Rat Oral Mucosal Ulcer. J. Dent. 2016, 17, 43–48. [Google Scholar]
- Sperandio, F.F.; Simões, A.; Aranha, A.C.C.; Corre, L.; Sousa, S.C.O.M. Photodynamic Therapy Mediated by Methylene Blue Dye in Wound Healing. Photomed. Laser Surg. 2010, 28, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Kübler, A.; Finley, R.K., III; Born, I.A.; Mang, T.S. Effect of photodynamic therapy on the healing of a rat skin flap and its implication for head and neck reconstructive surgery. Lasers Surg. Med. 1996, 18, 397–405. [Google Scholar] [CrossRef]
- Murray, R.Z.; West, Z.E.; Cowin, A.J.; Farrugia, B.L. Development and use of biomaterials as wound healing therapies. Burns Trauma 2019, 7, s41038-018. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Yildirimer, L.; Thanh, N.T.K.; Seifalian, A.M. Skin regeneration scaffolds: A multimodal bottom-up approach. Trends Biotechnol. 2012, 30, 638–648. [Google Scholar] [CrossRef]
- Lambrechts, S.A.G.; Demidova, T.N.; Aalders, M.C.G.; Hasan, T.; Hamblin, M.R. Photodynamic therapy for Staphylococcus aureus infected burn wounds in mice. Photochem. Photobiol. Sci. 2005, 4, 503–509. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.; Tomé, J.P. Photodynamic inactivation of bacteria: Finding the effective targets. Future Med. Chem. 2015, 7, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Tome, J.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, M.; Rocha, S.; Cunha, Â.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Effect of Photodynamic Therapy on the Virulence Factors of Staphylococcus aureus. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- de Vasconcelos Catão, M.H.C.; Nonaka, C.F.W.; de Albuquerque, R.L.C.; Bento, P.M.; de Oliveira Costa, R. Effects of red laser, infrared, photodynamic therapy, and green LED on the healing process of third-degree burns: Clinical and histological study in rats. Lasers Med. Sci. 2015, 30, 421–428. [Google Scholar] [CrossRef]
- Silva, J.C.E.; Lacava, Z.G.M.; Kuckelhaus, S.; Silva, L.P.; Neto, L.F.M.; Sauro, E.E.; Tedesco, A.C. Evaluation of the use of low level laser and photosensitizer drugs in healing. Lasers Surg. Med. 2004, 34, 451–457. [Google Scholar] [CrossRef]
