5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology
Abstract
1. Introduction
2. Databases, Exclusion and Inclusion Criteria
3. Natural Sources of 5-HTP
4. Qualitative and Quantitative Analysis of 5-HTP
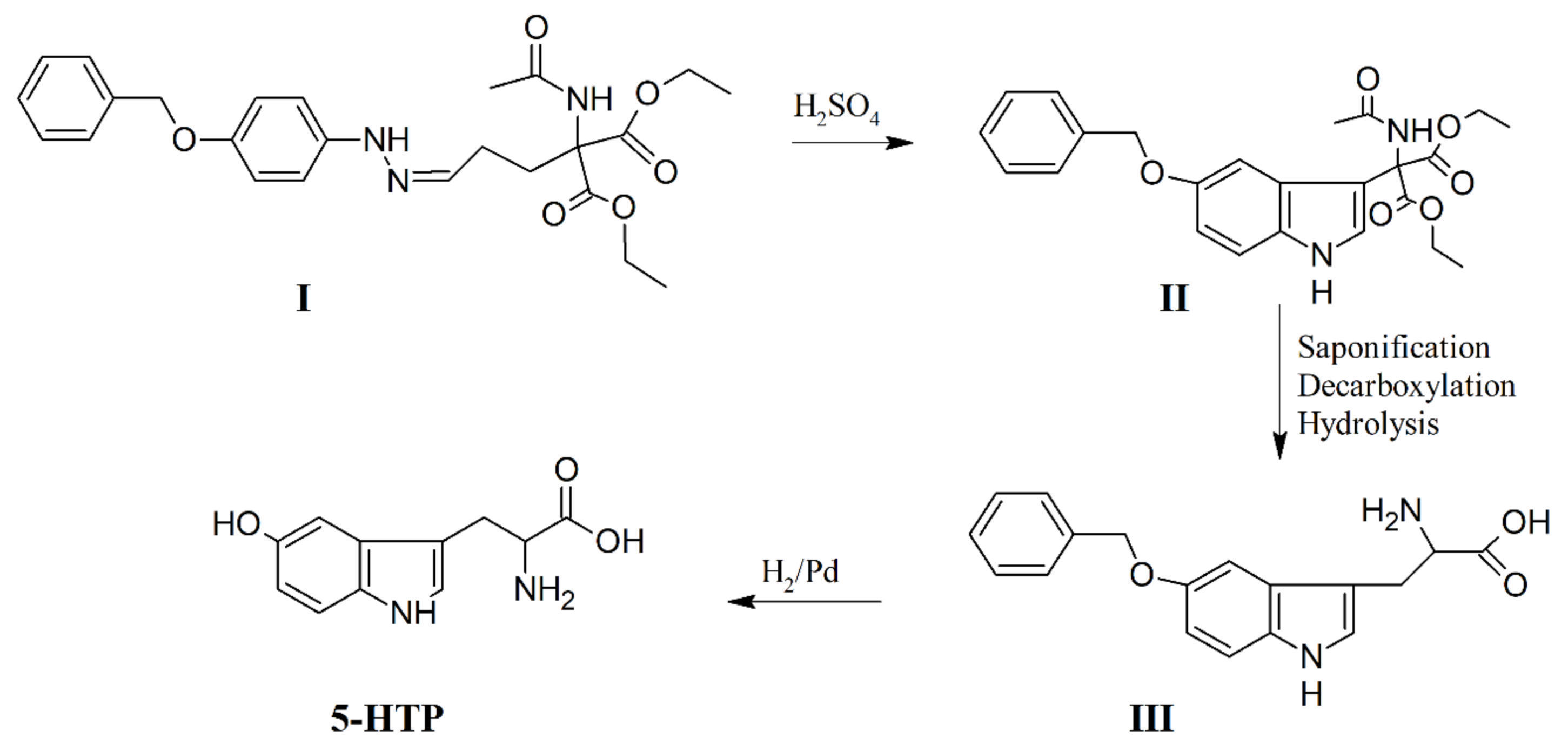
5. Chemical Synthesis of 5-HTP
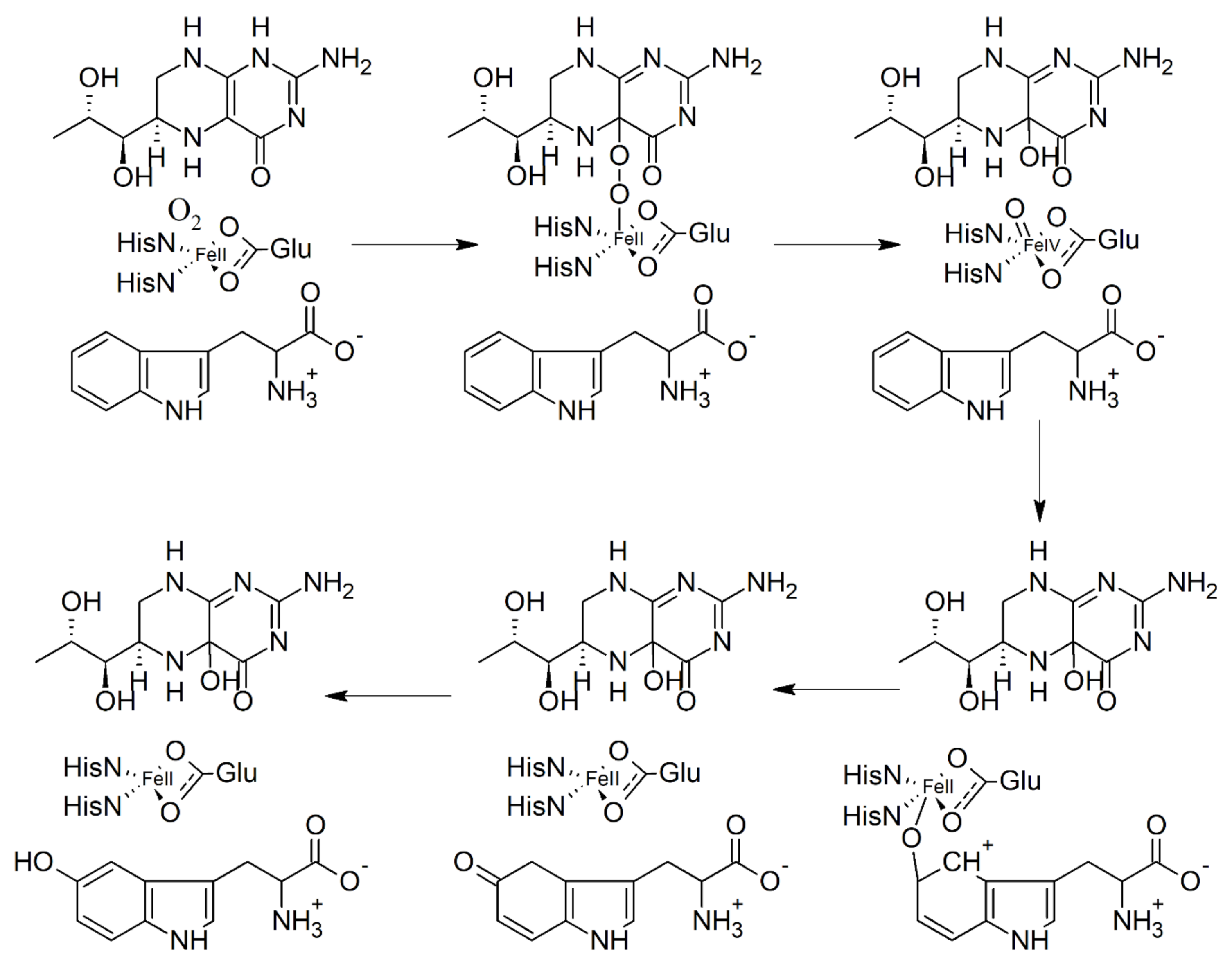
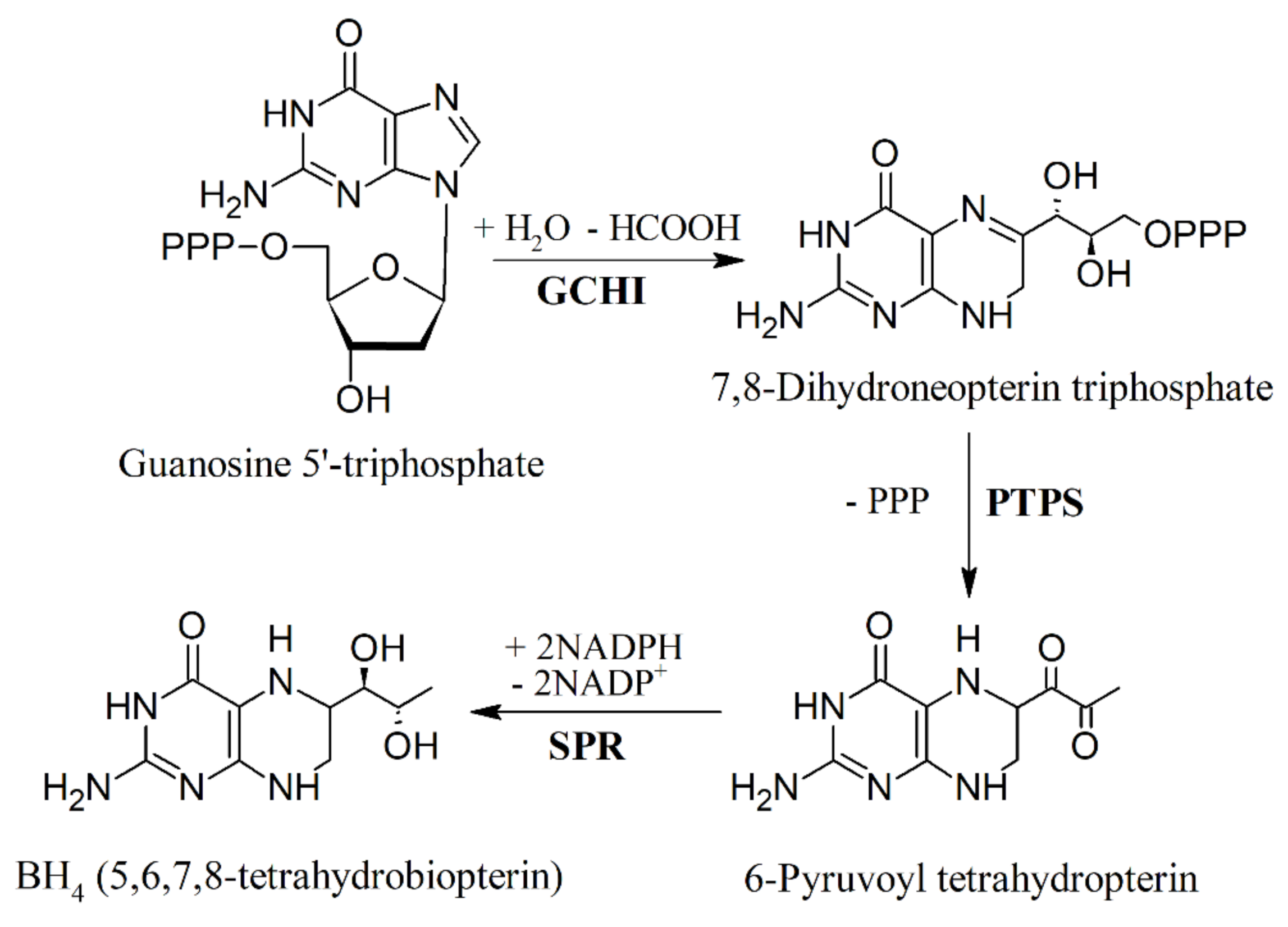
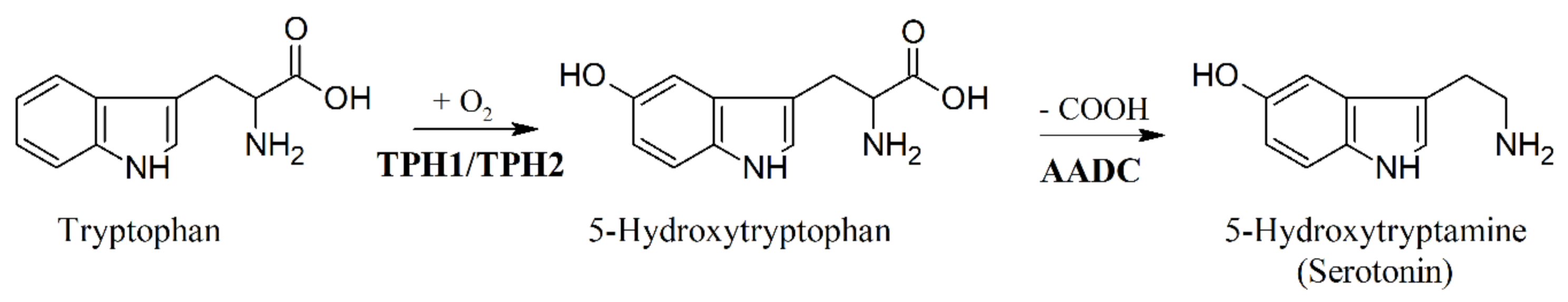
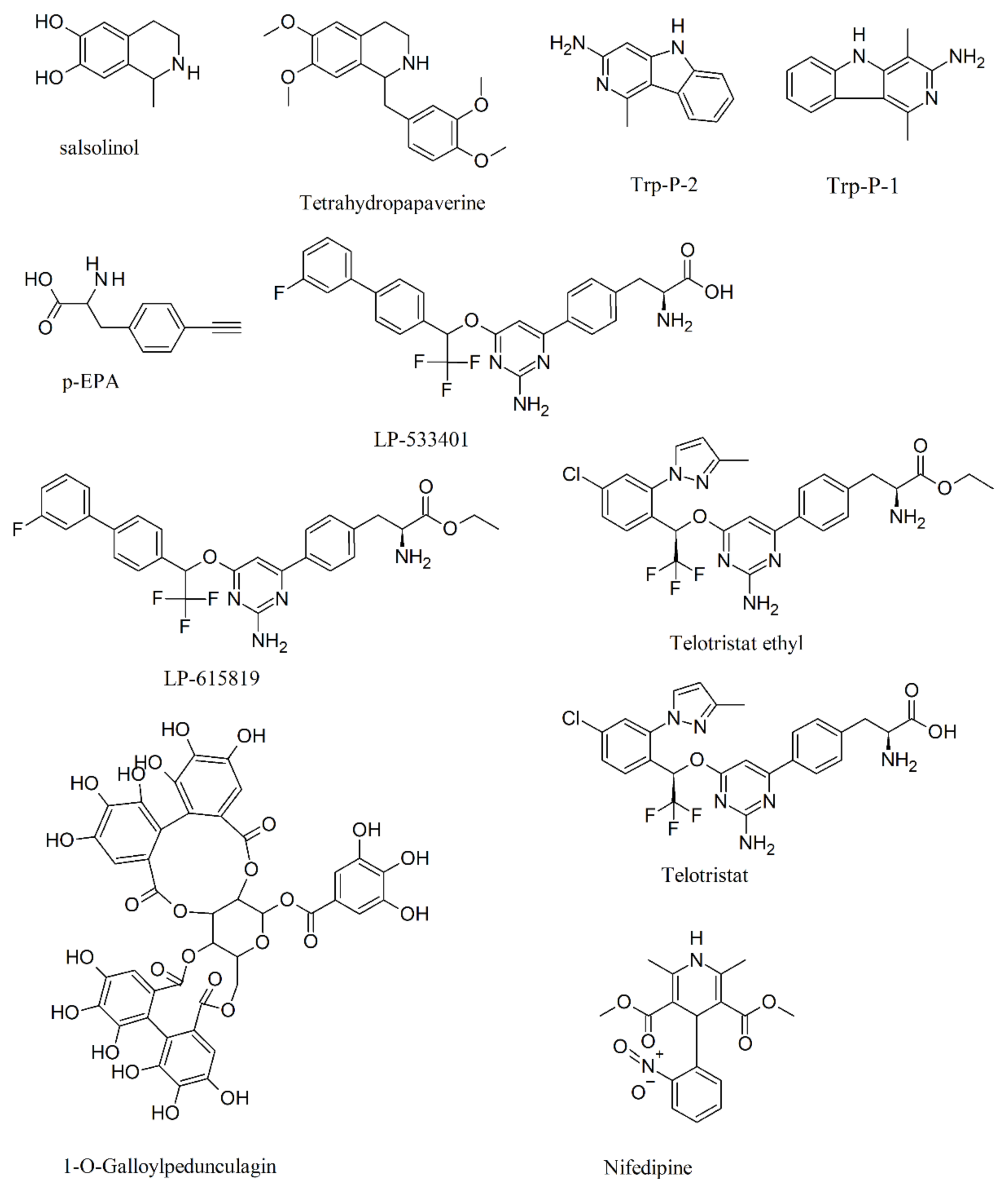
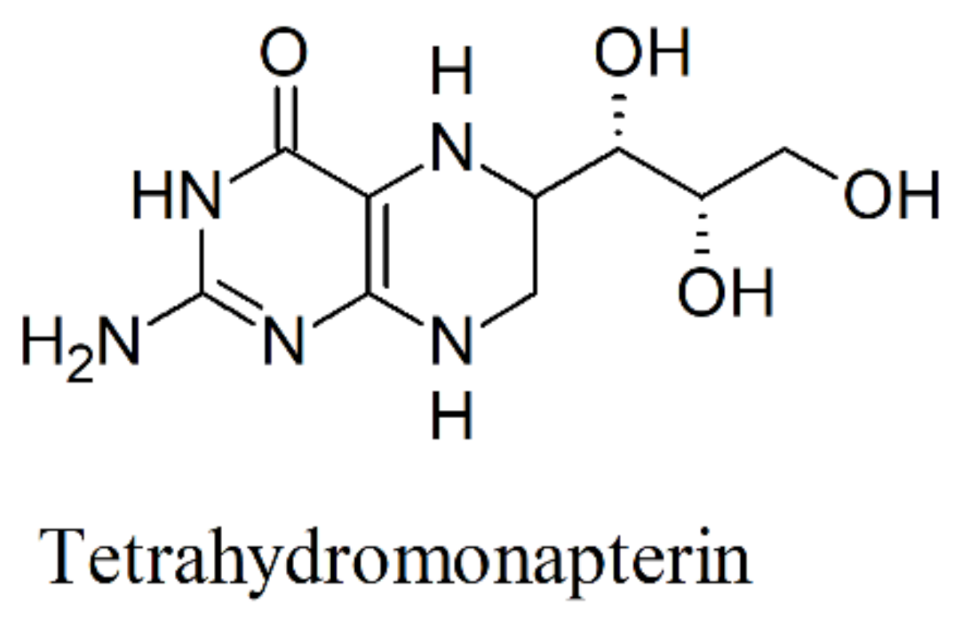
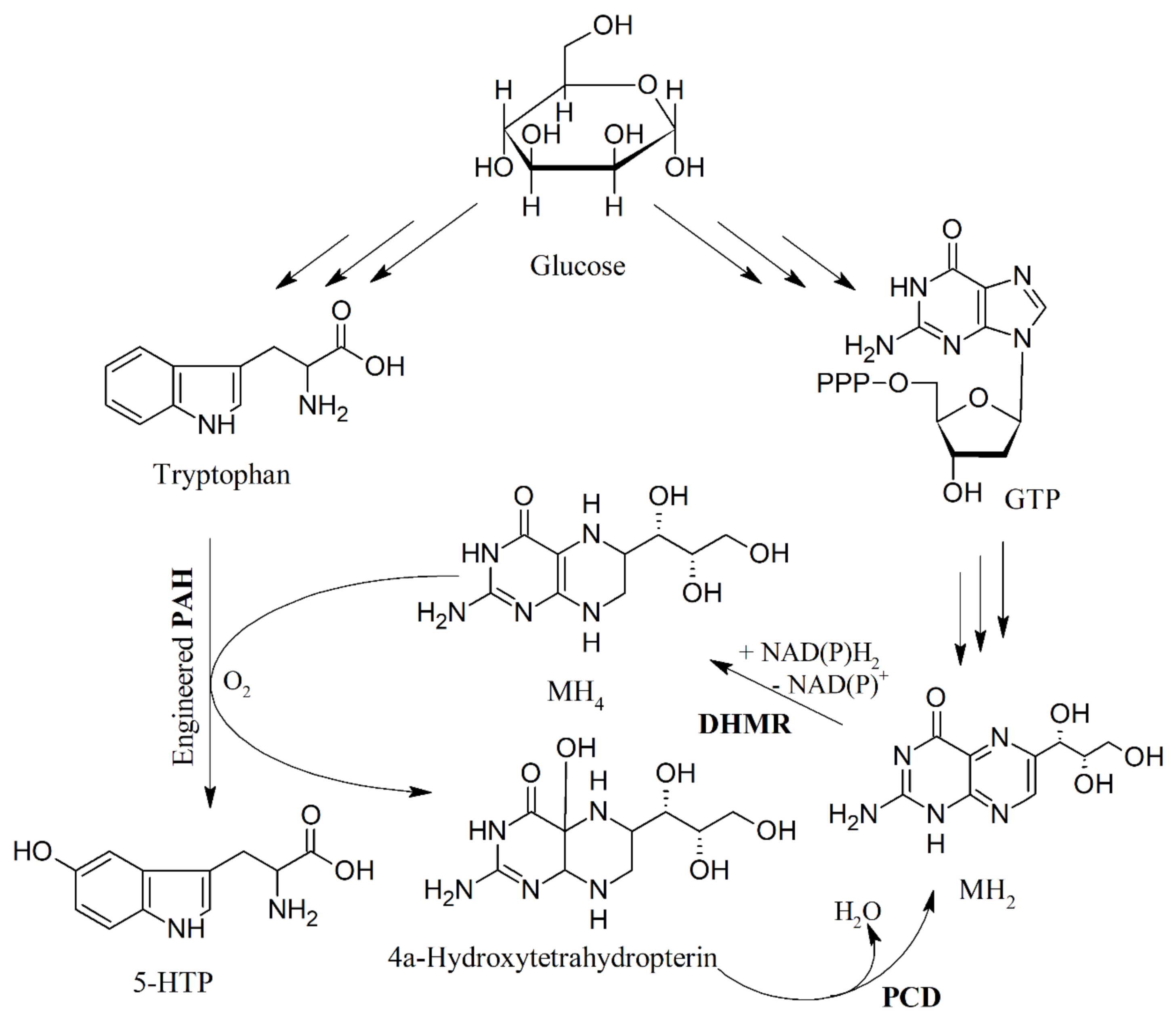
6. Biosynthesis of 5-HTP and Inhibition of Tryptophan Hydroxylase (TPH)
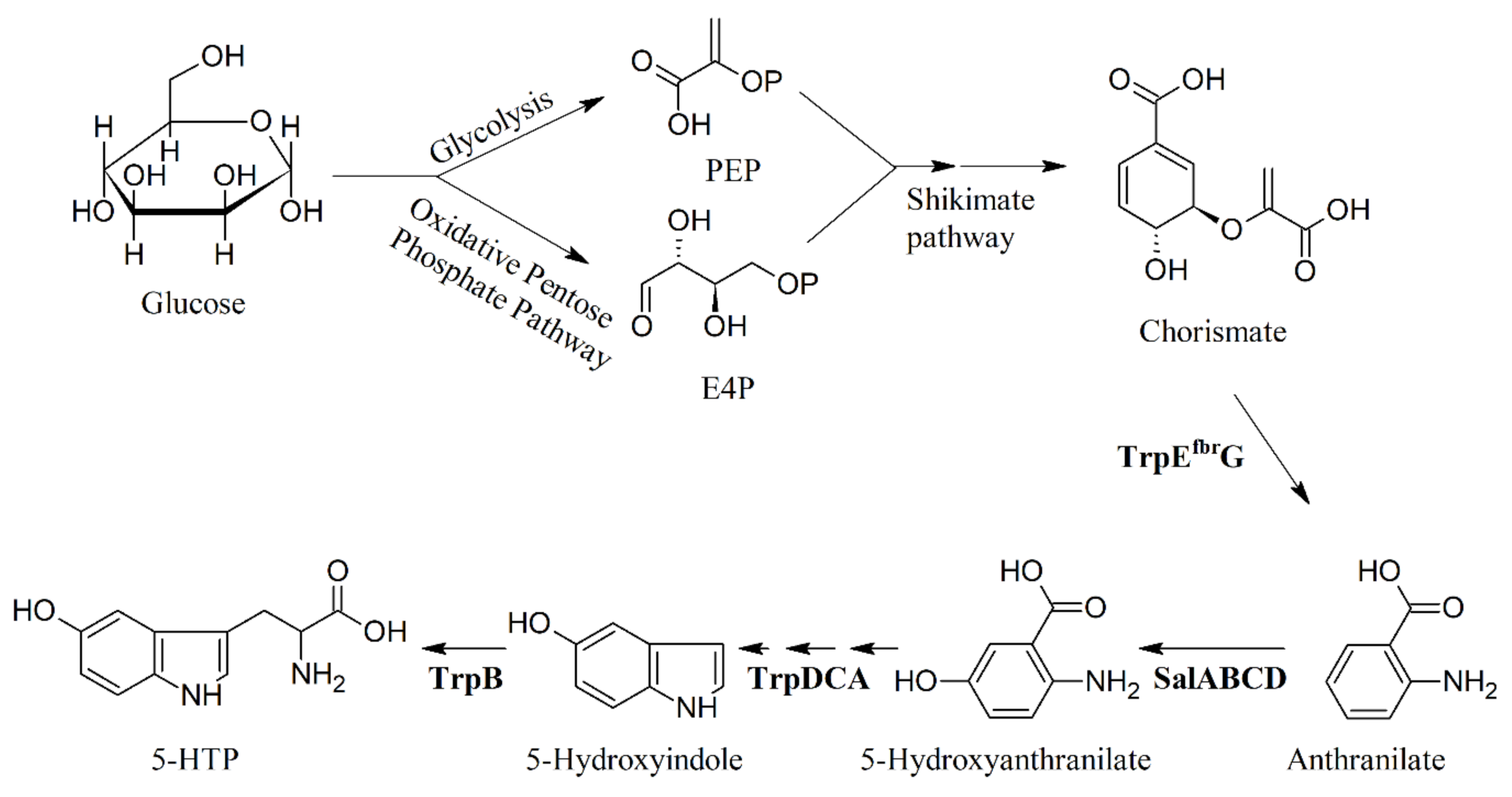
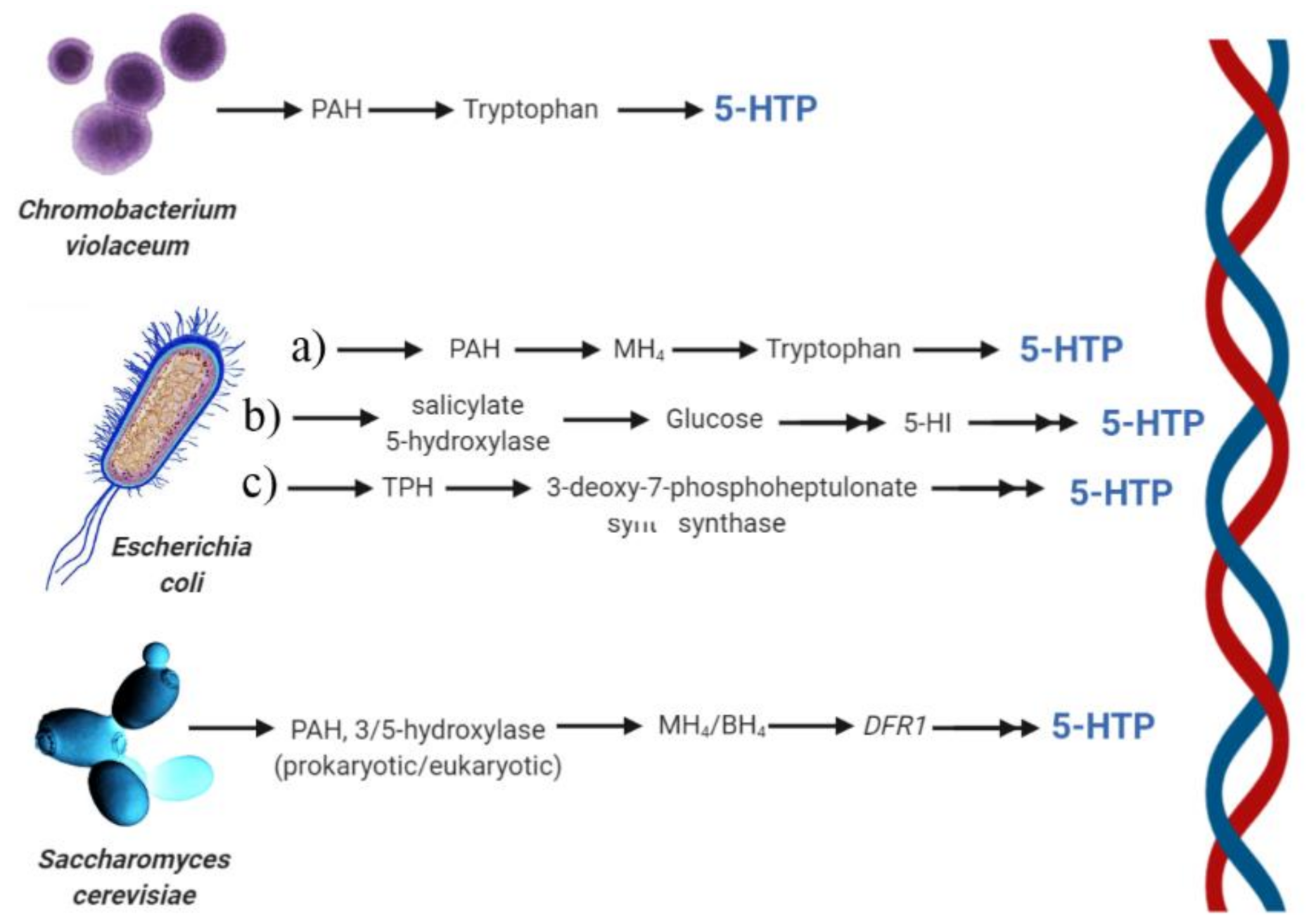
7. Metabolic Engineering and Heterologous Production of 5-HTP
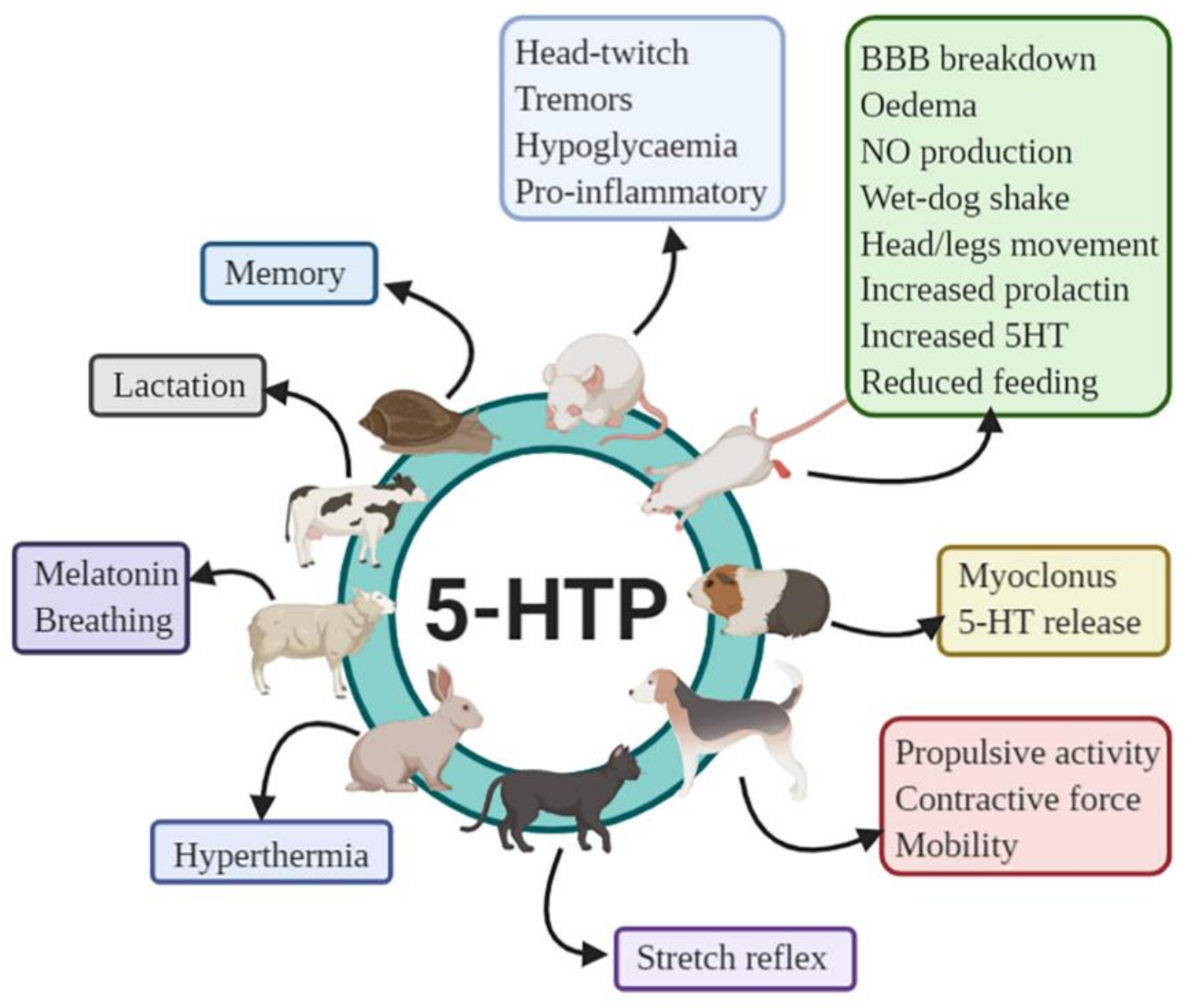
8. Physiological Effects of 5-HTP
8.1. Animal Studies
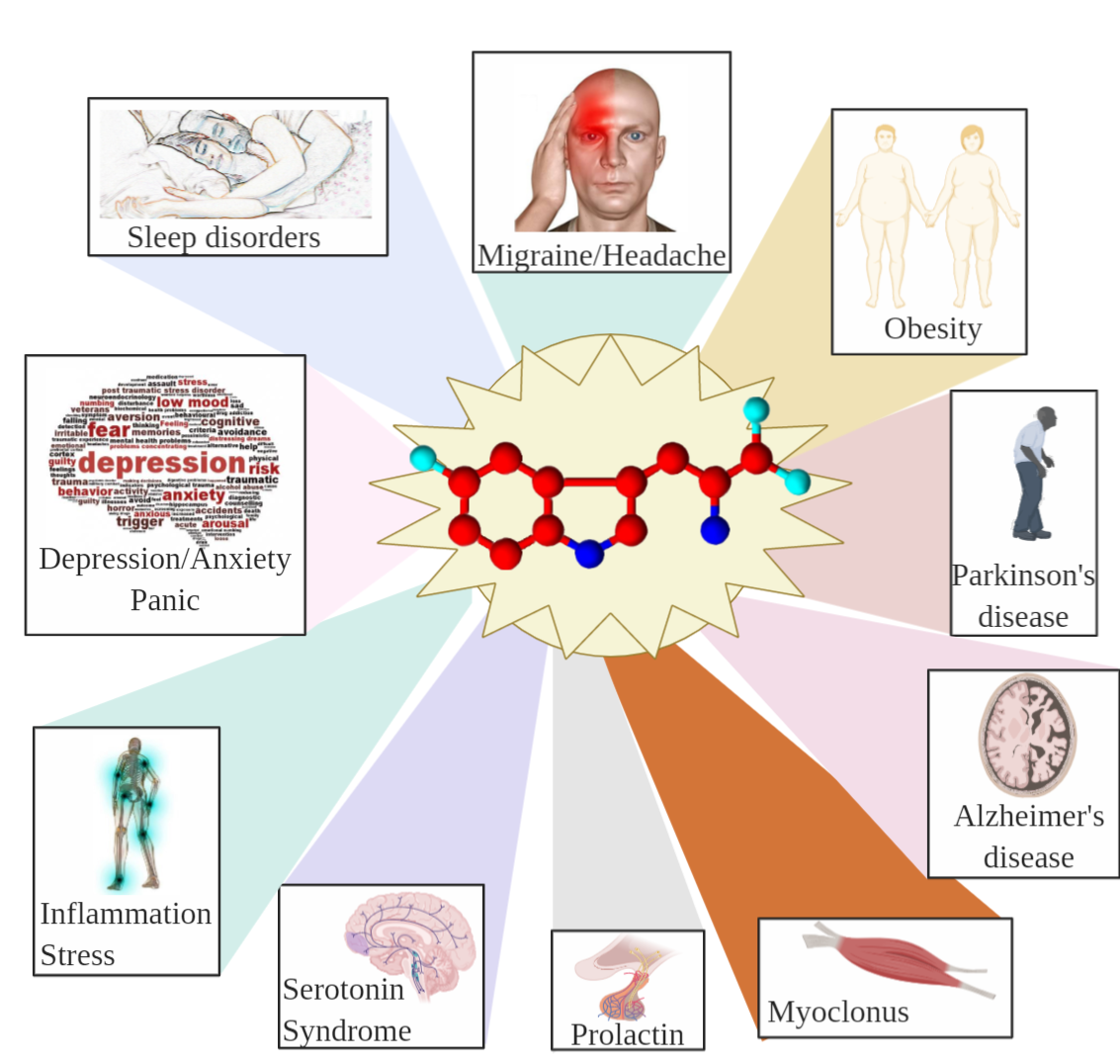
8.2. Effects of 5-HTP on Humans
8.2.1. Serotonin Syndrome
8.2.2. Effect of 5-HTP on Depression, Anxiety, Dystonia, and Panic Disorders
8.2.3. Effect of 5-HTP on Sleep Disorders
8.2.4. Effects of 5-HTP on Migraine, Ataxia, Fibromyalgia, Alzheimer’s, and Parkinson’s Disease
8.2.5. Effect of 5-HTP on Myoclonus
8.2.6. Effect of 5-HTP on Obesity
8.2.7. Effect of 5-HTP on Prolactin
8.2.8. Antioxidant, Anti-inflammatory, and Analgesic Effects of 5-HTP
9. Toxicology of 5-HTP
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4a-HOPH3 | 4a-Hydroxypterin |
| 5-HI | 5-Hydroxyanthranilate |
| 5-HT | 5-Hydroxytryptamine, Serotonin |
| 5-HTP | L-5-Hydroxytryptophan |
| AADC | Aromatic Amino Acid Decarboxylase |
| BH3OH | Pterin-4α-carbinolamine |
| BH4 | Tetrahydrobiopterin |
| BMI | Body Mass Index |
| CE | Capillary Electrophoresis |
| CNS | Central Nervous System |
| COX-2 | Cyclo oxygenase-2 |
| CREB | cAMP Response Element-Binding Protein |
| CSF | Cerebrospinal Fluid |
| CviPAH | C. violaceum L-phenylalanine 4-hydroxylase |
| DAD | Diode Array Detector |
| DAT | Dementia of the Alzheimer’s type |
| DHMR | Dihydromonapterin Reductase |
| DHPR | Dihydropteridine Reductase |
| E4P | Erythrose-4-phosphate |
| EI | Electrospray Ionization |
| EMS | Eosinophilia–Myalgia Syndrome |
| FD | Fluorescence Detector |
| FM | Fibromyalgia |
| GI | Gastrointestinal |
| HPLC | High-Performance Liquid Chromatography |
| IL-6 | Interleukin-6 |
| LP-533401 | [(2S)-2-amino-3-(4-(2-amino-6-(2,2,2-trifluoro-1-(3′-fluorobiphenyl-4-yl)ethoxy)pyrimidin-4-yl)phenyl)propanoic acid] |
| LP-615819 | [(2S)-ethyl 2-amino-3-(4-(2-amino-6-(2,2,2-trifluoro-1-(3′-fluorobiphenyl-4-yl)ethoxy)pyrimidin-4-yl)phenyl)propanoic acid] |
| LPS | Lipopolysaccharide |
| MAO | Monoamine oxidase |
| MH2 | Dihydromonapterin |
| MH4 | Tetrahydromonapterin |
| MS | Mass Spectrometry |
| PAH | L-Phenylalanine 4-hydroxylase |
| PCD | Pterin-4α-carbinolamine Dehydratase |
| PD | Parkinson’s Disease |
| Peak AAA1 | (S)-2-amino-3-(2((S,E)-7-methylnon-1-en-1-yl)-1H-indol-3-yl) propanoic acid |
| Peak AAA2 | (S)-2-amino-3-(2-((E)-dec-1-en-1-yl)-1H-indol-3-yl) propanoic acid |
| Peak E | [1,1′-Ethylidenebis(L-tryptophan)] |
| Peak X | 4,5-Tryptophan-dione |
| Peak-UV5 | 3-anilinoalanine |
| PEP | Phosphoenol Pyruvate |
| p-EPA | p-Ethynylphenylalanine |
| PKA | Protein Kinase A |
| PTPS | 6-Pyruvate-tetrahydropterin Synthase |
| REM | Rapid Eye Movement |
| RFLP | Restriction Fragment Length Polymorphism |
| ROS | Reactive Oxygen Species |
| SalABCD | Salicylate 5-hydroxylase |
| SCL-90 | 90-Item Symptoms Checklist |
| SIM | Selective Ion Monitoring |
| SNRI | Serotonin–Norepinephrine Reuptake Inhibitor |
| SPR | Sepiapterin Reductase |
| SSRI | Selective Serotonin Reuptake Inhibitor |
| TCM | Traditional Chinese Medicine |
| TDO | Tryptophan 2,3-dioxygenase |
| TPH | Tryptophan Hydroxylase |
| TrpEfbrG | Anthranilate Synthase |
| Trp-P-1 | 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole |
| Trp-P-2 | 3-Amino-1-methyl-5H-pyrido[4,3-b]indole |
References
- Strac, D.S.; Pivac, N.; Muck-Seler, D. The serotonergic system and cognitive function. Transl. Neurosci. 2016, 7, 35–49. [Google Scholar]
- Ye, T.T.; Yin, X.M.; Yu, L.; Zheng, S.J.; Cai, W.J.; Wu, Y.; Feng, Y.Q. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-mass spectrometry. J. Pineal Res. 2019, 66, 11. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S. Physiological and pharmacological perspectives of melatonin. Arch. Physiol. Biochem. 2020, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Bader, M. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 2003, 66, 1673–1680. [Google Scholar] [CrossRef]
- Das, Y.T.; Bagchi, M.; Bagchi, D.; Preuss, H.G. Safety of 5-hydroxy-l-tryptophan. Toxicol. Lett. 2004, 150, 111–122. [Google Scholar] [CrossRef]
- Klarskov, K.; Gagnon, H.; Boudreault, P.L.; Normandin, C.; Plancq, B.; Marsault, E.; Gleich, G.J.; Naylor, S. Structure determination of disease associated peak aaa from l-tryptophan implicated in the eosinophilia-myalgia syndrome. Toxicol. Lett. 2018, 282, 71–80. [Google Scholar] [CrossRef]
- Klarskov, K.; Johnson, K.L.; Benson, L.M.; Cragun, J.D.; Gleich, G.J.; Wrona, M.; Jiang, X.R.; Dryhurst, G.; Naylor, S. Structural characterization of a case-implicated contaminant, “peak x,” in commercial preparations of 5-hydroxytryptophan. J. Rheumatol. 2003, 30, 89–95. [Google Scholar]
- Klarskov, K.; Johnson, K.L.; Benson, L.M.; Gleich, G.J.; Naylor, S. Eosinophilia-myalgia syndrome case-associated contaminants in commercially available 5-hydroxytryptophan. In Tryptophan, Serotonin and Melatonin: Basic Aspects and Applications; Huether, G., Kochen, W., Simat, T.J., Steinhart, H., Eds.; Springer: New York, NY, USA, 1999; pp. 461–468. [Google Scholar]
- Esposito, M.; Precenzano, F.; Sorrentino, M.; Avolio, D.; Carotenuto, M. A medical food formulation of griffonia simplicifolia/magnesium for childhood periodic syndrome therapy: An open-label study on motion sickness. J. Med. Food 2015, 18, 916–920. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Faliva, M.; Bucci, M.; Perna, S. Relationship between the absorption of 5-hydroxytryptophan from an integrated diet, by means of griffonia simplicifolia extract, and the effect on satiety in overweight females after oral spray administration. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2012, 17, E22–E28. [Google Scholar]
