Different Roles of Auxins in Somatic Embryogenesis Efficiency in Two Picea Species
Abstract
1. Introduction
2. Results
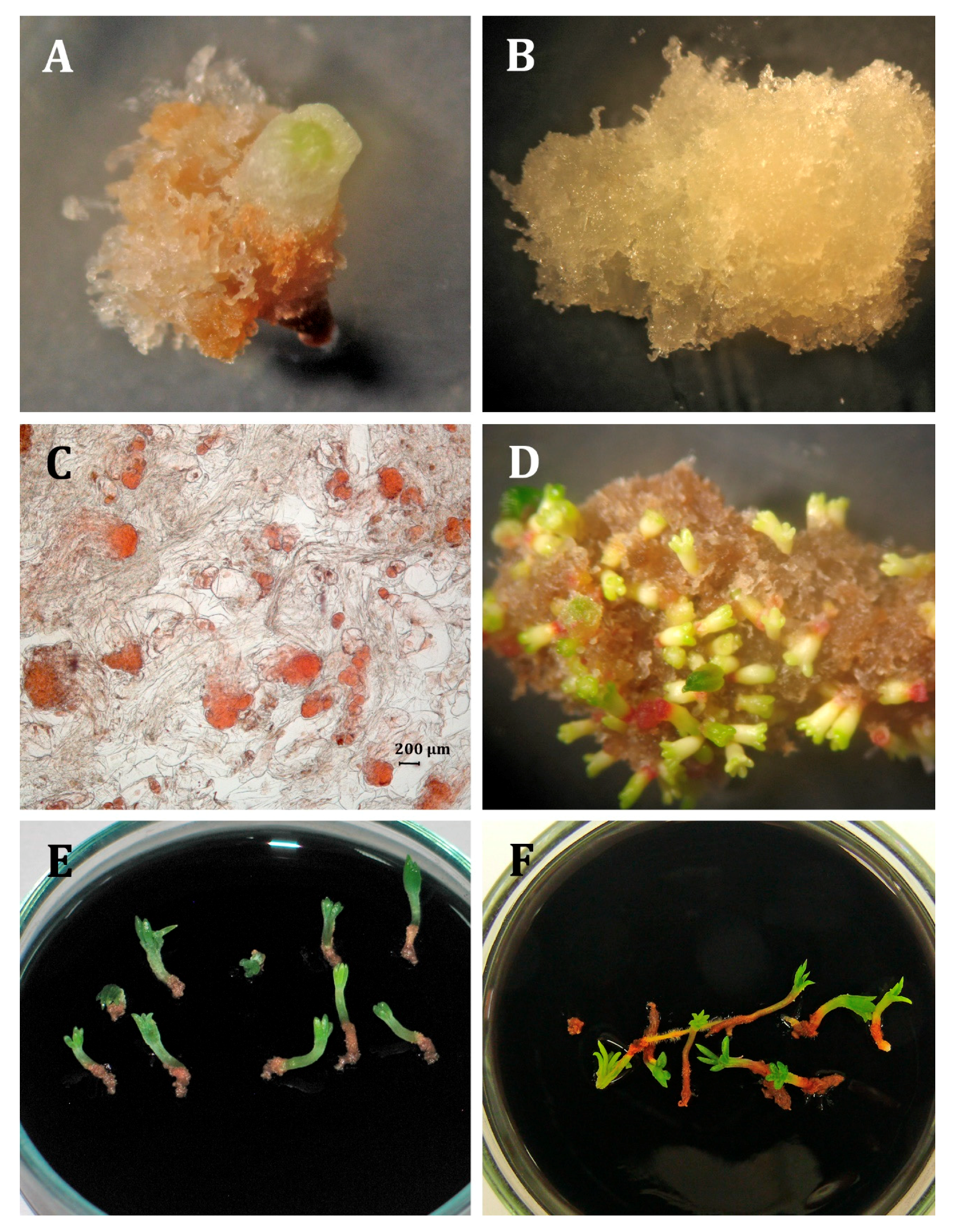
2.1. Initiation and Maintenance of Embryogenic Cultures
2.2. Effects of Auxin Treatment on the Physiological Condition of the ET Lines and the Levels of Oxidative Stress and Guaiacol Peroxidase (POX) Activity
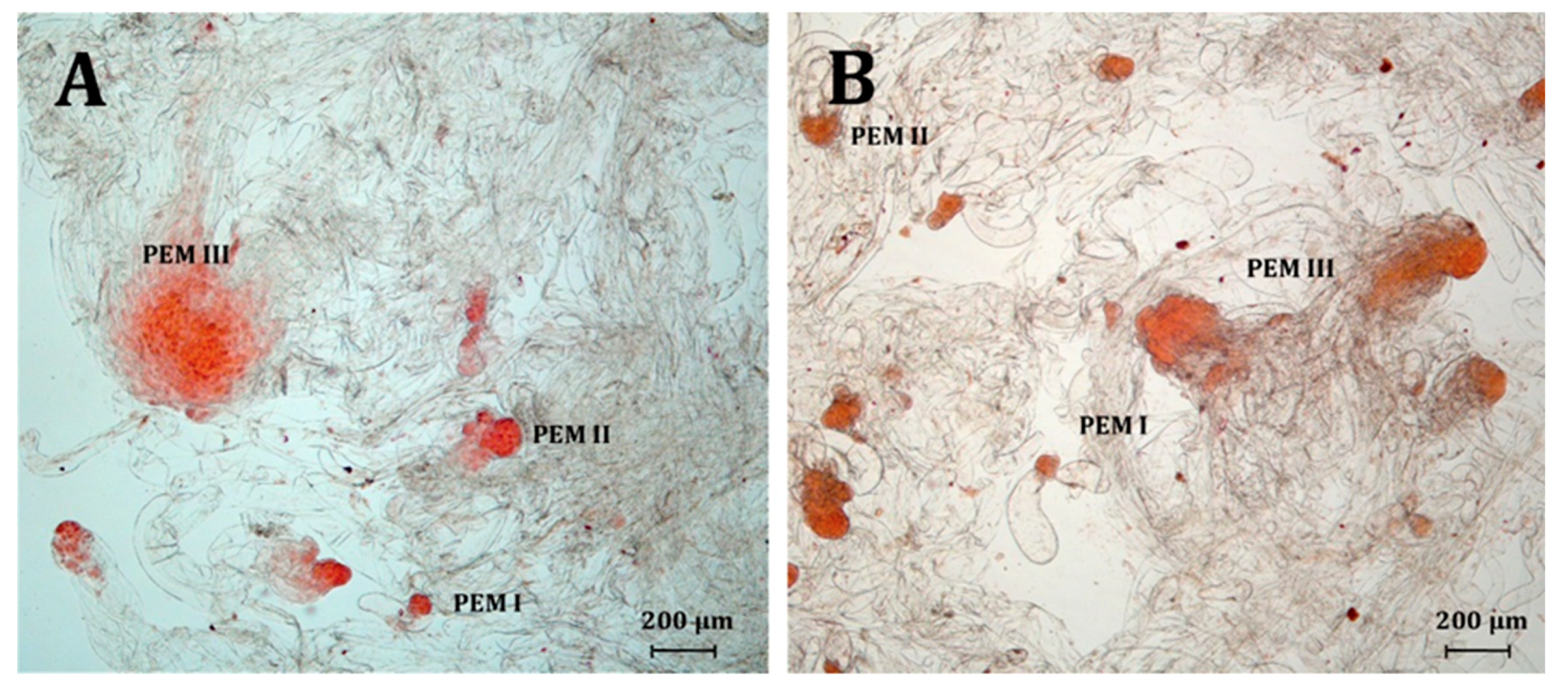
2.3. Somatic Embryo Production
2.4. Germination and Acclimatization
3. Discussion
3.1. Initiation and Maintenance of Embryogenic Cultures
3.2. Effect of Auxin Treatment on the Physiological Condition of ET Lines and the Levels of Oxidative Stress and Guaiacol Peroxidase Activity
3.3. Somatic Embryo Production
3.4. Germination and Acclimatization
4. Materials and Methods
4.1. Plant Material
4.2. Initiation and Maintenance of Embryogenic Cultures
4.3. Effects of Auxin Treatment on the Physiological Condition of the ET Lines and the Levels of Oxidative Stress and Guaiacol Peroxidase Activity
4.4. Somatic Embryo Production
4.5. Germination and Acclimatization
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| BA | benzyladenine |
| BSA | bovine serum albumin |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| EDTA | ethylenedinitrilotetraacetic acid |
| ET | embryogenic tissue |
| H2O2 | hydrogen peroxide |
| LM | Litvay et al. (1985) medium |
| NAA | 1-naphtaleneacetic acid |
| PEG | polyethylene glycol |
| PEM I | embryogenic stucture type I |
| PGR | plant growth regulator |
| Picloram | 4-amino-3,5,6-trichloropicolinic acid |
| POX | guaiacol peroxidase |
| PVPP | polyvinylpolypyrrolidone |
| ROS | reactive oxygen species |
| SE | somatic embryogenesis |
| TCA | trichloroacetic acid |
References
- Bonga, J.M. Conifer clonal propagation in tree improvement programs. In Vegetative Propagation of Forest Trees; Park, Y.S., Bonga, J.M., Moon, H.K., Eds.; National Institute of Forest Science: Seoul, Korea, 2016; pp. 3–31. [Google Scholar]
- Bozhkov, P.V.; Filonova, L.H.; von Arnold, S. A key developmental switch during Norway spruce somatic embryogenesis is induced by a withdrawal of growth regulators and is associated with cell death and extracellular acidification. Biotechnol. Bioeng. 2002, 77, 658–667. [Google Scholar] [CrossRef]
- Vondráková, Z.; Krajňáková, J.; Fischerová, L.; Vágner, M.; Eliášová, K. Physiology and role of plant growth regulators in somatic embryogenesis. In Vegetative Propagation of Forest Trees; Park, Y.S., Bonga, J.M., Moon, H.K., Eds.; National Institute of Forest Science: Seoul, Korea, 2016; pp. 123–169. [Google Scholar]
- Simon, S.; Petrášek, J. Why plants need more than one type of auxin. Plant Sci. 2011, 180, 454–460. [Google Scholar] [CrossRef]
- Harry, I.S.; Thorpe, T.A. In vitro culture of forest trees. In Plant Cell and Tissue Culture; Vasil, I.K., Thorpe, T., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 539–560. [Google Scholar]
- Fehér, A. The initiation phase of somatic embryogenesis: what we know and what we don’t. Acta Biol. Szeged. 2008, 52, 53–56. [Google Scholar]
- Teixteira da Silva, J.A.; Malabadi, R.B. Factors affecting somatic embryogenesis in conifers. J. For. Res. 2012, 23, 503–515. [Google Scholar] [CrossRef]
- Fehér, A.; Pasternak, T.P.; Dudits, D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss. Org. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Jiménez, V.M. Regulation of in vitro somatic embryogenesis with emphasis on the role of endogenous hormones. R. Bras. Fiziol. Veg. 2001, 13, 196–223. [Google Scholar] [CrossRef]
