Abstract
Biomaterials have been the subject of numerous studies to pursue potential therapeutic interventions for a wide variety of disorders and diseases. The physical and chemical properties of various materials have been explored to develop natural, synthetic, or semi-synthetic materials with distinct advantages for use as drug delivery systems for the central nervous system (CNS) and non-CNS diseases. In this review, an overview of popular biomaterials as drug delivery systems for neurogenerative diseases is provided, balancing the potential and challenges associated with the CNS drug delivery. As an effective drug delivery system, desired properties of biomaterials are discussed, addressing the persistent challenges such as targeted drug delivery, stimuli responsiveness, and controlled drug release in vivo. Finally, we discuss the prospects and limitations of incorporating extracellular vesicles (EVs) as a drug delivery system and their use for biocompatible, stable, and targeted delivery with limited immunogenicity, as well as their ability to be delivered via a non-invasive approach for the treatment of neurodegenerative diseases.
1. Introduction
Neurodegenerative diseases are characterized by malfunctions and the progressive death of neural cells with time. These diseases also exhibit complex and mixed clinical phenomena, including oxidative stress, neuroinflammation, and cell death [1]. The most commonly reported neurodegenerative diseases are Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), HIV-associated neurocognitive disorder (HAND), and traumatic brain injury (TBI) [2,3]. Current treatments for neurodegenerative diseases are not adequate to cure or defer disease development and progression [4]. The main reason for unmet medical needs is the presence of the complex and sophisticated blood–brain barrier (BBB). The human BBB is a highly selective, semipermeable brain barrier that offers a protective mechanism for the human brain. With the presence of multiple efflux transporters and tight junctions, certain drug molecules have difficulty crossing the BBB [5,6]. Therefore, it is critically important to design and develop biomaterial-based drug delivery systems to combat neuronal diseases.
2. Challenges and Implications of Biomaterials-Based Drug Delivery Approach in Neurodegenerative Diseases
Biomaterials have essential roles in the treatment of neurodegenerative diseases. Nanoparticles in particular have been used with higher frequency in research and development (Table 1) [7]. Among nanoparticles, different biomaterials were investigated in depth for their advantages and disadvantages [8]. Differentiated by monomer source, researchers worked on metal nanoparticles, metalloid nanoparticles, solid lipid nanoparticles (SLNs), polymeric nanoparticles, liposomes, and extracellular vesicles (EVs) [9]. There are many misunderstandings regarding nanoparticle nomenclature. “Nanoparticle” is a general name for drug delivery particles with a size between 10 and 1000 nm. Nanoparticles may be named after their shapes, e.g., nanocubes, nanoplates, nanorods, nanospheres, nanotetrapods, nanoprisms, and nanobelts [7]. Nanoparticles may also be named according to the specific materials used in the assembly, such as with solid lipid nanoparticles, Poly Lactic-co-Glycolic Acid (PLGA) nanoparticles, and metal nanoparticles. Hydrogels and macroscopic polymer matrix drug delivery systems have also been explored for the treatment of neurodegenerative diseases [10,11].

Table 1.
Summary of popular biomaterials used in neurodegenerative diseases and related classification.
Metal nanoparticles are simple biomaterials with a straightforward structure. Gold nanoparticles (AuNPs) are mainly used as trackers rather than as drug delivery systems [13]. In stem cell therapies, AuNPs were used to visualize cell integration, cell proliferation, and tissue recovery [12]. Silver nanoparticles (AgNPs) have similar structures compared to AuNPs. However, AgNPs are used preferably as a therapeutic entity rather than as a drug delivery carrier. AgNPs can also trigger the anti-inflammatory reaction inside microglia, which can be beneficial in neurodegenerative diseases [14]. This mechanism may also be used to treat HAND, since microglia are a targeting site for antiretroviral drug delivery [5]. Metal oxide nanoparticles, such as iron oxide, cerium oxide, and zinc oxide, are developed for imaging and decreasing oxidative stress. These NPs respond to the magnetic field, which benefits researchers and health care providers [15]. Moreover, short hairpin RNA (shRNA) was reported to be immobilized onto iron oxide NPs in a PD model [16].
Quantum dots (QDs) have been widely used in the imaging and diagnosis of neurodegenerative diseases such as AD. QDs coated with amyloid-beta (Aβ) on the surface are used to track Aβ plaques [21]. Similar to AuNPs, QDs are also useful in tracking transplant cells and cell proliferation [22]. Metalloid nanoparticles are primarily made from cadmium-selenium QDs and silica. Silica nanoparticles loaded with or without drug were reported to incorporate into living neuronal cells [23] and the BBB in vivo [24,25].
SLNs and liposomes are made of similar materials with different structures [8]. SLNs use a single-layer spherical structure to encapsulate cargo molecules. In SLNs, lipids and cargos are uniformly mixed to form the core and then stabilized by surfactants. The final SLN usually has lipids and cargos in the core, with the surfactant’s hydrophilic segments facing outward to form the closure. SLNs can improve the stability and delivery of hydrophobic/lipophilic molecules [33]. In contrast with SLNs, liposomes utilize a bilayer spherical structure to encapsulate cargo molecules. Liposomes have a hydrophilic core surrounded by a hydrophobic/lipophilic layer, allowing liposomes to carry cargos with different physicochemical properties [27]. Several liposome-based drug products have been approved by the United States Food and Drug Administration (USFDA) [26]. In 2017, the USFDA approved a liposome-encapsulated combination of daunorubicin-cytarabine (Vyxeos) for adults with newly diagnosed therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC) (https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209401s000lbl.pdf). More importantly, the USFDA approved this product through the facilitated regulatory pathway, indicating the innovative nature of this drug delivery system [34,35]. Both SLNs and liposomes can be modified to provide additional features, such as site-specific targeting, lack of immunogenicity, and low toxicity. Micelles are another form of lipid nanoparticle. Micelles are very similar to liposomes and SLNs, but micelles have only one lipid layer without a lipid core. Micelle solution has been used to encapsulate curcumin for the potential treatment of several neurodegenerative diseases including AD, PD, and MS [28]. However, their application in humans is limited by their bioavailability, and therefore, the development of new nanoformulations with curcumin is necessary to treat neurodegenerative diseases. EVs, natural drug delivery systems, combine all the advantages from SLNs, liposomes, and micelles. EVs have been investigated in depth regarding targeted delivery, no/low toxicity, and personalized medicine [36]. The Covid-19 pandemic also brings EVs to researchers’ attention. Researchers are planning to take advantage of EVs to increase the dose of certain drugs while avoiding severe systemic toxicity [30].
Polymeric nanoparticles are the most advanced and well-developed drug delivery systems used in the treatment of central nervous system (CNS) diseases. Polymeric nanoparticles are made of polymers, which are macromolecules with monomers linked together to form a chain or chain-like structures [37]. Most monomers are naturally available in the human body. Hence, polymeric nanoparticles can be degraded and cleared out of the human body without triggering an immune reaction. The most popular polymeric nanoparticles are made of polylactic acid (PLA), PLGA, and polyethylene glycol (PEG) [7,9,37]. The porous structure of polymeric nanoparticles provides a large space to encapsulate cargos. PLGA nanoparticle-based drug formulations have been shown to cross the BBB and deliver cargos into both macrophages [6] and microglia [5]. Similarly, paclitaxel-loaded PLGA-NPs were also reported to cross the BBB with the assistance of glutathione coating [38]. Loperamide and rhodamine-123 coated NPs were also reported to be delivered into the brain [39].
Preferably, nanoparticle-based drug delivery systems for treating neurodegenerative diseases should be biocompatible, stable, and biodegradable. Production should be cost-effective, scalable, reproducible, and amenable to sterilization and functional modifications. Conversely, these nanoparticle systems should not be cytotoxic, neurotoxic, or provoke an immune response. Common inorganic materials used to form nanoparticle systems include iron, gold, silica, silver, titanium, and zinc [40]. Nanoparticles from these materials are amenable to different sizes and shapes and can be designed for diagnostic and therapeutic applications. These inorganic nanoparticle systems can cross the BBB. Their surfaces can be modified covalently or non-covalently to impart desired properties, such as targeted drug delivery and stimuli-responsive drug release. Inorganic nanoparticles can form reactive oxygen species and are less biodegradable [40]. With poor clearance, accumulation of the inorganic material or metabolites could lead to adverse effects. Inorganic nanoparticles fabricated from iron, silver, titanium, and zinc nanoparticle are less biocompatible due to their potential neurotoxicity [41,42,43]. Silica nanoparticles have been reported to reduce oxidative stress and dopamine levels in the striatum in rat models [44]. Animal model studies have shown that a decrease of the neurotransmitter dopamine in the striatum led to the loss of motor functions characterized by PD [45]. QDs are smaller nanoparticles typically made from a combination of metals and metalloids. QDs can also be made from organic materials such as graphene. QDs are mostly employed in bioanalytical applications, biological detection, and diagnosis, due to photoactivity and semi conductivity. The therapeutic applications of QDs are less explored. Some have reported the application of QDs as photosensitizers for photodynamic therapy (PDT) [46,47]. The generation of reactive oxygen species that are suspected of promoting neurodegeneration is typical during the PDT procedure, blocking the broad application of PDT [48]. To our knowledge, studies investigating the biodegradability of inorganic nanoparticles mainly focus on systemic clearance. Investigations into their distribution and long-term toxicity in the brain are essential for their deployment for therapeutic applications against neurodegenerative diseases.
