Pro-Inflammatory Effect of Gliadins and Glutenins Extracted from Different Wheat Cultivars on an In Vitro 3D Intestinal Epithelium Model
Abstract
1. Introduction
2. Results
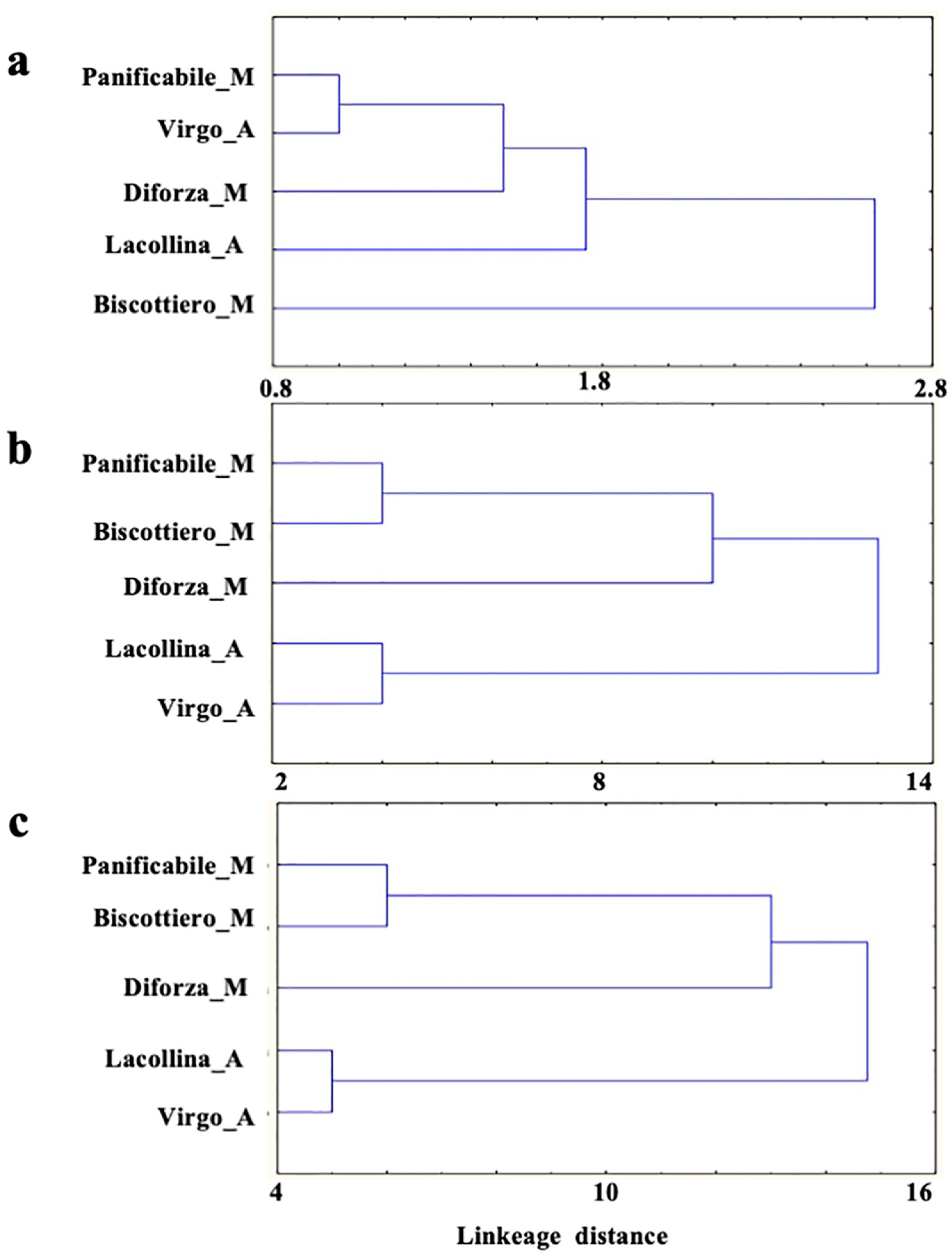
2.1. Protein Content, Composition, and Rheological Properties
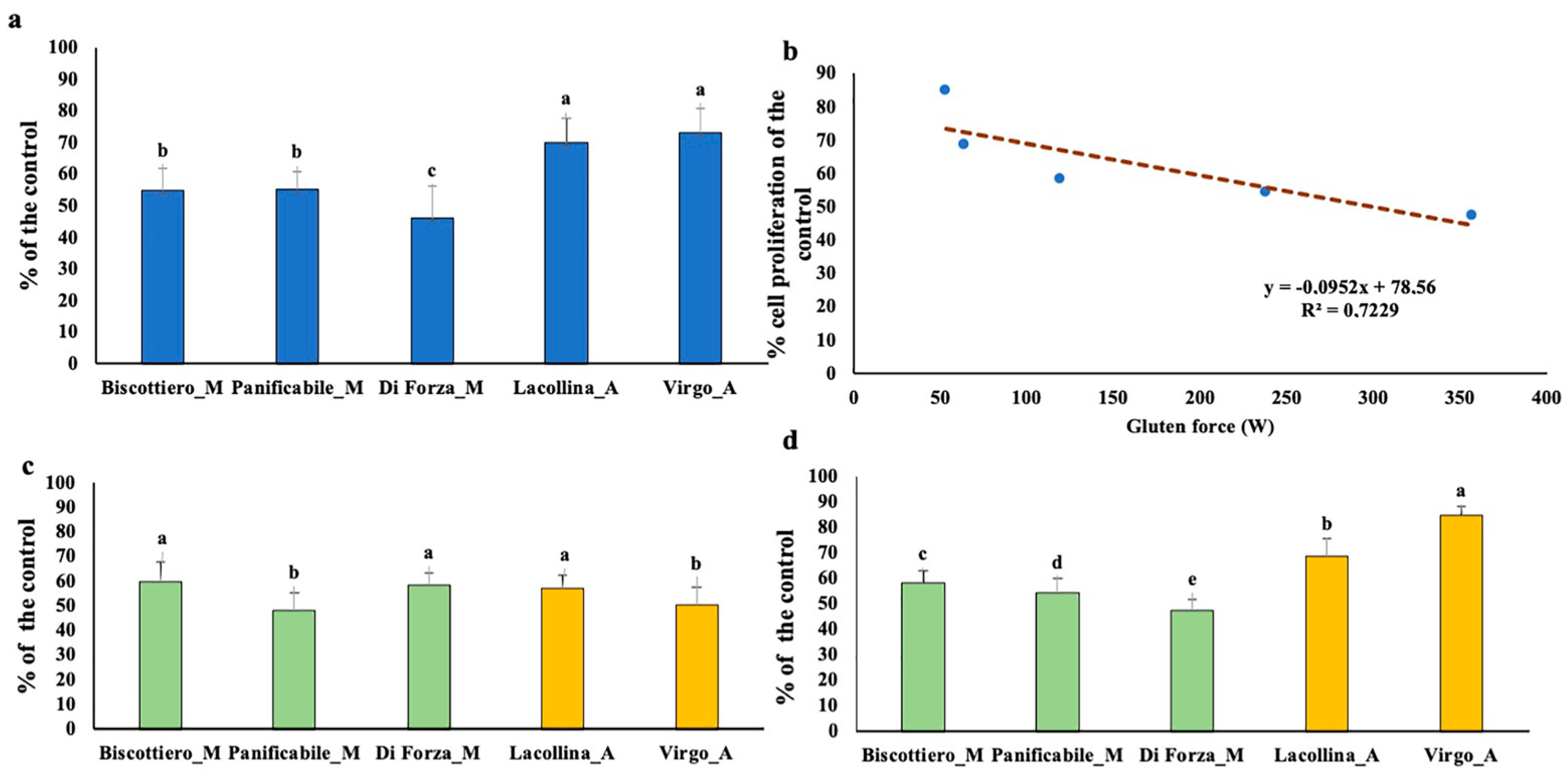
2.2. In Vitro Effects of Modern and Old Common Wheat Protein Mixes on Caco-2 and U937 Cell Lines
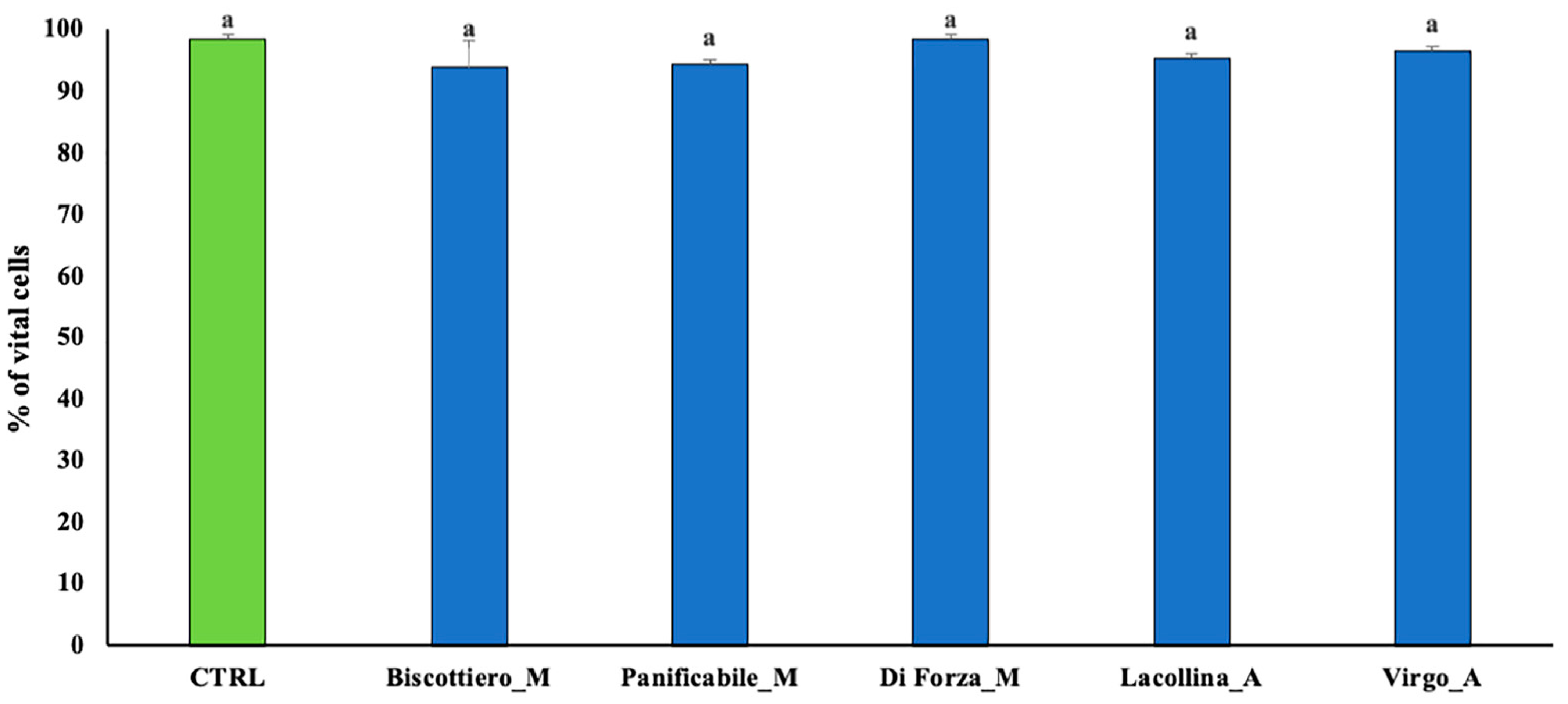
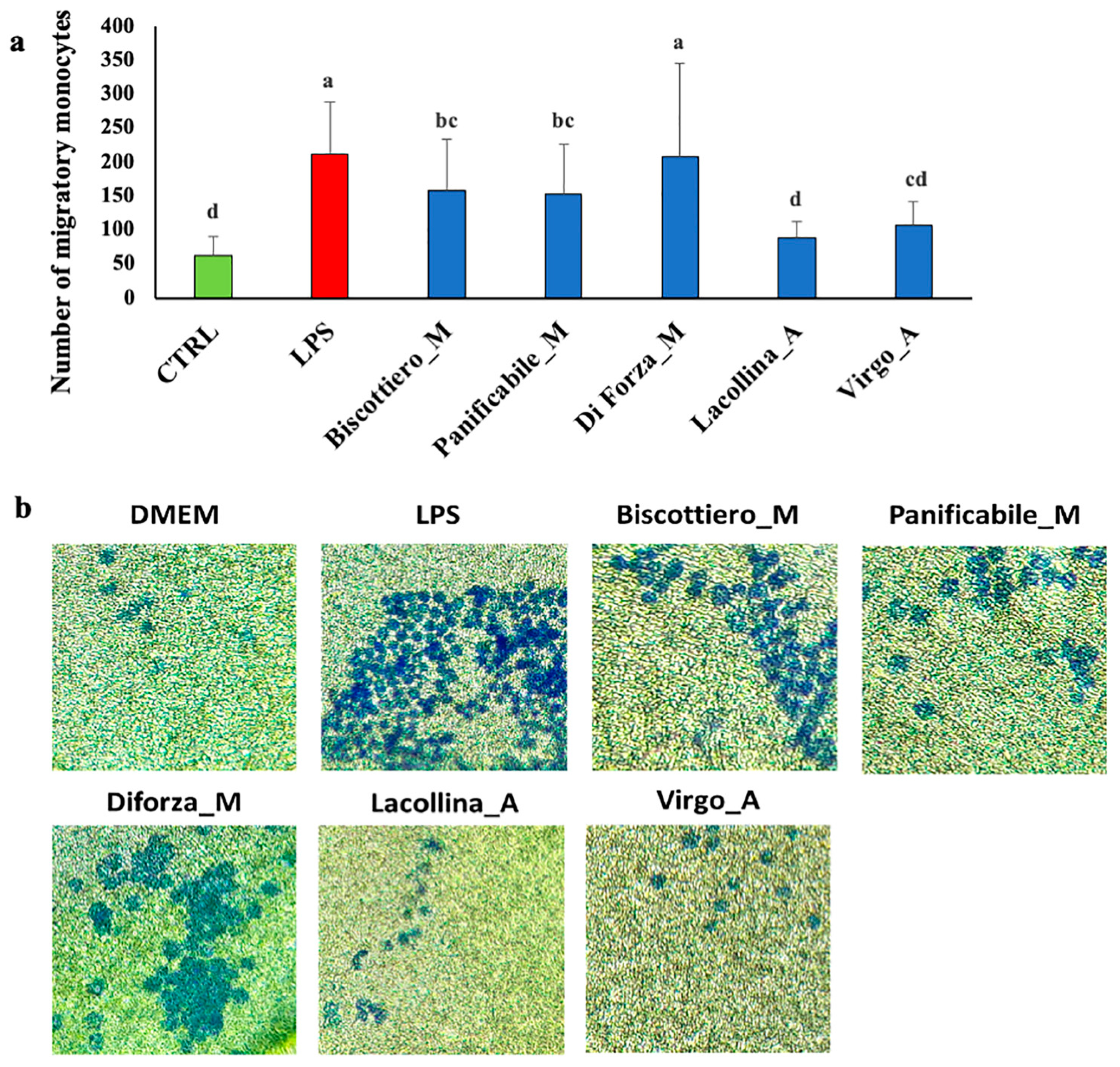
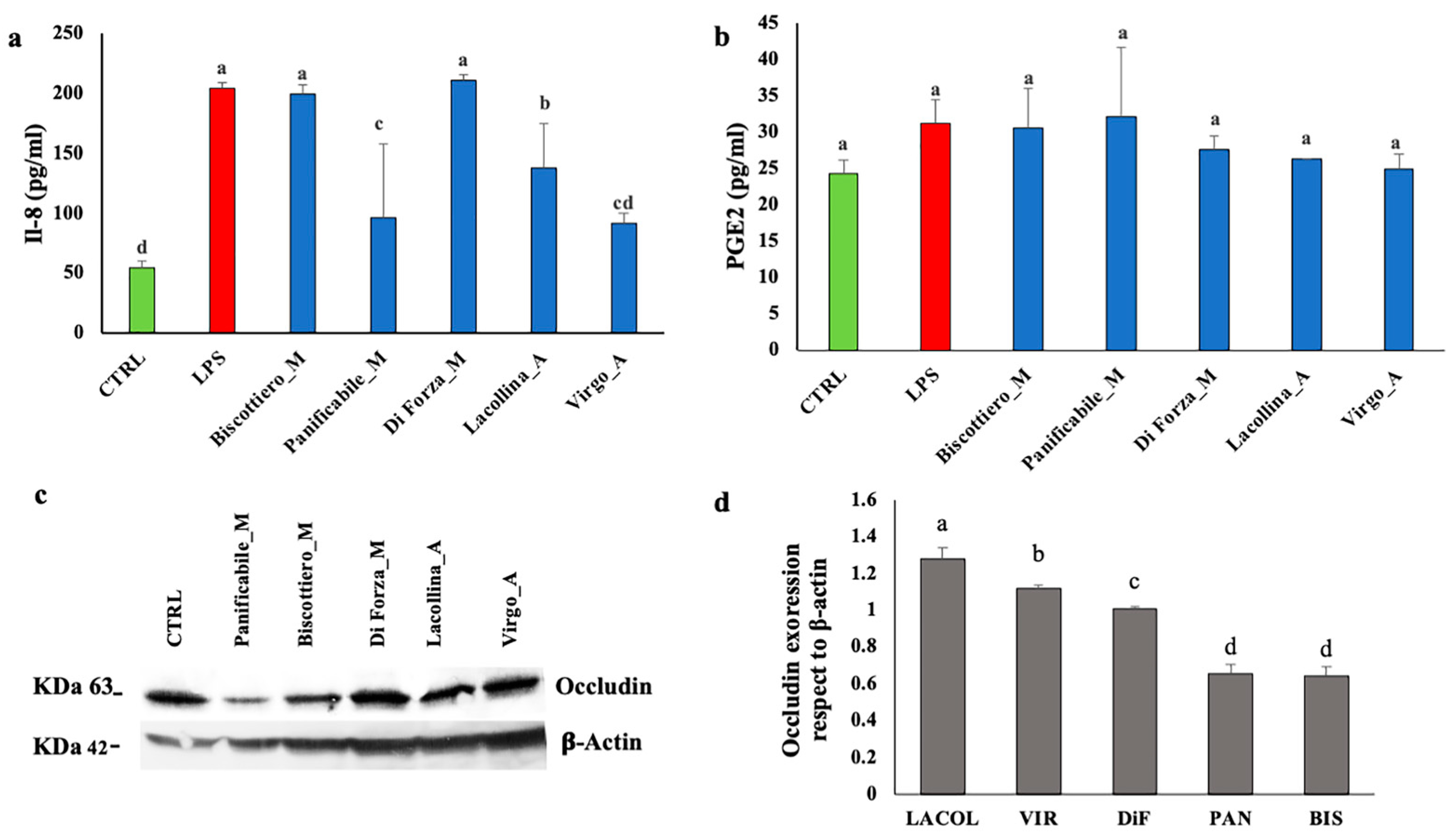
2.3. Wheat Protein Effects on Vitality, Migration, Interleukin-8, Prostaglandin E2, and Occludin Expression in Two-Dimensional Caco-2/U937 Co-Cultures
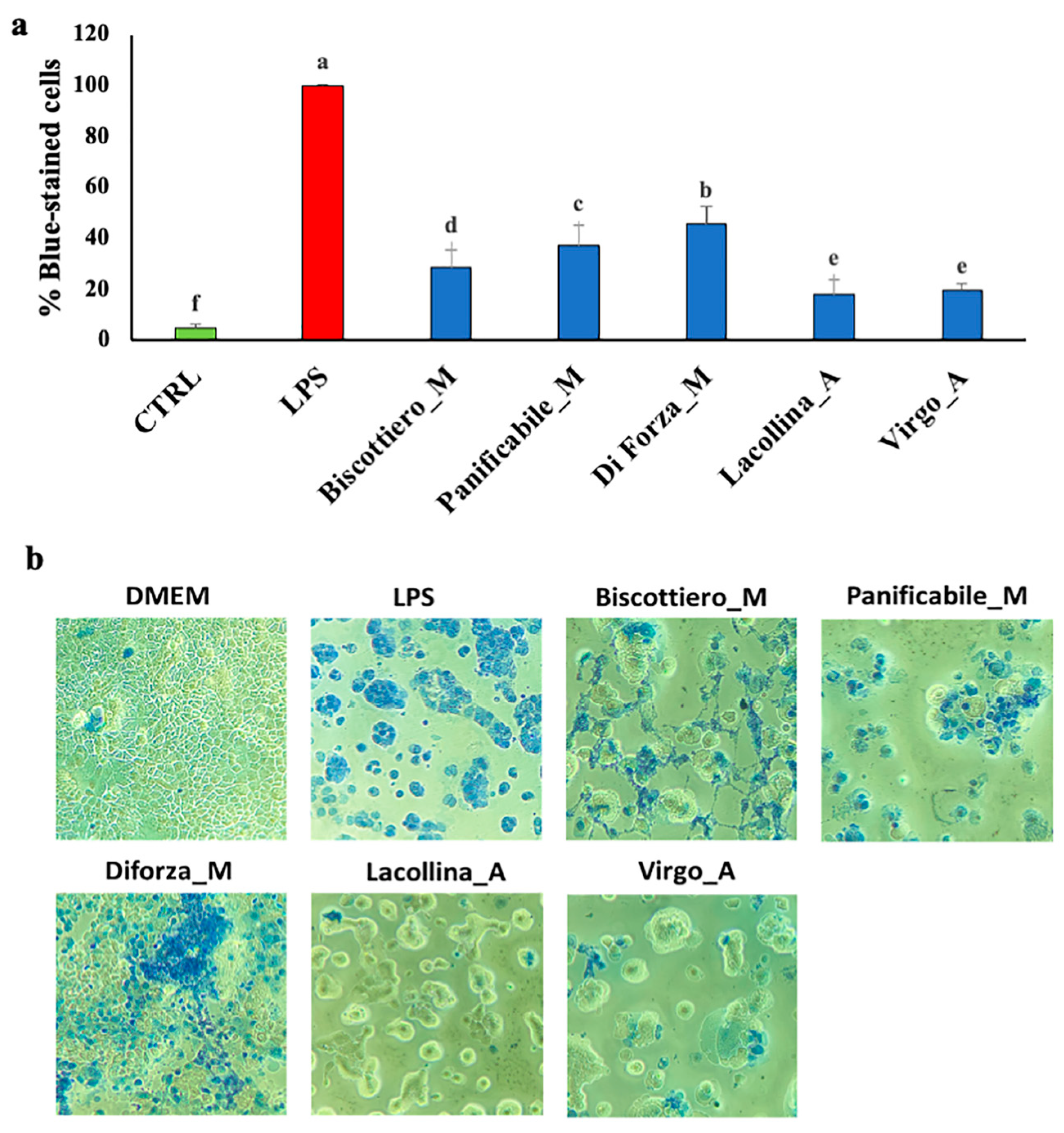
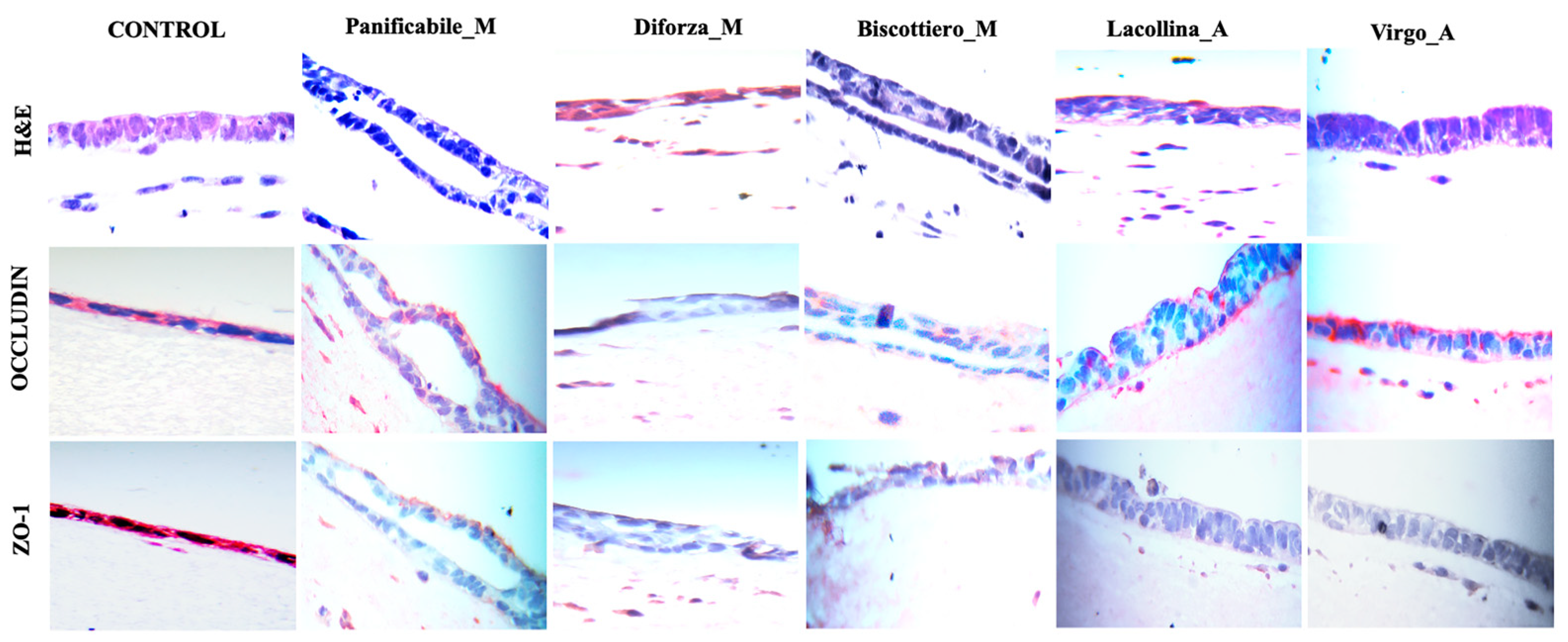
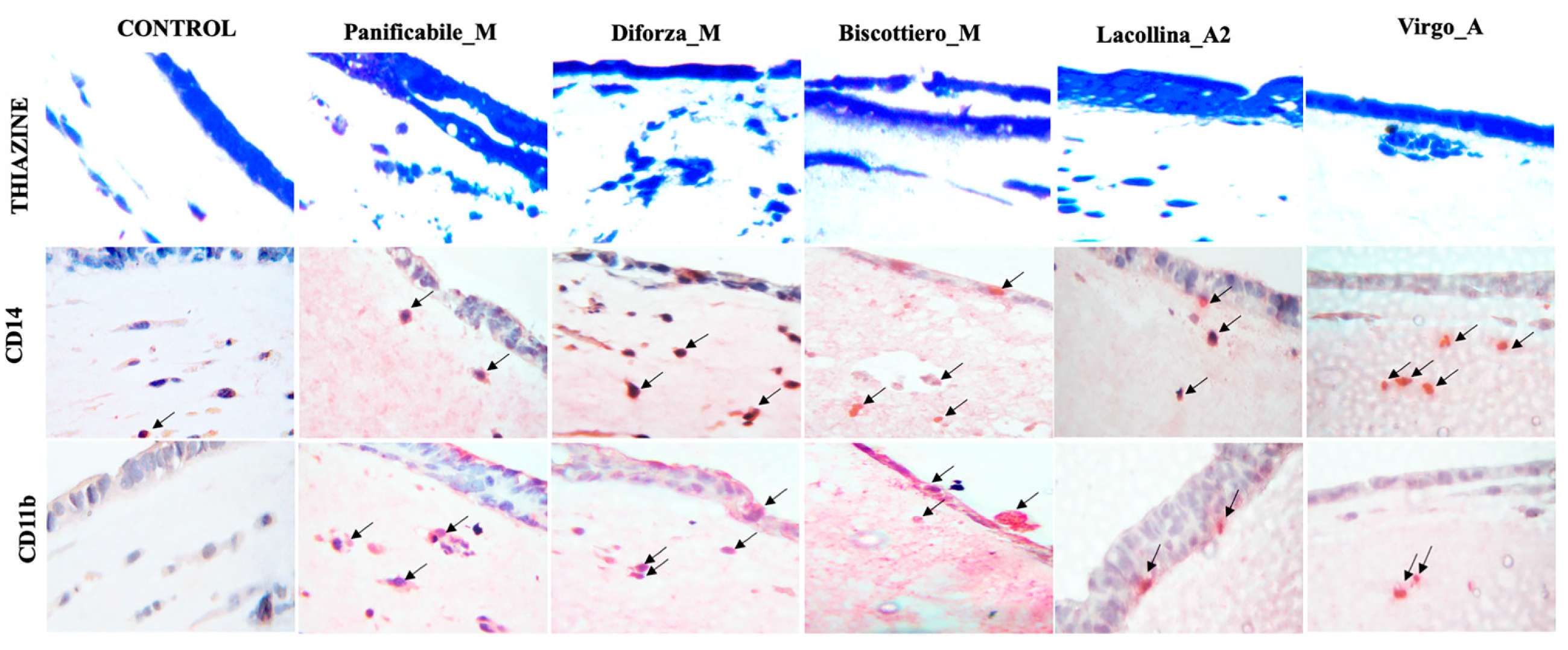
2.4. Wheat Protein Effects on Epithelial Structure, Tight Junction Protein Localization, Monocyte Migration, and Macrophage Differentiation in Three-Dimensional Caco-2/U937/L929 Co-Cultures
3. Discussion
4. Materials and Methods
4.1. Wheat Samples
4.2. Quality Parameters
4.3. Protein Fraction Extraction and Quantification
4.4. Cell Model Systems and Growth Maintenance Conditions
4.5. Monoculture Experiments Using Caco-2 and U937 Cell Lines
4.6. Two-Dimensional Co-Culture Experiments Using Caco-2 and U937 Cell Lines
4.7. Three-Dimensional Reconstituted Intestinal Equivalents Using Caco-2, U937, and L929 Cells
4.8. Cell Proliferation Measurements
4.9. Determination of Cell Vitality
4.10. Cell Migration Measurements
4.11. Measurement of Interleukin-8 (IL-8) and Prostaglandin E2 (PGE2)
4.12. Western Blot Analysis of Occludin Expression Levels
4.13. Immunohistochemical Analysis
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GS | Gluten strength |
| 2D | Two dimensional |
| 3D | Three dimensional |
| CD14 | Cluster of differentiation 14 |
| CD11b | Cluster of differentiation 11b |
| HMW-GluS | High molecular weight glutenin subunits |
| LMW-GluS | Low molecular weight glutenin subunits |