- Freitas, E.O.; Monteiro, T.R.; Nogueira, G.F.; Scherwinski-Pereira, J.E. Somatic embryogenesis from immature and mature zygotic embryos of the açaí palm (Euterpe oleracea): Induction of embryogenic cultures, morphoanatomy and its morphological characteristics. Sci. Hortic.- Amst. 2016, 212, 126–135. [Google Scholar] [CrossRef]
- Venkataiah, P.; Bhanuprakash, P.; Suman, K.S.; Subhash, K. Somatic embryogenesis and plant regeneration of Capsicum baccatum L. J. Genet. Eng. Biotech. 2016, 14, 55–60. [Google Scholar] [CrossRef]
- Lu, C.Y.; Thorpe, T. Somatic embryogenesis and plantlet regeneration in cultured immature embryos of Picea glauca. J. Plant Physiol. 1987, 128, 297–302. [Google Scholar] [CrossRef]
- Ramarosandratana, A.V.; van Staden, J. Changes in competence for somatic embryogenesis in Norway spruce zygotic embryos segments. Plant Cell Tiss. Org. 2003, 74, 249–255. [Google Scholar] [CrossRef]
- Hazubska-Przybył, T.; Bojarczuk, K. Somatic embryogenesis of selected spruce species (Picea abies, P. omorika, P. pungens ‘Glauca’ and P. breweriana). Acta Soc. Bot. Pol. 2008, 77, 189–199. [Google Scholar]
- Kim, Y.W. Initiation of embryogenic callus from mature zygotic embryos in Japanese larch (Larix kaempferi). J. Plant Biotechnol. 2015, 42, 223–227. [Google Scholar] [CrossRef]
- Li, C.H.; Liu, B.G.; Kim, T.D.; Moon, H.K.; Choi, Y.E. Somatic embryogenesis and plant regeneration in elite genotypes of Picea koraiensis. Plant Biotechnol. Rep. 2008, 2, 259–265. [Google Scholar] [CrossRef]
- Warchoł, M.; Skrzypek, E.; Kusibab, T.; Dubert, F. Induction of somatic embryogenesis and biochemical characterization of Cordyline australis (G. Forst.) Endl. ‘Red Star‘ callus. Sci. Hortic.-Amst. 2015, 192, 338–345. [Google Scholar]
- Filonova, L.H.; Bozhkov, P.V.; von Arnold, S. Developmental pathway of somatic embryogenesis in Picea abies as revealed by time–lapse trackind. J. Exp. Bot. 2000, 51, 249–264. [Google Scholar] [CrossRef]
- Salaj, T.; Klubicová, K.; Matusova, R.; Salaj, J. Somatic embryogenesis in selected conifer trees Pinus nigra Arn. and Abies hybrids. Front. Plant Sci. 2019, 10, 13. [Google Scholar] [CrossRef]
- von Aderkas, P.; Lelu, M.A.; Label, P. Plant growth regulator levels during maturation of larch somatic embryogenesis. Plant Physiol. Biochem. 2001, 39, 495–502. [Google Scholar] [CrossRef]
- Klubicová, K.; Uvá cková, L.; Danchenko, M.; Nemecek, P.; Skultéty, L.; Salaj, J.; Salaj, T. Insight into the early stage of Pinus nigra Arn., somatic embryogenesis using discovery proteomics. J. Proteomics 2017, 169, 99–111. [Google Scholar]
- Zhang, S.G.; Han, S.Y.; Yang, W.H.; Wei, H.L.; Zhang, M.; Qi, L.W. Changes in H2O2 content and antioxidant enzyme gene expression during the somatic embryogenesis of Larix leptolepis. Plant Cell Tiss. Org. 2010, 100, 21–29. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Potters, G.; Caubergs, R.; Jansen, M.A.K. Complementary interactions between oxidative stress and auxin control plant growth responses at plant, organ and cellular level. J. Exp. Bot. 2005, 56, 1991–2001. [Google Scholar] [CrossRef]
- Tian, M.; Gu, Q.; Zhu, M. The involvement of hydrogen peroxide and antioxidant enzymes in the process of shoot organogenesis of strawberry callus. Plant Sci. 2003, 165, 701–707. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Los, D.A.; Allakhverdiev, S.I.; Kuznetov, V. Signaling role of reactive oxygen species in plants under stress. Russ. J. Plant Physiol. 2012, 59, 141–154. [Google Scholar] [CrossRef]
