Abstract
Calcium (Ca2+) plays an important role in regulating the differentiation and function of osteoclasts. Calcium oscillations (Ca oscillations) are well-known phenomena in receptor activator of nuclear factor kappa B ligand (RANKL)-induced osteoclastogenesis and bone resorption via calcineurin. Many modifiers are involved in the fine-tuning of Ca oscillations in osteoclasts. In addition to macrophage colony-stimulating factors (M-CSF; CSF-1) and RANKL, costimulatory signaling by immunoreceptor tyrosine-based activation motif-harboring adaptors is important for Ca oscillation generation and osteoclast differentiation. DNAX-activating protein of 12 kD is always necessary for osteoclastogenesis. In contrast, Fc receptor gamma (FcRγ) works as a key controller of osteoclastogenesis especially in inflammatory situation. FcRγ has a cofactor in fine-tuning of Ca oscillations. Some calcium channels and transporters are also necessary for Ca oscillations. Transient receptor potential (TRP) channels are well-known environmental sensors, and TRP vanilloid channels play an important role in osteoclastogenesis. Lysosomes, mitochondria, and endoplasmic reticulum (ER) are typical organelles for intracellular Ca2+ storage. Ryanodine receptor, inositol trisphosphate receptor, and sarco/endoplasmic reticulum Ca2+ ATPase on the ER modulate Ca oscillations. Research on Ca oscillations in osteoclasts has still many problems. Surprisingly, there is no objective definition of Ca oscillations. Causality between Ca oscillations and osteoclast differentiation and/or function remains to be examined.
1. Introduction
Calcium (Ca2+) is a simple molecule, but has various cellular functions [1]. It acts as a common second messenger in many biological process [2,3]. For example, small changes in Ca2+ can induce dynamic cellular functions, including synapse transduction in neural cells [4], muscle contraction [5], and fertilization in oocytes [6].
Hematopoietic stem cell-derived osteoclasts are electrically stable cells, and the Ca2+ concentrations in bone marrow macrophages (BMMs) and osteoclasts are maintained at almost constant levels. However, subtle changes in the Ca2+ levels with and without extracellular stimuli, so-called calcium oscillations (Ca oscillations), play important roles in the cellular differentiation, function, and death of osteoclasts.
The effect of Ca oscillations on the differentiation process of osteoclasts remains under debate. Receptor activator of nuclear factor kappa B ligand (RANKL) is an essential inducer of osteoclast differentiation [7]. Nuclear factor of activated T-cells 1 (NFATc1) is a master regulator gene for osteoclastogenesis [8]. In addition to these essential factors for osteoclastogenesis, appropriate Ca oscillations are necessary depending on the situation for RANKL-induced NFATc1 auto-amplification.
In osteoclast differentiation, calcineurin is an important activator of NFATc1 converting Ca oscillations signals under RANKL transduction pathway [9]. Recent study clarified PICK1 is a positive regulator of calcineurin B [10]. Another group showed that mTORC1 impedes NFATc1 activation by calcineurin [11]. Cyclosporine A, which is an inhibitor of calcineurin, inhibited bone resorption by osteoclast in vitro [12,13], however, another group reported cyclosporine A did not affect bone resorption significantly [14]. The role of calcineurin in bone resorption might be controversial.
In this review, we will explain comprehensively important parts for inducing intracellular Ca oscillations (Figure 1). Initially, we will focus on costimulatory signals of osteoclastogenesis in the upstream of Ca oscillations. Next, we will discuss Ca2+ channels, especially transient receptor potential (TRP) channels. Intracellular storage also plays an important role in fine-tuning of Ca oscillations. Furthermore, environmental factors around osteoclasts affect intracellular Ca2+ concentration. Finally, we will present research perspectives on Ca oscillations in osteoclasts.
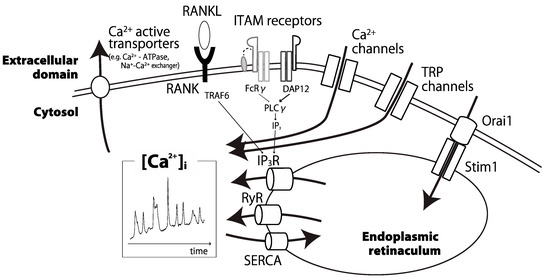
Figure 1.
Schematic representation of the main modifiers of calcium oscillations in osteoclasts. Some calcium channels, pumps, ITAM receptors, and intracellular organelles coordinate intracellular calcium oscillations in a proper manner. [Ca2+]i: intracellular calcium ion concentration; RANK: receptor activator of nuclear factor kappa B; RANKL: RANK ligand; ITAM: immunoreceptor tyrosine-based activation motif; FcRγ: Fc receptor gamma; DAP12: DNAX-activating protein of 12 kD; TRP: transient receptor potential; IP3: inositol 1,4,5-trisphosphate; RyR: ryanodine receptor; SERCA: sarco/endoplasmic reticulum Ca2+ ATPase.
2. Costimulatory Signals during Osteoclast Development
Appropriate Ca oscillations are necessary for NFATc1 auto-amplification in osteoclastogenesis [8]. Koga et al. reported that immunoreceptor tyrosine-based activation motif (ITAM)-harboring adaptors transduced costimulatory signals during osteoclastogenesis. ITAM receptor-adaptor complexes acted as key modulators of Ca oscillations in BMMs [15]. Phosphorylated ITAM adaptor proteins recruit Syk tyrosine kinase and induce Ca oscillations through activation of PLCγ [16]. Syk, c-Src, and αvβ3 integrin cooperate under costimulatory signaling [17]. Protein tyrosine kinase inhibitors disrupt actin organization and osteoclast activity [18].
Monocytes and BMMs have two different types of ITAM adapter proteins, DNAX activating protein of 12 kD (DAP12) and Fc receptor gamma (FcRγ). These two types of ITAM proteins have different roles in osteoclastogenesis.
Measurement methods of Ca oscillations in Section 2 were summarized on Table 1 in order of description.

Table 1.
Ca oscillations studies related to costimulatory signals in osteoclast differentiation.
2.1. DAP12
DAP12 was reported as a disease gene for Nasu–Hakola disease, and DAP12 KO mice show increased bone mass (osteopetrosis) [22]. Under DAP12 depletion, Ca oscillations are deactivated in osteoclast precursor cells [15]. The homolog DAP10 regulates osteoclastogenesis by cooperating with myeloid DAP12-associating lectin-1 (MDL-1) [23].
TREM-2 is a receptor associated with DAP12 on myeloid cells [24]. TREM-2 regulates osteoclast differentiation and function. In clinical settings, TREM-2-deficient patients exhibit increased immature osteoclasts and impaired bone resorption [25]. Interestingly, TREM-2 gene expression is not regulated by NFATc1 [26], and the anti-inflammatory cytokine interleukin-10 inhibits TREM-2 expression and costimulatory signals in osteoclasts [19].
Siglec proteins are plasmalemmal receptors that recognize sialylated glycans. Siglec-15, a receptor associated with DAP12, regulates osteoclast differentiation [27], and Siglec-15-deficient mice show osteopetrosis [28]. As DAP12-associated receptors, TREM-2 and Siglec-15 have important roles in osteoclastogenesis. However, the different roles for Ca oscillations among the receptors and DAP12 remain unclear.
