Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors
Abstract
1. Introduction
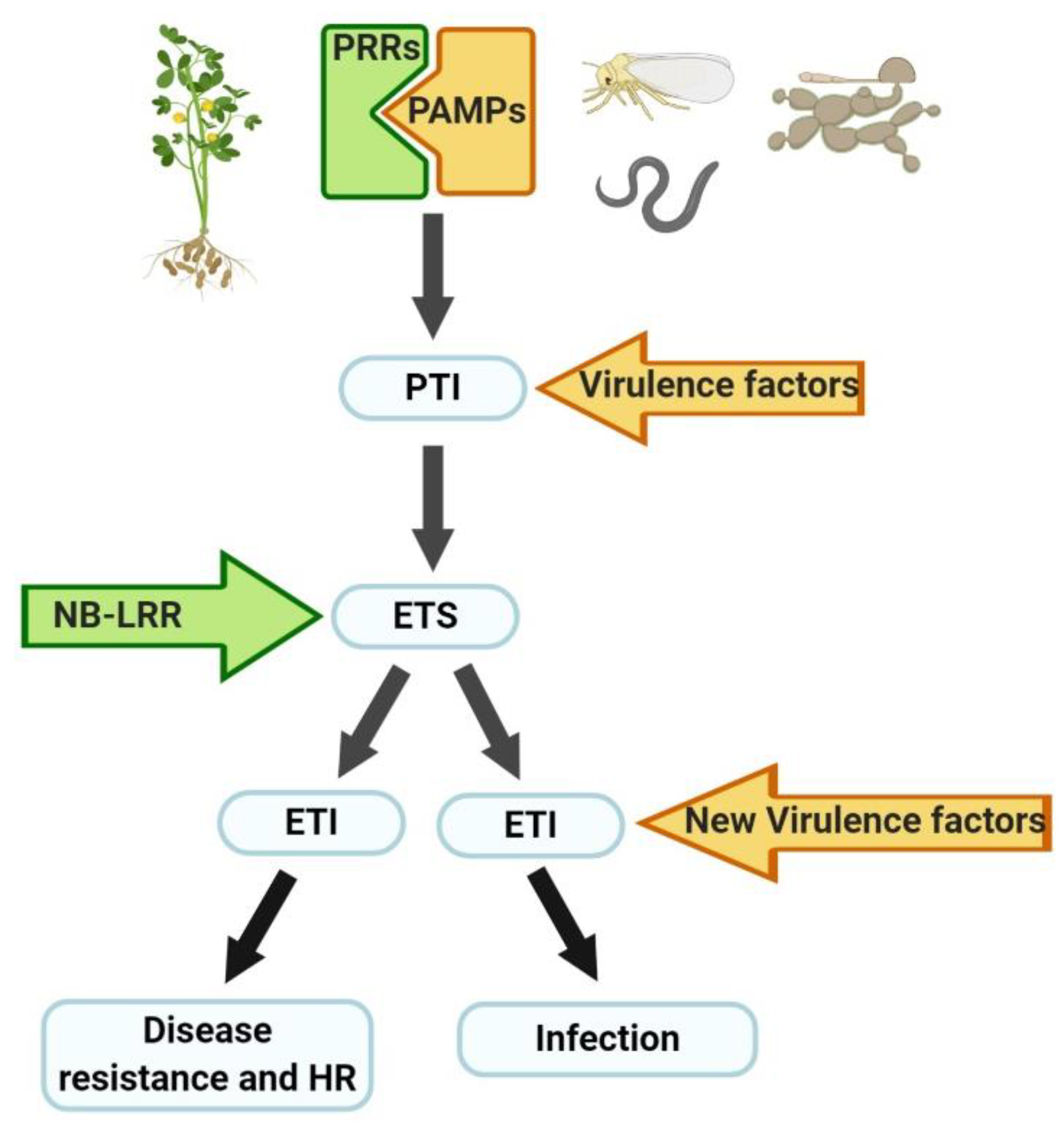
2. Legume Responses to Pathogen Attack
3. Phytohormones and PIs in Legumes
4. PIs Present in Legumes
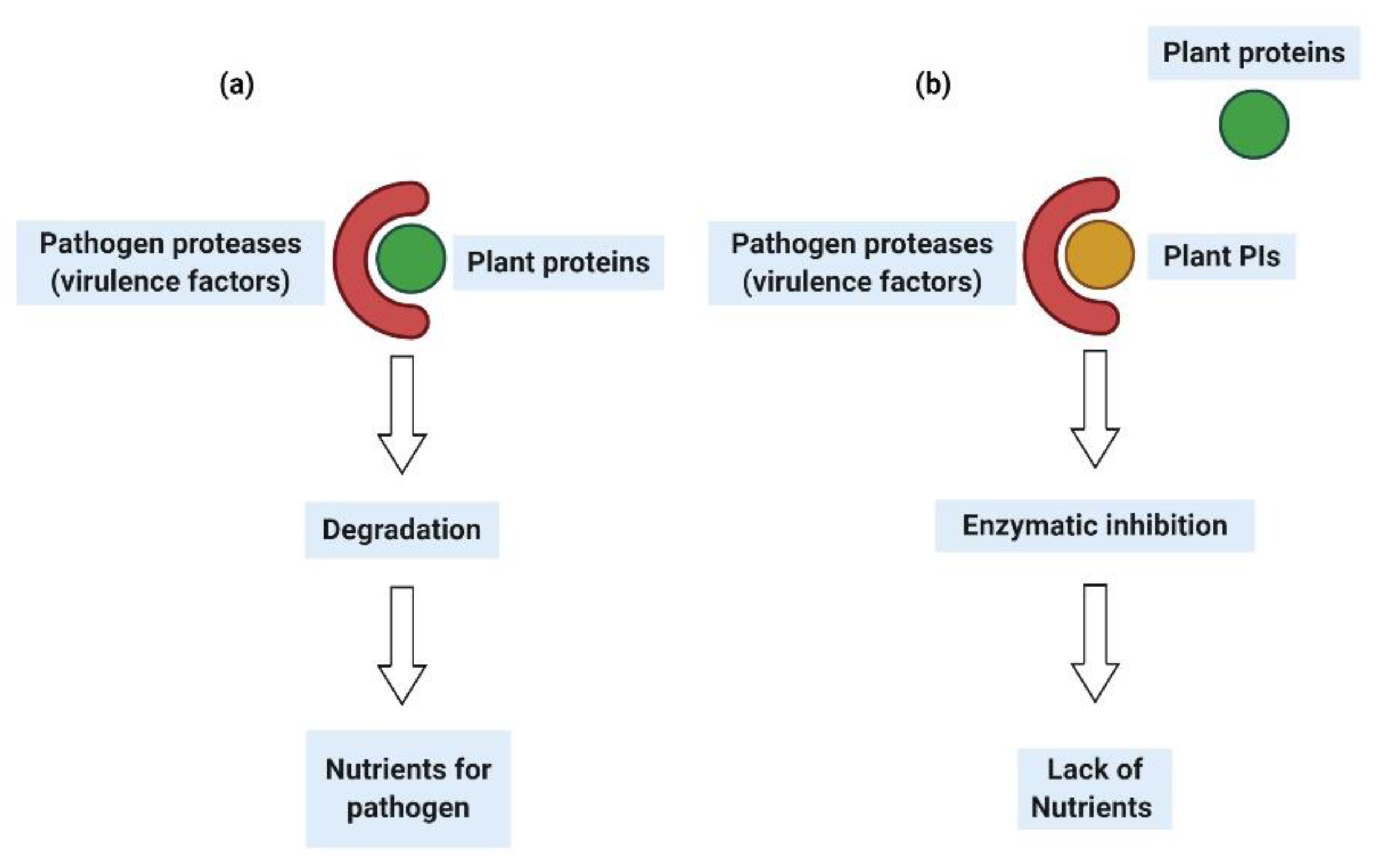
5. Legume PIs as a Biopesticide against Insects and Nematodes
6. Legume PIs as a Biopesticide against Phytopathogenic Fungi and Bacteria
7. Recombinant PIs for Biotechnological Application
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ANFs | Anti-nutritional factors |
| ET | Ethylene |
| ETI | Effector-triggered immunity |
| ETS | Effector-triggered susceptibility |
| HGPs | H. armigera gut trypsin-like protease |
| HR | Hypersensitive cell death |
| JA | Jasmonic acid |
| NB-LRR | Nucleotide-binding and leucine-rich repeat domains |
| PA | Phosphatidic acid |
| PAMPs | Pathogen-associated molecular patterns |
| PIs | Protease inhibitors |
| PR | Pathogenesis-related |
| PRRs | Pathogen recognition receptor |
| PTI | PAMP-triggered immunity |
| SA | Salicylic acid |
References
- Smýkal, P.; Coyne, C.J.; Ambrose, M.J.; Maxted, N.; Schaefer, H.; Blair, M.W.; Berger, J.; Greene, S.L.; Nelson, M.N.; Besharat, N.; et al. Legume crops phylogeny and genetic diversity for science and breeding. CRC Crit. Rev. Plant Sci. 2015, 34, 43–104. [Google Scholar] [CrossRef]
- Kamboj, R.; Nanda, V. Proximate composition, nutritional profile and health benefits of legumes—A review. Legum. Res. 2018, 41, 325–332. [Google Scholar] [CrossRef]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. A review of the nutritional value of legumes and their effects on obesity and its related co-morbidities. Obes. Rev. 2014, 15, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E.; et al. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. CRC Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Franke, A.C.; van den Brand, G.J.; Vanlauwe, B.; Giller, K.E. Sustainable intensification through rotations with grain legumes in Sub-Saharan Africa: A review. Agric. Ecosyst. Environ. 2018, 261, 172–185. [Google Scholar] [CrossRef]
- Rubiales, D.; Fondevilla, S.; Chen, W.; Gentzbittel, L.; Higgins, T.J.V.; Castillejo, M.A.; Singh, K.B.; Rispail, N. Achievements and Challenges in Legume Breeding for Pest and Disease Resistance. CRC Crit. Rev. Plant Sci. 2015, 34, 195–236. [Google Scholar] [CrossRef]
- Dhandaydham, M.; Charles, L.; Zhu, H.; Starr, J.L.; Huguet, T.; Cook, D.R.; Prosperi, J.M.; Opperman, C. Characterization of root-knot nematode resistance in Medicago truncatula. J. Nematol. 2008, 40, 46–54. [Google Scholar]