| CD | Celiac disease |
| WA | wheat allergy |
| NCGS | Non-celiac gluten sensitivity |
| NCWS | Non-celiac wheat sensitivity |
| ATIs | Amylase-trypsin inhibitors |
| P/L | gluten strength/extensibility |
| A + G | Albumins + globulins |
| LPS | Lipopolysaccharide |
| IL-8 | Interleukin-8 |
| PGE2 | Prostaglandin E2 |
| COX-2 | Cyclooxygenase-2 |
| TJ | Tight junction |
| ECM | Extracellular matrix |
| H & E | Hematoxylin and eosin |
| ZO-1 | Zonula occludin-1 |
| MAPK | Mitogen-activated protein kinase |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
References
- Kucek, L.K.; Veenstra, L.D.; Amnuaycheewa, P.; Sorrells, M.E. A Grounded Guide to Gluten: How Modern Genotypes and Processing Impact Wheat Sensitivity. Compr. Rev. Food Sci. Food Saf. 2015, 14, 285–302. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P. Gluten and wheat intolerance today: Are modern wheat strains involved? Int. J. Food Sci. Nutr. 2014, 65, 577–581. [Google Scholar] [CrossRef]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef]
- Ierardi, E.; Losurdo, G.; Piscitelli, D.; Giorgio, F.; Amoruso, A.; Iannone, A.; Principi, M.; Di Leo, A. Biological markers for non-celiac gluten sensitivity: A question awaiting for a convincing answer. Gastroenterol. Hepatol. Bed Bench 2018, 2018 11, 203–208. [Google Scholar]
- Sapone, A.; Lammers, K.M.; Casolaro, V.; Cammarota, M.; Giuliano, M.; De Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I.; et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC Med. 2011, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Altenbach, S.B.; Chang, H.-C.; Simon-Buss, A.; Jang, Y.-R.; Denery-Papini, S.; Pineau, F.; Gu, Y.Q.; Huo, N.; Lim, S.-H.; Kang, C.-S.; et al. Towards reducing the immunogenic potential of wheat flour: Omega gliadins encoded by the D genome of hexaploid wheat may also harbor epitopes for the serious food allergy WDEIA. BMC Plant Biol. 2018, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ozuna, C.V.; Barro, F. Characterization of gluten proteins and celiac disease-related immunogenic epitopes in the Triticeae: Cereal domestication and breeding contributed to decrease the content of gliadins and gluten. Mol. Breed. 2018, 38, 22. [Google Scholar] [CrossRef]