Compared to inorganic nanoparticles, lipid and polymeric nanoparticles are inherently more biocompatible. In addition to the advantages over inorganic NPs, lipids and polymer nanoparticle surfaces can also be modified to impart desired functionalities. Natural polymer nanoparticles are generally less toxic and more biodegradable compared to synthetic polymers. The degradation of synthetic polymers can lead to increased toxicity. Without modifications, the hydrophobicity of some polymeric systems limits their application to the delivery of hydrophilic drugs. Hydrogels, on the other hand, are hydrophilic and thus are incompatible with hydrophobic drugs. If a controlled/long-term drug release is desired, polymeric nanoparticle systems are not recommended due to the burst release effect. The use of toxic organic solvents to produce polymer nanoparticle systems is sometimes unavoidable, thus limiting scalability. While most inorganic nanoparticle systems are used for diagnosis, several reports have demonstrated the use of lipid-based nanoparticle systems for therapeutic applications. The application of lipid nanoparticles toward the treatment of neurodegenerative diseases can be hindered by limited drug-loading capacity, especially with hydrophilic drugs and peptide/proteins, and reduced bioavailability due to faster clearance [49]. Lipid-based nanoparticle systems can also be susceptible to thermal, oxidative, or hydrolytic degradation. The surface modification capability of lipid-based nanoparticle systems is also limited due to the unavailability of diverse chemical functionalities and steric hindrance.
Naturally occurring EVs are a special kind of lipid-based nanoparticle that can be harvested from cell culture media, blood, plasma, and other bodily fluids such as cerebrospinal fluid (CSF), saliva, milk, and urine [50]. EVs can be considered biological products, and this categorization necessitates a special classification and regulatory scrutiny for approved clinical use. EVs consist of various biomolecules, including proteins and nucleic acids, which must be evaluated for cytotoxicity and immunogenicity. For EVs to be employed for therapeutic applications, their origin and biologic functions must be fully understood. The deployment of EVs for pharmaceuticals is also limited by challenges with the scalability of isolation techniques, inadequacies of current characterization techniques to address pharmaceutical and regulatory-relevant properties, and poor production reproducibility [51]. The application of EVs for therapeutics is in its infancy. Extensive investigations are needed to better understand their potential in drug development and unlock possible advantages over other drug delivery systems.
3. Extracellular Vesicle-Based Drug Delivery Systems
EVs are small membranous vesicles that are naturally produced and excreted from numerous cell types. EVs circulate through all bodily fluids and play a major role in intracellular and intercellular communication due to their multi-functional components such as proteins, DNAs, microRNAs, and mRNAs [52,53,54]. EVs’ ability to exchange genetic material with recipient cells can induce phenotypic modifications, making them a potential candidate in drug delivery systems for therapy [55]. Since EVs mediate cell-to-cell communication, they play critical roles in regulating the multiple facets involved in the pathogenesis of numerous diseases [56,57,58]. EVs may pathologically function as vehicles in drug treatment to disrupt communication pathways in pathogenesis to slow or alter disease progression [59].
3.1. EV Background
EVs are classified based on the size and biogenesis pathway. The three major classified subgroups of EVs are exosomes, microvesicles (MVs, also called microparticles), and apoptotic bodies, and their internal contents consist of lipids, proteins, and nucleic acids [60,61]. Exosomes, MVs, and apoptotic bodies all contain distinct proteins that indicate their biogenic pathways and specific functions [62]. Exosomes range from about 30–150 nm in diameter. However, exosome size distribution and zeta potential can vary significantly among preparations from different isolation methods [63]. Exosomes are formed by the inward budding of the membrane of early endosomes that eventually mature into multivesicular bodies [62]. Since exosomes originate in the endosomal pathway, they are enhanced with protein chaperones, scaffolding proteins, and proteins for endosomal trafficking [64]. Researchers initially believed that exosomes’ biological purpose was to expel unwanted material from cells. However, it has been discovered that they participate in cell maintenance, cell-to-cell communication, and tumor progression [59,62,65,66]. Microvesicles are derived from the outward budding of a cell’s plasma membrane [64,67], while apoptotic bodies are formed only during programmed cell death and generated through cell fragmentation and plasma membrane blebbing of apoptotic cells [67,68]. MVs can range from 50 to 1000 nm in size, and their formation is not well understood, but it is hypothesized that cytoskeleton components are required for their formation. Similar to exosomes, the biological purpose of MVs is to participate in cellular communication between local and distant cell types. Apoptotic bodies range from 50 to 5000 nm in size and are excreted by dying cells. They are formed when the cytoskeleton and plasma membrane separates due to increased hydrostatic pressure induced by cell contraction [62]. Various isolation methods have been developed to potentially overcome the challenges associated with EV isolation, such as EV heterogeneity and biochemical property overlap, that may inhibit effective EV isolation [69,70,71,72,73,74,75]. Some of the developed techniques include ultracentrifugation, density gradient centrifugation, exosome precipitation, antibody-based immunoaffinity purification, tangential flow filtration, and nano-flow cytometry. EV differentiation in various extracellular environments remains the main challenge of inefficient EV isolation in clinical settings and has to be overcome for these techniques to be clinically reproducible [62].
3.2. Drug Loading in EVs
Parental cells determine the biological structure and function of EVs in vivo. While some biological features of EVs are currently known, further exploration, especially about ensuring the safety and efficacy of drug-loaded EVs, must precede future therapeutic applications. The specific approaches used to load EVs will also affect drug-loading capacity and the lifetime of drug-loaded EVs Based on the understanding of the biological features of EVs, there are two major methods of drug loading: (I) endogenous drug loading and (II) exogenous drug loading. To minimize the elimination and degradation of EVs, the selection of drug loading method is pivotal.
3.2.1. Endogenous Drug Loading
Endogenous drug loading methods aim to optimize drug cargo compartmentalization into EVs through the nonspecific binding of drugs to the cytoplasmic membrane of donor cells [76]. In this method, desired cargos are simply incubated with EV-secreting cells. Readily, cargos may passively diffuse across the cell membrane and these cells then secrete EVs loaded with the desired cargo [77,78]. This drug-loading technique is a relatively straightforward strategy that involves a step-by-step process to load drugs by manipulating the donor cells [76]. Endogenous drug-loading strategies rely on the natural mechanisms of EVs to package drug cargo more efficiently. For endogenous loading, donor cells are exposed to the drug of interest, which is followed by stimuli such as heat or hypoxia to induce the release of drug-loaded EVs [76]. EVs are hypothesized to be proficient candidates for drug delivery systems due to their endogenous origin and internal structures. EVs contain intrinsic biological functions and internal cage-like structures that are ideal for containing and delivering drug loads to specific molecular targets [79]. They also possess an aqueous core and lipophilic shell formed by the lipid bilayer, creating two internal compartments. The lipid bilayer gives EVs the amphiphilic nature that allows them to store and dissolve hydrophobic and hydrophilic compounds, making them desirable for use in drug delivery systems [80].
3.2.2. Exogenous Drug Loading
To utilize EVs as drug carriers, an alternative approach for loading desired cargos into EVs can be achieved after EV isolation as an exogenous drug loading method. This method involves the isolation of EVs and subsequent drug loading or desired cargos in EV using mechanical approaches. Exogenous drug loading techniques vary depending on the target molecules of interest such as proteins, small molecules, and nucleic acids-specifically miRNA. Some of the mechanical methods used to load desired cargos into EVs include incubation at room temperature, electroporation, sonication, transfection, saponin permeabilization, and mechanical extrusion [78,81,82,83,84]. Electroporation, the guidance of proteins and signature sequences, producing hybrid EVs with lysosomes, transfecting donor cells, and transfection with commercialized reagents, are methods to load nucleic acids into EVs [81]. Drug-loading strategies include incubation, ultrasonic treatment, eddy current oscillation, and direct mixing [78,83,84]. Ultrasonic treatment was discovered to have increased the drug paclitaxel’s load capacity and supported the release of EVs excreted by macrophages [81]. The disadvantage of passive loading methods includes the degradation of exosomes due to multiple purification steps. The physicochemical properties of drug molecules can affect the stability and bioactivity of EVs [81].
While most cells produce EVs, not all cell-derived EVs are ideal drug carriers. Drug capacity and efficient delivery depend on the size, yield, intracavitary composition, and surface protein(s) [85]. EV biogenesis is a major factor determining the drug-loading capacity of the various types of cell-derived EVs. Since EVs encompass some of their parent cell contents during biogenesis, there is limited space for endogenous and exogenous drug loading [85]. Passive drug-loading strategies used in concentration gradient-based strategies, such as electroporation or sonication, result in low loading efficiency. To compensate for the low loading efficiency, researchers have opted for active loading strategies to target exosome membranes during exosome biogenesis [86]. Exosomes are enhanced with transmembrane proteins that can be fused to cargo molecules to localize these molecules in exosomal cytosol [86]. The heterogeneous internal components and chemical lipid composition can influence drug compatibility with EVs, affecting the drug-loading capacity. Pre-loading methods, post-loading methods, and drug hydrophobicity are factors influencing drug-loading capacity. For instance, researchers have found an 11-fold increase in drug-loading efficiency with the use of membrane permeabilizer saponin and hypotonic dialysis [85,87]. Researchers have taken a comparative approach to assess which method results in a higher loading efficiency. Porphyrins (of different hydrophobicities) served as the model drug that was encapsulated and loaded into EVs via dialysis, extrusion, electroporation method, and using saponin [87]. Hydrophobic compounds were loaded more efficiently in EVs via active methods than by using a passive incubation loading method [87]. Loading drugs into EVs resulted in a cellular uptake greater than 60%, and the photodynamic effect of hydrophobic porphyrins was greater in comparison to drug-loaded liposomes [87].
3.3. EV-Based Drug Delivery for Neurodegenerative Diseases
As we learn more about EVs and their potential roles in the human body, new opportunities for the use of EVs as therapeutic agents have risen. The ability of EVs to penetrate the BBB and transfer cellular components between the CNS and the peripheral circulatory system suggests promising applications in many neurodegenerative diseases, such as AD, PD, MS, HAND, TBI, as well as COVID-19-associated brain damage [53,88,89,90,91].