- Jang, Y.H.; Koo, G.-B.; Kim, J.-Y.; Kim, Y.-S.; Kim, Y.C. Prolonged Activation of ERK Contributes to the Photorejuvenation Effect in Photodynamic Therapy in Human Dermal Fibroblasts. J. Investig. Dermatol. 2013, 133, 2265–2275. [Google Scholar] [CrossRef]
- Nelson, K.K.; Melendez, J.A. Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 2004, 37, 768–784. [Google Scholar] [CrossRef]
- Rosique, R.G.; Rosique, M.J.; Farina Junior, J.A. Curbing Inflammation in Skin Wound Healing: A Review. Int. J. Inflamm. 2015, 2015, 316235. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Medical Applications of Rose Bengal- and Riboflavin-Photosensitized Protein Crosslinking. Photochem. Photobiol. 2019, 95, 1097–1115. [Google Scholar] [CrossRef]
- Tsao, S.; Yao, M.; Tsao, H.; Henry, F.P.; Zhao, Y.; Kochevar, J.J.; Redmond, R.W.; Kochevar, I.E. Light-activated tissue bonding for excisional wound closure: A split-lesion clinical trial. Br. J. Dermatol. 2012, 166, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Jaszczyszyn, A.; Gąsiorowski, K.; Świątek, P.; Malinka, W.; Cieślik-Boczula, K.; Petrus, J.; Czarnik-Matusewicz, B. Chemical structure of phenothiazines and their biological activity. Pharmacol. Rep. 2012, 64, 16–23. [Google Scholar] [CrossRef]
- Wilson, B.; Fernández, M.-J.; Lorente, A.; Grant, K.B. Synthesis and DNA interactions of a bis-phenothiazinium photosensitizer. Org. Biomol. Chem. 2008, 6, 4026–4035. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Mohr, H.; Walker, W.H. Phenothiazinium derivatives for pathogen inactivation in blood products. J. Photochem. Photobiol. B Biol. 2007, 86, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Byrne, M.N.; Gattrell, M.A. Phenothiazinium-based photobactericidal materials. J. Photochem. Photobiol. B Biol. 2006, 84, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Parekh, S.G.; Trauner, K.B.; Zarins, B.; Foster, T.E.; Anderson, R.R. Photodynamic modulation of wound healing with BPD-MA and CASP. Lasers Surg. Med. 1999, 24, 375–381. [Google Scholar] [CrossRef]