- De Santis, M.A.; Giuliani, M.M.; Giuzio, L.; De Vita, P.; Lovegrove, A.; Shewry, P.R.; Flagella, Z. Differences in gluten protein composition between old and modern durum wheat genotypes in relation to 20th century breeding in Italy. Eur. J. Agron. 2017, 87, 19–29. [Google Scholar] [CrossRef]
- Pronin, D.; Börner, A.; Weber, H.; Scherf, K.A. Wheat (Triticum aestivum L.) Breeding from 1891 to 2010 Contributed to Increasing Yield and Glutenin Contents but Decreasing Protein and Gliadin Contents. J. Agric. Food Chem. 2020, 68, 13247–13256. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, H.C.; De Jong, H.C.; Salentijn, E.M.J.; Dekking, L.; Bosch, D.; Hamer, R.J.; Gilissen, L.J.W.J.; Van Der Meer, I.M.; Smulders, M.J.M. Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: Wheat breeding may have contributed to increased prevalence of celiac disease. Theor. Appl. Genet. 2010, 121, 1527–1539. [Google Scholar] [CrossRef]
- Valerii, M.C.; Ricci, C.; Spisni, E.; Di Silvestro, R.; De Fazio, L.; Cavazza, E.; Lanzini, A.; Campieri, M.; Dalpiaz, A.; Pavan, B.; et al. Responses of peripheral blood mononucleated cells from non-celiac gluten sensitive patients to various cereal sources. Food Chem. 2015, 176, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Prandi, B.; Tedeschi, T.; Folloni, S.; Galaverna, G.; Sforza, S. Peptides from gluten digestion: A comparison between old and modern wheat varieties. Food Res. Int. 2017, 91, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.B.; Upadhyay, S.; Saini, R.G.; Mantha, A.K.; Dhiman, M. Inflammatory response of gliadin protein isolated from various wheat varieties on human intestinal cell line. J. Cereal Sci. 2018, 81, 91–98. [Google Scholar] [CrossRef]