3.3.1. AD
AD is a progressive neurodegenerative disease that is associated with dementia in the elderly [92]. AD pathogenesis is not yet completely clear. Many studies suggest that AD is characterized by the coexistence of two hallmark pathways that lead to the functional loss of synapses and neurons: the accumulation and disposition of insoluble Aβ plaques and the hyper-phosphorylation of tau proteins (P-tau), in addition to oxidative stress, cholinergic dysfunction, and inflammation [88,93,94]. Aβ plaque formation hinders synaptic plasticity, leading to neuronal apoptosis. This usually begins years before the appearance of any symptoms [92]. P-tau spread occurs after Aβ plaques are formed, and it is shown to affect specific sensors or motor functions in the brain, which is responsible for the loss of cognitive skills in AD patients [88,92]. Accumulating evidence suggests that EVs have a neurotoxic role in the propagation of AD, since amyloid precursor protein (APP)-metabolites, including Aβ, were found tied to exosomes, which are a subset of EVs [95].
Furthermore, Aβ plaques were found to be enriched with proteins that are associated with EV composition [95]. Studies have also indicated that the extent of neuronal loss is associated with EV levels in CSF [95]. EVs were also investigated in AD for their protective role as potential therapeutic agents [95,96]. For instance, cystatin C-loaded EVs have a neuroprotective role, and low serum cystatin C was detected in sporadic AD clinical presentation [95]. Different studies have suggested that EVs derived from human CSF may reverse the synaptic plasticity by disrupting the activity of Aβ plaques [95]. Another suggestion was to use short interfering RNA (siRNA)-loaded EVs against beta-secretase 1 to help decrease Aβ plaque formation [93,95]. Finally, studies have shown that the inhibition of EV release in AD animals using a neutral sphingomyelinase inhibitor (GW4869) has therapeutic benefits [97,98,99]. However, it also has undesired side effects associated with EV inhibition [97,100,101]. Nevertheless, the evidence is promising for EV use as early diagnostic biomarkers and potential therapeutic agents in AD.
3.3.2. PD
PD is the second most common neurodegenerative disease among the elderly [102]. PD is a progressive movement disorder associated with neuronal death in different regions of the brain [103]. PD is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta and over-expression and aggregation of misfiled α-Synuclein (α-Syn) proteins, which is the primary constituent of Lewy bodies [104,105]. α-Syn can be transmitted from either the CNS or peripheral blood monocytes to the brain via EVs. Even though PD is a neurodegenerative disease, α-Syn levels in blood erythrocytes were higher than in the CSF by about 10-fold [103]. The α-Syn proteins within peripheral red blood cells (RBCs) can cross the BBB and be taken up by microglia into the brain parenchyma via EVs (RBC-EVs) [103]. The same study has indicated that systemic inflammation increases BBB permeability, which in turn increases the RBC-EV influx, leading to the development of PD. Further, the uptake of RBC-EVs by microglia enhances microglial inflammatory responses, leading to an increase in neurodegeneration [103]. In a 6-hydroxydopamine (6-OHDA)-treated mouse model of PD, significant anti-inflammatory and neuroprotective effects were observed following the intranasal delivery of catalase-loaded EVs. The same approach can be explored to deliver therapeutic proteins across the BBB for the treatment of various neurodegenerative diseases [106].
To date, there is no curative treatment for PD, which is one reason why early diagnosis using EVs as biomarkers has been a major topic of interest in disorders such as PD [102,107]. The promising accumulation of evidence suggests that EVs can be loaded with therapeutic agents and engineered to target a specific neuronal population [102,108].
3.3.3. MS
MS is a demyelinating autoimmune disease for which there is currently no effective re-myelination therapy [109]. However, a recent study found that in addition to EVs’ ability to cross the BBB, EVs are mediators in the axon myelination process [110]. In this study, EVs derived from mesenchymal stem cells (MSC-EVs) were demonstrated as feasible potential immunomodulatory agents and tissue repair mediators. Another study in 2019 has discussed the potential use of EVs as prognostic and diagnostic biomarkers for MS [111]. In the same study, an immune marker array was used to identify EV surface proteins to differentiate MS patients and healthy controls. Toll-like receptor-3 (TLR3) was found in lower concentrations in MS patients than in controls, while TLR4 was higher in MS patients [111]. Although it is still too early to say that EVs can be drug delivery agents or even diagnostic biomarkers in MS, the evidence is promising.
3.3.4. HAND
HAND, which comprises different forms of neurocognitive impairments, is a growing concern among HIV populations [112]. Although the lifespan of people living with HIV has been prolonged since the discovery of antiretroviral therapy, HIV infection still promotes premature aging due to its persistent infection in the CNS glial cells, which can induce HIV-1 associated dementia [113,114,115]. EVs from HIV-1 infected cells were found to carry viral neurotoxins such as gp120, Nef, and Tat, which can cause cell death, BBB disturbance, as well as induce HAND [88,116,117,118,119,120]. EVs carrying HIV-1 Nef released from infected cells have been shown to promote latent HIV-1 reactivation [121,122]. Platelet and megakaryocyte-derived EVs render the cells susceptible to HIV-1 infection by transferring HIV co-receptors such as CXCR4 and CCR5 [123,124]. HAND presentation is very similar to AD, especially because AD hallmarks such as Aβ plaque accumulation and hyper-phosphorylated P-tau were detected in both AD and HAND [125]. However, in HAND, EVs carrying Tat may induce Aβ plaque accumulation, increase BBB permeability, inhibit the effect of Aβ peptide degrading enzyme (neprilysin), and inhibit the Aβ clearance mechanism by inhibiting the phagocytic activity of microglia [126,127,128].
EVs are being investigated to be used in HAND patients due to their ability to cross the BBB [129]. For instance, Tat-induced EV-Aβ and EV-tau could be drug targets for caffeine, as caffeine was studied to inhibit both Tat-induced Aβ production and tau phosphorylation [130,131]. Another example and potential intervention is the use of rapamycin-loaded EVs to modulate autophagy in the CNS in HAND patients. Early autophagy induction via rapamycin treatment showed promising results in reducing plaques, tangles, and cognitive deficits in preclinical AD models [132]. EVs are also explored as a potential diagnostic marker for cognitive impairment. Neuron-derived EVs are shown to be present at a significantly lower quantity in HIV individuals with neurocognitive impairment. Furthermore, these individuals had higher levels of high mobility group box protein 1 and Aβ in neuron-derived EVs [133].
3.3.5. TBI
The use of EVs as diagnostic biomarkers also promises to fill the diagnostic accuracy gap in many different diseases, such as in TBI [134]. A recent study used miRNA EVs and GluR2+ EVs to identify TBI presence, severity, recovery, and history of prior injuries [135]. Moreover, AD biomarkers, such as Aβ and P-tau concentrations, were higher in EVs that were isolated from TBI patients than from controls [134,136]. Additionally, EV-tau and Aβ42 were higher in patients with a history of multiple TBIs. Furthermore, EV neurofilament neuropolypeptide and glial fibrillary acidic protein were associated with TBI diffusion and recovery [134]. Unresolved or dysregulated immune responses after TBI can contribute to chronic activation of neurotoxic microglia, eventually leading to progressive neuronal cell death [137]. Following TBI, activated microglia/macrophages release microparticles/microvesicles that propagate the injured brain’s neuroinflammatory responses by further activating the neighboring microglia [53]. Thus, inhibiting EV secretion is warranted to regulate the over-activated innate immune responses by microglia during brain injury. Studies indicate that glial cell activation and the regulation of innate immune responses can be achieved either by blocking EV biogenesis or by the neutralization of EVs using nSMase inhibitors and the novel surfactant polyethylene glycol telomere B, respectively [53,138].
3.3.6. COVID-19 Associated Brain Damage
Mounting evidence suggests that the novel 2019 coronavirus disease (COVID-19) is neurotropic, as it is a viscerotropic disease [139]. COVID-19-related brain damage is associated with cytokine overproduction and toxicity, perhaps by delivering the virus and/or inflammatory/oxidative components to the CNS via EVs [30,139,140]. COVID-19 causes a broad variety of neurologic complications, such as hemorrhagic and/or ischemic strokes, seizures, and encephalopathy, which predicts a direct relationship between viral tropism and CNS injuries [139]. Presently, treatment options for COVID-19 are still being investigated, including the use of EVs as unique drug targets and carriers [30]. Moreover, EVs can be used in the treatment of COVID-19-associated brain damage due to their unique ability to penetrate the BBB and their potential to be engineered and targeted to a specific part of the CNS.
3.4. EV-Based Therapeutic Approach
EVs as therapeutic drug carriers are currently undergoing clinical trials for the treatment of pathogenic diseases such as cancer, autoimmune diseases, and neurodegenerative diseases [36,141]. EV-based drug delivery systems harness promising results due to their diverse cell-based origins and their ability to modulate various cell communication pathways (Table 2). Cargo loading methods, tissue targeting, the functional delivery of cargo to recipient cells, and the promotion of EV stability are strategic issues that are considered when choosing therapeutic agents for disease treatment [142]. Some therapeutic strategies may utilize EVs’ natural properties, such as pathogen suppression, immune modulation, or regeneration promotion, to improve the outcome of treatment by slowing pathogenesis or weakening autoimmune responses [142]. Data from recent clinical trials highlight that the importance of EVs as therapeutic delivery systems lie in the EV features, including cellular interactions, bio-distribution, circulation time, different cargo loading methods, and administration [143]. Drugs that could specifically benefit from EV drug delivery systems include anti-inflammatory agents and small RNA therapeutics [36,143,144,145,146,147,148,149].

Table 2.
Extracellular vesicles-based therapies in preclinical setting.
Several human tumors originate in the epithelium and exhibit high epidermal growth factor receptor (EGFR) expression, hinting that EGFR could be a target in cancer drug delivery systems [150]. Nucleic acid drugs have promising therapeutic potential, but there are limits to their clinical application due to a lack of efficient drug delivery systems [151,152,153]. Ohno et al. demonstrated that exosomes can act as an effective drug delivery system of miRNA to EGFR-expressing breast cancer cells [150]. The profile and biocompatibility of exosomes make them ideal in miRNA drug delivery because they are the natural carriers of miRNA. In a preclinical study, MSC-EVs have been shown to promote neurogenesis, neurite remodeling, and synaptic plasticity in an experimental rat model of ischemic stroke [154], as well as improvement in sciatic nerve regeneration in rats [155]. Given the beneficial effects of MSC-EVs in many preclinical models, MSC-EV therapy was given to patients with graft-versus-host disease (GvHD). Shortly after the start of MSC-exosome therapy, clinical GvHD symptoms were found to be significantly improved [156]. Systemically administered MSC-EVs improved impaired function and structural injury in the fetal ovine brain following hypoxia-ischemia [157].