- Cruz, L.P.; de Sá, L.F.R.; Santos, L.A.; Gravina, G.A.; Carvalho, A.O.; Fernandes, K.V.S.; Freire Filho, F.R.; Gomes, V.M.; Oliveira, A.E.A. Evaluation of resistance in different cowpea cultivars to Callosobruchus maculatus infestation. J. Pest Sci. (2004) 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Mainali, B.P.; Kim, H.J.; Park, C.G.; Heon Kim, J.; Yoon, Y.N.; Oh, I.S.; Bae, S. Do Oviposition preference and development of azuki bean weevil, Callosobruchus chinensis, on five different leguminous seeds. J. Stored Prod. Res. 2015, 61, 97–101. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldívar, S.O. Inactivation methods of trypsin inhibitor in legumes: A review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, L.X.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Heredia, J.B. Effect of cooking and germination on bioactive compounds in pulses and their health benefits. J. Funct. Foods 2017, 38, 624–634. [Google Scholar] [CrossRef]
- Srinivasan, A.; Giri, A.P.; Harsulkar, A.M.; Gatehouse, J.A.; Gupta, V.S. A Kunitz trypsin inhibitor from chickpea (Cicer arietinum L.) that exerts anti-metabolic effect on podborer (Helicoverpa armigera) larvae. Plant Mol. Biol. 2005, 57, 359–374. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Sels, J.; Mathys, J.; De Coninck, B.M.A.; Cammue, B.P.A.; De Bolle, M.F.C. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Duxbury, Z.; Ma, Y.; Furzer, O.J.; Huh, S.U.; Cevik, V.; Jones, J.D.G.; Sarris, P.F. Pathogen perception by NLRs in plants and animals: Parallel worlds. BioEssays 2016, 38, 769–781. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Katagiri, F.; Tsuda, K. Understanding the plant immune system. Mol. Plant-Microbe Interact. 2010, 23, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N.; Sofyin, A.V.; Revina, T.A.; Gvozdeva, E.L.; Ievleva, E.V.; Valueva, T.A. Secretion of proteolytic enzymes by three phytopathogenic microorganisms. Appl. Biochem. Microbiol. 2013, 49, 514–520. [Google Scholar] [CrossRef]
- Yarullina, L.G.; Akhatova, A.R.; Kasimova, R.I. Hydrolytic enzymes and their proteinaceous inhibitors in regulation of plant–pathogen interactions. Russ. J. Plant Physiol. 2016, 63, 193–203. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Zeng, R. Insect response to plant defensive protease inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Koiwa, H.; Bressan, R.A.; Hasegawa, P.M. Regulation of protease inhibitors and plant defense. Trends Plant Sci. 1997, 2, 379–384. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Giacometti, R.; Barneto, J.; Barriga, L.G.; Sardoy, P.M.; Balestrasse, K.; Andrade, A.M.; Pagano, E.A.; Alemano, S.G.; Zavala, J.A. Early perception of stink bug damage in developing seeds of field-grown soybean induces chemical defences and reduces bug attack. Pest Manag. Sci. 2016, 72, 1585–1594. [Google Scholar] [CrossRef]
- Lee, S.; Hirt, H.; Lee, Y. Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J. 2001, 26, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Paudel, J.R.; Bede, J.C. Ethylene signaling modulates herbivore-induced defense responses in the model legume Medicago truncatula. Mol. Plant-Microbe Interact. 2015, 28, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Ma, Y.; Yang, W.; Yang, Q.; Yu, D. Identification of soybean herbivory-regulated genes and a transgenic investigation of their potential in insect resistance. Plant Cell. Tissue Organ Cult. 2015, 123, 321–340. [Google Scholar] [CrossRef]
- Gao, L.L.; Anderson, J.P.; Klingler, J.P.; Nair, R.M.; Edwards, O.R.; Singh, K.B. Involvement of the octadecanoid pathway in bluegreen aphid resistance in Medicago truncatula. Mol. Plant-Microbe Interact. 2007, 20, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, L.G.; Guo, S.M.; Gao, L.L.; Singh, K.B. Genetic mapping of a major resistance gene to pea aphid (Acyrthosipon pisum) in the model legume Medicago truncatula. Int. J. Mol. Sci. 2016, 17, 1224. [Google Scholar] [CrossRef] [PubMed]
- Yamchi, A.; Ben, C.; Rossignol, M.; Zareie, S.R.; Mirlohi, A.; Sayed-Tabatabaei, B.E.; Pichereaux, C.; Sarrafi, A.; Rickauer, M.; Gentzbittel, L. Proteomics analysis of Medicago truncatula response to infection by the phytopathogenic bacterium Ralstonia solanacearum points to jasmonate and salicylate defence pathways. Cell. Microbiol. 2018, 20, e12796. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, B.N.; Brooks, D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Umemoto, N.; Kakitani, M.; Iwamatsu, A.; Yoshikawa, M.; Yamaoka, N.; Ishida, I. The structure and function of a soybean β-glucan-elicitor-binding protein. Proc. Natl. Acad. Sci. USA 1997, 94, 1029–1034. [Google Scholar] [CrossRef]
- Haq, S.K.; Atif, S.M.; Khan, R.H. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: Natural and engineered phytoprotection. Arch. Biochem. Biophys. 2004, 431, 145–159. [Google Scholar] [CrossRef]
- Sharma, K. Protease inhibitors in crop protection from insects. Int. J. Curr. Res. Acad. Rev. 2015, 3, 55–70. [Google Scholar]