- Jefferis, A.F.; Chevretton, E.B.; Berenbaum, M.C. Muscle Damage and Recovery in the Rabbit Tongue Following Photodynamic Therapy with Haematoporphyrin Derivative. Acta Oto-Laryngol. 1991, 111, 153–160. [Google Scholar] [CrossRef]
- Stern, S.J.; Flock, S.T.; Small, S.; Thomsen, S.; Jacques, S. Photodynamic therapy with chloroaluminum sulfonated phthalocyanine in the rat window chamber. Am. J. Surg. 1990, 160, 360–364. [Google Scholar] [CrossRef]
- Freire, F.; Ferraresi, C.; Jorge, A.O.C.; Hamblin, M.R. Photodynamic therapy of oral Candida infection in a mouse model. J. Photochem. Photobiol. B Biol. 2016, 159, 161–168. [Google Scholar] [CrossRef]
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblin, M.R. Bacterial Photodynamic Inactivation Mediated by Methylene Blue and Red Light Is Enhanced by Synergistic Effect of Potassium Iodide. Antimicrob. Agents Chemother. 2015, 59, 5203–5212. [Google Scholar] [CrossRef]
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight into the Potentiation Effect of Potassium Iodide on aPDT Efficacy. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.G.; de Lima, M.A.; Okamoto, T.; Milanezi, L.A.; Júnior, E.C.G.; Fernandes, L.A.; de Almeida, J.M.; Theodoro, L.H. Effect of photodynamic therapy on the healing of cutaneous third-degree-burn: Histological study in rats. Lasers Med. Sci. 2010, 25, 221–228. [Google Scholar] [CrossRef] [PubMed]
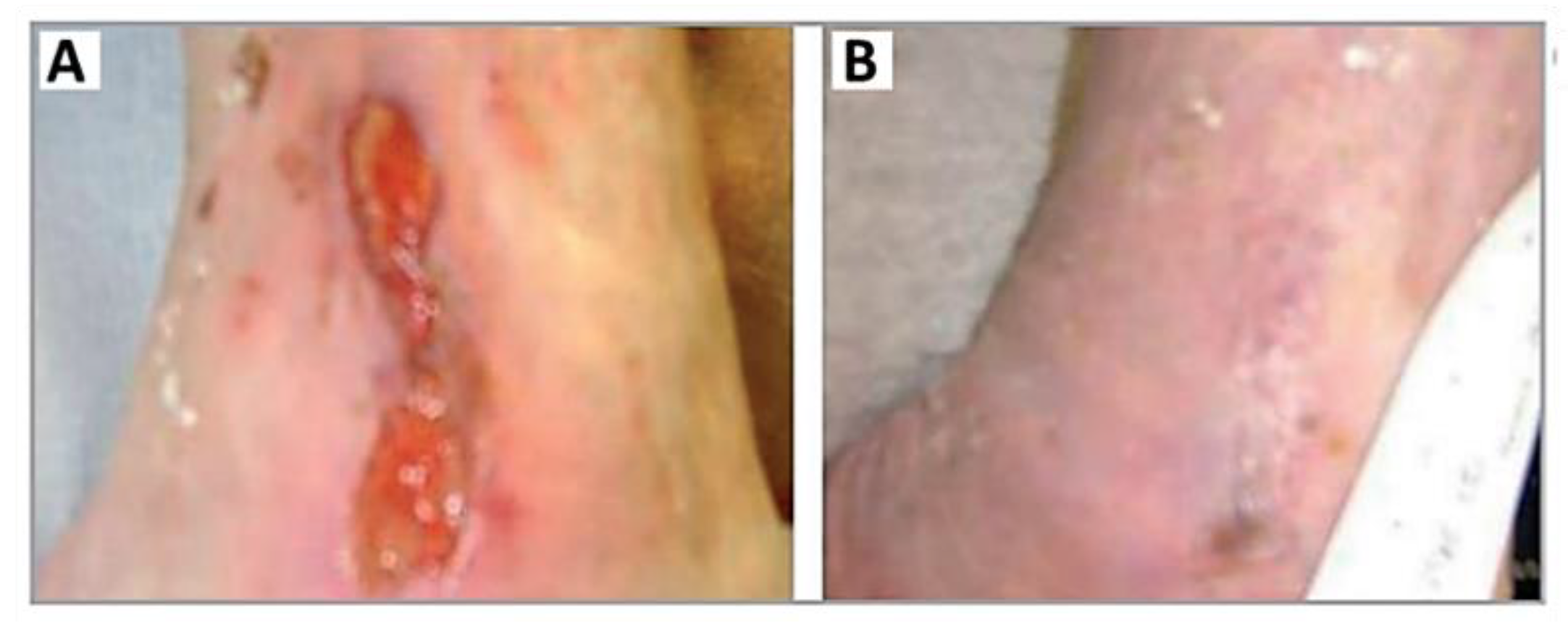
- Morley, S.; Griffiths, J.; Philips, G.; Moseley, H.; O’Grady, C.; Mellish, K.; Lankester, C.L.; Faris, B.; Young, R.J.; Brown, S.B.; et al. Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: A new approach to antimicrobial therapy. Br. J. Dermatol. 2013, 168, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-J.; Zhu, Y.-Q.; Peng, Y.-B.; Chen, Y.-S.; Hu, Y.-P.; Lu, H.-X.; Yu, W.-R.; Fang, Y.; Du, J.