Monocyte-derived myeloid cells play a central role in inflammatory/inflammation-related autoimmune diseases. Studies have been conducted to test the efficiency of exosomes as drug delivery vehicles of anti-inflammatory agents [158]. Sun et al. examined EVs, specifically exosomes, as promising agents for delivering curcumin, an anti-inflammatory drug, to target inflammatory cells [158]. Curcumin is a natural polyphenol derived from the rhizome of Curcuma longa (turmeric) and known for its chemopreventative, antineoplastic, anti-inflammatory, and antioxidant activity [158,159]. However, curcumin’s low solubility remains a major issue due to its hydrophobic properties. To increase exosome encapsulation efficiency, curcumin was mixed with EL-4-derived exosomes, resulting in the solubility of exosomal curcumin five-fold higher than free curcumin [158]. In order to develop an exosomal-based delivery system to treat PD, a potent antioxidant enzyme catalase was loaded into exosomes ex vivo. Following intranasal administration, catalase-loaded exosomes demonstrated significant neuroprotective effects in an in vitro and in vivo model of PD [106].
4. Limitations of EV-Based Drug Delivery Systems and Current Advancements to Counter these Limitations
Exosomes have potential advantages in drug delivery. While the application of exosomes as drug delivery systems appears realistic in humans, there are still some challenges that lie ahead. For example, the problem of manufacturing large-scale batches of exosomes for clinical use remains unsolved. Furthermore, the production of homogenous EVs is challenging, as EVs produced from the same cell source have varied sizes [78]. These limitations need to be overcome in order to improve the feasibility of using EV-based drug delivery systems.
4.1. Interaction of Drugs with EV Components
Since EVs may serve as an ideal drug delivery system in CNS disease by crossing the BBB and delivering proteins, RNAs, DNA, and chemical drugs, various techniques are used for the loading of therapeutic agents inside EVs. However, various drug-metabolizing enzymes and drug transporters have been shown to be expressed in EVs [162]. These enzymes pose challenges in terms of the stability of drugs, especially small molecules, which can be metabolized and effluxed out of the EVs. Cytochrome P450 (CYP) enzymes are the major metabolizers of xenobiotics, including therapeutic drugs [162]. Recently, Kumar et al. have shown a significant expression of functional CYP enzymes in EVs derived from human plasma [163]. Further studies have demonstrated that various CYP enzymes, mostly expressed in the liver and immune cells, are packaged in EVs and eventually secreted in plasma [163]. Plasma EVs circulate in the periphery and perhaps in the CNS. They likely interact with other cell types by releasing these CYP enzymes. The further induction of CYP enzymes in cell-derived EVs, upon exposure to xenobiotics such as tobacco and alcohol, suggests the role of these EV CYP enzymes in drug metabolism in extrahepatic cells [164,165,166]. Similarly, efflux transporters, such as p-glycoprotein (P-gp) are expressed in cell-derived EVs and circulated via plasma [30]. These studies suggest that CYP enzymes and efflux transporters not only metabolize/transport xenobiotics in the liver and gut but can also clear toxic compounds, endogenous compounds, and therapeutic drugs in other extrahepatic cells/organs and the peripheral circulation. Therefore, a complete understanding of various EV components, including CYP enzymes and efflux transporters, is important to predict the stability of drugs encapsulated in EVs derived from various sources. Upon understanding the role of CYPs and transporters in EVs of various cell types, EVs can be isolated from a source that is devoid of these enzymes. Alternatively, drugs can be loaded along with CYP and P-gp inhibitors as pharmacoenhancers to inhibit/reduce the effects of these enzymes on the metabolic stability of loaded drugs. For example, being strong inhibitors of the CYP3A4 enzyme, ritonavir and cobicistat are known pharmacoenhancers of antiretroviral drugs [167], which can be used to co-formulate drugs that are CYP3A4 substrates.
In addition to efflux and metabolic stability, it is also important to study the interaction of small molecules and metals present in EVs with various drugs. For example, it is known that metals such as calcium interact with antibiotics such as doxycycline or minocycline, leading to reduced drug bioavailability [168]. Thus, antibiotics encapsulated in EVs isolated from milk (an easy source of EVs) are likely to interact with calcium and reduce the chemical stability of the drug. Similarly, EVs derived from lung alveolar macrophages may have various inhaled xenobiotics such as air pollutants, EVs derived from liver may have numerous stable xenobiotics obtained from food, and EVs derived from immune cells may have many small immune molecules. These small molecules are likely to interact with various drugs encapsulated in EVs isolated from their respective cells. Therefore, before developing EV-drug formulations, it is imperative to examine whether small molecules and metals present in EVs can interact with encapsulated drugs and reduce their stability. Thus, drug loading can be tailored to EVs isolated from cell types that do not have small molecules with the potential to interact with specific drugs.
Using multiple drugs simultaneously confers a high likelihood of producing drug–drug interactions (DDI) via CYP enzymes present in EVs. Many drugs are not only substrates, but they are also inhibitors and/or inducers/activators of CYP enzymes. Thus, DDIs occur as a result of the inhibition or induction/activation of CYP enzymes. Although most CYP enzymes are predominantly expressed in the liver, they are found to be packaged in EVs/exosomes and circulate with plasma. For example, CYP2E1, which is highly abundant in plasma EVs [168,169], causes a DDI between alcohol and acetaminophen, leading to hepatotoxicity. An overdose of alcohol and/or acetaminophen, as well as drinking alcohol while taking acetaminophen, will increase the risk of adverse drug reactions. Toxicity is not only expected in hepatocytes but also in extrahepatic cells, including CNS cells, via the circulation of EV CYP2E1. The expression of CYP enzymes also shows genetic variability. Thus, when using EVs/exosomes to deliver a drug, the interactions between drugs and the presence of polymorphic CYP enzymes should also be cautiously considered.
4.2. In Vivo Pharmacokinetics of EV Drugs
With greater attention paid to the potential of EV drug applications, a few EV drugs are currently undergoing clinical trials. The pharmacokinetics of EV drugs limit the efficacy and efficiency of EV drugs. Therefore, the administration method of EV drugs is an important step that governs the distribution of EVs into targeted sites or cells.
4.2.1. Intravenous Injection (IV)
The size of EVs is one of the factors that determines the pharmacokinetics of EV drugs. When intravenous injection is performed, only EVs smaller than 100 nm diameter can move with RBCs, with a small fraction of accumulation near the vascular wall. This indicates that smaller EVs are more appropriate for drug delivery purposes. After intravenous injection into the mouse model, EV levels immediately reduce and aggregate in the liver [169]. As a result, the majority of EVs circulating with RBCs in vivo are cleared by the liver and the spleen.
DiD lipid dye-labeled MSC-EVs were injected into the tail vein of mice and were mainly captured by the liver, spleen, and bone marrow as observed in vivo imaging, with the strongest fluorescence in the liver and spleen of mice [170]. The biodistribution results from a different group show that the source of EVs is related to the distribution sites in mice [171]. The diameter of EVs has been shown to affect biodistribution, with EVs < 100 nm able to move through the liver without significant uptake by hepatocytes [172]. Blood concentrations and circulation kinetics of EVs also limit their pharmacokinetics. Macrophages identify the apoptotic signal on the EV membranes and promote clearance. Inhibiting the clearance of macrophages can maintain certain concentrations of EVs and prolong the circulation time of EVs in vivo [173].
4.2.2. Intraperitoneal Injections, Subcutaneous Injections, and Oral Administration
Compared to intravenous injection, after the intraperitoneal or subcutaneous delivery of HEK293T exosomes, they accumulate in the liver, gastrointestinal tract, or pancreas [174]. It is feasible to increase the concentration and duration of exosome efficacy on the target organ by the local administration. The direct injection of EVs through intranasal delivery can prevent EVs from entering the systemic circulation and being cleared in large amounts by the liver and spleen [175]. Oral administration is one of the potential methods for EV administration without invasive injury. In contrast to intravenous injection, the oral administration of milk-derived exosomes improved immune function and reduced arthritis in mice [176].
4.3. Delivery of Drugs to the Target
The drug delivery system design depends on the structure of a drug molecule, its formulation, administration approach, dosage form, and other related techniques. The recent development in drug delivery systems focused on nanoparticles can increase the drug concentration in targeted parts of the body. Exosomes can be widely detected in many body fluids. Unlike other artificial nanoparticles, exosomes are naturally generated in vivo, which makes them an ideal drug delivery vehicle with a longer half-life, lower toxicity, and more specificity to targeted tissues [177,178]. Compared with synthetic drugs, drug-loaded exosomes are safer, stable, and biocompatible, due to their phospholipid bilayer structure [179]. The bio functional cargoes of exosomes range from nucleic acids, proteins, and lipids to synthetic drugs [180,181]. The uptake of exosomes has three steps: interactions between receptor and ligand, membrane fusion, and endocytosis/ phagocytosis [182]. The uptake of exosomes relies on one cell type and surface proteins of exosomes [183]. Some studies show that receptor–ligand interactions enhance the biological efficacy of exosomes, especially in cancer therapy [184] and modulating immune responses [182].
Researchers showed that exosomes delivered miRNA efficiently to EGFR-expressing breast tumor cells in a mouse study [150]. Due to this feature, exosomes can be potentially developed as diagnostic biomarkers. On the other hand, it has been proven that exosomes from neural stem cells recognized by the Ifbgr1 receptor on target cells are indispensable to maintaining the Signal transducer and activator of transcription 1 pathway’s activation [185].