- Smirnow, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Zavattieri, M.A. Induction of somatic embryogenesis as an example of stress-related plants reactions. Electr. J. Biotech. 2010, 13, 1–9. [Google Scholar] [CrossRef]
- Correia, S.I.; Pinto, G.; Canhoto, J.M. Molecular biology of somatic embryogenesis in hardwoods. In Vegetative Propagation of Forest Trees; Park, Y.S., Bonga, J.M., Moon, H.K., Eds.; National Institute of Forest Science: Seoul, Korea, 2016; pp. 97–122. [Google Scholar]
- Ślesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signaling in response to environmental stresses. Acta Bioch. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef]
- Cheng, W.H.; Wang, F.L.; Cheng, X.Q.; Zhu, Q.H.; Sun, Y.Q.; Zhu, H.G.; Sun, J. Polyamine and its metabolite H2O2 play a key role in the conversion of embryogenic callus into somatic embryos in upland cotton (Gossypium hirsutum L.). Front. Plant Sci. 2015, 6, 1063. [Google Scholar] [CrossRef]
- Stasolla, C.; Yeung, E.C. Cellular ascorbic acid regulates the activity of major peroxidases in the apical poles of germinating white spruce (Picea glauca) somatic embryos. Plant Physiol. Biochem. 2007, 45, 188–198. [Google Scholar] [CrossRef]
- Brownleader, M.D.; Hopkins, J.; Mobasheri, A.; Dey, P.M.; Jackson, P.; Trevan, M. Role of extension peroxidase in tomato (Lycopersicon esculentum Mill.) seedling growth. Planta 2000, 210, 668–676. [Google Scholar] [CrossRef]
- Hazubska-Przybył, T.; Ratajczak, E.; Kalemba, E.M.; Bojarczuk, K. Growth regulators and guaiacol peroxidase activity during the induction phase of somatic embryogenesis in Picea species. Dendrobiology 2013, 69, 77–86. [Google Scholar] [CrossRef][Green Version]
- Kormutak, A.; Salaj, T.; Matušova, R.; Vookova, B. Biochemistry of zygotic and somatic embryogenesis in silver fir (Abies alba Mill.). Acta Biol. Crac. Ser. Bot. 2003, 45, 59–62. [Google Scholar]
- Sharma, S.K.; Bryan, G.J.; Millam, S. Auxin pulse treatment holds the potential to enhance efficiency and practicability of somatic embryogenesis in potato. Plant Cell Rep. 2007, 26, 945–950. [Google Scholar] [CrossRef]
- Cook, T.J.; Racusen, R.H.; Cohen, J.D. The role of auxin in plant embryogenesis. Plant Cell 1993, 5, 1494–1495. [Google Scholar] [CrossRef]
- Kawahara, R.; Komamine, A. Molecular basis of somatic embryogenesis. In Biotechnology in Agriculture and Forestry, Somatic Embryogenesis and Synthetic Seed; Bajaj, Y.P.S., Ed.; Springer-Verlag: Berlin, Germany, 1995; Volume 30, pp. 30–40. [Google Scholar]
- Malabadi, R.B.; Choudhury, H.; Tandon, P. Initiation, maintenance and maturation of somatic embryos from thin apical dome sections in Pinus kesiya (Royle ex. Gord) promoted by partial desiccation and Gellan gum. Sci. Hortic. 2004, 102, 449–459. [Google Scholar] [CrossRef]
- Pullman, G.S.; Skryabina, A. Liquid medium and liquid overlays improve embryogenic tissue initiation in conifers. Plant Cell Rep. 2007, 26, 873–887. [Google Scholar] [CrossRef]
- Gupta, P.K. Methods of Initiating Plant Somatic Embryos. U.S. Patent No 9374954, 2014. New York, NY. [Google Scholar] [CrossRef]
- Ilashi, T. Induction of somatic embryogenesis in woody plants. Acta Physiol. Plant 2016, 38, 118. [Google Scholar] [CrossRef]
- Pais, M.S. Somatic Embryogenesis Induction in Woody Species: The Future After OMICs Data Assessment. Front. Plant Sci. 2019, 10, 240. [Google Scholar] [CrossRef]