2.2. FcRγ
In contrast to DAP12 and its coupling receptors, the role of FcRγ in osteoclastogenesis is ambiguous and environmentally dependent. Grevers et al. reported that Fcγ receptor activation by immune complexes inhibited osteoclastogenesis [29]. Meanwhile, Seeling et al. reported that IgG autoantibody binding to Fcγ receptors promoted osteoclast differentiation and bone resorption [30]. Moreover, Negishi-Koga et al. reported that osteoclastogenesis is regulated in accordance with the environmental inflammatory state. In the physiological state, FcRγ couples with PIR-A or OSCAR and promotes osteoclastogenesis. While, in the pathological inflammatory situation with an abundance with immune complex, FcRγ binds to several types of Fcγ receptors. FcγR I/III/IV strengthen FcRγ signaling, in contrast, FcγRIIB weaken FcRγ signaling. The coupling patterns of Fcγ receptors with FcRγ modify the strength of FcRγ signaling and affect osteoclastogenesis [20].
Our group showed that a rheumatic drug, cytotoxic T-lymphocyte antigen 4 (CTLA4)-Ig, inhibits osteoclastogenesis by interfering with Ca2+ signaling via FcRγ, representing the first report of a cofactor that affects costimulatory signals during osteoclast differentiation [21]. Osteoclastogenesis may be finely-tuned via FcRγ with immunoglobulins and related cofactors according to immunological situations.
DAP12 delivers costimulatory signals in a direct and straightforward manner during osteoclast differentiation. In contrast, the costimulatory signals through FcRγ are complicated and environment-dependent. It remains to be solved why the downstream mechanisms including Ca oscillations differ according to ITAM types.
3. Calcium Channels and Transporters in Osteoclast
Ca oscillations under costimulatory signal are necessary for osteoclastogenesis [31]. However, receptors of costimulatory signals themselves do not affect directly intracellular Ca concentration like Ca channels or transporters. Ca oscillations are evoked by membranous and intracellular organs in a coordinated manner. In this section, plasmalemmal components inducing Ca oscillations are discussed.

Table 2.
Ca oscillations studies related to Ca2+ channels and transporters.
3.1. TRP Family
Some ionic channels on osteoclasts are activated by Ca2+, voltage, and even cellular stretching [51]. In addition, outside fluid flow induces different Ca2+ alterations according to the osteoclast differentiation stage [52].
TRP channels are well-known to work as environmental sensors for factors such as environmental pressure, acid, taste, and temperature [53]. The TRP family members highly contribute to osteoclast differentiation and function.
TRP vanilloid 1 (TRPV1) was identified as a capsaicin receptor [54] and is sensitive to heat [55]. In TRPV1 knockout (KO) mice, osteoclast differentiation is attenuated by decreasing Ca oscillations. Osteoblast differentiation is also disrupted and fracture healing is delayed [56]. Pharmacological blockade of TRPV1 channels inhibits osteoclast and osteoblast differentiation, and alleviates bone loss induced by ovariectomy [57] and tail suspension [58].
TRPV2 is a 50% homolog of TRPV1 that mediates high-threshold noxious heat sensation [59]. RANKL induces TRPV2 expression, activates Ca oscillations, and induces osteoclastogenesis through Ca2+-NFAT pathway [32]. In multiple myeloma, TRPV2 enhanced Ca2+-calcineurin-NFAT signaling [33].
TRPV4, an approximate 40% homolog of TRPV1, transduces warm stimuli [60]. TRPV4 cooperates with STIM1 and mediates fluid flow-induced Ca oscillations in osteoclast differentiation [34]. TRPV4 induces Ca2+ influx, activates calmodulin signaling, and regulates late differentiation of osteoclasts [35,36]. TRPV4 depletion suppresses osteoclastogenesis through the Ca2+-calcineurin-NFAT pathway [61].
TRPV5 and TRPV6 are homomeric and heteromeric epithelial channels that exhibit the highest Ca2+ selectivity among the TRP channels [62]. TRPV5 mediates RANKL-induced intracellular Ca2+ increases and reduces bone resorption due to a negative feedback mechanism to reduce the bone resorptive activity of mature osteoclasts [37]. Estrogen increases TRPV5 expression and inhibits osteoclast differentiation [38]. Although TRPV6 is abundant in bone cells, it is not crucial for mineralization [63]. Meanwhile, TRPV6 depletion promotes osteoclastic differentiation and function, and results in osteopenia [64].
Within the TRP family, TRPV channels are highly involved in osteoclast differentiation and function. Focusing on TRP family members other than TRPV, TRP canonical 1 (TRPC1) [65], TRPC3, and TRPC6 [39] regulate Ca2+ storage in osteoclasts.
3.2. Voltage-Gated Ca2+ Channels
The plasmalemmal voltage altered the activities of some ionic channels, including Ca2+ channels, in electrophysiological experiments in osteoclasts [40,51]. For example, the T-type Ca2+ channel Cav3.2, a target of the anticonvulsant drug diphenylhydantoin, positively regulates Ca signaling, NFATc1 activation, and osteoclastogenesis [41].
Voltage-gated Ca2+ channels also control osteoclast podosome formation and bone resorption [42]. Electrical Ca2+ entry and store refilling also define osteoclast survival [66].
Some Ca2+ channel modulators alter osteoclast function. Ca2+ channel agonists open Ca2+ channels on osteoclasts and decrease bone resorption [67]. Intracellular elevation of cytosolic Ca2+ induces osteoclast migration [68].
Ca2+ channels are modulated by certain plasmalemmal proteins. For example, regulator of G protein signaling 12 (RGS12) is involved in late differentiation of osteoclasts by Ca oscillations via N-type Ca2+ channels [43]. RGS12 promotes osteoclastogenesis and results in pathological bone loss [44]. RGS12 also controls osteoblast differentiation via Ca oscillations and the Gαi-ERK pathway [69]. RGS10 is necessary for Ca oscillations, NFATc1 signaling, and osteoclastogenesis [45].
3.3. K+ Channels
High extracellular Ca2+ and H+ induce voltage-gated outward efflux of potassium (K+) [70]. The Ca2+-dependent K+ current activates osteoclast spreading kinetics [71]. In a recent paper, the Ca2+-activated K+ channel KCa3.1 is shown to regulate Ca2+-dependent NFATc1 expression in the inflammatory situation [46].
Meanwhile, K+ channels can restrict osteoclast differentiation. K+ channel subfamily K member 1 (KCNK1) inhibits osteoclastogenesis by blocking Ca oscillations [47]. High-K+ solution depolarizes the osteoclast membrane potential through K+ channels. As a result, the driving force for Ca2+ influx into the cells is diminished, and the intracellular Ca2+ concentration decreases [48].
3.4. Ca2+-ATPase and Na+-Ca2+ Exchanger
Ca2+-ATPase is a calcium transporter associated with ATP hydrolysis. Ca2+-ATPase regulates bone mass in vivo through osteoclast differentiation and survival. Ca2+-ATPase inhibitors increase intracellular Ca2+ and induce osteoclast formation in a coculture system [72]. Plasmalemmal Ca2+-ATPase maintains bone mass by reducing Ca oscillations and limiting osteoclast differentiation and survival [49]. Meanwhile, Na+-Ca2+ exchanger (NCX) is an active transporter that excretes Ca2+ extracellularly in exchange for Na+ uptake. NCX1 and NCX3 are expressed in mature osteoclasts and significantly increase the intracellular Ca2+ concentration by removing extracellular Na+ [50].