4.4. Immune Clearance
Although the phospholipid bilayer structure of EVs makes them more biocompatible, elimination can happen before EVs arrive at the target cells. Most of the elimination is initiated by innate immunity. When exosomes are intravenously injected into mice, they are instantly cleared by the reticuloendothelial system before they reach the target tumor tissue [186]. Sinus CD169+ macrophages have been shown to suppress cancer progression by eliminating the tumor-derived exosomes before they interacted with B cells [187]. Exosomes can also regulate innate immunity. Tumor-derived exosomes can negatively regulate T cell immunity by raising adenosine levels when expressing CD39 and CD73 [188]. Thus, further study to understand the immune clearance of EVs, as well as the development of necessary steps to counteract immune clearance, is needed. One such approach could be the isolation of EVs from the plasma of patients, drug loading in these EVs, and injecting drug-loaded EVs back to the same patients. This personalized medicine approach may have the ability to eliminate/reduce unwanted and adverse immune reactions.
5. Conclusions
This review presents recent advances in a biomaterial-based drug delivery approach for the controlled delivery of drugs or desired cargos to prevent or treat neurodegeneration in the CNS. As previously stated, biomaterials exist in different types; however, their use in clinical application is limited due to intensive interaction with the body tissue. Meanwhile, EVs possess numerous advantages over biomaterials in the context of a safe and effective drug delivery approach (Figure 1). In contrast to liposome-assisted drug delivery, EVs manifest higher loading efficiency and loading capacity for chemical drugs, and being natural nanoparticles, they are biodegradable and not expected to have adverse effects. The natural ability of exosomes to carry biological molecules such as long and short nucleic acid, proteins, and small molecules, as well as their ability to regulate gene expression and the phenotypic modifications of recipient cells make them an ideal drug-delivery modality. EVs not only demonstrate lower toxicity and lower immunogenicity than other drug delivery strategies but also bear specific surface proteins that can guide themselves to target organs. Due to the physiological role in various cellular functions, EVs have the potential to act as an ideal drug delivery system for neurodegenerative diseases by crossing the BBB and delivering desired cargos that include chemical drugs to the CNS. Under appropriate conditions, drugs that possess a hydrophobic or lipophilic nature or molecules such as antioxidants, anticancer, or anti-inflammatory drugs can be encapsulated into EVs. However, to intensify the bioavailability and efficacy of drugs with complicated properties, the successful integration of these drugs into EVs is required. In several studies, the delivery of target genes and selective silencing of genes aided by siRNA-loaded EVs has been validated. However, before we realize the full potential of EV-loaded drugs for therapeutic applications, certain limitations involving drug stability, in vivo pharmacokinetics, drug targeting, immune clearance, and the production of large-scale sterile preparations must be overcome.
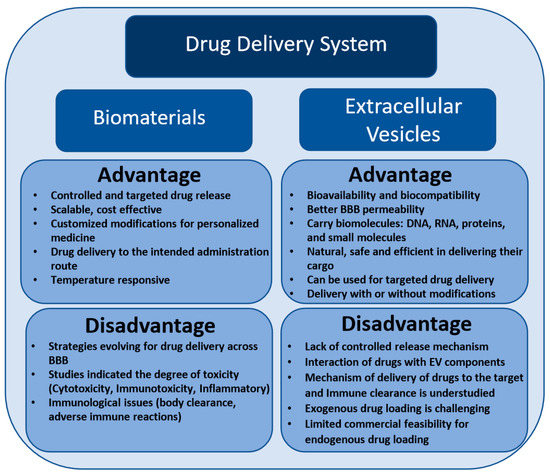
Figure 1.
Possible advantages and disadvantages of biomaterials and extracellular vesicles as a drug delivery system.
Funding
This study is partially supported by funding from the National Institute of Health (DA047178 and CA213232) and Plough Center for Sterile Drug Delivery Solutions, University of Tennessee Health Science Center.
Acknowledgments
The authors are grateful to Kelli Anne Gerth (University of Tennessee Health Science center) for critical reading and editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Tweedie, D.; Scerba, M.T.; Greig, N.H. Neuroinflammation as a factor of neurodegenerative disease: Thalidomide analogs as treatments. Front. Cell Dev. Biol. 2019, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Maher, P. The potential of flavonoids for the treatment of neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 3056. [Google Scholar] [CrossRef]
- Gong, Y.; Zhi, K.; Nagesh, P.K.B.; Sinha, N.; Chowdhury, P.; Chen, H.; Gorantla, S.; Yallapu, M.M.; Kumar, S. An elvitegravir nanoformulation crosses the blood–brain barrier and suppresses HIV-1 replication in Microglia. Viruses 2020, 12, 564. [Google Scholar] [CrossRef]
- Gong, Y.; Chowdhury, P.; Nagesh, P.K.B.; Rahman, M.A.; Zhi, K.; Yallapu, M.M.; Kumar, S. Novel elvitegravir nanoformulation for drug delivery across the blood-brain barrier to achieve HIV-1 suppression in the CNS macrophages. Sci. Rep. 2020, 10, 3835. [Google Scholar] [CrossRef]
- Ramanathan, S.; Archunan, G.; Sivakumar, M.; Tamil Selvan, S.; Fred, A.L.; Kumar, S.; Gulyás, B.; Padmanabhan, P. Theranostic applications of nanoparticles in neurodegenerative disorders. Int. J. Nanomed. 2018, 13, 5561–5576. [Google Scholar] [CrossRef]
- Li, Y.; Liu, R.; Ji, W.; Li, Y.; Liu, L.; Zhang, X. Delivery systems for theranostics in neurodegenerative diseases. Nano Res. 2018, 11, 5535–5555. [Google Scholar] [CrossRef]
- Vissers, C.; Ming, G.-L.; Song, H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Adv. Drug Deliv. Rev. 2019, 148, 239–251. [Google Scholar] [CrossRef]
- Jain, A.; Kim, Y.-T.; McKeon, R.J.; Bellamkonda, R.V. In situ gelling hydrogels for conformal repair of spinal cord defects, and local delivery of BDNF after spinal cord injury. Biomaterials 2006, 27, 497–504. [Google Scholar] [CrossRef]
- Fon, D.; Al-Abboodi, A.; Chan, P.P.Y.; Zhou, K.; Crack, P.; Finkelstein, D.I.; Forsythe, J.S. Effects of GDNF-loaded injectable gelatin-based hydrogels on endogenous neural progenitor cell migration. Adv. Healthc. Mater. 2014, 3, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, N.; Arifin, D.R.; Shats, I.; Janowski, M.; Walczak, P.; Hyeon, T.; Bulte, J.W.M. In vivo micro-CT imaging of human mesenchymal stem cells labeled with gold-poly-L-lysine nanocomplexes. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef] [PubMed]
- Sintov, A.C.; Velasco-Aguirre, C.; Gallardo-Toledo, E.; Araya, E.; Kogan, M.J. Metal nanoparticles as targeted carriers circumventing the blood-brain barrier. Int. Rev. Neurobiol. 2016, 130, 199–227. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Carter, D.A.; Leo, B.F.; Ruenraroengsak, P.; Chen, S.; Goode, A.E.; Theodorou, I.G.; Chung, K.F.; Carzaniga, R.; Shaffer, M.S.P.; Dexter, D.T.; et al. Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes. Sci. Rep. 2017, 7, 42871. [Google Scholar] [CrossRef]
- Kong, S.D.; Lee, J.; Ramachandran, S.; Eliceiri, B.P.; Shubayev, V.I.; Lal, R.; Jin, S. Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Control. Release Off. J. Control. Release Soc. 2012, 164, 49–57. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, L.-K.; Zhang, L.; Zhuang, S.; Zhan, X.; Chen, W.-Y.; Du, S.; Yin, L.; You, R.; Li, C.-H.; et al. Inhibition by multifunctional magnetic nanoparticles loaded with alpha-synuclein RNAi plasmid in a Parkinson’s disease model. Theranostics 2017, 7, 344–356. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. 2009, 48, 2308–2312. [Google Scholar] [CrossRef]
- Hirst, S.M.; Karakoti, A.S.; Tyler, R.D.; Sriranganathan, N.; Seal, S.; Reilly, C.M. Anti-inflammatory properties of cerium oxide nanoparticles. Small 2009, 5, 2848–2856. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Tokuraku, K.; Marquardt, M.; Ikezu, T. Real-time imaging and quantification of amyloid-beta peptide aggregates by novel quantum-dot nanoprobes. PLoS ONE 2009, 4, e8492. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Domowicz, M.S.; Schwartz, N.B.; Henry, J.; Medintz, I.; Delehanty, J.B.; Stewart, M.H.; Susumu, K.; Huston, A.L.; Deschamps, J.R.; et al. Delivery and tracking of quantum dot peptide bioconjugates in an intact developing avian brain. ACS Chem. Neurosci. 2015, 6, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Barandeh, F.; Nguyen, P.-L.; Kumar, R.; Iacobucci, G.J.; Kuznicki, M.L.; Kosterman, A.; Bergey, E.J.; Prasad, P.N.; Gunawardena, S. Organically modified silica nanoparticles are biocompatible and can be targeted to neurons in vivo. PLoS ONE 2012, 7, e29424. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lin, B.; Shao, W.; Zhu, Z.; Ji, T.; Yang, C. In vitro and in vivo studies on the transport of PEGylated silica nanoparticles across the blood-brain barrier. ACS Appl. Mater. Interfaces 2014, 6, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Zaruba, K.; Oravec, M.; Kunes, M.; Babula, P.; Ulbrich, P.; Brezaniova, I.; Opatrilova, R.; Triska, J.; Suchy, P. Preparation of silica nanoparticles loaded with nootropics and their in vivo permeation through blood-brain barrier. Biomed. Res. Int. 2015, 2015, 812673. [Google Scholar] [CrossRef]
- Kohli, A.G.; Kierstead, P.H.; Venditto, V.J.; Walsh, C.L.; Szoka, F.C. Designer lipids for drug delivery: From heads to tails. J. Control. Release Off. J. Control. Release Soc. 2014, 190, 274–287. [Google Scholar] [CrossRef]