- Bonga, J.M.; Klimaszewska, K.K.; von Aderkas, P. Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tiss. Org. 2010, 100, 241–254. [Google Scholar] [CrossRef]
- Astarita, L.V.; Guerra, M.P. Early somatic embryogenesis in A. angustifolia – induction and maintenance of embryonal-suspensor mass cultures. Braz. J. Plant Physiol. 1998, 10, 113–118. [Google Scholar]
- Hosoi, Y.; Maruyama, T.E. Plant regeneration from embryogenic tissue of Pinus luchuensis Mayr, an endemic species in Ryukyu Island, Japan. Plant Biotechnol. 2012, 29, 401–406. [Google Scholar] [CrossRef]
- Kim, Y.; Moon, H.K. Enhancement of somatic embryogenesis and plant regeneration in Japanese larch (Larix leptolepis). Plant Cell Tiss. Org. 2007, 88, 241–245. [Google Scholar] [CrossRef]
- McKey, J.J.; Becwar, M.R.; Park, Y.-S.; Corderro, J.P.; Pullman, G.S. Genetic control of somatic embryogenesis initiation in loblolly pine and implications for breeding. Tree Genet. Genomes 2006, 2, 1–9. [Google Scholar] [CrossRef]
- Mo, L.H.; von Arnold, S. Origin and development of embryogenic cultures from seedlings of Norway spruce (Picea abies). J. Plant Physiol. 1991, 138, 223–230. [Google Scholar] [CrossRef]
- Salopek, B.; Tramisak–Milaković, T.; Mihaljević, S.; Jelaska, S. Storage product accumulation during the maturation of Picea omorika (Panc.) Purk. somatic embryos. Period. Biol. 1997, 99, 117–124. [Google Scholar]
- Webb, D.T.; Webster, F.; Flinn, B.S.; Roberts, D.R.; Ellis, D.D. Factors influencing the induction of embryogenic callus from embryos of Picea glauca and P. engelmanii. Can. J. For. Res. 1989, 19, 1303–1308. [Google Scholar] [CrossRef]
- Maruyama, E.; Hosoi, Y.; Ishii, K. Somatic embryogenesis and plant regeneration in Yakutanegoyou, Pinus armandii Franch. var. amamiana (Koidz.) Hatusima, an endemic and endangered species in Japan. In Vitro Cell Dev. Biol. Plant 2007, 43, 28–34. [Google Scholar] [CrossRef]
- Maruyama, E.; Hosoi, Y. Progress in Somatic Embryogenesis of Japanese Pines. Front. Plant Sci. 2019, 10, 31. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Cyr, D.R. Conifer somatic embryogenesis: I. Development. Dendrobiology 2002, 48, 31–39. [Google Scholar]
- Klimaszewska, K.; Hargreaves, C.; Lelu-Walter, A.M.; Trontin, J.F. Advances in conifer somatic embryogenesis since year 2000. In In Vitro Embryogenesis in Higher Plants, Methods in Molecular Biology; Germanà, M.A., Lambardi, M., Eds.; © Springer Science + Business Media: New York, NY, USA, 2016; Volume 1359, pp. 131–166. [Google Scholar] [CrossRef]
- Salajova, T.; Jasik, J.; Salaj, J. Somatic embryogenesis in hybrid firs Abies alba x Abies cephalonica and Abies alba x Abies numidica. In In Vitro Cultures of Conifers; Salajova, T., Jasik, J., Salaj, J., Eds.; Veda Publishing House of the Slovak Academy of Sciences: Bratislava, Slovak Republic, 1998; pp. 63–69. [Google Scholar]
- Stasolla, C.; Kong, L.; Yeung, E.C.; Thorpe, T.A. Maturation of somatic embryos in conifers: Morphogenesis, physiology, biochemistry and molecular biology. In Vitro Cell. Dev. Biol. – Plant 2002, 38, 93–105. [Google Scholar] [CrossRef]
- Brenton, D.; Harvengt, L.; Trontin, J.F.; Bouvet, A.; Favre, J.M. High subculture frequency maltose-based and hormone-free medium sustained early development of somatic embryos in Maritime pine. In Vitro Cell Dev. Biol. Plant. 2005, 41, 494–504. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen peroxidase and nitric oxide as signalling molecules in plants. J Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef]