4. Intracellular Calcium Storage in Osteoclast
In addition to Ca2+ exchange with the extracellular domain, intracellular organelles that store Ca2+ are involved in cytoplasmic alterations to the intracellular Ca2+ concentration [66]. Ca2+ store refilling is closely related to osteoclast function. On electrochemical microscopy, bone-resorbing osteoclasts show intracellular functional Ca2+ compartments [73]. In this section, we will focus on the internal storehouse of Ca.

Table 3.
Ca oscillations studies related to intracellular calcium storage.
4.1. Endoplasmic Reticulum
The largest intracellular Ca2+ storage organelle is the endoplasmic reticulum (ER). Several calcium receptors on the ER membrane modulate the cytosolic Ca2+ concentration. Ryanodine receptor is localized on not only excitable cells such as muscle and nerve cells, but also non-excitable cells such as BMMs [86]. Ryanodine has an inhibitory effect on osteoclast function [74].
Inositol 1,4,5-trisphosphate (IP3) produced by phospholipase C (PLC) binds to IP3 receptors and releases Ca2+ from the ER to the cytoplasm. Although Ca oscillations do not occur in IP3 receptor type 2 KO mice, osteoclasts are generated. There is a complementary differentiation pathway that is independent of the Ca2+-NFATc1 axis [75].
Sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) is a calcium uptake pump on the ER. In SERCA2 heterozygote mice, RANKL-induced Ca oscillations do not occur and osteoclastogenesis is diminished [76].
Transmembrane (Tmem) proteins on the ER membrane are novel therapeutic targets for bone loss. For example, Tmem178, which is down-regulated by PLCγ2, is a negative regulator of Ca oscillations and osteoclastogenesis through modulation of the NFATc1 axis [77]. Meanwhile, Tmem64 is a positive regulator of osteoclastogenesis via SERCA2-dependent Ca2+ signaling [78].
Ca2+ release-activated Ca2+ (CRAC) channels are composed of plasmalemmal Orai1 and Stim1 on the ER membrane. Direct influx from extracellular domain into ER through CRAC channels occurs after RANKL stimulation [32,79,87]. CRAC channels are necessary in the late phase for cell fusion to produce multinucleated osteoclasts [80]. Another group showed CRAC channels are necessary for NFATc1 activation in the early phase of osteoclast differentiation [81]. Orai1-depleted mice exhibit skeletal impairments through inhibition of osteoclast and osteoblast differentiation [82,88]. Stim1 mutations are associated with immunodeficiency and dentition defects [89].
4.2. Lysosome and Mitochondria, and Nucleus
Lysosomes and mitochondria are typical Ca2+ storage organelles. Lysosomal Ca2+ release mediated by TRP family member TRPML1 is necessary for osteoclastogenesis [83]. Mitochondrial granules in bone-resorbing osteoclasts contain abundant Ca2+ [90].
The nucleus is another Ca2+ storage organelle. In stimulated osteoclasts, nuclear Ca2+ increases in a similar manner to cytosolic Ca2+ [84], and integrin receptors mediate the intranuclear Ca2+ concentration [85].
5. Environmental Factors Affecting Intracellular Calcium of Osteoclast
Ca channels, transporters, and intracellular organs are major components inducing Ca oscillations. However, we cannot miss environmental factors when explaining Ca oscillations. In this section, we will discuss on important outsiders affecting intracellular Ca concentration.

Table 4.
Ca oscillations studies related to environmental factors.
5.1. Extracellular Calcium and Calcium-Sensing Receptor
The extracellular free Ca2+ concentration is much higher than the intracellular concentration, especially in the bone microenvironment. Although there is a large difference between the Ca2+ concentrations inside and outside cells, intracellular Ca2+ is maintained within a narrow range, and subtle intracellular Ca2+ changes are commonly used for second messenger signaling. In osteoclasts, intracellular Ca oscillations are known to affect their differentiation and function according to the surrounding environment.
The extracellular Ca2+ concentration also affects osteoclast differentiation and function. Zaidi et al. reported that the high Ca2+ concentration at bone resorption sites led to a high Ca2+ concentration in osteoclasts and directly limited osteoclast function [91]. In contrast, other reports described that a high Ca2+ concentration stimulated osteoclast differentiation and bone-resorption function in a coculture system with osteoblasts [96,97,98]. The extracellular Ca2+ concentration also affects osteoclast migration [99]. Interestingly, Xiang et al. showed osteoclasts became attached to the bone surface when the Ca2+ concentration was low [100]. Furthermore, the external Ca2+ concentration has effects on osteoclast survival [101] and apoptosis [102].
Calcium-sensing receptor (CaSR) is a major transducer of information on the extracellular Ca2+ concentration to osteoclast precursors and mature osteoclasts. A high Ca2+ concentration directly promotes osteoclastogenesis via CaSR [103]. CaSR is also present in mature osteoclasts [104]. In bone growth plate maturation, CaSR is necessary for 25-hydroxyvitamin D-1α-hydroxylase, which modulates systemic Ca2+ concentration, to evoke calcium-stimulated bone erosion [105]. In addition, CaSR is involved in osteoclast apoptosis [106,107]. The downstream of CaSR contains the phosphoinositide 3-kinase/Akt pathway [108] and RANKL signaling pathway [92].
The systemic serum Ca2+ level is maintained by hormonal regulation, including parathyroid hormone and vitamin D. Calcitonin secreted by the thyroid gland also regulates systemic Ca2+. Calcitonin inhibits bone resorption and Ca2+ release from bone through calcitonin receptors on osteoclasts [109]. In addition, calcitonin-induced Ca2+ decreases affect osteoclast shape and bone resorption [110,111]. In a recent paper, calcitonin was shown to induce bone formation by interrupting sphingosine 1-phosphate release from osteoclasts [112].
5.2. Protons and Reactive Oxygen Species
The extracellular proton level measured by pH is the simplest factor involved in the intracellular Ca2+ concentration. Acid-sensing ion channel 1a promotes acid-induced osteoclastogenesis [93]. Kato and Matsushita showed that protons contributed to osteoclast and osteoblast differentiation independently of bicarbonate ions [113].
In the acidified situation, osteoclasts decrease their cytosolic Ca2+, synthesize extracellular matrix [94], and promote bone resorption [114]. In addition, accumulation of acids and Ca2+ acts as negative feedback for vacuolar-type H+-ATPase [115].
Another environmental factor involved in the fine-tuning of Ca oscillations is oxidative stress. Reactive oxygen species promotes osteoclast differentiation via NF-κB activation and Ca2+ efflux from endoplasmic reticulum [116]. RANKL induced reactive oxygen species production and enduring Ca oscillations [87]. Osteoclast differentiation decreased with the disruption of oxidative stress and Ca2+ signaling by asperpyrone A [95].
6. Perspectives for Research on Ca Oscillations in Osteoclast
In this review, we have described the key players that influence intracellular Ca oscillations. In the last section, we will focus on unsolved questions around Ca oscillations in osteoclast differentiation, function, and apoptosis.
6.1. No Consensus for the Definition of Ca Oscillations or Spikes
There is no common definition of Ca oscillations. This is the most critical problem associated with discussing Ca oscillations in osteoclasts. Many papers have shown line graphs for fluorescence ratios of Ca2+ indicators, Fura-2, or Fluo-4 and Fura Red. Qualitative assessment of Ca oscillations by appearance has been separately performed by the authors and their readers. Although some papers tried quantitative assessment with frequency and amplitude of spike or peak, these methods are in a minority (tables).