- Carnevale, G.; Di Viesti, V.; Zavatti, M.; Benelli, A.; Zanoli, P. Influence of griffonia simplicifolia on male sexual behavior in rats: Behavioral and neurochemical study. Phytomedicine 2011, 18, 947–952. [Google Scholar] [CrossRef]
- Carnevale, G.; Di Viesti, V.; Zavatti, M.; Zanoli, P. Anxiolytic-like effect of griffonia simplicifolia baill. Seed extract in rats. Phytomedicine 2011, 18, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.K.; Ramakrishna, T.; Subbaraju, G.V. Hplc estimation of 5-hydroxytryptophan in griffonia simplicifolia extracts. Asian J. Chem. 2005, 17, 506–510. [Google Scholar]
- Lemaire, P.A.; Adosraku, R.K. An hplc method for the direct assay of the serotonin precursor, 5-hydroxytrophan, in seeds of griffonia simplicifolia. Phytochem. Anal. 2002, 13, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Vigliante, I.; Mannino, G.; Maffei, M.E. Chemical characterization and DNA fingerprinting of griffonia simplicifolia baill. Molecules 2019, 24, 1032. [Google Scholar] [CrossRef] [PubMed]
- Glinwood, R.; Pettersson, J.; Ahmed, E.; Ninkovic, V.; Birkett, M.; Pickett, J. Change in acceptability of barley plants to aphids after exposure to allelochemicals from couch-grass (elytrigia repens). J. Chem. Ecol. 2003, 29, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Hagin, R.D. Isolation and identification of 5-hydroxyindole-3-acetic acid and 5-hydroxytryptophan, major allelopathic aglycons in quackgrass (agropyron-repens l beauv). J. Agric. Food Chem. 1989, 37, 1143–1149. [Google Scholar] [CrossRef]
- Murch, S.J.; KrishnaRaj, S.; Saxena, P.K. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated st. John’s wort (hypericum perforatum l. Cv. Anthos) plants. Plant Cell Rep. 2000, 19, 698–704. [Google Scholar] [CrossRef]
- Fernandez-Cruz, E.; Cerezo, A.B.; Cantos-Villar, E.; Troncoso, A.M.; Garcia-Parrilla, M.C. Time course of l-tryptophan metabolites when fermenting natural grape musts: Effect of inoculation treatments and cultivar on the occurrence of melatonin and related indolic compounds. Aust. J. Grape Wine Res. 2019, 25, 92–100. [Google Scholar] [CrossRef]
- Diamante, M.S.; Borges, C.V.; da Silva, M.B.; Minatel, I.O.; Correa, C.R.; Gomez, H.A.G.; Lima, G.P.P. Bioactive amines screening in four genotypes of thermally processed cauliflower. Antioxidants 2019, 8, 311. [Google Scholar] [CrossRef]
- Lysek, N.; Kinscherf, R.; Claus, R.; Lindel, T. L-5-hydroxytryptophan: Antioxidant and anti-apoptotic principle of the intertidal sponge hymeniacidon heliophila. Z. Fur Nat. C J. Biosci. 2003, 58, 568–572. [Google Scholar] [CrossRef]
- Muszynska, B.; Sulkowska-Ziaja, K.; Ekiert, H. Indole compounds in fruiting bodies of some edible basidiomycota species. Food Chem. 2011, 125, 1306–1308. [Google Scholar] [CrossRef]
- Muszynska, B.; Sulkowska-Ziaja, K.; Ekiert, H. Analysis of indole compounds in methanolic extracts from the fruiting bodies of cantharellus cibarius (the chanterelle) and from the mycelium of this species cultured in vitro. J. Food Sci. Technol. Mysore 2013, 50, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, L.S. Indole-derivatives in certain panaeolus species from east europe and siberia. Mycol. Res. 1993, 97, 251–254. [Google Scholar] [CrossRef]
- Koppisetti, G.; Siriki, A.; Sukala, K.; Subbaraju, G.V. Estimation of l-5-hydroxytryptophan in rat serum and griffonia seed extracts by liquid chromatography-mass spectrometry. Anal. Chim. Acta 2005, 549, 129–133. [Google Scholar] [CrossRef]
- Guillen-Casla, V.; Rosales-Conrado, N.; Leon-Gonzalez, M.E.; Perez-Arribas, L.V.; Polo-Diez, L.M. Determination of serotonin and its precursors in chocolate samples by capillary liquid chromatography with mass spectrometry detection. J. Chromatogr. A 2012, 1232, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, I. Effects of carbidopa on the cerebral accumulation of exogenous l-5-hydroxytryptophan in mice. Acta Pharm. Toxicol. (Copenh) 1984, 55, 199–202. [Google Scholar] [CrossRef]
- Boulet, L.; Faure, P.; Flore, P.; Monteremal, J.; Ducros, V. Simultaneous determination of tryptophan and 8 metabolites in human plasma by liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1054, 36–43. [Google Scholar] [CrossRef]
- Konieczna, L.; Roszkowska, A.; Niedzwiecki, M.; Baczek, T. Hydrophilic interaction chromatography combined with dispersive liquid-liquid microextraction as a preconcentration tool for the simultaneous determination of the panel of underivatized neurotransmitters in human urine samples. J. Chromatogr. A 2016, 1431, 111–121. [Google Scholar] [CrossRef]
- Morgan, L.D.; Baker, H.; Yeoman, M.S.; Patel, B.A. Chromatographic assay to study the activity of multiple enzymes involved in the synthesis and metabolism of dopamine and serotonin. Analyst 2012, 137, 1409–1415. [Google Scholar] [CrossRef]
- Fickbohm, D.J.; Lynn-Bullock, C.P.; Spitzer, N.; Caldwell, H.K.; Katz, P.S. Localization and quantification of 5-hydroxytryptophan and serotonin in the central nervous systems of tritonia and aplysia. J. Comp. Neurol. 2001, 437, 91–105. [Google Scholar] [CrossRef]
- Coelho, A.G.; Aguiar, F.P.C.; de Jesus, D.P. A rapid and simple method for determination of 5-hydroxytryptophan in dietary supplements by capillary electrophoresis. J. Braz. Chem. Soc. 2014, 25, 783–787. [Google Scholar] [CrossRef]
- Peterson, Z.D.; Lee, M.L.; Graves, S.W. Determination of serotonin and its precursors in human plasma by capillary electrophoresis-electrospray ionization-time-of-flight mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 810, 101–110. [Google Scholar] [CrossRef]
- Wise, D.D.; Shear, J.B. Quantitation of nicotinamide and serotonin derivatives and detection of flavins in neuronal extracts using capillary electrophoresis with multiphoton-excited fluorescence. J. Chromatogr. A 2006, 1111, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.D.; Song, P.S.; Yan, X.; Jiang, S.X. Separation of biogenic amines by micellar electrokinetic chromatography. Chin. J. Anal. Chem. 2007, 35, 95–98. [Google Scholar]