- Konieczny, R.; Libik, M.; Tuleja, M.; Niewiadomska, E.; Miszalski, Z. Oxidative events during in vitro regeneration of sunflower. Acta Physiol. Plant. 2008, 30, 71–79. [Google Scholar] [CrossRef]
- Libik, M.; Konieczny, R.; Pater, B.; Ślesak, I.; Miszalski, Z. Differences in the activities of some antioxidant enzymes and in H2O2 content during rhizogenesis and somatic embryogenesis in callus cultures of the ice plant. Plant Cell Rep. 2005, 23, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Inzé, D.; Van Breusegem, F. Signal transduction during oxidative stress. J. Exp. Bot. 2002, 53, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Prudente, D.; Batista de Souza, L.; Paiva, R. Plant Somatic Embryogenesis: Modulatory Role of Oxidative Stress. Proc. Natl. Acadcademy Sci. India Sect. B Biol. Sci. 2019. [Google Scholar] [CrossRef]
- Kairong, C.; Gengsheng, X.; Xinmin, L.; Gengmei, X.; Yafu, W. Effect of hydrogen peroxide on somatic embryogenesis of Lycium barbarum L. Plant Sci. 1999, 146, 9–16. [Google Scholar] [CrossRef]
- Karpiński, S.; Reynolds, H.; Karpińska, B.; Wingsle, G.; Creissen, G.; Mullineaux, P. Systemic signalling and acclimation in response to excess excitation Energy in Arabidopsis. Science 1999, 284, 654–657. [Google Scholar] [CrossRef]
- Guan, L.; Zhao, J.; Scandalios, J.G. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 2000, 22, 87–95. [Google Scholar] [CrossRef]
- Blazquez, S.; Olmos, E.; Hernández, J.A.; Fernández-García, N.; Fernández, J.A.; Piqueras, A. Somatic embryogenesis in saffron (Crocus sativus L.) histological differentiation and implication of some components of the antioxidant enzymatic system. Plant Cell Tiss. Org. 2009, 97, 49–57. [Google Scholar] [CrossRef]
- Martin, F.; Clément-Vidal, A.; Burel, A.; Farinas, B.; Sanier, C.; Fabre, D.; Leclercq, J.; Rio, M.; Woraathasin, N.; Montoro, P.; et al. Overexpression of EcGSH1 induces glutathione production and alters somatic embryogenesis and plant development in Hevea brasiliensis. Ind. Crops Prod. 2018, 112, 803–814. [Google Scholar] [CrossRef]
- Rose, R.J. Somatic Embryogenesis in the Medicago trancatula Model: Cellular and Molecular Mechanisms. Front. Plant Sci. 2019, 10, 267. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, X.; Gou, K.; Deng, J.; Xu, J.; Gao, W.; Lindsey, K.; Zhang, X. ROS Homeostasis Regulates Somatic Embryogenesis via the Regulation of Auxin Signaling in Cotton. Mol. Cell. Proteomics 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Đorđewić, B.; Prášková, M.; Hampel, D.; Havel, L. Effects of cadmium and lead stress on somatic embryogenesis of coniferous species. Part II: Changes of thiol substances. Acta Physiol. Plant. 2017, 39, 141. [Google Scholar] [CrossRef]
- Dorigan de Matos Furlanetto, A.L.; Valente, C.; Martinez, G.R.; Merlin Rocha, M.E.; Maurer, J.B.B.; Cadena, S.M.S.C. Cold stress on Araucaria angustifolia embryogenic cells results on oxidative stress and induces adaptation: implications for conservation and propagation. Free Radic. Res. 2019, 53, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Jo, L.; Dos Santos, A.L.W.; Bueno, C.A.; Barbosa, H.R.; Floh, E.I.S. Proteomic analysis and polyamines, ethylene and reactive oxygen species levels of Araucaria angustifolia (Brazilian pine) embryogenic cultures with different embryogenic potential. Tree Physiol. 2014, 34, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Egertsdotter, U. Plant physiological and genetical aspects of the somatic embryogenesis process in conifers. Scan. J. Forest Res. 2019, 34, 360–369. [Google Scholar] [CrossRef]