A lack of intracellular Ca2+ changes is not a physiological condition for viable cells. However, Ca oscillations were often binarized and regarded as all-or-none phenomena. Knockout of specific molecules often abolished Ca oscillations. Ca oscillations tended to be underestimated, and subtle changes in oscillations were omitted.
Our group proposed a new analytical method for Ca oscillations in a recent paper [21]. Concretely speaking, time-series Ca oscillations can be transformed into a frequency domain and evaluated in terms of speed. Fast Fourier transformation and wavelet analysis may be useful for detecting quick responses to environmental stimuli. A gold standard for Ca oscillation analysis should be established with modern computer technology and physio-mathematical methods.
6.2. Ca oscillation Alterations According to the Differentiation Time Course
Another problem is the variation in focused timing of osteoclastogenesis in different research. Experimental setting differs by each research (tables). In addition, Ca oscillations have not been well examined in the early phase of osteoclast differentiation, because Ca2+ signaling is considered weak in the immature phase. However, even in BMMs or osteoclast precursor cells, spontaneous Ca oscillations can be observed [21]. The Ca oscillation changes during the time course of osteoclast differentiation from BMMs to mature osteoclasts should be discussed in detail.
6.3. Whether Macrophage Colony-Stimulating Factors (M-CSF) or RANKL Can Evoke Ca Oscillations?
Surprisingly, it remains unclear whether RANKL can evoke Ca oscillations in electrically stable cells such as BMMs. Though some papers showed Ca2+ spike by acute RANKL stimulation, some research did not.
In our experiments, RANKL did not induce rapid responses to intracellular Ca oscillations [21]. It is difficult to separate the effect of RANKL on Ca oscillations from those of other environmental factors including extracellular Ca2+ itself, pH, temperature, and macrophage colony-stimulating factors (M-CSF; CSF-1), as other stimuli required for osteoclastogenesis. Accumulation of well-controlled observations would solve the simple but profound question of the contribution ratio of each component.
6.4. Direct Relationships between ITAM Receptors and Ca2+ Channels, Transporters, and Storage Organelles?
Immunoreceptor tyrosine-based activation motifs (ITAMs) are regarded as necessary costimulatory signals during osteoclastogenesis [15]. However, it remains unclear how costimulatory signals including downstream phosphorylation of Syk and PLC are directly linked to quick Ca oscillations and calcineurin activity changes. The frequency conversion mechanism for costimulatory signals should be examined with modern technology. For example, a visualization technique for the phosphorylation status of single molecules would provide clues. Appropriate usage of calcium indicators and imaging techniques is considerably important.
6.5. Identification of the Conductor of Finely-Tuned Ca Oscillations and Clarification of the True Causal Relationship between Ca Oscillations and Osteoclast Differentiation
Previous papers have shown that many molecules are involved in fine-tuning of Ca oscillations in a cooperative manner. Ca oscillations in osteoclastogenesis appear to act in a coordinated and orchestrated manner through proper regulation of calcium channels, pumps, and intracellular organelles. However, we do not have evidence on the mechanism for the integrated coordination of the components and their contributions to the harmony of the process. This is partly because Ca oscillations in osteoclastogenesis have mainly been examined in deteriorating, rather than physiological, situations in some KO mice. To reveal the underlying mechanism for the orchestration, novel approaches other than the use of single inhibitors or knockdown may be necessary.
Furthermore, we cannot reach a conclusion on whether Ca oscillations are a cause or a result of osteoclastogenesis. If we can create proper Ca2+ changes and accomplish the induction of osteoclasts without M-CSF and RANKL stimuli, Ca oscillations may be proven as a cause of osteoclast differentiation. However, we cannot realize such artificial osteoclast induction with the currently available technology.
7. Conclusions
In osteoclast differentiation, many actors play to maintain Ca oscillations (Figure 1). Recent studies showed that the balance and the strength of costimulatory signals producing optimal Ca oscillations are important. Ca2+ channels and transporters work in harmony to alter intracellular Ca2+ concentration. Transient receptor potential (TRP) channels on cell membrane are key tuners of Ca oscillations in adaptation to the environment.
Osteoclast differentiation has not yet been resolved, because the fundamental mechanism underlying the well-known phenomena of Ca oscillations remains unclear. There may be hidden therapeutic target points in the electrophysiological regulation of osteoclast differentiation and function. Further investigations on Ca oscillations will lead to comprehensive understanding of osteoclasts.
Author Contributions
Writing—original draft preparation, H.O.; art-work, H.O. and K.O.; review, editing, H.O., K.O., and S.T.; conceptualization, H.O. and K.O.; supervision K.O. and S.T.; All authors made direct and intellectual contributions to this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by JSPS KAKENHI (grant number: JP 18K09017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Ca | Calcium |
| BMM | Bone marrow macrophage |
| M-CSF | Macrophage-colony stimulating factor |
| CSF-1 | Colony stimulating factor-1 |
| RANKL | Receptor activator of nuclear factor kappa B ligand |
| NFATc1 | Nuclear factor of activated T-cells 1 |
| TRP | Transient receptor potential |
| IP3 | Inositol 1,4,5-trisphosphate |
| RyR | Ryanodine receptor |
| SERCA | Sarco/endoplasmic reticulum Ca2+ ATPase |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| FcRγ | Fc receptor gamma |
| DAP12 | DNAX-activating protein of 12 kD |
| CTLA4 | Cytotoxic T-lymphocyte antigen 4 |
| KO | Knock out |
| TRPV | Transient receptor potential vanilloid |
| TRPC | Transient receptor potential canonical |
| RGS | Regulator of G protein signaling |
| KD | Knock down |
| KCNK | K+ channel subfamily K member |
| NCX | Na+-Ca2+ exchanger |
| BM | Bone marrow |
| ER | Endoplasmic reticulum |
| PLC | Phospholipase C |
| Tmem | Transmembrane |
| CRAC | Ca2+ release-activated Ca2+ |
| CaSR | Calcium-sensing receptor |
References