- Shi, H.M.; Wang, B.; Niu, L.M.; Cao, M.S.; Kang, W.J.; Lian, K.Q.; Zhang, P.P. Trace level determination of 5-hydroxytryptamine and its related indoles in amniotic fluid by gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2017, 143, 176–182. [Google Scholar] [CrossRef]
- Hinterholzer, A.; Stanojlovic, V.; Regl, C.; Huber, C.G.; Cabrele, C.; Schubert, M. Identification and quantification of oxidation products in full-length biotherapeutic antibodies by nmr spectroscopy. Anal. Chem. 2020, 92, 9666–9673. [Google Scholar] [CrossRef]
- Kuo, T.R.; Chen, J.S.; Chiu, Y.C.; Tsai, C.Y.; Hu, C.C.; Chen, C.C. Quantitative analysis of multiple urinary biomarkers of carcinoid tumors through gold-nanoparticle-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chim. Acta 2011, 699, 81–86. [Google Scholar] [CrossRef]
- Bisby, R.H.; Arvanitidis, M.; Botchway, S.W.; Clark, I.P.; Parker, A.W.; Tobin, D. Investigation of multiphoton-induced fluorescence from solutions of 5-hydroxytryptophan. Photochem. Photobiol. Sci. 2003, 2, 157–162. [Google Scholar] [CrossRef][Green Version]
- Tunna, I.J.; Patel, B.A. Analysis of 5-hydroxytryptophan in the presence of excipients from dietary capsules: Comparison between cyclic voltammetry and uv visible spectroscopy. Anal. Methods 2013, 5, 2523–2528. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, G.K.; Hu, Y.F. A sensitive electrochemical method for the determination of 5-hydroxytryptophan in rats’ brain tissue based on a carbon nanosheets-modified electrode. Anal. Methods 2015, 7, 1971–1976. [Google Scholar] [CrossRef]
- Ranganathan, D.; Zamponi, S.; Berrettoni, M.; Mehdi, B.L.; Cox, J.A. Oxidation and flow-injection amperometric determination of 5-hydroxytryptophan at an electrode modified by electrochemically assisted deposition of a sol-gel film with templated nanoscale pores. Talanta 2010, 82, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Kalachar, H.C.B.; Arthoba Naik, Y.; Basavanna, S.; Viswanatha, R.; Venkatesha, T.G.; Sheela, T. Amperometric and differential pulse voltammetric determination of 5-hydroxy-l-tryptophan in pharmaceutical samples using gold modified pencil graphite electrode. J. Chem. Pharm. Res. 2011, 3, 530–539. [Google Scholar]
- Chen, L.; Lian, H.T.; Sun, X.Y.; Liu, B. Sensitive detection of l-5-hydroxytryptophan based on molecularly imprinted polymers with graphene amplification. Anal. Biochem. 2017, 526, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goyal, R.N. Simultaneous determination of melatonin and 5-hydroxytrptophan at the disposable poly-(melamine)/poly-(o-aminophenol) composite modified screen printed sensor. J. Electro. Anal. Chem. 2020, 874, 114458. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, R.; Goyal, R.N. Palladium nano particles decorated multi-walled carbon nanotubes modified sensor for the determination of 5-hydroxytryptophan in biological fluids. Sens. Actuators B Chem. 2017, 239, 1060–1068. [Google Scholar] [CrossRef]
- Li, M.D.; Tseng, W.L.; Cheng, T.L. Ultrasensitive detection of indoleamines by combination of nanoparticle-based extraction with capillary electrophoresis/laser-induced native fluorescence. J. Chromatogr. A 2009, 1216, 6451–6458. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Bayat, M. Pyrolytic graphite electrode modified with a thin film of a graphite/diamond nano-mixture for highly sensitive voltammetric determination of tryptophan and 5-hydroxytryptophan. Microchim. Acta 2011, 174, 361–366. [Google Scholar] [CrossRef]
- Seo, K.D.; Hossain, M.M.D.; Gurudatt, N.G.; Choi, C.S.; Shiddiky, M.J.A.; Park, D.S.; Shim, Y.B. Microfluidic neurotransmitters sensor in blood plasma with mediator-immobilized conducting polymer/n, s-doped porous carbon composite. Sens. Actuators B Chem. 2020, 313, 128017. [Google Scholar] [CrossRef]
- Hamlin, K.E.; Fischer, F.E. The synthesis of 5-hydroxytryptamine. J. Am. Chem. Soc. 1951, 73, 5007–5008. [Google Scholar] [CrossRef]
- Ek, A.; Witkop, B. The synthesis of labile hydroxytryptophan metabolites1. J. Am. Chem. Soc. 1954, 76, 5579–5588. [Google Scholar] [CrossRef]
- Snyder, H.R.; Smith, C.W. A convenient synthesis of dl-tryptophan. J. Am. Chem. Soc. 1944, 66, 350–351. [Google Scholar] [CrossRef]
- Koo, J.; Avakian, S.; Martin, G.J. Synthesis in the 5-hydroxyindole series. N-acetyl-5-hydroxytryptophan and related compounds. J. Org. Chem. 1959, 24, 179–183. [Google Scholar] [CrossRef]
- Frangatos, G.; Chubb, F.L. A new synthesis of 5-hydroxytryptophan. Can. J. Chem. 1959, 37, 1374–1376. [Google Scholar] [CrossRef]
- Moe, O.A.; Warner, D.T. 1,4-addition reactions. I. The addition of acylamidomalonates to acrolein1a. J. Am. Chem. Soc. 1948, 70, 2763–2765. [Google Scholar] [CrossRef]
- Fitzpatrick, P.F. Tetrahydropterin-dependent amino acid hydroxylases. Annu. Rev. Biochem. 1999, 68, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Eser, B.E.; Barr, E.W.; Frantorn, P.A.; Saleh, L.; Bollinger, J.M.; Krebs, C.; Fitzpatrick, P.F. Direct spectroscopic evidence for a high-spin fe(iv) intermediate in tyrosine hydroxylase. J. Am. Chem. Soc. 2007, 129, 11334. [Google Scholar] [CrossRef] [PubMed]
- Pavon, J.A.; Eser, B.; Huynh, M.T.; Fitzpatrick, P.F. Single turnover kinetics of tryptophan hydroxylase: Evidence for a new intermediate in the reaction of the aromatic amino acid hydroxylases. Biochemistry 2010, 49, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Hara, R.; Kino, K. Enhanced synthesis of 5-hydroxy-l-tryptophan through tetrahydropterin regeneration. AMB Express 2013, 3, 7. [Google Scholar] [CrossRef]
- Wang, H.J.; Liu, W.Q.; Shi, F.; Huang, L.; Lian, J.Z.; Qu, L.; Cai, J.; Xu, Z.A. Metabolic pathway engineering for high-level production of 5-hydroxytryptophan in escherichia coli. Metab. Eng. 2018, 48, 279–287. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kataoka, E.; Miyamoto, N.; Furukawa, K.; Ohsuye, K.; Yabuta, M. Genetic engineering of escherichia coli for production of tetrahydrobiopterin. Metab. Eng. 2003, 5, 246–254. [Google Scholar] [CrossRef]
- Moran, G.R.; Fitzpatrick, P.F. A continuous fluorescence assay for tryptophan hydroxylase. Anal. Biochem. 1999, 266, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Flatmark, T.; Stevens, R.C. Structural insight into the aromatic amino acid hydroxylases and their disease-related mutant forms. Chem. Rev. 1999, 99, 2137–2160. [Google Scholar] [CrossRef] [PubMed]