-Z.; Yao, M. Enzyme activated photodynamic therapy for methicillin-resistant Staphylococcus aureus infection both in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2014, 136, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Judy, M.; Jackson, R.; Nosir, H.; Matthews, J.L.; Loyd, J.; Lewis, D.; Utecht, R.; Yuan, D. Healing Results in Meniscus and Articular Cartilage Photochemically Welded with 1,8-Naphthalimide Dyes; SPIE: Cardiff, UK, 1997; Volume 2970. [Google Scholar]
- Judy, M.; Jackson, R.; Nosir, H.; Matthews, J.L.; Lewis, D.; Utecht, R.; Yuan, D. Repair of Articular Cartilage and Meniscal Tears by Photoactive Dyes: In-Vivo Study; SPIE: Cardiff, UK, 1996; Volume 2922. [Google Scholar]
- Judy, M.; Nosir, H.; Jackson, R.; Matthews, J.L.; Lewis, D.; Utecht, R.; Yuan, D. Bonding of Human Meniscal and Articular Cartilage with Photoactive 1,8-Naphthalimide Dyes; SPIE: Cardiff, UK, 1996; Volume 2671. [Google Scholar]
- Judy, M.; Matthews, J.L.; Boriak, R.; Burlacu, A.; Lewis, D.; Utecht, R. Heat-Free Photochemical Tissue Welding with 1,8-Naphthalimide Dyes Using Visible (420 nm) Light; SPIE: Cardiff, UK, 1993; Volume 1876. [Google Scholar]
- Judy, M.; Nosir, H.; Jackson, R.; Matthews, J.L.; Utecht, R.; Lewis, D.; Yuan, D. Photochemical Bonding of Skin with 1,8-Naphthalimide Dyes; SPIE: Cardiff, UK, 1998; Volume 3195. [Google Scholar]
- Wong, T.W.; Wu, E.C.; Ko, W.C.; Lee, C.C.; Hor, L.I.; Huang, I.H. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus by indocyanine green and near infrared light. Dermatol. Sin. 2018, 36, 8–15. [Google Scholar] [CrossRef]
- Gharesi, S.; Pourhajibagher, M.; Chiniforush, N.; Raoofian, R.; Hashemi, M.; Shahabi, S.; Bahador, A. Effect of photodynamic therapy based on indocyanine green on expression of apoptosis-related genes in human gingival fibroblast cells. Photodiagnosis Photodyn. Ther. 2017, 19, 33–36. [Google Scholar] [CrossRef]
- Wang, S.; Hüttmann, G.; Rudnitzki, F.; Diddens-Tschoeke, H.; Zhang, Z.; Rahmanzadeh, R. Indocyanine green as effective antibody conjugate for intracellular molecular targeted photodynamic therapy. J. Biomed. Opt. 2016, 21. [Google Scholar] [CrossRef]