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016, 291, 20849–20857. [Google Scholar] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [PubMed]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Kistamas, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [PubMed]
- Swann, K. The role of Ca2+ in oocyte activation during In Vitro fertilization: Insights into potential therapies for rescuing failed fertilization. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1830–1837. [Google Scholar] [PubMed]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar]
- Hirotani, H.; Tuohy, N.A.; Woo, J.T.; Stern, P.H.; Clipstone, N.A. The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J. Biol. Chem. 2004, 279, 13984–13992. [Google Scholar]
- Kamano, Y.; Watanabe, J.; Iida, T.; Kondo, T.; Okawa, H.; Yatani, H.; Saeki, M.; Egusa, H. Binding of PICK1 PDZ domain with calcineurin B regulates osteoclast differentiation. Biochem. Biophys. Res. Commun. 2018, 496, 83–88. [Google Scholar]
- Huynh, H.; Wan, Y. mTORC1 impedes osteoclast differentiation via calcineurin and NFATc1. Commun. Biol. 2018, 1, 29. [Google Scholar] [PubMed]
- Stewart, P.J.; Green, O.C.; Stern, P.H. Cyclosporine A inhibits calcemic hormone-induced bone resorption in vitro. J. Bone Miner. Res. 1986, 1, 285–291. [Google Scholar] [PubMed]
- Awumey, E.M.; Moonga, B.S.; Sodam, B.R.; Koval, A.P.; Adebanjo, O.A.; Kumegawa, M.; Zaidi, M.; Epstein, S. Molecular and functional evidence for calcineurin-A alpha and beta isoforms in the osteoclast: Novel insights into cyclosporin A action on bone resorption. Biochem. Biophys. Res. Commun. 1999, 254, 248–252. [Google Scholar] [PubMed]
- Williams, J.P.; McKenna, M.A.; Thames, A.M., 3rd; McDonald, J.M. Effects of cyclosporine on osteoclast activity: Inhibition of calcineurin activity with minimal effects on bone resorption and acid transport activity. J. Bone Miner. Res. 2003, 18, 451–457. [Google Scholar] [PubMed]
- Koga, T.; Inui, M.; Inoue, K.; Kim, S.; Suematsu, A.; Kobayashi, E.; Iwata, T.; Ohnishi, H.; Matozaki, T.; Kodama, T.; et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 2004, 428, 758–763. [Google Scholar] [PubMed]
- Mocsai, A.; Humphrey, M.B.; Van Ziffle, J.A.; Hu, Y.; Burghardt, A.; Spusta, S.C.; Majumdar, S.; Lanier, L.L.; Lowell, C.A.; Nakamura, M.C. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 6158–6163. [Google Scholar] [PubMed]
- Zou, W.; Kitaura, H.; Reeve, J.; Long, F.; Tybulewicz, V.L.; Shattil, S.J.; Ginsberg, M.H.; Ross, F.P.; Teitelbaum, S.L. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J. Cell Biol. 2007, 176, 877–888. [Google Scholar]
- Kajiya, H.; Okabe, K.; Okamoto, F.; Tsuzuki, T.; Soeda, H. Protein tyrosine kinase inhibitors increase cytosolic calcium and inhibit actin organization as resorbing activity in rat osteoclasts. J. Cell. Physiol. 2000, 183, 83–90. [Google Scholar]
- Park-Min, K.H.; Ji, J.D.; Antoniv, T.; Reid, A.C.; Silver, R.B.; Humphrey, M.B.; Nakamura, M.; Ivashkiv, L.B. IL-10 suppresses calcium-mediated costimulation of receptor activator NF-kappa B signaling during human osteoclast differentiation by inhibiting TREM-2 expression. J. Immunol. 2009, 183, 2444–2455. [Google Scholar]
- Negishi-Koga, T.; Gober, H.J.; Sumiya, E.; Komatsu, N.; Okamoto, K.; Sawa, S.; Suematsu, A.; Suda, T.; Sato, K.; Takai, T.; et al. Immune complexes regulate bone metabolism through FcRgamma signalling. Nat. Commun. 2015, 6, 6637. [Google Scholar]
- Okada, H.; Kajiya, H.; Omata, Y.; Matsumoto, T.; Sato, Y.; Kobayashi, T.; Nakamura, S.; Kaneko, Y.; Nakamura, S.; Koyama, T.; et al. CTLA4-Ig Directly Inhibits Osteoclastogenesis by Interfering With Intracellular Calcium Oscillations in Bone Marrow Macrophages. J. Bone Miner. Res. 2019, 34, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Kaifu, T.; Nakahara, J.; Inui, M.; Mishima, K.; Momiyama, T.; Kaji, M.; Sugahara, A.; Koito, H.; Ujike-Asai, A.; Nakamura, A.; et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Investig. 2003, 111, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Inui, M.; Kikuchi, Y.; Aoki, N.; Endo, S.; Maeda, T.; Sugahara-Tobinai, A.; Fujimura, S.; Nakamura, A.; Kumanogoh, A.; Colonna, M.; et al. Signal adaptor DAP10 associates with MDL-1 and triggers osteoclastogenesis in cooperation with DAP12. Proc. Natl. Acad. Sci. USA 2009, 106, 4816–4821. [Google Scholar] [CrossRef] [PubMed]
- Daws, M.R.; Lanier, L.L.; Seaman, W.E.; Ryan, J.C. Cloning and characterization of a novel mouse myeloid DAP12-associated receptor family. Eur. J. Immunol. 2001, 31, 783–791. [Google Scholar] [CrossRef]
- Cella, M.; Buonsanti, C.; Strader, C.; Kondo, T.; Salmaggi, A.; Colonna, M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J. Exp. Med. 2003, 198, 645–651. [Google Scholar] [CrossRef]
- Kim, Y.; Sato, K.; Asagiri, M.; Morita, I.; Soma, K.; Takayanagi, H. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J. Biol. Chem. 2005, 280, 32905–32913. [Google Scholar] [CrossRef]
- Hiruma, Y.; Hirai, T.; Tsuda, E. Siglec-15, a member of the sialic acid-binding lectin, is a novel regulator for osteoclast differentiation. Biochem. Biophys. Res. Commun. 2011, 409, 424–429. [Google Scholar] [CrossRef]
- Hiruma, Y.; Tsuda, E.; Maeda, N.; Okada, A.; Kabasawa, N.; Miyamoto, M.; Hattori, H.; Fukuda, C. Impaired osteoclast differentiation and function and mild osteopetrosis development in Siglec-15-deficient mice. Bone 2013, 53, 87–93. [Google Scholar] [CrossRef]
- Grevers, L.C.; de Vries, T.J.; Everts, V.; Verbeek, J.S.; van den Berg, W.B.; van Lent, P.L. Immune complex-induced inhibition of osteoclastogenesis is mediated via activating but not inhibitory Fcgamma receptors on myeloid precursor cells. Ann. Rheum. Dis. 2013, 72, 278–285. [Google Scholar] [CrossRef]
- Seeling, M.; Hillenhoff, U.; David, J.P.; Schett, G.; Tuckermann, J.; Lux, A.; Nimmerjahn, F. Inflammatory monocytes and Fcgamma receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 10729–10734. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, H.; Okamoto, F.; Nemoto, T.; Kimachi, K.; Toh-Goto, K.; Nakayana, S.; Okabe, K. RANKL-induced TRPV2 expression regulates osteoclastogenesis via calcium oscillations. Cell Calcium 2010, 48, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Zhu, H.; Yan, Q.; Shen, X.; Lu, X.; Wang, J.; Li, J.; Chen, L. TRPV2-induced Ca2+-calcineurin-NFAT signaling regulates differentiation of osteoclast in multiple myeloma. Cell Commun. Signal. 2018, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Bian, X.; Liu, C.; Wang, S.; Guo, M.; Tao, Y.; Huo, B. STIM1 and TRPV4 regulate fluid flow-induced calcium oscillation at early and late stages of osteoclast differentiation. Cell Calcium 2018, 71, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, R.; Vriens, J.; Voets, T.; Karashima, Y.; Owsianik, G.; Vennekens, R.; Lieben, L.; Torrekens, S.; Moermans, K.; Vanden Bosch, A.; et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008, 8, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, R.; Mizuno, A.; Komori, H.; Kajiya, H.; Uekawa, A.; Kitaura, H.; Okabe, K.; Ohyama, K.; Komori, T. Calcium/calmodulin-signaling supports TRPV4 activation in osteoclasts and regulates bone mass. J. Bone Miner. Res. 2012, 27, 1708–1721. [Google Scholar] [CrossRef] [PubMed]