- Ehret, M.; Cash, C.D.; Hamon, M.; Maitre, M. Formal demonstration of the phosphorylation of rat brain tryptophan hydroxylase by ca2+/calmodulin-dependent protein kinase. J. Neurochem. 1989, 52, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Matthes, S.; Bader, M. Peripheral serotonin synthesis as a new drug target. Trends Pharmacol. Sci. 2018, 39, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Hoglund, E.; Overli, O.; Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Sakowski, S.A.; Geddes, T.J.; Thomas, D.M.; Levi, E.; Hatfield, J.S.; Kuhn, D.M. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res. 2006, 1085, 11–18. [Google Scholar] [CrossRef]
- Ota, M.; Dostert, P.; Hamanaka, T.; Nagatsu, T.; Naoi, M. Inhibition of tryptophan hydroxylase by (r)- and (s)-1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinolines (salsolinols). Neuropharmacology 1992, 31, 337–341. [Google Scholar] [CrossRef]
- Kim, E.I.; Kang, M.H.; Lee, M.K. Inhibitory effects of tetrahydropapaverine on serotonin biosynthesis in murine mastocytoma p815 cells. Life Sci. 2004, 75, 1949–1957. [Google Scholar] [CrossRef]
- Naoi, M.; Hosoda, S.; Ota, M.; Takahashi, T.; Nagatsu, T. Inhibition of tryptophan hydroxylase by food-derived carcinogenic heterocyclic amines, 3-amino-1-methyl-5h-pyrido[4,3-b]indole and 3-amino-1,4-dimethyl-5h-pyrido[4,3-b]indole. Biochem. Pharmacol. 1991, 41, 199–203. [Google Scholar] [CrossRef]
- Zimmer, L.; Luxen, A.; Giacomelli, F.; Pujol, J.F. Short- and long-term effects of p-ethynylphenylalanine on brain serotonin levels. Neurochem. Res. 2002, 27, 269–275. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Yang, Q.; Sun, W.M.; Vogel, P.; Heydorn, W.; Yu, X.Q.; Hu, Z.X.; Yu, W.S.; Jonas, B.; Pineda, R.; et al. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J. Pharmacol. Exp. Ther. 2008, 325, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Cianchetta, G.; Stouch, T.; Yu, W.; Shi, Z.C.; Tari, L.W.; Swanson, R.V.; Hunter, M.J.; Hoffman, I.D.; Liu, Q. Mechanism of inhibition of novel tryptophan hydroxylase inhibitors revealed by co-crystal structures and kinetic analysis. Curr. Chem. Genom. 2010, 4, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Telotristat ethyl: First global approval. Drugs 2017, 77, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.R.; De Lombaert, S.; Aiello, R.; Bourassa, P.; Barucci, N.; Zhang, Q.; Paralkar, V.; Stein, A.J.; Valentine, J.; Zavadoski, W. Discovery of acyl guanidine tryptophan hydroxylase-1 inhibitors. Bioorganic Med. Chem. Lett. 2016, 26, 2855–2860. [Google Scholar] [CrossRef]
- Goldberg, D.R.; De Lombaert, S.; Aiello, R.; Bourassa, P.; Barucci, N.; Zhang, Q.; Paralkar, V.; Stein, A.J.; Holt, M.; Valentine, J.; et al. Optimization of spirocyclic proline tryptophan hydroxylase-1 inhibitors. Bioorganic Med. Chem. Lett. 2017, 27, 413–419. [Google Scholar] [CrossRef]
- Shi, H.; Cui, Y.; Qin, Y. Discovery and characterization of a novel tryptophan hydroxylase 1 inhibitor as a prodrug. Chem. Biol. Drug Des. 2018, 91, 202–212. [Google Scholar] [CrossRef]
- Barbosa, R.; Scialfa, J.H.; Terra, I.M.; Cipolla-Neto, J.; Simonneaux, V.; Afeche, S.C. Tryptophan hydroxylase is modulated by l-type calcium channels in the rat pineal gland. Life Sci. 2008, 82, 529–535. [Google Scholar] [CrossRef]
- Braga, A.; Faria, N. Bioprocess optimization for the production of aromatic compounds with metabolically engineered hosts: Recent developments and future challenges. Front. Bioeng. Biotechnol. 2020, 8, 18. [Google Scholar] [CrossRef]
- Xu, P.; Gu, Q.; Wang, W.Y.; Wong, L.; Bower, A.G.W.; Collins, C.H.; Koffas, M.A.G. Modular optimization of multi-gene pathways for fatty acids production in e. Coli. Nat. Commun. 2013, 4, 1409. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Jiang, H.; Mao, X.Z. Biotechnological production of lycopene by microorganisms. Appl. Microbiol. Biotechnol. 2020, 104, 10307–10324. [Google Scholar] [CrossRef]
- Chen, X.X.; Zhang, C.Q.; Lindley, N.D. Metabolic engineering strategies for sustainable terpenoid flavor and fragrance synthesis. J. Agric. Food Chem. 2020, 68, 10252–10264. [Google Scholar] [CrossRef] [PubMed]
- Anarat-Cappillino, G.; Sattely, E.S. The chemical logic of plant natural product biosynthesis. Curr. Opin. Plant Biol. 2014, 19, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, C.; Weissbach, H.; Udenfriend, S. 5-hydroxytryptophan formation and tryptophan metabolism in chromobacterium violaceum. Arch. Biochem. Biophys. 1956, 63, 122–130. [Google Scholar] [CrossRef]
- Letendre, C.H.; Dickens, G.; Guroff, G. The tryptophan hydroxylase of chromobacterium violaceum. J. Biol. Chem. 1974, 249, 7186–7191. [Google Scholar] [PubMed]
- Kino, K.; Hara, R.; Nozawa, A. Enhancement of l-tryptophan 5-hydroxylation activity by structure-based modification of l-phenylalanine 4-hydroxylase from chromobacterium violaceum. J. Biosci. Bioeng. 2009, 108, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Pribat, A.; Blaby, I.K.; Lara-Nunez, A.; Gregory, J.F.; de Crecy-Lagard, V.; Hanson, A.D. Folx and folm are essential for tetrahydromonapterin synthesis in escherichia coli and pseudomonas aeruginosa. J. Bacteriol. 2010, 192, 475–482. [Google Scholar] [CrossRef]
- Lin, Y.H.; Sun, X.X.; Yuan, Q.P.; Yan, Y.J. Engineering bacterial phenylalanine 4-hydroxylase for microbial synthesis of human neurotransmitter precursor 5-hydroxytryptophan. Acs Synth. Biol. 2014, 3, 497–505. [Google Scholar] [CrossRef]
- Mora-Villalobos, J.A.; Zeng, A.P. Synthetic pathways and processes for effective production of 5-hydroxytryptophan and serotonin from glucose in escherichia coli. J. Biol. Eng. 2018, 12, 3. [Google Scholar] [CrossRef]
- Sun, X.X.; Lin, Y.H.; Yuan, Q.P.; Yan, Y.J. Precursor-directed biosynthesis of 5-hydroxytryptophan using metabolically engineered e. Coli. Acs Synth. Biol. 2015, 4, 554–558. [Google Scholar] [CrossRef]
- Luo, H.; Schneider, K.; Christensen, U.; Lei, Y.; Herrgard, M.J.; Palsson, B.O. Microbial synthesis of human-hormone melatonin at gram scales. Acs Synth. Biol. 2020, 9, 1240–1245. [Google Scholar] [CrossRef]