- Lim, H.-J.; Oh, C.-H. Indocyanine green-based photodynamic therapy with 785 nm light emitting diode for oral squamous cancer cells. Photodiagnosis Photodyn. Ther. 2011, 8, 337–342. [Google Scholar] [CrossRef]
- Shemesh, C.S.; Moshkelani, D.; Zhang, H. Thermosensitive Liposome Formulated Indocyanine Green for Near-Infrared Triggered Photodynamic Therapy: In Vivo Evaluation for Triple-Negative Breast Cancer. Pharm. Res. 2015, 32, 1604–1614. [Google Scholar] [CrossRef]
- Wong, T.-W.; Liao, S.-Z.; Ko, W.-C.; Wu, C.-J.; Wu, S.B.; Chuang, Y.-C.; Huang, I.-H. Indocyanine Green—Mediated Photodynamic Therapy Reduces Methicillin-Resistant Staphylococcus aureus Drug Resistance. J. Clin. Med. 2019, 8, 411. [Google Scholar] [CrossRef]
- Topaloglu, N.; Güney, M.; Yuksel, S.; Gülsoy, M. Antibacterial photodynamic therapy with 808-nm laser and indocyanine green on abrasion wound models. J. Biomed. Opt. 2015, 20, 028003. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.T.; Tran, T.T.V.; Pan, S.C.; Huang, H.K.; Chen, Y.C.; Wong, T.W. Cystic Fibrosis Transmembrane Conductance Regulator: A Possible New Target for Photodynamic Therapy Enhances Wound Healing. Adv. Wound Care 2019, 8, 476–486. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, C.D.; Cortes, D.C.; Lazurko, C.; Amrani, S.; Rosales-Rojas, R.; Zuñiga-Bustos, M.; Sedlakova, V.; Poblete, H.; Stamplecoskie, K.; Suuronen, E.J.; et al. Light-Activated Peptide-Based Materials for Sutureless Wound Closure. ACS Appl. Mater. Interfaces 2019, 11, 45007–45015. [Google Scholar] [CrossRef] [PubMed]
- Kamegaya, Y.; Farinelli, W.A.; Vila Echague, A.V.; Akita, H.; Gallagher, J.; Flotte, T.J.; Anderson, R.R.; Redmond, R.W.; Kochevar, I.E. Evaluation of photochemical tissue bonding for closure of skin incisions and excisions. Lasers Surg. Med. 2005, 37, 264–270. [Google Scholar] [CrossRef]
- Chan, B.P.; Kochevar, I.E.; Redmond, R.W. Enhancement of Porcine Skin Graft Adherence Using a Light-Activated Process. J. Surg. Res. 2002, 108, 77–84. [Google Scholar] [CrossRef]