- Chamoux, E.; Bisson, M.; Payet, M.D.; Roux, S. TRPV-5 mediates a receptor activator of NF-kappaB (RANK) ligand-induced increase in cytosolic Ca2+ in human osteoclasts and down-regulates bone resorption. J. Biol. Chem. 2010, 285, 25354–25362. [Google Scholar] [CrossRef]
- Chen, F.; Ouyang, Y.; Ye, T.; Ni, B.; Chen, A. Estrogen inhibits RANKL-induced osteoclastic differentiation by increasing the expression of TRPV5 channel. J. Cell. Biochem. 2014, 115, 651–658. [Google Scholar] [CrossRef]
- Klein, S.; Mentrup, B.; Timmen, M.; Sherwood, J.; Lindemann, O.; Fobker, M.; Kronenberg, D.; Pap, T.; Raschke, M.J.; Stange, R. Modulation of Transient Receptor Potential Channels 3 and 6 Regulates Osteoclast Function with Impact on Trabecular Bone Loss. Calcif. Tissue Int. 2020, 106, 655–664. [Google Scholar] [CrossRef]
- Pazianas, M.; Zaidi, M.; Huang, C.L.; Moonga, B.S.; Shankar, V.S. Voltage sensitivity of the osteoclast calcium receptor. Biochem. Biophys. Res. Commun. 1993, 192, 1100–1105. [Google Scholar] [CrossRef]
- Koide, M.; Kinugawa, S.; Ninomiya, T.; Mizoguchi, T.; Yamashita, T.; Maeda, K.; Yasuda, H.; Kobayashi, Y.; Nakamura, H.; Takahashi, N.; et al. Diphenylhydantoin inhibits osteoclast differentiation and function through suppression of NFATc1 signaling. J. Bone Miner. Res. 2009, 24, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, A.; Hruska, K.A.; Greenfield, E.M.; Duncan, R.; Alvarez, J.; Barattolo, R.; Colucci, S.; Zambonin-Zallone, A.; Teitelbaum, S.L.; Teti, A. Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J. Cell Biol. 1990, 111, 2543–2552. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.P. RGS12 is essential for RANKL-evoked signaling for terminal differentiation of osteoclasts in vitro. J. Bone Miner. Res. 2007, 22, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Cao, J.; Liu, T.; Li, Y.P.; Scannapieco, F.; He, X.; Oursler, M.J.; Zhang, X.; Vacher, J.; Li, C.; et al. Regulators of G protein signaling 12 promotes osteoclastogenesis in bone remodeling and pathological bone loss. Cell Death Differ. 2015, 22, 2046–2057. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.P. RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca2+]i oscillation regulation. Genes Dev. 2007, 21, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Grossinger, E.M.; Kang, M.; Bouchareychas, L.; Sarin, R.; Haudenschild, D.R.; Borodinsky, L.N.; Adamopoulos, I.E. Ca2+-Dependent Regulation of NFATc1 via KCa3.1 in Inflammatory Osteoclastogenesis. J. Immunol. 2018, 200, 749–757. [Google Scholar] [CrossRef]
- Yeon, J.T.; Kim, K.J.; Chun, S.W.; Lee, H.I.; Lim, J.Y.; Son, Y.J.; Kim, S.H.; Choi, S.W. KCNK1 inhibits osteoclastogenesis by blocking the Ca2+ oscillation and JNK-NFATc1 signaling axis. J. Cell Sci. 2015, 128, 3411–3419. [Google Scholar] [CrossRef]
- Kajiya, H.; Okamoto, F.; Fukushima, H.; Takada, K.; Okabe, K. Mechanism and role of high-potassium-induced reduction of intracellular Ca2+ concentration in rat osteoclasts. Am. J. Physiol. Cell Physiol. 2003, 285, C457–C466. [Google Scholar] [CrossRef]
- Kim, H.J.; Prasad, V.; Hyung, S.W.; Lee, Z.H.; Lee, S.W.; Bhargava, A.; Pearce, D.; Lee, Y.; Kim, H.H. Plasma membrane calcium ATPase regulates bone mass by fine-tuning osteoclast differentiation and survival. J. Cell Biol. 2012, 199, 1145–1158. [Google Scholar] [CrossRef]
- Li, J.P.; Kajiya, H.; Okamoto, F.; Nakao, A.; Iwamoto, T.; Okabe, K. Three Na+/ Ca2+ exchanger (NCX) variants are expressed in mouse osteoclasts and mediate calcium transport during bone resorption. Endocrinology 2007, 148, 2116–2125. [Google Scholar] [CrossRef]
- Ypey, D.L.; Weidema, A.F.; Hold, K.M.; Van der Laarse, A.; Ravesloot, J.H.; Van Der Plas, A.; Nijweide, P.J. Voltage, calcium, and stretch activated ionic channels and intracellular calcium in bone cells. J. Bone Miner. Res. 1992, 7 (Suppl. 2), S377–S387. [Google Scholar] [CrossRef]
- Li, P.; Hu, M.; Sun, S.; Zhang, Y.; Gao, Y.; Long, M.; Huo, B.; Zhang, D. Fluid flow-induced calcium response in early or late differentiated osteoclasts. Ann. Biomed. Eng. 2012, 40, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.-E.; Julius, D. Molecular Basis for Species-Specific Sensitivity to “Hot” Chili Peppers. Cell 2002, 108, 421–430. [Google Scholar] [CrossRef]
- He, L.H.; Liu, M.; He, Y.; Xiao, E.; Zhao, L.; Zhang, T.; Yang, H.Q.; Zhang, Y. TRPV1 deletion impaired fracture healing and inhibited osteoclast and osteoblast differentiation. Sci. Rep. 2017, 7, 42385. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; Landao-Bassonga, E.; Ralston, S.H. The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone 2010, 46, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Hanaka, M.; Iba, K.; Dohke, T.; Kanaya, K.; Okazaki, S.; Yamashita, T. Antagonists to TRPV1, ASICs and P2X have a potential role to prevent the triggering of regional bone metabolic disorder and pain-like behavior in tail-suspended mice. Bone 2018, 110, 284–294. [Google Scholar] [CrossRef]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Güler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-Evoked Activation of the Ion Channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef]
- Cao, B.; Dai, X.; Wang, W. Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca2+-calcineurin-NFATc1 pathway. J. Cell. Physiol. 2019, 234, 6831–6841. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.; Voets, T.; Hoefs, S.; Weidema, F.; Prenen, J.; Nilius, B.; Bindels, R.J. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 2003, 22, 776–785. [Google Scholar] [CrossRef] [PubMed]
- van der Eerden, B.C.; Weissgerber, P.; Fratzl-Zelman, N.; Olausson, J.; Hoenderop, J.G.; Schreuders-Koedam, M.; Eijken, M.; Roschger, P.; de Vries, T.J.; Chiba, H.; et al. The transient receptor potential channel TRPV6 is dynamically expressed in bone cells but is not crucial for bone mineralization in mice. J. Cell. Physiol. 2012, 227, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ni, B.; Yang, Y.O.; Ye, T.; Chen, A. Knockout of TRPV6 causes osteopenia in mice by increasing osteoclastic differentiation and activity. Cell. Physiol. Biochem. 2014, 33, 796–809. [Google Scholar] [CrossRef]