- Pilolli, R.; Gadaleta, A.; Mamone, G.; Nigro, D.; De Angelis, E.; Montemurro, N.; Monaci, L. Scouting for Naturally Low-Toxicity Wheat Genotypes by a Multidisciplinary Approach. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R. Do ancient types of wheat have health benefits compared with modern bread wheat? J. Cereal Sci. 2018, 79, 469–476. [Google Scholar] [CrossRef]
- Luongo, D.; Treppiccione, L.; Maurano, F.; Rossi, M.; Bergamo, P. The murine enterocyte cell line Mode-K is a novel and reliable in vitro model for studies on gluten toxicity. Food Chem. Toxicol. 2020, 140, 111331. [Google Scholar] [CrossRef]
- Iftikhar, M.; Iftikhar, A.; Zhang, H.; Gong, L.; Gong, L. Transport, metabolism and remedial potential of functional food extracts (FFEs) in Caco-2 cells monolayer: A review. Food Res. Int. 2020, 136, 109240. [Google Scholar] [CrossRef]
- Qazi, B.S.; Tang, K.; Qazi, A. Recent Advances in Underlying Pathologies Provide Insight into Interleukin-8 Expression-Mediated Inflammation and Angiogenesis. Int. J. Inflamm. 2011, 2011, 908468. [Google Scholar] [CrossRef]
- Capozzi, A.; Vincentini, O.; Gizzi, P.; Porzia, A.; Longo, A.; Felli, C.; Mattei, V.; Paolini, R.; Silano, M.; Sorice, M.; et al. Modulatory Effect of Gliadin Peptide 10-mer on Epithelial Intestinal CACO-2 Cell Inflammatory Response. PLoS ONE 2013, 8, e66561. [Google Scholar] [CrossRef] [PubMed]
- Agard, M.; Asakrah, S.; Morici, L. PGE2 suppression of innate immunity during mucosal bacterial infection. Front. Cell. Infect. Microbiol. 2013, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sander, G.R.; Cummins, A.G.; Powell, B.C. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 2005, 579, 4851–4855. [Google Scholar] [CrossRef] [PubMed]
- Drago, S.; El Asmar, R.; Di Pierro, M.; Clemente, M.G.; Sapone, A.T.A.; Thakar, M.; Iacono, G.; Carroccio, A.; D’Agate, C.; Not, T.; et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 2006, 41, 408–419. [Google Scholar] [CrossRef]