- Han, S.; Hwang, B.W.; Jeon, E.Y.; Jung, D.; Lee, G.H.; Keum, D.H.; Kim, K.S.; Yun, S.H.; Cha, H.J.; Hahn, S.K. Upconversion Nanoparticles/Hyaluronate–Rose Bengal Conjugate Complex for Noninvasive Photochemical Tissue Bonding. ACS Nano 2017, 11, 9979–9988. [Google Scholar] [CrossRef]
- Pupkaite, J.; Ahumada, M.; McLaughlin, S.; Temkit, M.; Alaziz, S.; Seymour, R.; Ruel, M.; Kochevar, I.; Griffith, M.; Suuronen, E.J.; et al. Collagen-Based Photoactive Agent for Tissue Bonding. ACS Appl. Mater. Interfaces 2017, 9, 9265–9270. [Google Scholar] [CrossRef]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- Jacobson, J.M.; Feinman, L.; Liebes, L.; Ostrow, N.; Koslowski, V.; Tobia, A.; Cabana, B.E.; Lee, D.-H.; Spritzler, J.; Prince, A.M. Pharmacokinetics, Safety, and Antiviral Effects of Hypericin, a Derivative of St. John’s Wort Plant, in Patients with Chronic Hepatitis C Virus Infection. Antimicrob. Agents Chemother. 2001, 45, 517–524. [Google Scholar] [CrossRef]
- Kadam, N.; Chaudhari, H.; Parikh, J.; Modi, V.; Kokil, S.; Balaramnavar, V. De novo Combination Therapy in Retroviral Infection. Int. J. Virol. 2010, 6, 219–223. [Google Scholar] [CrossRef]
- Nafee, N.; Youssef, A.; El-Gowelli, H.; Asem, H.; Kandil, S. Antibiotic-free nanotherapeutics: Hypericin nanoparticles thereof for improved in vitro and in vivo antimicrobial photodynamic therapy and wound healing. Int. J. Pharm. 2013, 454, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J.; et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, G.; Yang, P.; Lv, R.; Gai, S.; Li, C.; He, F.; Lin, J. Assembly of Au Plasmonic Photothermal Agent and Iron Oxide Nanoparticles on Ultrathin Black Phosphorus for Targeted Photothermal and Photodynamic Cancer Therapy. Adv. Funct. Mater. 2017, 27, 1700371. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Li, Z.; Zhu, S.; Zheng, Y.; Yeung, K.W.K.; Wu, S. Repeatable Photodynamic Therapy with Triggered Signaling Pathways of Fibroblast Cell Proliferation and Differentiation to Promote Bacteria-Accompanied Wound Healing. ACS Nano 2018, 12, 1747–1759. [Google Scholar] [CrossRef]