- Ong, E.C.; Nesin, V.; Long, C.L.; Bai, C.X.; Guz, J.L.; Ivanov, I.P.; Abramowitz, J.; Birnbaumer, L.; Humphrey, M.B.; Tsiokas, L. A TRPC1 protein-dependent pathway regulates osteoclast formation and function. J. Biol. Chem. 2013, 288, 22219–22232. [Google Scholar] [CrossRef]
- Mentaverri, R.; Kamel, S.; Brazier, M. Involvement of capacitive calcium entry and calcium store refilling in osteoclastic survival and bone resorption process. Cell Calcium 2003, 34, 169–175. [Google Scholar] [CrossRef]
- Ritchie, C.K.; Maercklein, P.B.; Fitzpatrick, L.A. Direct effect of calcium channel antagonists on osteoclast function: Alterations in bone resorption and intracellular calcium concentrations. Endocrinology 1994, 135, 996–1003. [Google Scholar] [CrossRef]
- Wheal, B.D.; Beach, R.J.; Tanabe, N.; Dixon, S.J.; Sims, S.M. Subcellular elevation of cytosolic free calcium is required for osteoclast migration. J. Bone Miner. Res. 2014, 29, 725–734. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Gilmore, A.; Gomez, N.M.; Fu, C.; Lim, J.; Yang, S.; Mitchell, C.H.; Li, Y.P.; Oursler, M.J.; et al. Regulator of G Protein Signaling Protein 12 (Rgs12) Controls Mouse Osteoblast Differentiation via Calcium Channel/Oscillation and Galphai-ERK Signaling. J. Bone Miner. Res. 2019, 34, 752–764. [Google Scholar] [CrossRef]
- Arkett, S.A.; Dixon, S.J.; Sims, S.M. Effects of extracellular calcium and protons on osteoclast potassium currents. J. Membr. Biol. 1994, 140, 163–171. [Google Scholar] [CrossRef]
- Espinosa, L.; Paret, L.; Ojeda, C.; Tourneur, Y.; Delmas, P.D.; Chenu, C. Osteoclast spreading kinetics are correlated with an oscillatory activation of a calcium-dependent potassium current. J. Cell Sci. 2002, 115, 3837–3848. [Google Scholar] [CrossRef] [PubMed]
- Takami, M.; Woo, J.T.; Takahashi, N.; Suda, T.; Nagai, K. Ca2+-ATPase inhibitors and Ca2+-ionophore induce osteoclast-like cell formation in the cocultures of mouse bone marrow cells and calvarial cells. Biochem. Biophys. Res. Commun. 1997, 237, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.E.; Rathod, H.; Gillespie, J.I.; Horrocks, B.R.; Datta, H.K. Scanning electrochemical microscopy at the surface of bone-resorbing osteoclasts: Evidence for steady-state disposal and intracellular functional compartmentalization of calcium. J. Bone Miner. Res. 2001, 16, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.K.; Strei, T.A.; Maercklein, P.B.; Fitzpatrick, L.A. Antithetic effects of ryanodine and ruthenium red on osteoclast-mediated bone resorption and intracellular calcium concentrations. J. Cell Biochem. 1995, 59, 281–289. [Google Scholar] [CrossRef]
- Kuroda, Y.; Hisatsune, C.; Nakamura, T.; Matsuo, K.; Mikoshiba, K. Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 8643–8648. [Google Scholar] [CrossRef]
- Yang, Y.M.; Kim, M.S.; Son, A.; Hong, J.H.; Kim, K.H.; Seo, J.T.; Lee, S.I.; Shin, D.M. Alteration of RANKL-induced osteoclastogenesis in primary cultured osteoclasts from SERCA2+/− mice. J. Bone Miner. Res. 2009, 24, 1763–1769. [Google Scholar] [CrossRef]
- Decker, C.E.; Yang, Z.; Rimer, R.; Park-Min, K.H.; Macaubas, C.; Mellins, E.D.; Novack, D.V.; Faccio, R. Tmem178 acts in a novel negative feedback loop targeting NFATc1 to regulate bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 15654–15659. [Google Scholar] [CrossRef]
- Kim, H.; Kim, T.; Jeong, B.C.; Cho, I.T.; Han, D.; Takegahara, N.; Negishi-Koga, T.; Takayanagi, H.; Lee, J.H.; Sul, J.Y.; et al. Tmem64 modulates calcium signaling during RANKL-mediated osteoclast differentiation. Cell Metab. 2013, 17, 249–260. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Q.; Feng, Z.; Zheng, L. STIM1 controls calcineurin/Akt/mTOR/NFATC2-mediated osteoclastogenesis induced by RANKL/M-CSF. Exp. Ther. Med. 2020, 20, 736–747. [Google Scholar] [CrossRef]
- Zhou, Y.; Lewis, T.L.; Robinson, L.J.; Brundage, K.M.; Schafer, R.; Martin, K.H.; Blair, H.C.; Soboloff, J.; Barnett, J.B. The role of calcium release activated calcium channels in osteoclast differentiation. J. Cell. Physiol. 2011, 226, 1082–1089. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Putney, J.W. Orai1-mediated calcium entry plays a critical role in osteoclast differentiation and function by regulating activation of the transcription factor NFATc1. FASEB J. 2012, 26, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.Y.; Foley, J.; Numaga-Tomita, T.; Petranka, J.G.; Bird, G.S.; Putney, J.W., Jr. Deletion of Orai1 alters expression of multiple genes during osteoclast and osteoblast maturation. Cell Calcium 2012, 52, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Erkhembaatar, M.; Gu, D.R.; Lee, S.H.; Yang, Y.M.; Park, S.; Muallem, S.; Shin, D.M.; Kim, M.S. Lysosomal Ca2+ Signaling is Essential for Osteoclastogenesis and Bone Remodeling. J. Bone Miner. Res. 2017, 32, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, N.; Bolsover, S.; Mason, W. Nuclear and cytosolic calcium changes in osteoclasts stimulated with ATP and integrin-binding peptide. Cell Calcium 1998, 24, 213–221. [Google Scholar] [CrossRef]
- Shankar, G.; Davison, I.; Helfrich, M.H.; Mason, W.T.; Horton, M.A. Integrin receptor-mediated mobilisation of intranuclear calcium in rat osteoclasts. J. Cell Sci. 1993, 105 Pt 1, 61–68. [Google Scholar]
- Huang, C.L.; Sun, L.; Fraser, J.A.; Grace, A.A.; Zaidi, M. Similarities and contrasts in ryanodine receptor localization and function in osteoclasts and striated muscle cells. Ann. N. Y. Acad. Sci. 2007, 1116, 255–270. [Google Scholar] [CrossRef]
- Kim, M.S.; Yang, Y.M.; Son, A.; Tian, Y.S.; Lee, S.I.; Kang, S.W.; Muallem, S.; Shin, D.M. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J. Biol. Chem. 2010, 285, 6913–6921. [Google Scholar] [CrossRef]
- Robinson, L.J.; Mancarella, S.; Songsawad, D.; Tourkova, I.L.; Barnett, J.B.; Gill, D.L.; Soboloff, J.; Blair, H.C. Gene disruption of the calcium channel Orai1 results in inhibition of osteoclast and osteoblast differentiation and impairs skeletal development. Lab. Investig. 2012, 92, 1071–1083. [Google Scholar] [CrossRef]
- Picard, C.; McCarl, C.A.; Papolos, A.; Khalil, S.; Luthy, K.; Hivroz, C.; LeDeist, F.; Rieux-Laucat, F.; Rechavi, G.; Rao, A.; et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N. Engl. J. Med. 2009, 360, 1971–1980. [Google Scholar] [CrossRef]
- Kawahara, I.; Koide, M.; Tadokoro, O.; Udagawa, N.; Nakamura, H.; Takahashi, N.; Ozawa, H. The relationship between calcium accumulation in osteoclast mitochondrial granules and bone resorption. Bone 2009, 45, 980–986. [Google Scholar] [CrossRef]