- Stasolla, C.; Yeung, E.C. Recent advances in conifer somatic embryogenesis: improving somatic embryo quality. Plant Cell Tiss. Org. 2003, 74, 15–35. [Google Scholar] [CrossRef]
- Egertsdotter, U.; Clapham, D. Method for maturing and synchronizing conifer somatic embryos. U.S. Patent No. 9125,352, 8 September 2015. [Google Scholar]
- Gerdakaneh, M.; Zohori, M. The Effect of Picloram on Somatic Embryogenesis of Different Explants of Strawberry (Fragaria ananassa Duch.). Br. Biotechnol. J. 2013, 3, 133–142. [Google Scholar] [CrossRef]
- Liu, C.M.; Xu, Z.H.; Chua, N.H. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 1993, 5, 621–630. [Google Scholar] [CrossRef]
- Schiavone, F.M.; Cooke, T.J. Unusual patterns of somatic embryogenesis in the domesticated carrot: developmental effects of exogenous auxins and auxin transport inhibitors. Cell Differ. 1987, 21, 53–62. [Google Scholar] [CrossRef]
- Belmonte Attree, S.M.; Fowke, L.C. Embryogeny of gymnosperms: advances in synthetic seed technology of conifers. Plant Cell Tiss. Org. 1993, 35, 1–35. [Google Scholar] [CrossRef]
- Hazubska-Przybył, T.; Wawrzyniak, M. Stimulation of somatic embryo growth and development in Picea spp. by polyethylene glycol. Dendrobiology 2017, 78, 168–178. [Google Scholar] [CrossRef][Green Version]
- Tikkinen, M.; Varis, S.; Aronen, T. Development of Somatic Embryo Maturation and Growing Techniques of Norway Spruce Emblings towards Large-Scale Field Testing. Forests 2018, 9, 325. [Google Scholar] [CrossRef]
- von Aderkas, P.; Label, P.; Lelu, M.A. Charcoal affects early development and hormonal concentrations of somatic embryos of hybrid larch. Tree Physiol. 2002, 22, 431–434. [Google Scholar] [CrossRef]
- Belmonte, M.F.; Macey, J.; Yeung, E.C.; Stasolla, C. The effect of osmoticum on ascorbate and glutathione metabolism during white spruce (Picea glauca) somatic embryo development. Plant Physiol. Biochem. 2005, 43, 337–346. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, J.; Jing, D.; Kong, L.; Zhang, S.; Wang, J. Plant regeneration of Picea asperata Mast. by somatic embryogenesis. Trees 2016, 31, 299–312. [Google Scholar] [CrossRef]
- Hu, R.; Sun, Y.; Wu, B.; Duan, H.; Zheng, H.; Hu, D.; Lin, H.; Tong, Z.; Xu, J.; Li, Y. Somatic Embryogenesis of Immature Cunninghamia lanceolata (Lamb.) Hook Zygotic Embryos. Sci. Rep. 2017, 7, 56. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Park, Y.S.; Overton, C.; Maceacheron, I.; Bonga, J.M. Optimized somatic embryogenesis in Pinus strobus L. In Vitro Cell. Dev. Biol. – Plant 2001, 37, 392–399. [Google Scholar] [CrossRef]
- Leljak-Levanić, D.; Mihaljević, S.; Jelaska, S. Variations in DNA methylation in Picea omorika (Panć). Purk. Embryogenic tissue and the ability for embryo maturation. Propag. Ornam. Plants 2009, 9, 3–9. [Google Scholar]
- Hay, E.I.; Charest, P.J. Somatic embryo germination and desiccation tolerance in conifers. In Somatic Embryogenesis in Woody Plants; Mohan, J.S., Gupta, P.K., Newton, R.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; Volume 4, pp. 61–69. [Google Scholar]
- Fridborg, G.; Pedersen, M.; Landstrom, L.E.; Eriksson, T. Effects of activated charcoal on tissue cultures: adsorption of metabolites in plant tissue culture. Physiol. Plant. 1987, 43, 104–106. [Google Scholar] [CrossRef]