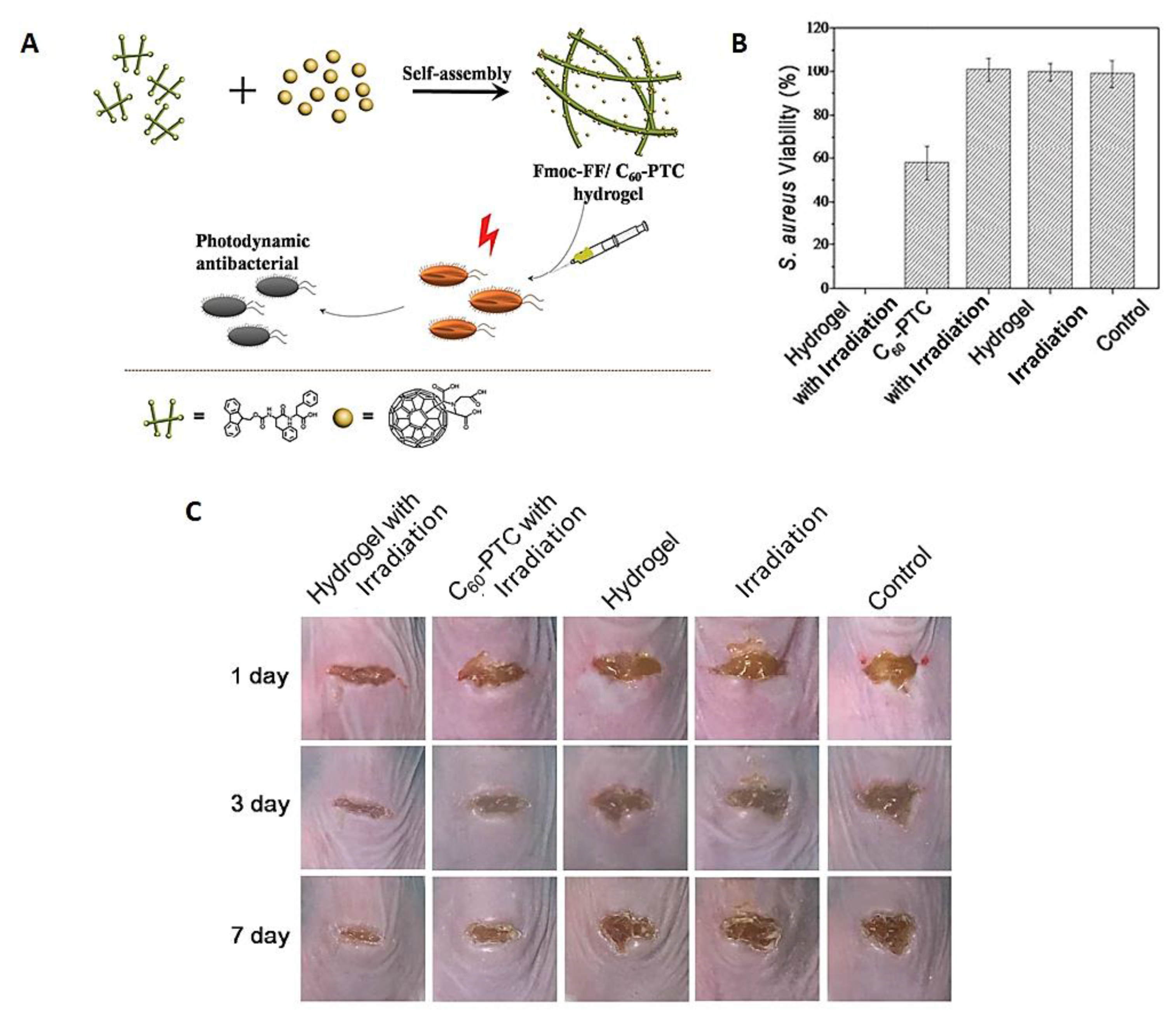
- Zhang, Y.; Zhang, H.; Zou, Q.; Xing, R.; Jiao, T.; Yan, X. An injectable dipeptide–fullerene supramolecular hydrogel for photodynamic antibacterial therapy. J. Mater. Chem. 2018, 6, 7335–7342. [Google Scholar] [CrossRef]
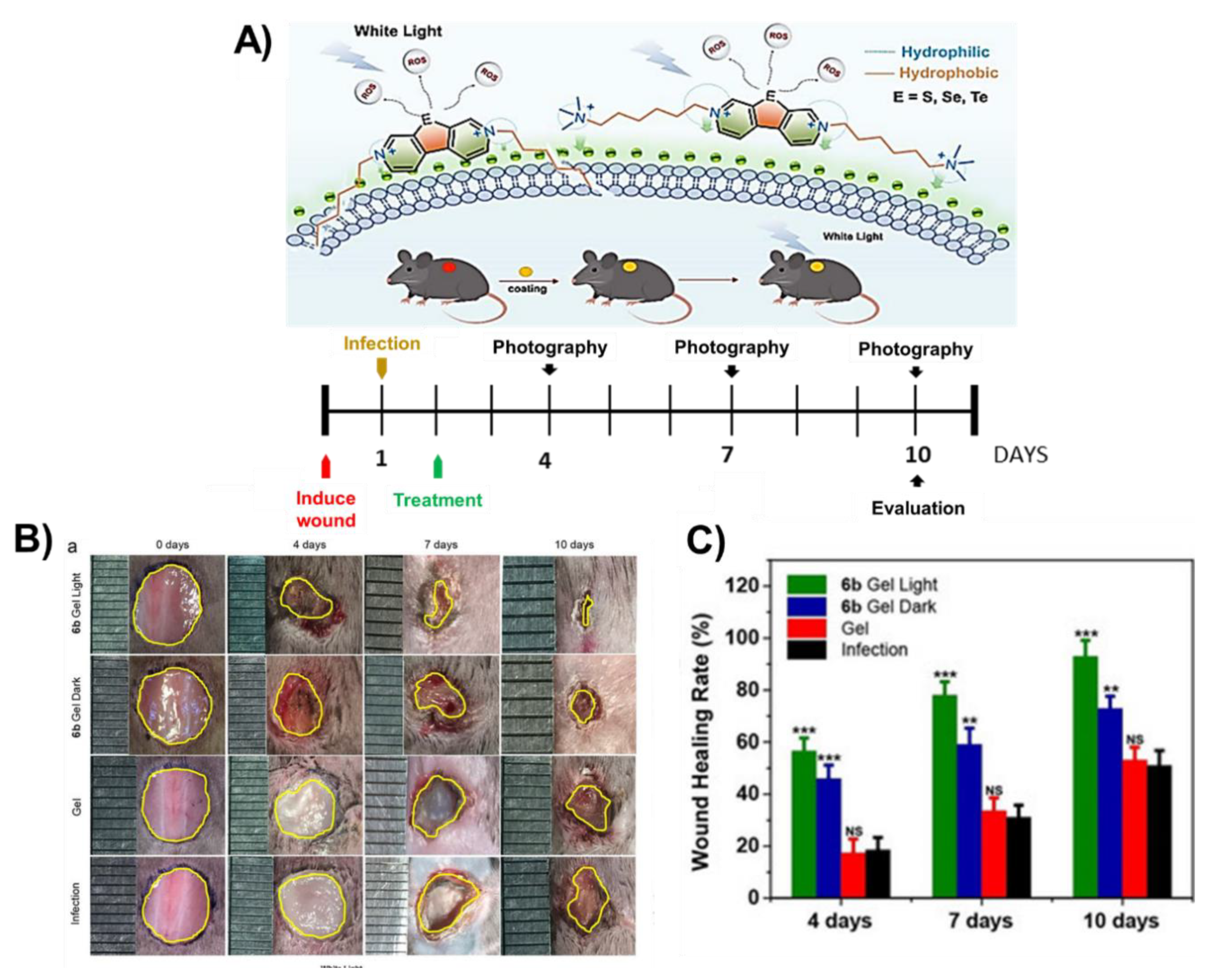
- Zhou, K.; Tian, R.; Li, G.; Qiu, X.; Xu, L.; Guo, M.; Chigan, D.; Zhang, Y.; Chen, X.; He, G. Cationic Chalcogenoviologen Derivatives for Photodynamic Antimicrobial Therapy and Skin Regeneration. Chem. Eur. J. 2019, 25, 13472–13478. [Google Scholar] [CrossRef]
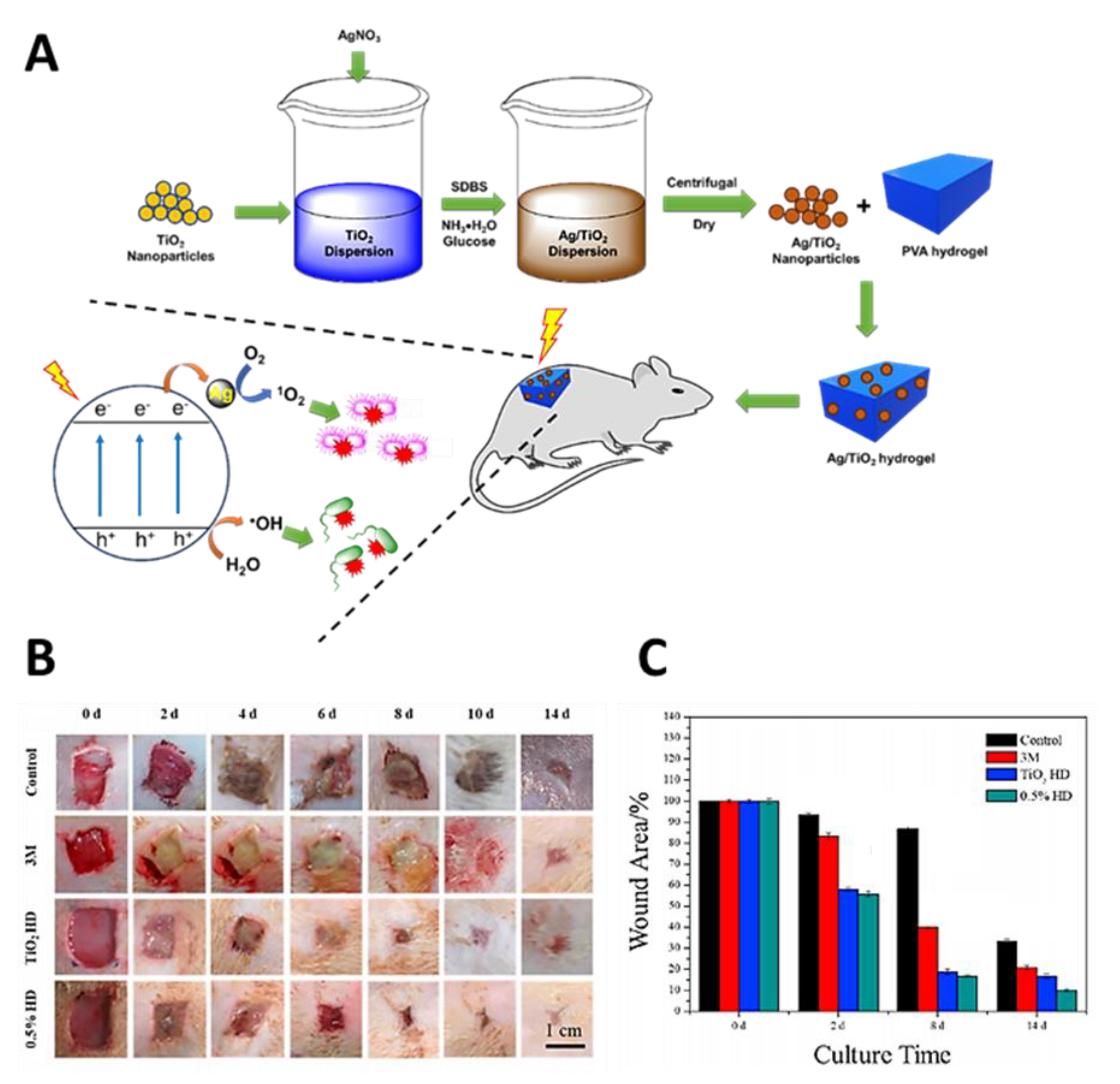
- Wang, J.; Zhang, C.; Yang, Y.; Fan, A.; Chi, R.; Shi, J.; Zhang, X. Poly (vinyl alcohol) (PVA) hydrogel incorporated with Ag/TiO2 for rapid sterilization by photoinspired radical oxygen species and promotion of wound healing. Appl. Surf. Sci. 2019, 494, 708–720. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
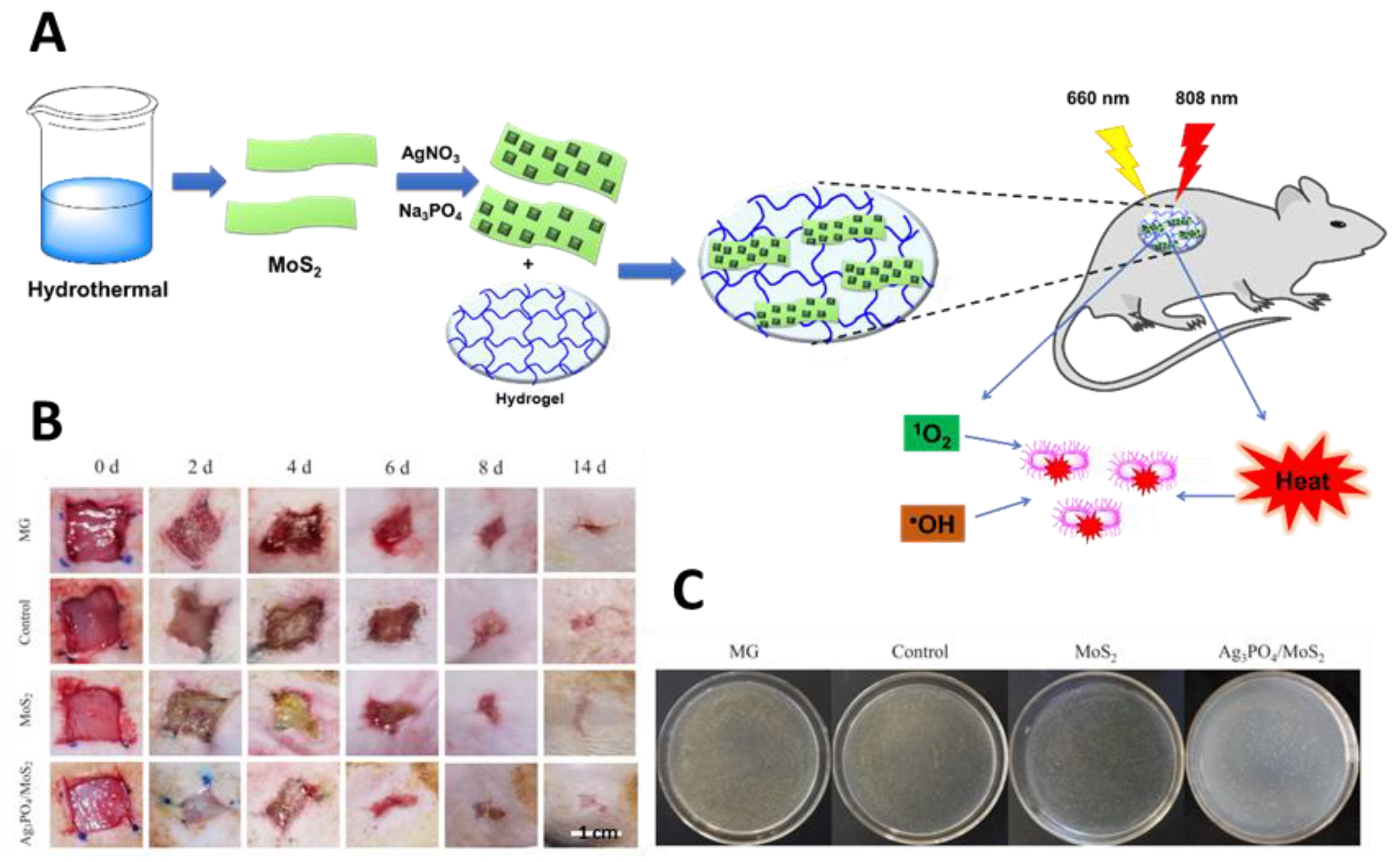
- Zhang, X.; Zhang, C.; Yang, Y.; Zhang, H.; Huang, X.; Hang, R.; Yao, X. Light-assisted rapid sterilization by a hydrogel incorporated with Ag3PO4/MoS2 composites for efficient wound disinfection. Chem. Eng. J. 2019, 374, 596–604. [Google Scholar] [CrossRef]
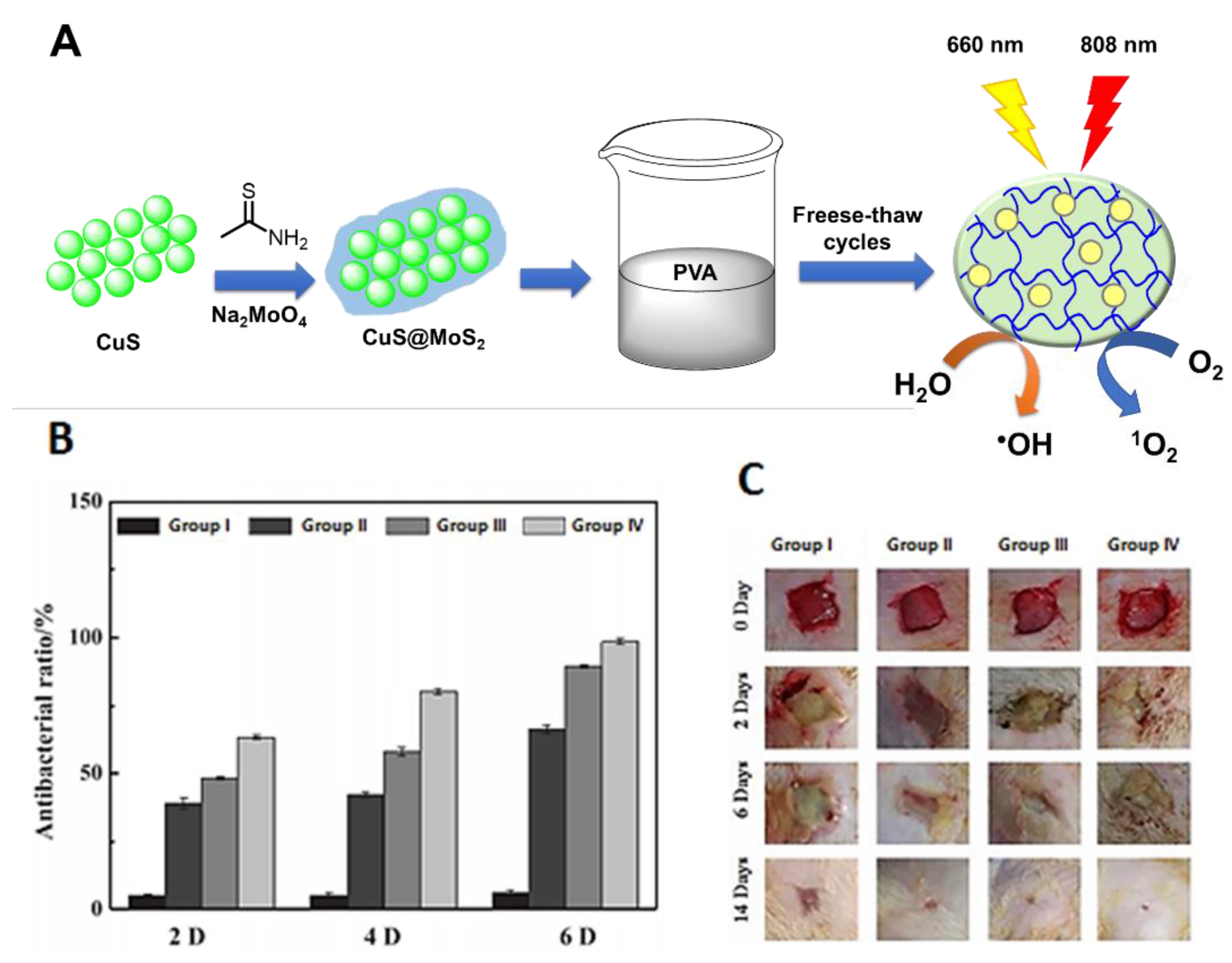
- Zhang, X.; Zhang, G.; Zhang, H.; Liu, X.; Shi, J.; Shi, H.; Yao, X.; Chu, P.K.; Zhang, X. A bifunctional hydrogel incorporated with CuS@MoS2 microspheres for disinfection and improved wound healing. Chem. Eng. J. 2020, 382, 122849. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Li, Z.; Zheng, Y.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Noninvasive rapid bacteria-killing and acceleration of wound healing through photothermal/photodynamic/copper ion synergistic action of a hybrid hydrogel. Biomater. Sci. 2018, 6, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
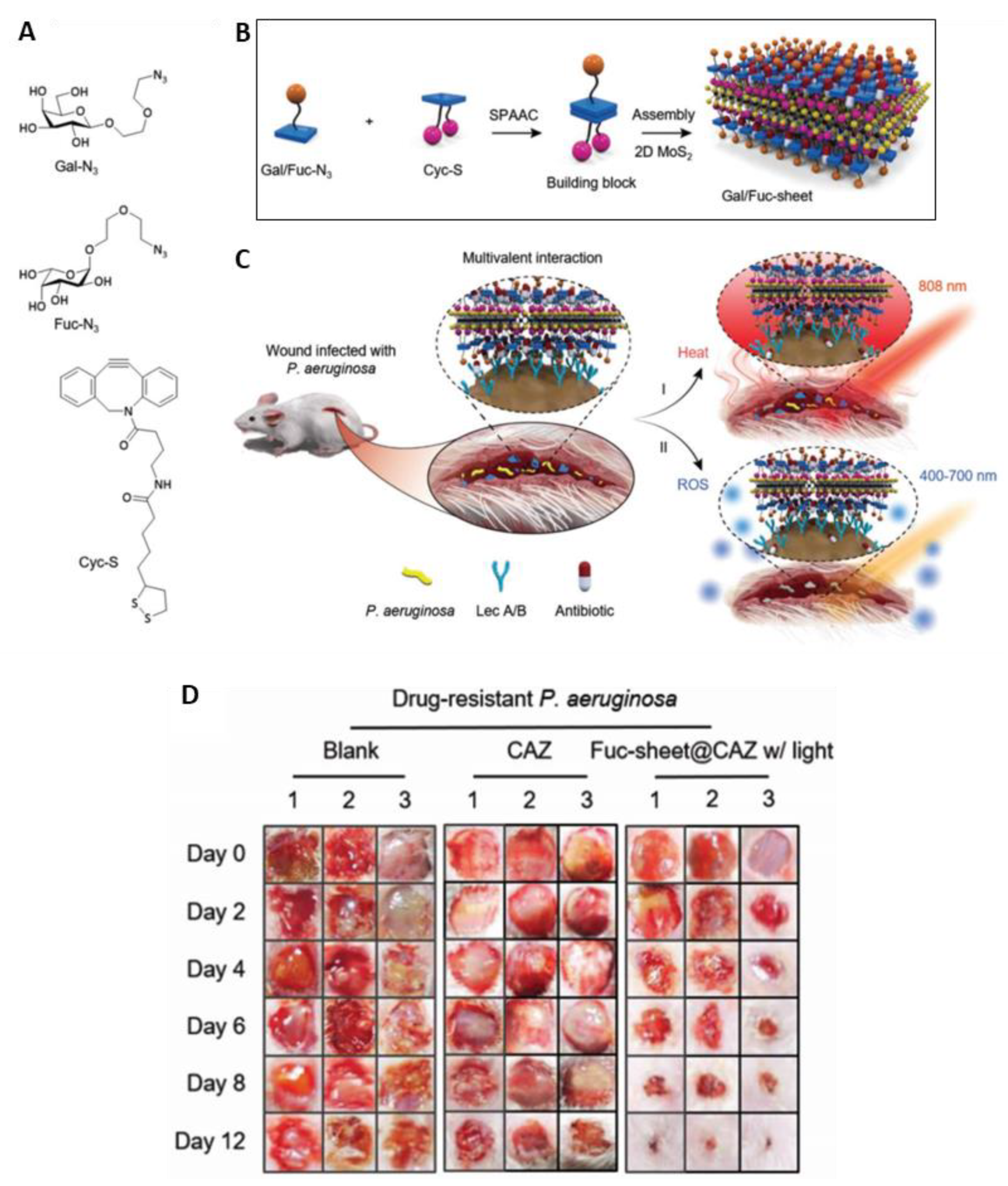
- Hu, X.-L.; Chu, L.; Dong, X.; Chen, G.-R.; Tang, T.; Chen, D.; He, X.-P.; Tian, H. Multivalent Glycosheets for Double Light–Driven Therapy of Multidrug-Resistant Bacteria on Wounds. Adv. Funct. Mater. 2019, 29, 1806986. [Google Scholar] [CrossRef]