- Tam, V.H.; Sosa, C.; Liu, R.; Yao, N.; Priestley, R.D. Nanomedicine as a non-invasive strategy for drug delivery across the blood brain barrier. Int. J. Pharm. 2016, 515, 331–342. [Google Scholar] [CrossRef]
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Kumar, S.; Zhi, K.; Mukherji, A.; Gerth, K. Repurposing antiviral protease inhibitors using extracellular vesicles for potential therapy of COVID-19. Viruses 2020, 12, 486. [Google Scholar] [CrossRef]
- Xiong, X.Y.; Tam, K.C.; Gan, L.H. Release kinetics of hydrophobic and hydrophilic model drugs from pluronic F127/poly (lactic acid) nanoparticles. J. Control. Release Off. J. Control. Release Soc. 2005, 103, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface functionalization of nanoparticles with Polyethylene Glycol: Effects on protein adsorption and cellular uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Maheshwari, R.; Tekade, M.; Chougule, M.B. Solid lipid nanoparticles for targeting and delivery of drugs and genes. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Elsevier: Cambridge, MA, USA, 2017; pp. 256–286. ISBN 978-0-12-809717-5. [Google Scholar]
- Liberti, L.; Breckenridge, A.; Hoekman, J.; Leufkens, H.; Lumpkin, M.; McAuslane, N.; Stolk, P.; Zhi, K.; Rägo, L. Accelerating access to new medicines: Current status of facilitated regulatory pathways used by emerging regulatory authorities. J. Public Health Policy 2016, 37, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Liberti, L.; Breckenridge, A.; Hoekman, J.; Leufkens, H.G.; Lumpkin, M.M.; Mcauslane, N.; Stolk, P.; Zhi, K.; Rago, L. Practical aspects of developing, implementing and using facilitated regulatory pathways in the emerging markets. In Proceedings of the Poster Drug Information Association Annual Meeting, Philadelphia, PA, USA, 28 June 2016. [Google Scholar]
- Zhi, K.; Kumar, A.; Raji, B.; Kochat, H.; Kumar, S. Formulation, manufacturing and regulatory strategies for extracellular vesicles-based drug products for targeted therapy of central nervous system diseases. Expert Rev. Precis. Med. Drug Dev. 2020, 1–13. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Geldenhuys, W.; Mbimba, T.; Bui, T.; Harrison, K.; Sutariya, V. Brain-targeted delivery of paclitaxel using glutathione-coated nanoparticles for brain cancers. J. Drug Target. 2011, 19, 837–845. [Google Scholar] [CrossRef]
- Tosi, G.; Costantino, L.; Rivasi, F.; Ruozi, B.; Leo, E.; Vergoni, A.V.; Tacchi, R.; Bertolini, A.; Vandelli, M.A.; Forni, F. Targeting the central nervous system: In vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. J. Control. Release Off. J. Control. Release Soc. 2007, 122, 1–9. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- Mishra, A.R.; Zheng, J.; Tang, X.; Goering, P.L. Silver nanoparticle-induced Autophagic-Lysosomal disruption and NLRP3-inflammasome activation in HepG2 cells is size-dependent. Toxicol. Sci. Off. J. Soc. Toxicol. 2016, 150, 473–487. [Google Scholar] [CrossRef]
- Petters, C.; Irrsack, E.; Koch, M.; Dringen, R. Uptake and metabolism of iron oxide nanoparticles in brain cells. Neurochem. Res. 2014, 39, 1648–1660. [Google Scholar] [CrossRef]
- Zeman, T.; Loh, E.-W.; Čierný, D.; Šerý, O. Penetration, distribution and brain toxicity of titanium nanoparticles in rodents’ body: A review. IET Nanobiotechnol. 2018, 12, 695–700. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Sun, J.; Xue, Y. Neurotoxicity of silica nanoparticles: Brain localization and dopaminergic neurons damage pathways. ACS Nano 2011, 5, 4476–4489. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Bakalova, R.; Ohba, H.; Zhelev, Z.; Ishikawa, M.; Baba, Y. Quantum dots as photosensitizers? Nat. Biotechnol. 2004, 22, 1360–1361. [Google Scholar] [CrossRef]
- Yaghini, E.; Seifalian, A.M.; MacRobert, A.J. Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy. Nanomedicine 2009, 4, 353–363. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Martins, S.; Sarmento, B.; Ferreira, D.C.; Souto, E.B. Lipid-based colloidal carriers for peptide and protein delivery--liposomes versus lipid nanoparticles. Int. J. Nanomed. 2007, 2, 595–607. [Google Scholar]
- Armstrong, D.; Wildman, D.E. Extracellular vesicles and the promise of continuous liquid biopsies. J. Pathol. Transl. Med. 2018, 52, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. Apl. Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kosaka, N.; Ochiya, T. Latest advances in extracellular vesicles: From bench to bedside. Sci. Technol. Adv. Mater. 2019, 20, 746–757. [Google Scholar] [CrossRef]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J. Neuroinflamm. 2017, 14, 47. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Somiya, M. Where does the cargo go?: Solutions to provide experimental support for the “extracellular vesicle cargo transfer hypothesis”. J. Cell Commun. Signal. 2020, 14, 135–146. [Google Scholar] [CrossRef]
- Vader, P.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Emerging targets for cancer therapy. Trends Mol. Med. 2014, 20, 385–393. [Google Scholar] [CrossRef]
- Devhare, P.B.; Ray, R.B. Extracellular vesicles: Novel mediator for cell to cell communications in liver pathogenesis. Mol. Asp. Med. 2018, 60, 115–122. [Google Scholar] [CrossRef]
- Wendler, F.; Stamp, G.W.; Giamas, G. Tumor-stromal cell communication: Small vesicles signal big changes. Trends Cancer 2016, 2, 326–329. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Petrovčíková, E.; Vičíková, K.; Leksa, V. Extracellular vesicles–biogenesis, composition, function, uptake and therapeutic applications. Biologia 2018, 73, 437–448. [Google Scholar] [CrossRef]
- Kumar, A.; Kodidela, S.; Tadrous, E.; Cory, T.J.; Walker, C.M.; Smith, A.M.; Mukherjee, A.; Kumar, S. Extracellular vesicles in viral replication and pathogenesis and their potential role in therapeutic intervention. Viruses 2020, 12, 887. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of extracellular vesicles, their origin, composition, purpose, and methods for Exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef] [PubMed]
- Zocco, D.; Ferruzzi, P.; Cappello, F.; Kuo, W.P.; Fais, S. Extracellular vesicles as shuttles of tumor biomarkers and anti-tumor drugs. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.V.; Heuser, J.E.; Stahl, P.D. Exosomes: Looking back three decades and into the future. J. Cell Biol. 2013, 200, 367–371. [Google Scholar] [CrossRef] [PubMed]
- White, I.J.; Bailey, L.M.; Aghakhani, M.R.; Moss, S.E.; Futter, C.E. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006, 25, 1–12. [Google Scholar] [CrossRef]
- Gustafson, D.; Veitch, S.; Fish, J.E. Extracellular vesicles as protagonists of diabetic cardiovascular pathology. Front. Cardiovasc Med. 2017, 4, 71. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.-L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef]
- Zhang, J.; Nguyen, L.T.; Hickey, R.; Walters, N.; Palmer, A.F.; Reátegui, E. Immunomagnetic Sequential Ultrafiltration (iSUF) platform for enrichment and purification of extracellular vesicles from biofluids. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tian, Y.; Gong, M.; Hu, Y.; Liu, H.; Zhang, W.; Zhang, M.; Hu, X.; Aubert, D.; Zhu, S.; Wu, L.; et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J. Extracell. Vesicles 2020, 9, 1697028. [Google Scholar] [CrossRef]
- Corso, G.; Mäger, I.; Lee, Y.; Görgens, A.; Bultema, J.; Giebel, B.; Wood, M.J.A.; Nordin, J.Z.; Andaloussi, S.E. Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography. Sci. Rep. 2017, 7, 11561. [Google Scholar] [CrossRef]
- DeMarino, C.; Pleet, M.L.; Cowen, M.; Barclay, R.A.; Akpamagbo, Y.; Erickson, J.; Ndembi, N.; Charurat, M.; Jumare, J.; Bwala, S.; et al. Antiretroviral drugs alter the content of extracellular vesicles from HIV-1-Infected cells. Sci. Rep. 2018, 8, 7653. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wong, D.K.; Hong, K.Y.; Raffai, R.L. Cushioned-density gradient ultracentrifugation (C-DGUC): A refined and high performance method for the isolation, characterization, and use of exosomes. Methods Mol. Biol. 2018, 1740, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baran, J.; Siedlar, M.; Baj-Krzyworzeka, M. Isolation of extracellular vesicles: Determining the correct approach (Review). Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef]
- Balachandran, B.; Yuana, Y. Extracellular vesicles-based drug delivery system for cancer treatment. Cogent. Med. 2019, 6. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharm. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Knez, M. Comparison of two endogenous delivery agents in cancer therapy: Exosomes and ferritin. Pharmacol. Res. 2016, 110, 1–9. [Google Scholar] [CrossRef]
- Ren, J.; He, W.; Zheng, L.; Duan, H. From structures to functions: Insights into exosomes as promising drug delivery vehicles. Biomater. Sci. 2016, 4, 910–921. [Google Scholar] [CrossRef]