- Orlando, A.; Linsalata, M.; Notarnicola, M.; Tutino, V.; Russo, F. Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: The role of cellular polyamines. BMC Microbiol. 2014, 14, 19. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Zamani, F.; Shahneh, F.Z.; Aghebati-Maleki, L.; Baradaran, B. Induction of CD14 Expression and Differentiation to Monocytes or Mature Macrophages in Promyelocytic Cell Lines: New Approach. Adv. Pharm. Bull. 2013, 3, 329–332. [Google Scholar]
- Yamamoto, T.; Sakaguchi, N.; Hachiya, M.M.; Nakayama, F.; Yamakawa, M.; Akashi, M. Role of catalase in monocytic dif-ferentiation of U937 cells by TPA: Hydrogen peroxide as a second messenger. Leukemia 2009, 23, 761–769. [Google Scholar] [CrossRef]
- Mandel, K.; Otte, A.; Hass, R. Involvement of CD11b integrin in the alteration of metabolic factors after phorbol ester stimulation of human myeloid leukemia cells. Cell Comm. Signal 2012, 10, 13. Available online: http://www.biosignaling.com/content/10/1/13 (accessed on 28 November 2020). [CrossRef]
- Peña, E.; Bernardo, A.; Soler, C.; Jouve, N. Relationship between common wheat (Triticum aestivum L.) gluten proteins and dough rheological properties. Euphytica 2005, 143, 169–177. [Google Scholar] [CrossRef]
- Oury, F.-X.; Chiron, H.; Faye, A.; Gardet, O.; Giraud, A.; Heumez, E.; Rolland, B.; Rousset, M.; Trottet, M.; Charmet, G.; et al. The prediction of bread wheat quality: Joint use of the phenotypic information brought by technological tests and the genetic information brought by HMW and LMW glutenin subunits. Euphytica 2009, 171, 87–109. [Google Scholar] [CrossRef]
- Dhaka, V.; Khatkar, B.S. Effects of Gliadin/Glutenin and HMW-GS/LMW-GS Ratio on Dough Rheological Properties and Bread-Making Potential of Wheat Varieties. J. Food Qual. 2015, 38, 71–82. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.-Q.; Wu, H.-Y.; Lu, C.-B.; Lu, G.-F.; Liu, D.-T.; Li, M.; Jiang, W.; Song, G.-H.; Gao, D.-R. Effect of high-molecular-weight glutenin subunit deletion on soft wheat quality properties and sugar-snap cookie quality estimated through near-isogenic lines. J. Integr. Agric. 2018, 17, 1066–1073. [Google Scholar] [CrossRef]