- Xu, D.; Fang, M.J.; Wang, H.J.; Huang, L.; Xu, Q.Y.; Xu, Z.N. Enhanced production of 5-hydroxytryptophan through the regulation of l-tryptophan biosynthetic pathway. Appl. Microbiol. Biotechnol. 2020, 104, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Wu, C.C.; Sheng, J.Y.; Feng, X.Y. Molecular basis of 5-hydroxytryptophan synthesis in saccharomyces cerevisiae. Mol. Biosyst. 2016, 12, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.N.; Black, J.W.; Fisher, E.W. Inhibitory effect of 5-hydroxytryptophan on acid gastric secretion. Nature 1957, 180, 1127. [Google Scholar] [CrossRef] [PubMed]
- Bulbring, E.; Lin, R.C. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-ht and its release in relation to intraluminal pressure and propulsive activity. J. Physiol. 1958, 140, 381–407. [Google Scholar] [PubMed]
- Udenfriend, S.; Weissbach, H.; Bogdanski, D.F. Increase in tissue serotonin following administration of its precursor 5-hydroxytryptophan. J. Biol. Chem. 1957, 224, 803–810. [Google Scholar] [PubMed]
- Bogdanski, D.F.; Weissbach, H.; Udenfriend, S. Pharmacological studies with the serotonin precursor, 5-hydroxytryptophan. J. Pharmacol. Exp. Ther. 1958, 122, 182–194. [Google Scholar]
- Corne, S.J.; Pickering, R.W.; Warner, B.T. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br. J. Pharmacol. Chemother. 1963, 20, 106–120. [Google Scholar] [CrossRef]
- Martin, P.; Frances, H.; Simon, P. Dissociation of head twitches and tremors during the study of interactions with 5-hydroxytryptophan in mice. J. Pharmacol. Methods 1985, 13, 193–200. [Google Scholar] [CrossRef]
- Endo, Y. Evidence that the accumulation of 5-hydroxytryptamine in the liver but not in the brain may cause the hypoglycemia induced by 5-hydroxytryptophan. Br. J. Pharmacol. 1985, 85, 591–598. [Google Scholar] [CrossRef]
- Endo, Y. Suppression and potentiation of 5-hydroxytryptophan-induced hypoglycemia by alpha-monofluoromethyldopa-correlation with the accumulation of 5-hydroxytryptamine in the liver. Br. J. Pharmacol. 1987, 90, 161–165. [Google Scholar]
- Yang, T.H.; Hsu, P.Y.; Meng, M.H.; Su, C.C. Supplement of 5-hydroxytryptophan before induction suppresses inflammation and collagen-induced arthritis. Arthritis Res. Ther. 2015, 17, 364. [Google Scholar] [CrossRef] [PubMed]
- Rogóz, Z.; Skuza, G.; Sowińska, H. The effect of the antihistaminic drugs on the central action of 5-hydroxytryptophan in mice. Polish J. Pharm. Pharm. 1981, 33, 459–465. [Google Scholar]
- Truscott, T.C. Effects of phenylalanine and 5-hydroxytryptophan on seizure severity in mice. Pharm. Biochem. Behav. 1975, 3, 939–941. [Google Scholar] [CrossRef]
- Carter, R.B.; Dykstra, L.A.; Leander, J.D.; Appel, J.B. Role of peripheral mechanisms in the behavioral effects of 5-hydroxytryptophan. Pharmacol. Biochem. Behav. 1978, 9, 249–253. [Google Scholar] [CrossRef]
- Sharma, A.; Castellani, R.J.; Smith, M.A.; Muresanu, D.F.; Dey, P.K.; Sharma, H.S. 5-hydroxytryptophan: A precursor of serotonin influences regional blood-brain barrier breakdown, cerebral blood flow, brain edema formation, and neuropathology. In New Therapeutic Strategies for Brain Edema and Cell Injury; Sharma, H.S., Sharma, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–44. [Google Scholar]
- Yap, C.Y.; Taylor, D.A. Involvement of 5-ht2 receptors in the wet-dog shake behaviour induced by 5-hydroxytryptophan in the rat. Neuropharmacology 1983, 22, 801–804. [Google Scholar] [CrossRef]
- Hadzović, S.; Ernst, A.M. The effect of 5-hydroxytryptamine and 5-hydroxytryptophan on extra-pyramidal function. Eur. J. Pharmacol. 1969, 6, 90–95. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Fang, V.S. Effect of apomorphine plus 5-hydroxytryptophan on plasma prolactin levels in male rats. Psychopharmacol. Commun. 1976, 2, 189–198. [Google Scholar]
- Ohgo, S.; Kato, Y.; Chihara, K.; Imura, H.; Maeda, K. Effect of hypothalamic surgery on prolactin release induced by 5-hydroxytryptophan (5-htp) in rats. Endocrinol. Jpn. 1976, 23, 485–491. [Google Scholar] [CrossRef]
- Preziosi, P.; Cerrito, F.; Vacca, M. Effects of naloxone on the secretion of prolactin and corticosterone induced by 5-hydroxytryptophan and a serotonergic agonist, mcpp. Life Sci. 1983, 32, 2423–2430. [Google Scholar] [CrossRef]
- Hingtgen, J.N.; Fuller, R.W.; Mason, N.R.; Aprison, M.H. Blockade of a 5-hydroxytryptophan-induced animal-model of depression with a potent and selective 5-ht2 receptor antagonist (ly53857). Biol. Psychiatry 1985, 20, 592–597. [Google Scholar] [CrossRef]
- Dreshfield-Ahmad, L.J.; Thompson, D.C.; Schaus, J.M.; Wong, D.T. Enhancement in extracelllar serotonin levels by 5-hydroxytryptophan loading after administration of way 100635 and fluoxetine. Life Sci. 2000, 66, 2035–2041. [Google Scholar] [CrossRef]
- Blundell, J.E.; Latham, C.J. Serotonergic influences on food intake: Effect of 5-hydroxytryptophan on parameters of feeding behaviour in deprived and free-feeding rats. Pharmacol. Biochem. Behav. 1979, 11, 431–437. [Google Scholar] [CrossRef]
- Oconnor, L.H.; Feder, H.H. Estradiol and progesterone influence l-5-hydroxytryptophan-induced myoclonus in male guinea-pigs-sex-differences in serotonin steroid interactions. Brain Res. 1985, 330, 121–125. [Google Scholar] [CrossRef]
- Pappert, E.J.; Goetz, C.G.; Stebbins, G.T.; Belden, M.; Carvey, P.M. 5-hydroxytryptophan-induced myoclonus in guinea pigs: Mediation through 5-ht1/2 receptor subtypes. Eur. J. Pharmacol. 1998, 347, 51–56. [Google Scholar] [CrossRef]
- Kojima, S.; Ikeda, M.; Kamikawa, Y. Investigation into the 5-hydroxytryptophan-evoked luminal 5-hydroxytryptamine release from the guinea pig colon. Jpn. J. Pharmacol. 2000, 84, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Zuzina, A.B.; Vinarskaya, A.K.; Balaban, P.M. Increase in serotonin precursor levels reinstates the context memory during reconsolidation. Invertebr. Neurosci. 2019, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, U.; Burks, T.F.; Feldberg, W.; Goodrich, C.A. Temperature responses and other effects of 5-hydroxytryptophan and 5-hydroxytryptamine when acting from the liquor space in unanaesthetized rabbits. Br. J. Pharmacol. 1970, 38, 688–701. [Google Scholar] [CrossRef]