- Martinez, M.; Santamaria, M.E.; Diaz-Mendoza, M.; Arnaiz, A.; Carrillo, L.; Ortego, F. Phytocystatins: Defense proteins against phytophagous insects and acari. Int. J. Mol. Sci. 2016, 17, 1747. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; dos Reis, S.; de Souza, C. Phytocystatins and their potential to control plant diseases caused by fungi. Protein Pept. Lett. 2015, 22, 104–111. [Google Scholar] [CrossRef] [PubMed]
- El-Latif, A.O.A. Protease purification and characterization of a serine protease inhibitor from Egyptian varieties of soybean seeds and its efficacy against Spodoptera littoralis. J. Plant Prot. Res. 2015, 55, 16–25. [Google Scholar] [CrossRef]
- Swathi, M.; Mohanraj, S.S.; Swaroop, V.; Gujjarlapudi, M.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Proteinase inhibitors from Cajanus platycarpus accessions active against pod borer Helicoverpa armigera. Acta Physiol. Plant. 2015, 37, 242. [Google Scholar] [CrossRef]
- Vasudev, A.; Sohal, S.K. Partially purified Glycine max proteinase inhibitors: Potential bioactive compounds against tobacco cutworm, Spodoptera litura (Fabricius, 1775) (Lepidoptera: Noctuidae). Turkish J. Zool. 2016, 40, 379–387. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, A.; Kaur, A.P.; Rup, P.J.; Sohal, S.K. Assessment of soybean inhibitor as a biopesticide against melon fruit fly, Bactrocera cucurbitae (Coquillett). J. Plant Dis. Prot. 2017, 124, 445–451. [Google Scholar] [CrossRef]
- Golla, S.K.; Rajasekhar, P.; Akbar, S.M.D.; Sharma, H.C. Proteolytic activity in the midgut of Helicoverpa armigera (Noctuidae: Lepidoptera) larvae fed on wild relatives of chickpea, Cicer arietinum. J. Econ. Entomol. 2018, 111, 2409–2415. [Google Scholar] [CrossRef]
- Negi, P.; Chand, S.; Thakur, N.; Nath, A.K. Biological Activity of Serine Protease Inhibitor Isolated from the Seeds of Phaseolus vulgaris. Agric. Res. 2018, 7, 265–270. [Google Scholar] [CrossRef]
- Rondoni, G.; Bertoldi, V.; Malek, R.; Djelouah, K.; Moretti, C.; Buonaurio, R.; Conti, E. Vicia faba plants respond to oviposition by invasive Halyomorpha halys activating direct defences against offspring. J. Pest Sci. (2004) 2018, 91, 671–679. [Google Scholar] [CrossRef]
- Ramalho, S.R.; Bezerra, C.D.S.; Lourenço De Oliveira, D.G.; Souza Lima, L.; Maria Neto, S.; Ramalho De Oliveira, C.F.; Valério Verbisck, N.; Rodrigues Macedo, M.L. Novel peptidase Kunitz inhibitor from Platypodium elegans seeds is active against Spodoptera frugiperda larvae. J. Agric. Food Chem. 2018, 66, 1349–1358. [Google Scholar] [CrossRef]