- Jourdain, I.; Lelu, M.A.; Label, P. Hormonal changes during growth of somatic masses in hybrid larch. Plant Physiol. Biochem. 1997, 35, 741–749. [Google Scholar]
- Vondrakova, Z.; Dobrev, P.I.; Pesek, B.; Fischerova, L.; Vagner, M.; Motyka, V. Profiles of Endogenous Phytohormones Over the Course of Norway Spruce Somatic Embryogenesis. Front. Plant Sci. 2018, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin-cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Möller, B.; Weijers, D. Auxin Control of Embryo Patterning. Cold Spring Harb. Perspect. Biol. 2009, 1, a001545. [Google Scholar]
- Palovaara, J.; Hakman, I. WOX2 and polar auxin transport during spruce embryo pattern formation. Plant Sign. Behav. 2009, 4, 153–155. [Google Scholar]
- Garcia, C.; Furtado de Almeida, A.A.; Costa, M.; Britto, D.; Valle, R.; Royaert, S.; Marelli, J.P. Abnormalities in somatic embryogenesis caused by 2,4-D: an overview. Plant Cell Tiss. Org. 2019, 137, 193–212. [Google Scholar] [CrossRef]
- Pasternak, T.P. The role of auxin, pH, and stress in the activation of embryogenic division in leaf protoplasts-derived cells of alfalfa. Plant Physiol. 2002, 129, 1807–1819. [Google Scholar] [CrossRef]
- Fehér, A. Somatic embryogenesis–stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta, Gene Regul. Mech. 2015, 1849, 385–402. [Google Scholar]
- Leljak-Levanić, D.; Bauer, N.; Mihaljević, S.; Jelaska, S. Changes in DNA methylation during somatic embryogenesis in Cucurbita pepo L. Plant Cell Rep. 2004, 23, 120–127. [Google Scholar] [CrossRef]
- Gaj, M. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul. 2004, 43, 27–47. [Google Scholar] [CrossRef]
- Vondráková, Z.; Eliášová, K.; Fischerová, L.; Vágner, M. The role of auxin in somatic embryogenesis of Abies alba. Open Life Sci. 2011, 6, 587–596. [Google Scholar] [CrossRef]
- Nissen, P.; Minocha, S.C. Inhibition by 2,4-D of somatic embryogenesis in carrot as explored by its reversal by difluoromethylornithine. Physiol Plant 1993, 89, 673–680. [Google Scholar] [CrossRef]
- Litvay, J.D.; Verma, D.C.; Johson, M.A. Influence of loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of wild carrot (Daucus carota L.). Plant Cell Rep. 1985, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Sagisaka, S. The occurrence of peroxide in a perennial plant Populus gelrica. Plant Physiol. 1976, 57, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. In Methods in Enzymology; Collowick, S.P., Kapplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; pp. 764–775. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

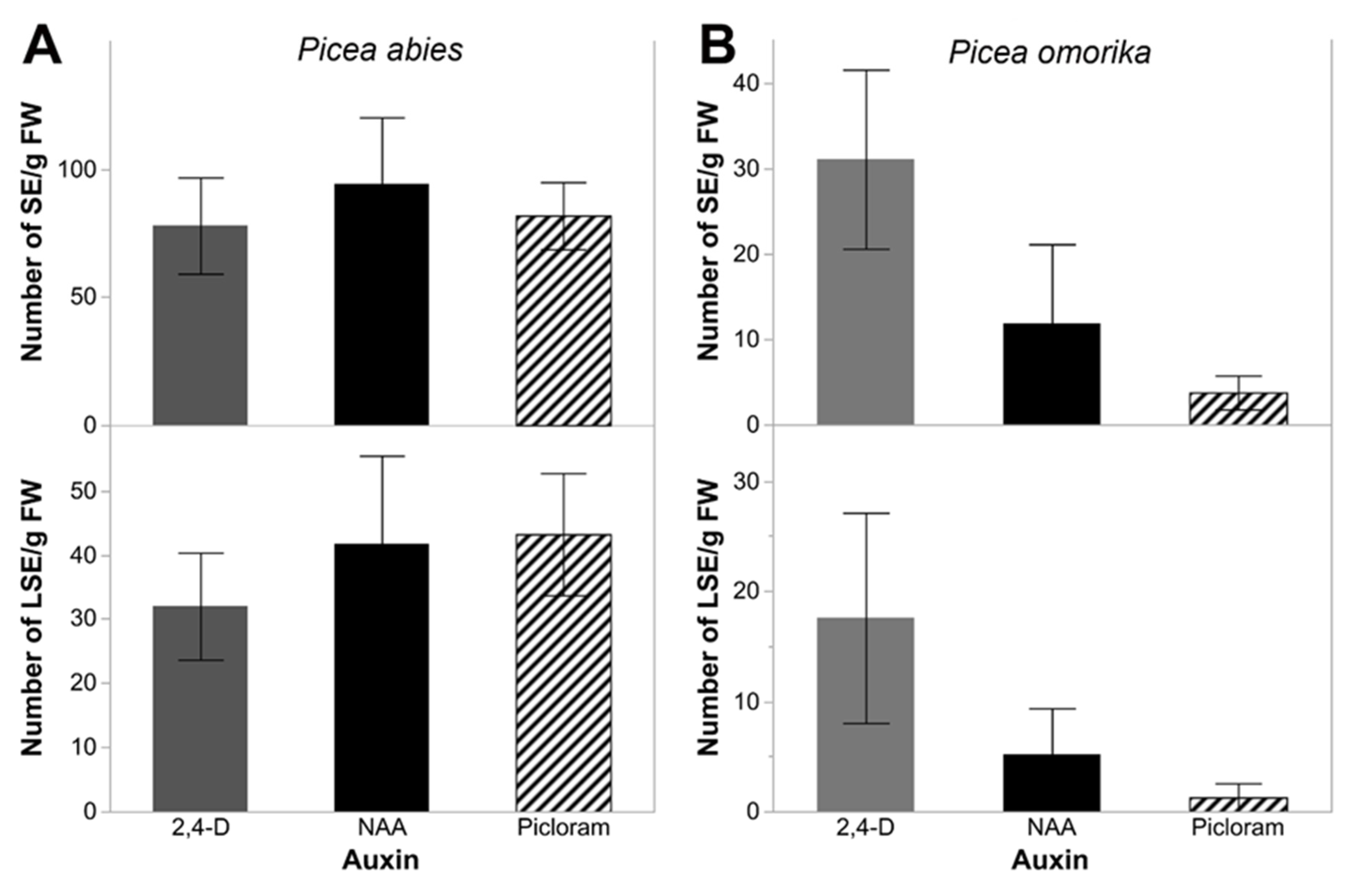

| Species | PGR Type | Total Number of Lines (100%) | Number of Explants with ET Initiation (%) |
|---|---|---|---|
| Picea abies | 2,4-D | 232 | 17 (7.33%) |
| NAA | 249 | 19 (7.63%) | |
| picloram | 229 | 24 (10.48%) | |
| Picea omorika | 2,4-D | 250 | 55 (22.00%) |
| NAA | 271 | 44 (16.24%) | |
| picloram | 264 | 40 (15.15%) |
| Species | PGRs Type | Number of ET Lines According to Number of the Passages (%) | |||
|---|---|---|---|---|---|
| ≤10 | >10 | Up to 40 | Total (100%) | ||
| Picea abies | 2,4-D | 12 (70.59%) | 5 (29.41%) | 5 (29.41%) | 17 |
| NAA | 16 (84.21%) | 3 (15.79%) | 3 (15.79%) | 19 | |
| picloram | 19 (79.17%) | 5 (20.83%) | 5 (20.83%) | 24 | |
| Picea omorika | 2,4-D | 48 (87.27%) | 7 (12.73%) | 5 (9.09%) | 55 |
| NAA | 34 (82.93%) | 7 (17.07%) | 7 (17.07%) | 41 | |
| picloram | 29 (76.32%) | 9 (23.68%) | 4 (10.53%) | 38 | |
| Species | PGRs Type | ET Growth [g] | H2O2 [nmol/gFW] | POX [mM/gFW] |
|---|---|---|---|---|