- Liu, C.; Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics 2019, 9, 1015–1028. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, Z.; Zhou, K.; Feng, N. Exosomes as carriers for antitumor therapy. Acs Biomater. Sci. Eng. 2019, 5, 4870–4881. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Q.; Sun, X.; Wang, Y. Recent advancements in the loading and modification of therapeutic exosomes. Front. Bioeng. Biotechnol. 2020, 8, 586130. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Baek, G.; Choi, H.; Kim, Y.; Lee, H.; Choi, C. Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl. Med. 2019, sctm.18-0226. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Kodidela, S.; Gerth, K.; Haque, S.; Gong, Y.; Ismael, S.; Singh, A.; Tauheed, I.; Kumar, S. Extracellular vesicles: A possible link between HIV and Alzheimer’s disease-like pathology in HIV subjects? Cells 2019, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, T.; Yang, N.; Han, D.; Mi, X.; Li, Y.; Liu, K.; Vuylsteke, A.; Xiang, H.; Guo, X. Neurological manifestations of patients with COVID-19: Potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front. Med. 2020. [Google Scholar] [CrossRef]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Marshall, M. How COVID-19 can damage the brain. Nature 2020, 585, 342–343. [Google Scholar] [CrossRef]
- Vella, L.J.; Hill, A.F.; Cheng, L. Focus on extracellular vesicles: Exosomes and their role in protein trafficking and biomarker potential in Alzheimer’s and Parkinson’s disease. Int. J. Mol. Sci. 2016, 17, 173. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zhao, B.; Zhao, J.; Li, S. Potential roles of exosomal MicroRNAs as diagnostic biomarkers and therapeutic application in Alzheimer’s disease. Neural Plast. 2017, 2017, 7027380. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Ciregia, F.; Urbani, A.; Palmisano, G. Extracellular vesicles in brain tumors and neurodegenerative diseases. Front. Mol. Neurosci. 2017, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Miller, B.L.; Carlson, O.D.; Mustapic, M.; Kapogiannis, D. Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2015, 2, 769–773. [Google Scholar] [CrossRef]
- Hill, A.F. Extracellular vesicles and neurodegenerative diseases. J. Neurosci. 2019, 39, 9269–9273. [Google Scholar] [CrossRef]
- Dinkins, M.B.; Enasko, J.; Hernandez, C.; Wang, G.; Kong, J.; Helwa, I.; Liu, Y.; Terry, A.V.; Bieberich, E. Neutral Sphingomyelinase-2 deficiency ameliorates Alzheimer’s disease pathology and improves cognition in the 5XFAD Mouse. J. Neurosci. 2016, 36, 8653–8667. [Google Scholar] [CrossRef]
- Dinkins, M.B.; Dasgupta, S.; Wang, G.; Zhu, G.; Bieberich, E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 1792–1800. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, W.; Yao, Q.; Zhang, H.; Dong, G.; Zhang, M.; Liu, Y.; Chen, J.-K.; Dong, Z. Exosome production and its regulation of EGFR during wound healing in renal tubular cells. Am. J. Physiol Ren. Physiol 2017, 312, F963–F970. [Google Scholar] [CrossRef]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2020, 9, 1703244. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, T.; Zhang, B. Exosomes in Parkinson’s disease. Neurosci. Bull. 2017, 33, 331–338. [Google Scholar] [CrossRef]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Peelaerts, W.; Bousset, L.; Van der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van den Haute, C.; Melki, R.; Baekelandt, V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Meissner, W.G.; Frasier, M.; Gasser, T.; Goetz, C.G.; Lozano, A.; Piccini, P.; Obeso, J.A.; Rascol, O.; Schapira, A.; Voon, V.; et al. Priorities in Parkinson’s disease research. Nat. Rev. Drug Discov. 2011, 10, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Sun, T.; An, J.; Wen, L.; Liu, F.; Bu, Z.; Cui, Y.; Feng, J. Potential roles of exosomes in Parkinson’s disease: From pathogenesis, diagnosis, and treatment to prognosis. Front. Cell Dev. Biol. 2020, 8, 86. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Osorio-Querejeta, I.; Alberro, A.; Muñoz-Culla, M.; Mäger, I.; Otaegui, D. Therapeutic potential of extracellular vesicles for Demyelinating diseases; challenges and opportunities. Front. Mol. Neurosci. 2018, 11, 434. [Google Scholar] [CrossRef]
- Laso-García, F.; Ramos-Cejudo, J.; Carrillo-Salinas, F.J.; Otero-Ortega, L.; Feliú, A.; Gómez-de Frutos, M.; Mecha, M.; Díez-Tejedor, E.; Guaza, C.; Gutiérrez-Fernández, M. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE 2018, 13, e0202590. [Google Scholar] [CrossRef]
- Bhargava, P.; Nogueras-Ortiz, C.; Chawla, S.; Bæk, R.; Jørgensen, M.M.; Kapogiannis, D. Altered levels of toll-like receptors in circulating extracellular vesicles in multiple sclerosis. Cells 2019, 8, 1058. [Google Scholar] [CrossRef]
- Clifford, D.B.; Ances, B.M. HIV-associated neurocognitive disorder. Lancet Infect. Dis. 2013, 13, 976–986. [Google Scholar] [CrossRef]
- Mollace, V.; Nottet, H.S.L.M.; Clayette, P.; Turco, M.C.; Muscoli, C.; Salvemini, D.; Perno, C.F. Oxidative stress and neuroAIDS: Triggers, modulators and novel antioxidants. Trends Neurosci. 2001, 24, 411–416. [Google Scholar] [CrossRef]
- High, K.P.; Brennan-Ing, M.; Clifford, D.B.; Cohen, M.H.; Currier, J.; Deeks, S.G.; Deren, S.; Effros, R.B.; Gebo, K.; Goronzy, J.J.; et al. HIV and aging: State of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J. Acquir. Immune. Defic Syndr. 2012, 60 (Suppl. 1), S1–S18. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, S.; Sakai, K.; Bresser, J.; Stevenson, M.; Evinger-Hodges, M.J.; Volsky, D.J. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J. Virol 1987, 61, 3774–3782. [Google Scholar] [CrossRef] [PubMed]
- Arakelyan, A.; Fitzgerald, W.; Zicari, S.; Vanpouille, C.; Margolis, L. Extracellular vesicles carry HIV Env and facilitate Hiv infection of human Lymphoid tissue. Sci. Rep. 2017, 7, 1695. [Google Scholar] [CrossRef] [PubMed]
- Sami Saribas, A.; Cicalese, S.; Ahooyi, T.M.; Khalili, K.; Amini, S.; Sariyer, I.K. HIV-1 Nef is released in extracellular vesicles derived from astrocytes: Evidence for Nef-mediated neurotoxicity. Cell Death Dis. 2017, 8, e2542. [Google Scholar] [CrossRef]
- Rahimian, P.; He, J.J. Exosome-associated release, uptake, and neurotoxicity of HIV-1 Tat protein. J. Neurovirol. 2016, 22, 774–788. [Google Scholar] [CrossRef]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef]
- Raymond, A.D.; Diaz, P.; Chevelon, S.; Agudelo, M.; Yndart-Arias, A.; Ding, H.; Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Roy, U.; et al. Microglia-derived HIV Nef+ exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J. Neurovirol. 2016, 22, 129–139. [Google Scholar] [CrossRef]
- Ali, S.A.; Huang, M.-B.; Campbell, P.E.; Roth, W.W.; Campbell, T.; Khan, M.; Newman, G.; Villinger, F.; Powell, M.D.; Bond, V.C. Genetic characterization of HIV type 1 Nef-induced vesicle secretion. Aids Res. Hum. Retrovir. 2010, 26, 173–192. [Google Scholar] [CrossRef]
- Arenaccio, C.; Anticoli, S.; Manfredi, F.; Chiozzini, C.; Olivetta, E.; Federico, M. Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1. Retrovirology 2015, 12, 87. [Google Scholar] [CrossRef]
- Rozmyslowicz, T.; Majka, M.; Kijowski, J.; Murphy, S.L.; Conover, D.O.; Poncz, M.; Ratajczak, J.; Gaulton, G.N.; Ratajczak, M.Z. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS 2003, 17, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.; Kleinschmidt, A.; Brühl, H.; Klier, C.; Nelson, P.J.; Cihak, J.; Plachý, J.; Stangassinger, M.; Erfle, V.; Schlöndorff, D. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 2000, 6, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Gisslén, M.; Krut, J.; Andreasson, U.; Blennow, K.; Cinque, P.; Brew, B.J.; Spudich, S.; Hagberg, L.; Rosengren, L.; Price, R.W.; et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. Bmc Neurol. 2009, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N. Metabolic regulation of brain Abeta by Neprilysin. Science 2001, 292, 1550–1552. [Google Scholar] [CrossRef]
- Giunta, B.; Zhou, Y.; Hou, H.; Rrapo, E.; Fernandez, F.; Tan, J. HIV-1 TAT inhibits microglial phagocytosis of Abeta peptide. Int. J. Clin. Exp. Pathol. 2008, 1, 260–275. [Google Scholar]
- Rempel, H.C.; Pulliam, L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS 2005, 19, 127–135. [Google Scholar] [CrossRef]
- Shahjin, F.; Chand, S.; Yelamanchili, S.V. Extracellular vesicles as drug delivery vehicles to the Central Nervous System. J. Neuroimmune Pharm. 2019. [Google Scholar] [CrossRef]
- Soliman, M.L.; Geiger, J.D.; Chen, X. Caffeine blocks HIV-1 Tat-induced Amyloid Beta production and Tau Phosphorylation. J. Neuroimmune Pharm. 2017, 12, 163–170. [Google Scholar] [CrossRef]
- Eskelinen, M.H.; Kivipelto, M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J. Alzheimers Dis. 2010, 20 (Suppl. 1), S167–S174. [Google Scholar] [CrossRef]
- Majumder, S.; Richardson, A.; Strong, R.; Oddo, S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS ONE 2011, 6, e25416. [Google Scholar] [CrossRef]
- Sun, B.; Dalvi, P.; Abadjian, L.; Tang, N.; Pulliam, L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS 2017, 31, F9–F17. [Google Scholar] [CrossRef]
- Guedes, V.A.; Devoto, C.; Leete, J.; Sass, D.; Acott, J.D.; Mithani, S.; Gill, J.M. Extracellular vesicle proteins and MicroRNAs as biomarkers for traumatic brain injury. Front. Neurol. 2020, 11, 663. [Google Scholar] [CrossRef]
- Ko, J.; Hemphill, M.; Yang, Z.; Sewell, E.; Na, Y.J.; Sandsmark, D.K.; Haber, M.; Fisher, S.A.; Torre, E.A.; Svane, K.C.; et al. Diagnosis of traumatic brain injury using miRNA signatures in nanomagnetically isolated brain-derived extracellular vesicles. Lab Chip 2018, 18, 3617–3630. [Google Scholar] [CrossRef]