- Fjalland, B. Neuroleptic influence on hyperthermia induced by 5-hydroxytryptophan and p-methoxy-amphetamine in maoi-pretreated rabbits. Psychopharmacology 1979, 63, 113–117. [Google Scholar] [CrossRef]
- Denoyer, M.; Kitahama, K.; Sallanon, M.; Touret, M.; Jouvet, M. 5-hydroxytryptophan uptake and decarboxylating neurons in the cat hypothalamus. Neuroscience 1989, 31, 203–211. [Google Scholar] [CrossRef]
- Ellaway, P.H.; Trott, J.R. The mode of action of 5-hydroxytryptophan in facilitating a stretch reflex in the spinal cat. Exp. Brain Res. 1975, 22, 145–162. [Google Scholar] [CrossRef]
- Haverback, B.J.; Bogdanski, D.; Hogben, C.A. Inhibition of gastric acid secretion in the dog by the precursor of serotonin, 5-hydroxytryptophan. Gastroenterology 1958, 34, 188–195. [Google Scholar] [CrossRef]
- Schemann, M.; Ehrlein, H.J. 5-hydroxytryptophan and cisapride stimulate propulsive jejunal motility and transit of chyme in dogs. Digestion 1986, 34, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Antonaccio, M.J.; Robson, R.D. Centrally-mediated cardiovascular effects of 5-hydroxytryptophan in mao-inhibited dogs: Modification by autonomic antagonists. Arch. Int. Pharmacodyn. Ther. 1975, 213, 200–210. [Google Scholar] [PubMed]
- Sugden, D.; Namboodiri, M.A.A.; Klein, D.C.; Grady, R.K.; Mefford, I.N. Ovine pineal indoles-effects of l-tryptophan or l-5-hydroxytryptophan administration. J. Neurochem. 1985, 44, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ma, C.; Zhao, G.D.; Wang, G.; Li, X.B.; Yang, K.L. Rumen-protected 5-hydroxytryptohan improves sheep melatonin synthesis in the pineal gland and intestinal tract. Med. Sci. Monit. 2019, 25, 3605–3616. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.J.; Hanson, M.A.; Moore, P.J.; Nijhuis, J.G.; Parkes, M.J. Stimulation of breathing movements by l-5-hydroxytryptophan in fetal sheep during normoxia and hypoxia. J. Physiol. Lond. 1988, 404, 575–589. [Google Scholar] [CrossRef]
- Weaver, S.R.; Prichard, A.S.; Maerz, N.L.; Prichard, A.P.; Endres, E.L.; Hernandez-Castellano, L.E.; Akins, M.S.; Bruckmaier, R.M.; Hernandez, L.L. Elevating serotonin pre-partum alters the holstein dairy cow hepatic adaptation to lactation. PLoS ONE 2017, 12, e0184939. [Google Scholar] [CrossRef]
- Martin, T.G. Serotonin syndrome. Ann. Emerg. Med. 1996, 28, 520–526. [Google Scholar] [CrossRef]
- Francescangeli, J.; Karamchandani, K.; Powell, M.; Bonavia, A. The serotonin syndrome: From molecular mechanisms to clinical practice. Int. J. Mol. Sci. 2019, 20, 2288. [Google Scholar] [CrossRef]
- Turner, E.H.; Loftis, J.M.; Blackwell, A.D. Serotonin a la carte: Supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol. Ther. 2006, 109, 325–338. [Google Scholar] [CrossRef]
- Agren, H.; Reibring, L.; Hartvig, P.; Tedroff, J.; Bjurling, P.; Hornfeldt, K.; Andersson, Y.; Lundqvist, H.; Langstrom, B. Low brain uptake of l- c-11 5-hydroxytryptophan in major depression-a positron emission tomography study on patients and healthy-volunteers. Acta Psychiatr. Scand. 1991, 83, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.D.; Birmaher, B.; Perel, J.M.; Dahl, R.E.; Meyer, V.; Alshabbout, M.; Iyengar, S.; Puigantich, J. Neuroendocrine response to l-5-hydroxytryptophan challenge in prepubertal major depression-depressed vs normal-children. Arch. Gen. Psychiatry 1992, 49, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Coppen, A.; Shaw, D.M.; Malleson, A. Changes in 5-hydroxytryptophan metabolism in depression. Br. J. Psychiatry J. Ment. Sci. 1965, 111, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Persson, T.; Roos, B.E. 5-hydroxytryptophan for depression. Lancet 1967, 2, 987–988. [Google Scholar] [CrossRef]
- Byerley, W.F.; Judd, L.L.; Reimherr, F.W.; Grosser, B.I. 5-hydroxytryptophan-a review of its antidepressant efficacy and adverse-effects. J. Clin. Psychopharmacol. 1987, 7, 127–137. [Google Scholar]
- Zmilacher, K.; Battegay, R.; Gastpar, M. L-5-hydroxytryptophan alone and in combination with a peripheral decarboxylase inhibitor in the treatment of depression. Neuropsychobiology 1988, 20, 28–35. [Google Scholar] [CrossRef]
- Smarius, L.; Jacobs, G.E.; Hoeberechts-Lefrandt, D.H.M.; de Kam, M.L.; van der Post, J.P.; de Rijk, R.; van Pelt, J.; Schoemaker, R.C.; Zitman, F.G.; van Gerven, J.M.A.; et al. Pharmacology of rising oral doses of 5-hydroxytryptophan with carbidopa. J. Psychopharmacol. 2008, 22, 426–433. [Google Scholar] [CrossRef]
- Van Praag, H.M. In search of the mode of action of antidepressants. 5-htp/tyrosine mixtures in depressions. Neuropharmacology 1983, 22, 433–440. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Umberkoman-Wiita, B.; Robertson, A.; Tricou, B.J.; Lowy, M.; Perline, R. Effect of 5-hydroxytryptophan on serum cortisol levels in major affective disorders. I. Enhanced response in depression and mania. Arch. Gen. Psychiatry 1984, 41, 366–374. [Google Scholar] [CrossRef]
- van Praag, H.M.; van den Burg, W.; Bos, E.R.; Dols, L.C. 5-hydroxytryptophan in combination with clomipramine in “therapy-resistant” depressions. Psychopharmacologia 1974, 38, 267–269. [Google Scholar] [CrossRef]
- Aliño, J.J.; Gutierrez, J.L.; Iglesias, M.L. 5-hydroxytryptophan (5-htp) and a maoi (nialamide) in the treatment of depressions. A double-blind controlled study. Int. Pharm. 1976, 11, 8–15. [Google Scholar]
- Mendlewicz, J.; Youdim, M.B. Antidepressant potentiation of 5-hydroxytryptophan by l-deprenil in affective illness. J. Affect Disord. 1980, 2, 137–146. [Google Scholar] [CrossRef]
- Kious, B.M.; Sabic, H.; Sung, Y.H.; Kondo, D.G.; Renshaw, P. An open-label pilot study of combined augmentation with creatine monohydrate and 5-hydroxytryptophan for selective serotonin reuptake inhibitor- or serotonin-norepinephrine reuptake inhibitor-resistant depression in adult women. J. Clin. Psychopharmacol. 2017, 37, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Westenberg, H.G.M. L-5-hydroxytryptophan in the treatment of anxiety disorders. J. Affect. Disord. 1985, 8, 197–200. [Google Scholar] [CrossRef]