| Picea abies | 2,4-D | 0.58 ± 0.1 b | 2.60 ± 0.2 a | 54.92 ± 5.8 ab |
| NAA | 1.06 ± 0.1 a | 2.25 ± 0.2 ab | 34.76 ± 7.5 b | |
| picloram | 0.78 ± 0.1 ab | 2.01 ± 0.12 b | 64.41 ± 5.8 a | |
| Picea omorika | 2,4-D | 0.90 ± 0.2 a | 2.01 ± 0.2 a | 32.97 ± 4.9 a |
| NAA | 1.21 ± 0.1 a | 1.61 ± 0.1 a | 28.78 ± 4.2 a | |
| picloram | 1.06 ± 0.2 a | 1.46 ± 0.2 a | 33.26 ± 5.5 a |
| Species | PGRs Type | PEM I | PEM II | PEM III |
|---|---|---|---|---|
| Number (Ratio) of Proembryogenic Structures | ||||
| Picea abies | 2,4-D | 125 (16.82%) | 309 (41.59%) b | 309 (41.59%) a |
| NAA | 129 (15.07%) | 445 (51.99%) a | 282 (32.94%) b | |
| picloram | 160 (15.53%) | 440 (42.72%) b | 430 (41.75%) a | |
| Picea omorika | 2,4-D | 592 (22.21%) ab | 1345 (50.47%) a | 728 (27.32%) b |
| NAA | 1353 (28.67%) a | 2352 (49.83%) a | 1015 (21.50%) c | |
| picloram | 251 (19.43%) b | 597 (46.21%) b | 444 (34.37%) a | |
| Size of the Embryogenic Region (µm, Mean ± SEM) | ||||
| Picea abies | 2,4-D | 48.71 ± 1.3 | 95.50 ± 1.3 | 212.62 ± 4.4 a |
| NAA | 49.99 ± 1.4 | 95.73 ± 1.5 | 179.30 ± 3.5 b | |
| picloram | 50.18 ± 1.3 | 95.04 ± 1.2 | 205.45 ± 3.5 a | |
| Picea omorika | 2,4-D | 48.49 ± 0.6 | 91.81 ± 0.7 b | 183.24 ± 3.3 |
| NAA | 48.55 ± 0.5 | 91.17 ± 0.6 b | 179.69 ± 2.8 | |
| picloram | 49.28 ± 0.7 | 94.47 ± 0.8 a | 190.89 ± 4.3 | |
| Species | PGRs Type | SE after Two Weeks in the Darkness | SE after Two Weeks on the Light | |||
|---|---|---|---|---|---|---|
| Hypocotyl Length (mm) | Radicle Length (mm) | Hypocotyl Length (mm) | Radicle Length (mm) | HL/RL Ratio | ||
| Picea abies | 2,4-D | 9.91 ± 0.4 a | 2.76 ± 0.1 a | 11.08 ± 0.4 a | 3.75 ± 0.2 b | 1.81 ± 0.1 a |
| NAA | 6.94 ± 0.3 c | 2.17 ± 0.1 b | 8.61 ± 0.3 b | 5.32 ± 0.4 a | 1.51 ± 0.1 b | |
| picloram | 8.46 ± 0.2 b | 2.71 ± 0.1 a | 10.37 ± 0.2 a | 4.53 ± 0.2 ab | 1.77 ± 0.1 a | |
| Picea omorika | 2,4-D | 3.79 ± 0.2 b | 2.52 ± 0.1 a | 4.98 ± 0.2 ab | 2.97 ± 0.1 a | 1.96 ± 0.4 b |
| NAA | 4.88 ± 0.3 a | 1.62 ± 0.1 b | 5.98 ± 0.4 a | 1.64 ± 0.1 b | 4.41 ± 0.4 a | |
| picloram | 4.42 ± 0.5 ab | 2.75 ± 0.3 a | 4.92 ± 0.5 b | 3.42 ± 0.5 a | 1.78 ± 0.1 b | |
| Species | PGRs Type | Number of SE | |
|---|---|---|---|
| Germinated in Vitro | Suitable for Acclimatization | ||
| Picea abies | 2,4-D | 234 | 31 |
| NAA | 296 | 123 | |
| picloram | 589 | 173 | |
| Picea omorika | 2,4-D | 166 | 10 |
| NAA | 58 | 0 | |
| picloram | 12 | 2 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazubska-Przybył, T.; Ratajczak, E.; Obarska, A.; Pers-Kamczyc, E. Different Roles of Auxins in Somatic Embryogenesis Efficiency in Two Picea Species. Int. J. Mol. Sci. 2020, 21, 3394. https://doi.org/10.3390/ijms21093394
Hazubska-Przybył T, Ratajczak E, Obarska A, Pers-Kamczyc E. Different Roles of Auxins in Somatic Embryogenesis Efficiency in Two Picea Species. International Journal of Molecular Sciences. 2020; 21(9):3394. https://doi.org/10.3390/ijms21093394
Chicago/Turabian StyleHazubska-Przybył, Teresa, Ewelina Ratajczak, Agata Obarska, and Emilia Pers-Kamczyc. 2020. "Different Roles of Auxins in Somatic Embryogenesis Efficiency in Two Picea Species" International Journal of Molecular Sciences 21, no. 9: 3394. https://doi.org/10.3390/ijms21093394
APA StyleHazubska-Przybył, T., Ratajczak, E., Obarska, A., & Pers-Kamczyc, E. (2020). Different Roles of Auxins in Somatic Embryogenesis Efficiency in Two Picea Species. International Journal of Molecular Sciences, 21(9), 3394. https://doi.org/10.3390/ijms21093394