- Xiang, Y.; Mao, C.; Liu, X.; Cui, Z.; Jing, D.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; et al. Rapid and Superior Bacteria Killing of Carbon Quantum Dots/ZnO Decorated Injectable Folic Acid-Conjugated PDA Hydrogel through Dual-Light Triggered ROS and Membrane Permeability. Small 2019, 15, 1900322. [Google Scholar] [CrossRef] [PubMed]
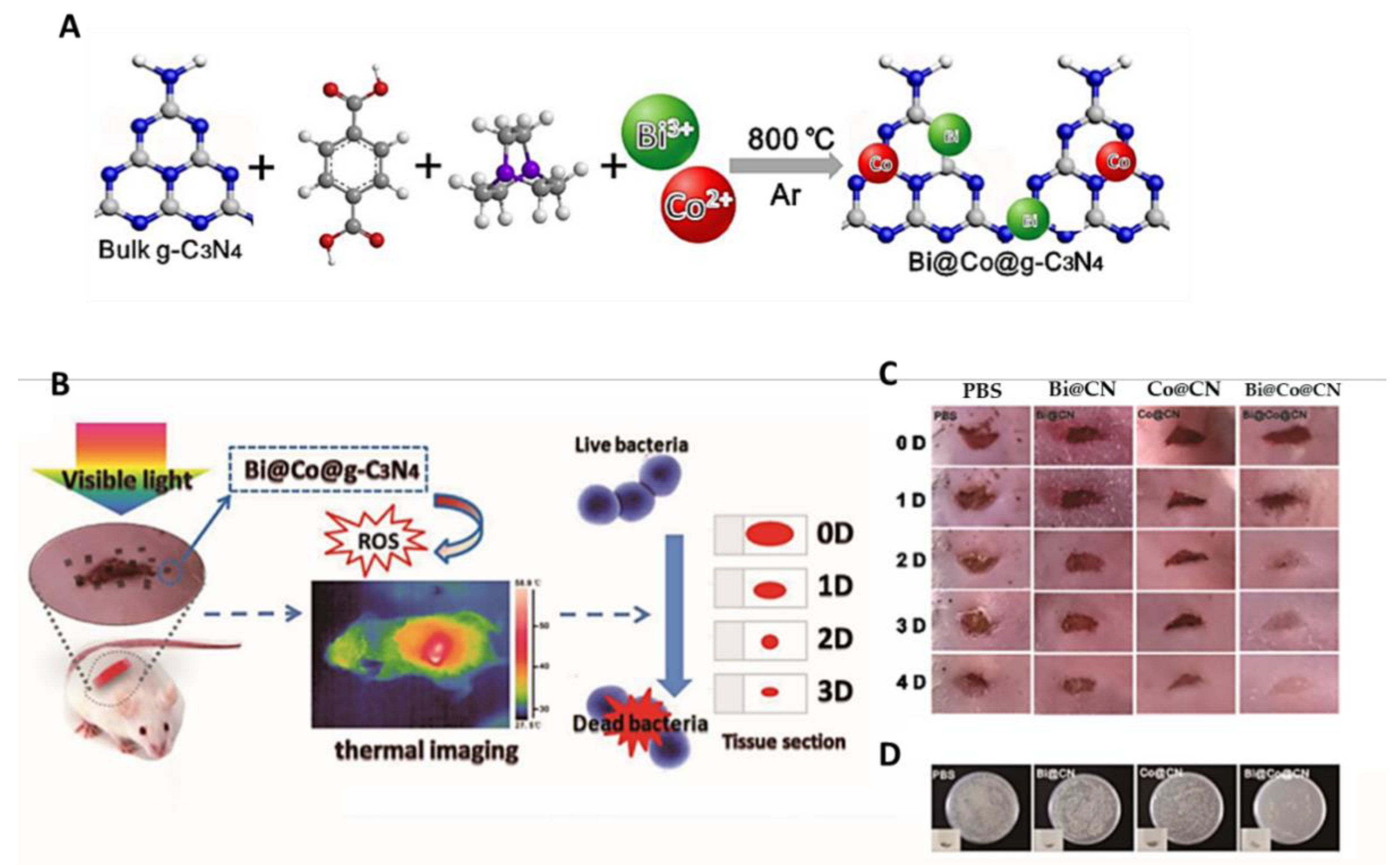
- Wang, R.; Zhang, B.; Liang, Z.; He, Y.; Wang, Z.; Ma, X.; Yao, X.; Sun, J.; Wang, J. Insights into rapid photodynamic inactivation mechanism of Staphylococcus aureus via rational design of multifunctional nitrogen-rich carbon-coated bismuth/cobalt nanoparticles. Appl. Catal. 2019, 241, 167–177. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallejo, M.C.S.; Moura, N.M.M.; Ferreira Faustino, M.A.; Almeida, A.; Gonçalves, I.; Serra, V.V.; Neves, M.G.P.M.S. An Insight into the Role of Non-Porphyrinoid Photosensitizers for Skin Wound Healing. Int. J. Mol. Sci. 2021, 22, 234. https://doi.org/10.3390/ijms22010234
Vallejo MCS, Moura NMM, Ferreira Faustino MA, Almeida A, Gonçalves I, Serra VV, Neves MGPMS. An Insight into the Role of Non-Porphyrinoid Photosensitizers for Skin Wound Healing. International Journal of Molecular Sciences. 2021; 22(1):234. https://doi.org/10.3390/ijms22010234
Chicago/Turabian StyleVallejo, Mariana C. S., Nuno M. M. Moura, Maria Amparo Ferreira Faustino, Adelaide Almeida, Idalina Gonçalves, Vanda V. Serra, and Maria Graça P. M. S. Neves. 2021. "An Insight into the Role of Non-Porphyrinoid Photosensitizers for Skin Wound Healing" International Journal of Molecular Sciences 22, no. 1: 234. https://doi.org/10.3390/ijms22010234
APA StyleVallejo, M. C. S., Moura, N. M. M., Ferreira Faustino, M. A., Almeida, A., Gonçalves, I., Serra, V. V., & Neves, M. G. P. M. S. (2021). An Insight into the Role of Non-Porphyrinoid Photosensitizers for Skin Wound Healing. International Journal of Molecular Sciences, 22(1), 234. https://doi.org/10.3390/ijms22010234