- Zaidi, M.; Datta, H.K.; Patchell, A.; Moonga, B.; MacIntyre, I. ‘Calcium-activated‘ intracellular calcium elevation: A novel mechanism of osteoclast regulation. Biochem. Biophys. Res. Commun. 1989, 163, 1461–1465. [Google Scholar] [CrossRef]
- Xu, J.; Wang, C.; Han, R.; Pavlos, N.; Phan, T.; Steer, J.H.; Bakker, A.J.; Joyce, D.A.; Zheng, M.H. Evidence of reciprocal regulation between the high extracellular calcium and RANKL signal transduction pathways in RAW cell derived osteoclasts. J. Cell. Physiol. 2005, 202, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, R.S.; Jiang, D.L.; He, X.L.; Jin, C.; Lu, W.G.; Su, Q.; Yuan, F.L. Acid-sensing ion channel 1a is involved in acid-induced osteoclastogenesis by regulating activation of the transcription factor NFATc1. FEBS Lett. 2013, 587, 3236–3242. [Google Scholar] [CrossRef] [PubMed]
- Teti, A.; Blair, H.C.; Schlesinger, P.; Grano, M.; Zambonin-Zallone, A.; Kahn, A.J.; Teitelbaum, S.L.; Hruska, K.A. Extracellular protons acidify osteoclasts, reduce cytosolic calcium, and promote expression of cell-matrix attachment structures. J. Clin. Investig. 1989, 84, 773–780. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Qiu, H.; Yuan, Y.; Chen, K.; Cao, Z.; Xiang Tan, R.; Tickner, J.; Xu, J.; Zou, J. Asperpyrone A attenuates RANKL-induced osteoclast formation through inhibiting NFATc1, Ca2+ signalling and oxidative stress. J. Cell. Mol. Med. 2019, 23, 8269–8279. [Google Scholar] [CrossRef]
- Kaji, H.; Sugimoto, T.; Kanatani, M.; Chihara, K. High extracellular calcium stimulates osteoclast-like cell formation and bone-resorbing activity in the presence of osteoblastic cells. J. Bone Miner. Res. 1996, 11, 912–920. [Google Scholar] [CrossRef]
- Shirai, Y.; Yoshimura, Y.; Yawaka, Y.; Hasegawa, T.; Kikuiri, T.; Takeyama, S.; Matsumoto, A.; Oguchi, H. Effect of extracellular calcium concentrations on osteoclast differentiation in vitro. Biochem. Biophys. Res. Commun. 1999, 265, 484–488. [Google Scholar] [CrossRef]
- Shin, M.M.; Kim, Y.H.; Kim, S.N.; Kim, G.S.; Baek, J.H. High extracellular Ca2+ alone stimulates osteoclast formation but inhibits in the presence of other osteoclastogenic factors. Exp. Mol. Med. 2003, 35, 167–174. [Google Scholar] [CrossRef]
- Xiang, B.; Liu, Y.; Zhao, W.; Zhao, H.; Yu, H. Extracellular calcium regulates the adhesion and migration of osteoclasts via integrin alphav beta 3 /Rho A/Cytoskeleton signaling. Cell Biol. Int. 2019, 43, 1125–1136. [Google Scholar] [CrossRef]
- Xiang, B.; Liu, Y.; Xie, L.; Zhao, Q.; Zhang, L.; Gan, X.; Yu, H. The osteoclasts attach to the bone surface where the extracellular calcium concentration decreases. Cell Biochem. Biophys. 2016, 74, 553–558. [Google Scholar] [CrossRef]
- Nielsen, R.H.; Karsdal, M.A.; Sorensen, M.G.; Dziegiel, M.H.; Henriksen, K. Dissolution of the inorganic phase of bone leading to release of calcium regulates osteoclast survival. Biochem. Biophys. Res. Commun. 2007, 360, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Lorget, F.; Kamel, S.; Mentaverri, R.; Wattel, A.; Naassila, M.; Maamer, M.; Brazier, M. High extracellular calcium concentrations directly stimulate osteoclast apoptosis. Biochem. Biophys. Res. Commun. 2000, 268, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, M.; Sugimoto, T.; Kanzawa, M.; Yano, S.; Chihara, K. High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochem. Biophys. Res. Commun. 1999, 261, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Mano, H.; Yamada, Y.; Takai, H.; Amizuka, N.; Kobori, M.; Izumi, N.; Kawashima, H.; Ozawa, H.; Ikeda, K.; et al. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem. Biophys. Res. Commun. 1998, 245, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Huo, R.; Samadfam, R.; Bolivar, I.; Miao, D.; Brown, E.M.; Hendy, G.N.; Goltzman, D. The calcium-sensing receptor and 25-hydroxyvitamin D-1alpha-hydroxylase interact to modulate skeletal growth and bone turnover. J. Bone Miner. Res. 2010, 25, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Mentaverri, R.; Yano, S.; Chattopadhyay, N.; Petit, L.; Kifor, O.; Kamel, S.; Terwilliger, E.F.; Brazier, M.; Brown, E.M. The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J. 2006, 20, 2562–2564. [Google Scholar] [CrossRef]
- Hurtel-Lemaire, A.S.; Mentaverri, R.; Caudrillier, A.; Cournarie, F.; Wattel, A.; Kamel, S.; Terwilliger, E.F.; Brown, E.M.; Brazier, M. The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis. New insights into the associated signaling pathways. J. Biol. Chem. 2009, 284, 575–584. [Google Scholar] [CrossRef]
- Boudot, C.; Saidak, Z.; Boulanouar, A.K.; Petit, L.; Gouilleux, F.; Massy, Z.; Brazier, M.; Mentaverri, R.; Kamel, S. Implication of the calcium sensing receptor and the Phosphoinositide 3-kinase/Akt pathway in the extracellular calcium-mediated migration of RAW 264.7 osteoclast precursor cells. Bone 2010, 46, 1416–1423. [Google Scholar] [CrossRef]
- Nicholson, G.C.; Moseley, J.M.; Sexton, P.M.; Mendelsohn, F.A.; Martin, T.J. Abundant calcitonin receptors in isolated rat osteoclasts. Biochemical and autoradiographic characterization. J. Clin. Investig. 1986, 78, 355–360. [Google Scholar] [CrossRef]
- Ikegame, M.; Ejiri, S.; Ozawa, H. Calcitonin-induced change in serum calcium levels and its relationship to osteoclast morphology and number of calcitonin receptors. Bone 2004, 35, 27–33. [Google Scholar] [CrossRef]
- Meleleo, D.; Picciarelli, V. Effect of calcium ions on human calcitonin. Possible implications for bone resorption by osteoclasts. Biometals 2016, 29, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Catala-Lehnen, P.; Huebner, A.K.; Jeschke, A.; Heckt, T.; Lueth, A.; Krause, M.; Koehne, T.; Albers, J.; Schulze, J.; et al. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat. Commun. 2014, 5, 5215. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Matsushita, M. Proton concentrations can be a major contributor to the modification of osteoclast and osteoblast differentiation, working independently of extracellular bicarbonate ions. J. Bone Miner. Metab. 2014, 32, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Meghji, S.; Morrison, M.S.; Henderson, B.; Arnett, T.R. pH dependence of bone resorption: Mouse calvarial osteoclasts are activated by acidosis. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E112–E119. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Kawawaki, J.; Moriura, Y.; Mori, H.; Morihata, H.; Kuno, M. pH dependence and inhibition by extracellular calcium of proton currents via plasmalemmal vacuolar-type H+-ATPase in murine osteoclasts. J. Physiol. 2006, 576, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).