- Dewar, D.H.; Amato, M.; Ellis, H.J.; Pollock, E.L.; Gonzalez-Cinca, N.; Wieser, H.; Ciclitira, P.J. The toxicity of high molecular weight glutenin subunits of wheat to patients with coeliac disease. Eur. J. Gastroenterol. Hepatol. 2006, 18, 483–491. [Google Scholar] [CrossRef]
- Howdle, P.D. Gliadin, glutenin or both? The search for the Holy Grail in coeliac disease. Eur. J. Gastroenterol. Hepatol. 2006, 18, 703–706. [Google Scholar] [CrossRef]
- Ellis, H.J.; Redondo, M.C.B.; Šuligoj, T.; Japelj, N.; Gonzalez-Cinca, N.; Côrte-Real, B.; Ciclitira, P.J. Monoclonal Antibodies to High Molecular weight Glutenin Subunits for Use in Measurement of Gluten in Foods. Food Nutr. Rep. 2015, 1, 10–18. [Google Scholar]
- Migliorini, P.; Ceccarelli, S.; Torri, L.; Arnoulet, M.; Lazzerini, G.; Ceccarelli, S. Agronomic and quality characteristics of old, modern and mixture wheat varieties and landraces for organic bread chain in diverse environments of northern Italy. Eur. J. Agron. 2016, 79, 131–141. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, R.; Hwang, S.-H.J.; Dutton, J.; Sims, C.E.; Allbritton, N.L. Analysis of Interleukin 8 Secretion by a Stem-Cell-Derived Human-Intestinal-Epithelial-Monolayer Platform. Anal. Chem. 2018, 90, 11523–11530. [Google Scholar] [CrossRef]
- Sánchez, J.P.O.; Mata-Haro, V.; Chávez, F.C.; De La Barca, A.M.C. Prolamins of maize and wheat differentially affect intestinal cells both in biopsies of celiac patients and CACO-2 cell line. Food Agric. Immunol. 2015, 27, 259–272. [Google Scholar] [CrossRef][Green Version]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Dong, Y.; Sun, J.; Sun, M.; Hou, Q.; Lai, Y.; Zhang, B. Protective Effect of Kaempferol on LPS-Induced Inflammation and Barrier Dysfunction in a Coculture Model of Intestinal Epithelial Cells and Intestinal Microvascular Endothelial Cells. J. Agric. Food Chem. 2020, 68, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Van De Walle, J.; Hendrickx, A.; Romier, B.; Larondelle, Y.; Schneider, Y.