- Kahn, R.S.; Westenberg, H.G.M.; Verhoeven, W.M.A.; Gispendewied, C.C.; Kamerbeek, W.D.J. Effect of a serotonin precursor and uptake inhibitor in anxiety disorders-a double-blind comparison of 5-hydroxytryptophan, clomipramine and placebo. Int. Clin. Psychopharmacol. 1987, 2, 33–45. [Google Scholar] [CrossRef]
- Ishida, A.; Takada, G.; Kobayashi, Y.; Toyoshima, I.; Takai, K. Effect of tetrahydrobiopterin and 5-hydroxytryptophan on hereditary progressive dystonia with marked diurnal fluctuation-a suggestion of the serotonergic system involvement. Tohoku J. Exp. Med. 1988, 154, 233–239. [Google Scholar] [CrossRef]
- Denboer, J.A.; Westenberg, H.G.M. Behavioral, neuroendocrine, and biochemical effects of 5-hydroxytryptophan administration in panic disorder. Psychiatry Res. 1990, 31, 267–278. [Google Scholar] [CrossRef]
- Schruers, K.; van Diest, R.; Overbeek, T.; Griez, E. Acute l-5-hydroxytryptophan administration inhibits carbon dioxide-induced panic in panic disorder patients. Psychiatry Res. 2002, 113, 237–243. [Google Scholar] [CrossRef]
- Maron, E.; Toru, I.; Vasar, V.; Shlik, J. The effect of 5-hydroxytryptophan on chotecystokinin-4-induced panic attacks in healthy volunteers. J. Psychopharmacol. 2004, 18, 194–199. [Google Scholar] [CrossRef]
- Wyatt, R.J.; Zarcone, V.; Engelman, K.; Dement, W.C.; Snyder, F.; Sjoerdsma, A. Effects of 5-hydroxytryptophan on the sleep of normal human subjects. Electroencephalogr. Clin. Neurophysiol. 1971, 30, 505–509. [Google Scholar] [CrossRef]
- Guilleminault, C.; Cathala, J.P.; Castaigne, P. Effects of 5-hydroxytryptophan on sleep of a patient with a brain-stem lesion. Electroencephalogr. Clin. Neurophysiol. 1973, 34, 177–184. [Google Scholar] [CrossRef]
- Zarcone, V.; Kales, A.; Scharf, M.; Tan, T.L.; Simmons, J.Q.; Dement, W.C. Repeated oral ingestion of 5-hydroxytryptophan. The effect on behavior and sleep processes in two schizophrenic children. Arch. Gen. Psychiatry 1973, 28, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Petre-Quadens, O.; De Lee, C. 5-hydroxytryptophan and sleep in down’s syndrome. J. Neurol. Sci. 1975, 26, 443–453. [Google Scholar] [CrossRef]
- Bruni, O.; Ferri, R.; Miano, S.; Verrillo, E. L-5-hydroxytryptophan treatment of sleep terrors in children. Eur. J. Pediatrics 2004, 163, 402–407. [Google Scholar] [CrossRef]
- Metz, J.T.; Holcomb, H.H.; Meltzer, H.Y. Effect of 5-hydroxytryptophan on h-reflex recovery curves in normal subjects and patients with affective-disorders. Biol. Psychiatry 1988, 23, 602–611. [Google Scholar] [CrossRef]
- Emanuele, E.; Bertona, M.; Minoretti, P.; Geroldi, D. An open-label trial of l-5-hydroxytryptophan in subjects with romantic stress. Neuroendocrinol. Lett. 2010, 31, 663–666. [Google Scholar]
- Titus, F.; Davalos, A.; Alom, J.; Codina, A. 5-hydroxytryptophan versus methysergide in the prophylaxis of migraine-randomized clinical-trial. Eur. Neurol. 1986, 25, 327–329. [Google Scholar] [CrossRef]
- Maissen, C.P.; Ludin, H.P. Comparative efficacy of 5-hydroxytryptophan and propranolol in interval treatment of migraine. Schweiz. Med. Wochenschr. 1991, 121, 1585–1590. [Google Scholar]
- Nicolodi, M.; Sicuteri, F. L-5-hydroxytryptophan can prevent nociceptive disorders in man. In Tryptophan, Serotonin and Melatonin: Basic Aspects and Applications; Huether, G., Kochen, W., Simat, T.J., Steinhart, H., Eds.; Springer: New York, NY, USA, 1999; pp. 177–182. [Google Scholar]
- Ribeiro, C.A.F.; Portuguese Headache, S. L-5-hydroxytryptophan in the prophylaxis of chronic tension-type headache: A double-blind, randomized, placebo-controlled study. Headache 2000, 40, 451–456. [Google Scholar] [CrossRef]
- Trouillas, P.; Brudon, F.; Adeleine, P. Improvement of cerebellar-ataxia with levorotatory form of 5-hydroxytryptophan-a double-blind-study with quantified data-processing. Arch. Neurol. 1988, 45, 1217–1222. [Google Scholar] [CrossRef]
- Trouillas, P.; Serratrice, G.; Laplane, D.; Rascol, A.; Augustin, P.; Barroche, G.; Clanet, M.; Degos, C.F.; Desnuelle, C.; Dumas, R.; et al. Levorotatory form of 5-hydroxytryptophan in friedreichs ataxia-results of a double-blind drug-placebo cooperative study. Arch. Neurol. 1995, 52, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Rus, A.; Molina, F.; Del Moral, M.L.; Ramirez-Exposito, M.J.; Martinez-Martos, J.M. Catecholamine and indolamine pathway: A case-control study in fibromyalgia. Biol. Res. Nurs. 2018, 20, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Fibromyalgia: Recent advances in diagnosis, classification, pharmacotherapy and alternative remedies. Int. J. Mol. Sci. 2020, 21, 7877. [Google Scholar] [CrossRef] [PubMed]
- Caruso, I.; Puttini, P.S.; Cazzola, M.; Azzolini, V. Double-blind-study of 5-hydroxytryptophan versus placebo in the treatment of primary fibromyalgia syndrome. J. Int. Med Res. 1990, 18, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Volicer, L.; Langlais, P.J.; Matson, W.R.; Mark, K.A.; Gamache, P.H. Serotoninergic system in dementia of the alzheimer type-abnormal forms of 5-hydroxytryptophan and serotonin in cerebrospinal-fluid. Arch. Neurol. 1985, 42, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Sano, I.; Taniguchi, K. [l-5-hydroxytryptophan(l-5-htp) therapy of parkinson’s disease. 2]. Munch. Med. Wochenschr. (1950) 1972, 114, 1717–1719. [Google Scholar]
- Chase, T.N. 5-hydroxytryptophan in parkinsonism. Lancet (London, England) 1970, 2, 1029–1030. [Google Scholar] [CrossRef]
- Magnussen, I.; Jensen, T.S.; Rand, J.H.; Van Woert, M.H. Plasma accumulation of metabolism of orally administered single dose l-5-hydroxytryptophan in man. Acta Pharmacol. Toxicol. (Copenh) 1981, 49, 184–189. [Google Scholar] [CrossRef]
- Meloni, M.; Puligheddu, M.; Carta, M.; Cannas, A.; Figorilli, M.; Defazio, G. Efficacy and safety of 5-hydroxytryptophan on depression and apathy in parkinson’s disease: A preliminary finding. Eur. J. Neurol. 2020, 27, 779–786. [Google Scholar] [CrossRef]
- Growdon, J.H.; Young, R.R.; Shahani, B.T. L-5-hydroxytryptophan in treatment of several different syndromes in which myoclonus is prominent. Neurology 1976, 26, 1135–1140. [Google Scholar] [CrossRef]