- Gill, J.; Mustapic, M.; Diaz-Arrastia, R.; Lange, R.; Gulyani, S.; Diehl, T.; Motamedi, V.; Osier, N.; Stern, R.A.; Kapogiannis, D. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 2018, 32, 1277–1284. [Google Scholar] [CrossRef]
- Loane, D.J.; Kumar, A.; Stoica, B.A.; Cabatbat, R.; Faden, A.I. Progressive neurodegeneration after experimental brain trauma: Association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 2014, 73, 14–29. [Google Scholar] [CrossRef]
- Kumar, A.; Henry, R.J.; Stoica, B.A.; Loane, D.J.; Abulwerdi, G.; Bhat, S.A.; Faden, A.I. Neutral Sphingomyelinase inhibition alleviates LPS-induced microglia activation and neuroinflammation after experimental traumatic brain injury. J. Pharm. Exp. 2019, 368, 338–352. [Google Scholar] [CrossRef]
- Aghagoli, G.; Gallo Marin, B.; Katchur, N.J.; Chaves-Sell, F.; Asaad, W.F.; Murphy, S.A. Neurological involvement in COVID-19 and potential mechanisms: A review. Neurocritical Care 2020. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezaie, J.; Nouri, M.; Panahi, Y. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2020, 85, 104422. [Google Scholar] [CrossRef]
- Tian, J.; Casella, G.; Zhang, Y.; Rostami, A.; Li, X. Potential roles of extracellular vesicles in the pathophysiology, diagnosis, and treatment of autoimmune diseases. Int. J. Biol. Sci. 2020, 16, 620–632. [Google Scholar] [CrossRef]
- György, B.; Hung, M.E.; Breakefield, X.O.; Leonard, J.N. Therapeutic applications of extracellular vesicles: Clinical promise and open questions. Annu. Rev. Pharm. Toxicol. 2015, 55, 439–464. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.d.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Vázquez-Ríos, A.J.; Molina-Crespo, Á.; Bouzo, B.L.; López-López, R.; Moreno-Bueno, G.; de la Fuente, M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. Nanobiotechnology 2019, 17, 85. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Aqil, F.; Kausar, H.; Agrawal, A.K.; Jeyabalan, J.; Kyakulaga, A.-H.; Munagala, R.; Gupta, R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016, 101, 12–21. [Google Scholar] [CrossRef]
- Didiot, M.-C.; Hall, L.M.; Coles, A.H.; Haraszti, R.A.; Godinho, B.M.; Chase, K.; Sapp, E.; Ly, S.; Alterman, J.F.; Hassler, M.R.; et al. Exosome-mediated delivery of hydrophobically modified siRNA for huntingtin mRNA silencing. Mol. Ther. 2016, 24, 1836–1847. [Google Scholar] [CrossRef]
- Shtam, T.A.; Kovalev, R.A.; Varfolomeeva, E.Y.; Makarov, E.M.; Kil, Y.V.; Filatov, M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013, 11, 88. [Google Scholar] [CrossRef]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor MicroRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef]
- Barba, A.A.; Bochicchio, S.; Dalmoro, A.; Lamberti, G. Lipid Delivery Systems for Nucleic-Acid-Based-Drugs: From Production to Clinical Applications. Pharmaceutics 2019, 11, 360. [Google Scholar] [CrossRef]
- Xu, L.; Anchordoquy, T. Drug delivery trends in clinical trials and translational medicine: Challenges and opportunities in the delivery of nucleic acid-based therapeutics. J. Pharm. Sci. 2011, 100, 38–52. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity after Stroke in Rats. J. Cereb. Blood Flow Metab. 2013, 33, 1711–1715. [Google Scholar] [CrossRef]
- Raisi, A.; Azizi, S.; Delirezh, N.; Heshmatian, B.; Farshid, A.A.; Amini, K. The Mesenchymal Stem Cell-Derived Microvesicles Enhance Sciatic Nerve Regeneration in Rat: A Novel Approach in Peripheral Nerve Cell Therapy. J. Trauma Acute Care Surg. 2014, 76, 991–997. [Google Scholar] [CrossRef]
- Kordelas, L.; Rebmann, V.; Ludwig, A.-K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-Derived Exosomes: A Novel Tool to Treat Therapy-Refractory Graft-versus-Host Disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef]
- Ophelders, D.R.M.G.; Wolfs, T.G.A.M.; Jellema, R.K.; Zwanenburg, A.; Andriessen, P.; Delhaas, T.; Ludwig, A.-K.; Radtke, S.; Peters, V.; Janssen, L.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect the Fetal Brain After Hypoxia-Ischemia. Stem Cells Transl. Med. 2016, 5, 754–763. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? Aaps J. 2009, 11, 495–510. [Google Scholar] [CrossRef]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Cooper, J.M.; Wiklander, P.B.O.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.V.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice: siRNA-EXOSOME decreased Alpha-Synuclein aggregates. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef]
- Gerth, K.; Kodidela, S.; Mahon, M.; Haque, S.; Verma, N.; Kumar, S. Circulating extracellular vesicles containing xenobiotic metabolizing CYP enzymes and their potential roles in extrahepatic cells via cell-cell interactions. Int. J. Mol. Sci. 2019, 20, 6178. [Google Scholar] [CrossRef]
- Kumar, S.; Sinha, N.; Gerth, K.A.; Rahman, M.A.; Yallapu, M.M.; Midde, N.M. Specific packaging and circulation of cytochromes P450, especially 2E1 isozyme, in human plasma exosomes and their implications in cellular communications. Biochem. Biophys. Res. Commun. 2017, 491, 675–680. [Google Scholar] [CrossRef]
- Kodidela, S.; Gerth, K.; Sinha, N.; Kumar, A.; Kumar, P.; Kumar, S. Circulatory Astrocyte and neuronal EVs as potential biomarkers of neurological dysfunction in HIV-infected subjects and alcohol/tobacco users. Diagnostics 2020, 10, 349. [Google Scholar] [CrossRef]
- Gong, Y.; Rao, P.S.S.; Sinha, N.; Ranjit, S.; Cory, T.J.; Kumar, S. The role of cytochrome P450 2E1 on ethanol-mediated oxidative stress and HIV replication in human monocyte-derived macrophages. Biochem. Biophys. Rep. 2019, 17, 65–70. [Google Scholar] [CrossRef]
- Haque, S.; Sinha, N.; Ranjit, S.; Midde, N.M.; Kashanchi, F.; Kumar, S. Monocyte-derived exosomes upon exposure to cigarette smoke condensate alter their characteristics and show protective effect against cytotoxicity and HIV-1 replication. Sci. Rep. 2017, 7, 16120. [Google Scholar] [CrossRef]
- Tseng, A.; Hughes, C.A.; Wu, J.; Seet, J.; Phillips, E.J. Cobicistat versus ritonavir: Similar pharmacokinetic enhancers but some important differences. Ann. Pharm. 2017, 51, 1008–1022. [Google Scholar] [CrossRef]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. 2013, 18, 601–610. [Google Scholar]
- Charoenviriyakul, C.; Takahashi, Y.; Morishita, M.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: Yield, physicochemical properties, and pharmacokinetics. Eur. J. Pharm. Sci. 2017, 96, 316–322. [Google Scholar] [CrossRef]
- Wen, S.; Dooner, M.; Papa, E.; Del Tatto, M.; Pereira, M.; Borgovan, T.; Cheng, Y.; Goldberg, L.; Liang, O.; Camussi, G.; et al. Biodistribution of Mesenchymal stem cell-derived extracellular vesicles in a radiation injury bone marrow murine model. Int. J. Mol. Sci. 2019, 20, 5468. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Lievens, J.; Snoeys, J.; Vekemans, K.; Van Linthout, S.; de Zanger, R.; Collen, D.; Wisse, E.; De Geest, B. The size of sinusoidal fenestrae is a critical determinant of hepatocyte transduction after adenoviral gene transfer. Gene Ther. 2004, 11, 1523–1531. [Google Scholar] [CrossRef]
- Imai, T.; Takahashi, Y.; Nishikawa, M.; Kato, K.; Morishita, M.; Yamashita, T.; Matsumoto, A.; Charoenviriyakul, C.; Takakura, Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J. Extracell. Vesicles 2015, 4, 26238. [Google Scholar] [CrossRef]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Pharmacokinetics of Exosomes-an important factor for elucidating the biological roles of Exosomes and for the development of exosome-based therapeutics. J. Pharm. Sci. 2017, 106, 2265–2269. [Google Scholar] [CrossRef]
- Betzer, O.; Perets, N.; Angel, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano 2017, 11, 10883–10893. [Google Scholar] [CrossRef]
- Arntz, O.J.; Pieters, B.C.H.; Broeren, M.G.A.; Bennink, M.B.; De Vries, M.; Van Lent, P.L.E.M.; Koenders, M.I.; Van den Berg, W.B.; Van der Kraan, P.M.; Van de Loo, F.A.J.; et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol. Nutr. Food Res. 2015, 59, 1701–1712. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- Jiang, X.-C.; Gao, J.-Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharm. 2017, 521, 167–175. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome theranostics: Biology and translational medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Lässer, C. Exosomes in diagnostic and therapeutic applications: Biomarker, vaccine and RNA interference delivery vehicle. Expert Opin. Biol. 2015, 15, 103–117. [Google Scholar] [CrossRef] [PubMed]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Cossetti, C.; Iraci, N.; Mercer, T.R.; Leonardi, T.; Alpi, E.; Drago, D.; Alfaro-Cervello, C.; Saini, H.K.; Davis, M.P.; Schaeffer, J.; et al. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol. Cell 2014, 56, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef]
- Pucci, F.; Garris, C.; Lai, C.P.; Newton, A.; Pfirschke, C.; Engblom, C.; Alvarez, D.; Sprachman, M.; Evavold, C.; Magnuson, A.; et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 2016, 352, 242–246. [Google Scholar] [CrossRef]
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 2011, 187, 676–683. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).