-J. Inflammatory parameters in Caco-2 cells: Effect of stimuli nature, concentration, combination and cell differentiation. Toxicol. Vitr. 2010, 24, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Turner, P.C.; Co, V.A.; Wang, M.F.; Amiri, K.M.A.; El-Nezami, H. Schisandrin A protects intestinal epithelial cells from deoxynivalenol-induced cytotoxicity, oxidative damage and inflammation. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Sheth, P.; Santos, N.D.; Seth, A.; LaRusso, N.F.; Rao, R. Lipopolysaccharide disrupts tight junctions in cholangiocyte monolayers by a c-Src-, TLR4-, and LBP-dependent mechanism. Am. J. Physiol. Liver Physiol. 2007, 293, G308–G318. [Google Scholar] [CrossRef]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef]
- Delbue, D.; Cardoso-Silva, D.; Branchi, F.; Itzlinger, A.; Letizia, M.; Siegmund, B.; Schumann, M. Celiac Disease Monocytes Induce a Barrier Defect in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2019, 20, 5597. [Google Scholar] [CrossRef]
- Shive, C.L.; Jiang, W.; Anthony, D.D.; Lederman, M.M. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015, 29, 1263–1265. [Google Scholar] [CrossRef]
- Thomas, K.E.; Sapone, A.; Fasano, A.; Vogel, S.N. Gliadin Stimulation of Murine Macrophage Inflammatory Gene Expression and Intestinal Permeability Are MyD88-Dependent: Role of the Innate Immune Response in Celiac Disease. J. Immunol. 2006, 176, 2512–2521. [Google Scholar] [CrossRef]
- AACC (American Association of Cereal Chemists) International. Adapted from Method 54-30A, Approved Methods, 10th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- Lookhart, G.; Bean, S. Separation and characterization of wheat protein fractions by high-performance capillary electropho-resis. Cereal Chem. 1995, 72, 527–532. [Google Scholar]