- Magnussen, I.; Dupont, E.; Prange-Hansen, A.; de Fine Olivarius, B. Palatal myoclonus treated with 5-hydroxytryptophan and a decarboxylase-inhibitor. Acta Neurol. Scand. 1977, 55, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Gascon, G.; Wallenberg, B.; Daif, A.K.; Ozand, P. Successful treatment of cherry red spot myoclonus syndrome with 5-hydroxytryptophan. Ann. Neurol. 1988, 24, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Hayakawa, T.; Shishikura, K.; Ohsawa, M.; Suzuki, H.; Fukuyama, Y. Improvement of action myoclonus by an administration of 5-hydroxytryptophan and carbidopa in a child with muscular subsarcolemmal hyperactivity. Brain Dev. 1990, 12, 516–520. [Google Scholar] [CrossRef]
- Jimenezjimenez, F.J.; Roldan, A.; Zancada, F.; Molinaarjona, J.A.; Fernandezballesteros, A.; Santos, J. Spinal myoclonus-successful treatment with the combination of sodium valproate and l-5-hydroxytryptophan. Clin. Neuropharmacol. 1991, 14, 186–190. [Google Scholar] [CrossRef]
- Ceci, F.; Cangiano, C.; Cairella, M.; Cascino, A.; Delben, M.; Muscaritoli, M.; Sibilia, L.; Fanelli, F.R. The effects of oral 5-hydroxytryptophan administration on feeding-behavior in obese adult female subjects. J. Neural. Transm. 1989, 76, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, C.; Ceci, F.; Cascino, A.; Delben, M.; Laviano, A.; Muscaritoli, M.; Antonucci, F.; Rossifanelli, F. Eating behavior and adherence to dietary prescriptions in obese adult subjects treated with 5-hydroxytryptophan. Am. J. Clin. Nutr. 1992, 56, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Nakai, Y.; Imura, H.; Chihara, K.; Ogo, S. Effect of 5-hydroxytryptophan (5-htp) on plasma prolactin levels in man. J. Clin. Endocrinol. Metab. 1974, 38, 695–697. [Google Scholar] [CrossRef]
- Sueldo, C.E.; Duda, M.; Kletzky, O.A. Influence of sequential doses of 5-hydroxytryptophan on prolactin-release. Am. J. Obstet. Gynecol. 1986, 154, 424–427. [Google Scholar] [CrossRef]
- Vlasses, P.H.; Rotmensch, H.H.; Swanson, B.N.; Clementi, R.A.; Ferguson, R.K. Effect of repeated doses of l-5-hydroxytryptophan and carbidopa on prolactin and aldosterone secretion in man. J. Endocrinol. Investig. 1989, 12, 87–91. [Google Scholar] [CrossRef]
- Keithahn, C.; Lerchl, A. 5-hydroxytryptophan is a more potent in vitro hydroxyl radical scavenger than melatonin or vitamin c. J. Pineal Res. 2005, 38, 62–66. [Google Scholar] [CrossRef]
- Derlacz, R.A.; Sliwinska, M.; Piekutowska, A.; Winiarska, K.; Drozak, J.; Bryla, J. Melatonin is more effective than taurine and 5-hydroxytryptophan against hyperglycemia-induced kidney-cortex tubules injury. J. Pineal Res. 2007, 42, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gonzales, M.C.; Fuentes-Broto, L.; Martinez-Ballarin, E.; Miana-Mena, F.J.; Berzosa, C.; Garcia-Gil, F.A.; Aranda, M.; Garcia, J.J. Effects of tryptophan and 5-hydroxytryptophan on the hepatic cell membrane rigidity due to oxidative stress. J. Membr. Biol. 2009, 231, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Lee, J.S.; Kim, J.M.; Lee, E.K.; Han, Y.K.; Kim, H.J.; Choi, J.; Ha, Y.M.; No, J.K.; Kim, Y.H.; et al. 5-hydroxytrytophan inhibits tert-butylhydroperoxide (t-bhp)-induced oxidative damage via the suppression of reactive species (rs) and nuclear factor-kappa b (nf-kappa b) activation on human fibroblast. J. Agric. Food Chem. 2010, 58, 6387–6394. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.S.; Kang, O.H.; Choi, J.G.; Oh, Y.C.; Lee, Y.S.; Jang, H.J.; Kim, J.H.; Park, H.; Jung, K.Y.; Sohn, D.H.; et al. 5-hydroxytryptophan acts on the mitogen-activated protein kinase extracellular-signal regulated protein kinase pathway to modulate cyclooxygenase-2 and inducible nitric oxide synthase expression in raw 264.7 cells. Biol. Pharm. Bull. 2009, 32, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Gwaltney-Brant, S.M.; Albretsen, J.C.; Khan, S.A. 5-hydroxytryptophan toxicosis in dogs: 21 cases (1989–1999). J. Am. Vet. Med Assoc. 2000, 216, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S. Tryptophan produced by showa denko and epidemic eosinophilia-myalgia syndrome-comment. J. Rheumatol. 1996, 23, 89–91. [Google Scholar]
- Kilbourne, E.M.; Philen, R.M.; Kamb, M.L.; Falk, H. Tryptophan produced by showa denko and epidemic eosinophilia-myalgia syndrome. J. Rheumatol. 1996, 23, 81–88. [Google Scholar]
- Preuss, H.G.; Echard, B.; Talpur, N.; Funk, K.A.; Bagchi, D. Does 5-hydroxytryptophan cause acute and chronic toxic perturbations in rats? Toxicol. Mech. Methods 2006, 16, 281–286. [Google Scholar] [CrossRef]
- Belongia, E.A.; Hedberg, C.W.; Gleich, G.J.; White, K.E.; Mayeno, A.N.; Loegering, D.A.; Dunnette, S.L.; Pirie, P.L.; MacDonald, K.L.; Osterholm, M.T. An investigation of the cause of the eosinophilia-myalgia syndrome associated with tryptophan use. N. Engl. J. Med. 1990, 323, 357–365. [Google Scholar] [CrossRef]
- Mayeno, A.N.; Lin, F.; Foote, C.S.; Loegering, D.A.; Ames, M.M.; Hedberg, C.W.; Gleich, G.J. Characterization of peak-e, a novel amino-acid associated with eosinophilia-myalgia-syndrome. Science 1990, 250, 1707–1708. [Google Scholar] [CrossRef]
- Goda, Y.; Suzuki, J.; Maitani, T.; Yoshihira, K.; Takeda, M.; Uchiyama, M. 3-anilino-l-alanine, structural determination of uv-5, a contaminant in ems-associated l-tryptophan samples. Chem. Pharm. Bull. 1992, 40, 2236–2238. [Google Scholar] [CrossRef] [PubMed]
- Michelson, D.; Page, S.W.; Casey, R.; Trucksess, M.W.; Love, L.A.; Milstien, S.; Wilson, C.; Massaquoi, S.G.; Crofford, L.J.; Hallett, M.; et al. An eosinophilia-myalgia-syndrome related disorder associated with exposure to l-5-hydroxytryptophan. J. Rheumatol. 1994, 21, 2261–2265. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2021, 22, 181. https://doi.org/10.3390/ijms22010181
Maffei ME. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. International Journal of Molecular Sciences. 2021; 22(1):181. https://doi.org/10.3390/ijms22010181
Chicago/Turabian StyleMaffei, Massimo E. 2021. "5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology" International Journal of Molecular Sciences 22, no. 1: 181. https://doi.org/10.3390/ijms22010181
APA StyleMaffei, M. E. (2021). 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. International Journal of Molecular Sciences, 22(1), 181. https://doi.org/10.3390/ijms22010181