- Truzzi, F.; Valerii, M.C.; Tibaldi, C.; Zhang, Y.; Abduazizova, V.; Spisni, E.; Dinelli, G. Are Supplements Safe? Effects of Gallic and Ferulic Acids on In Vitro Cell Models. Nutrients 2020, 12, 1591. [Google Scholar] [CrossRef] [PubMed]
| Biscottiero_M | Panificabile_M | Diforza_M | Lacollina_A | Virgo_A | |
|---|---|---|---|---|---|
| Ash (%) | 0.59 b | 0.60 b | 0.61 b | 1.06 a | 1.25 a |
| Protein (%) | 11.0 c | 11.0 c | 12.4 c | 15.6 a | 13.2 b |
| Moisture (%) | 11.6 a | 11.8 a | 11.8 a | 11.6 a | 12.0 a |
| Wet Gluten (%) | 19.7 b | 20.3 b | 22.5 b | 26.4 a | 24.4 a |
| Gluten strength W (10−4 J) | 119 c | 238 b | 389 a | 64 d | 53 d |
| Extensibility P/L (mm/mm) | 0.29 c | 0.48 b | 0.65 a | 0.46 b | 0.43 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truzzi, F.; Tibaldi, C.; Whittaker, A.; Dilloo, S.; Spisni, E.; Dinelli, G. Pro-Inflammatory Effect of Gliadins and Glutenins Extracted from Different Wheat Cultivars on an In Vitro 3D Intestinal Epithelium Model. Int. J. Mol. Sci. 2021, 22, 172. https://doi.org/10.3390/ijms22010172
Truzzi F, Tibaldi C, Whittaker A, Dilloo S, Spisni E, Dinelli G. Pro-Inflammatory Effect of Gliadins and Glutenins Extracted from Different Wheat Cultivars on an In Vitro 3D Intestinal Epithelium Model. International Journal of Molecular Sciences. 2021; 22(1):172. https://doi.org/10.3390/ijms22010172
Chicago/Turabian StyleTruzzi, Francesca, Camilla Tibaldi, Anne Whittaker, Silvia Dilloo, Enzo Spisni, and Giovanni Dinelli. 2021. "Pro-Inflammatory Effect of Gliadins and Glutenins Extracted from Different Wheat Cultivars on an In Vitro 3D Intestinal Epithelium Model" International Journal of Molecular Sciences 22, no. 1: 172. https://doi.org/10.3390/ijms22010172
APA StyleTruzzi, F., Tibaldi, C., Whittaker, A., Dilloo, S., Spisni, E., & Dinelli, G. (2021). Pro-Inflammatory Effect of Gliadins and Glutenins Extracted from Different Wheat Cultivars on an In Vitro 3D Intestinal Epithelium Model. International Journal of Molecular Sciences, 22(1), 172. https://doi.org/10.3390/ijms22010172