- Rocha, R.O.; Morais, J.K.S.; Oliveira, J.T.A.; Oliveira, H.D.; Sousa, D.O.B.; Souza, C.E.A.; Moreno, F.B.; Monteiro-Moreira, A.C.O.; Antonino De Souza, J.D.; Grossi De Sá, M.F.; et al. Proteome of soybean seed exudates contains plant defense-related proteins active against the root-knot nematode Meloidogyne incognita. J. Agric. Food Chem. 2015, 63, 5335–5343. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.B.D.S.; Oliveira, A.S.; Ribeiro, J.K.C.; Kiyota, S.; Vasconcelos, I.M.; De Oliveira, J.T.A.; De Sales, M.P. Effects of a novel pathogenesis-related class 10 (PR-10) protein from crotalaria pallida roots with papain inhibitory activity against root-knot nematode Meloidogyne incognita. J. Agric. Food Chem. 2010, 58, 4145–4152. [Google Scholar] [CrossRef] [PubMed]
- Dawei, L.; Shaoxu, Y.; Jingsheng, C.; Lijie, C.; Yuxi, D. Effects on trypsin inhibitor in roots of resistant soybeans after Heterodera glycines invasion. Int. J. Agric. Biol. 2016, 18, 965–968. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Wang, X.; Fang, W.; Bidochka, M.J.; She, R.; Xiao, Y.; Pei, Y. Psc-AFP, an antifungal protein with trypsin inhibitor activity from Psoralea corylifolia seeds. Peptides 2006, 27, 1726–1731. [Google Scholar] [CrossRef]
- Lopes, J.L.S.; Valadares, N.F.; Moraes, D.I.; Rosa, J.C.; Araújo, H.S.S.; Beltramini, L.M. Physico-chemical and antifungal properties of protease inhibitors from Acacia plumosa. Phytochemistry 2009, 70, 871–879. [Google Scholar] [CrossRef]
- Nair, M.; Sandhu, S.S. A Kunitz trypsin inhibitor from chickpea (Cicer arietinum L.) that exerts an antimicrobial effect on Fusarium oxysporum f.sp. ciceris. Agric. Sci. 2013, 4, 585–594. [Google Scholar] [CrossRef]
- Scarafoni, A.; Ronchi, A.; Prinsi, B.; Espen, L.; Assante, G.; Venturini, G.; Duranti, M. The proteome of exudates from germinating Lupinus albus seeds is secreted through a selective dual-step process and contains proteins involved in plant defence. FEBS J. 2013, 280, 1443–1459. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.; Bonacci, G.; Batthyany, C.; Amé, M.V.; Carrari, F.; Gieco, J.; Asis, R. Peanut seed cultivars with contrasting resistance to Aspergillus parasiticus colonization display differential temporal response of protease inhibitors. Phytopathology 2017, 107, 474–482. [Google Scholar] [CrossRef]
- Wang, S.; Shao, B.; Lu, W.; Hong, J.; Rao, P. Isolation of a trypsin-chymotrypsin inhibitor and its functional properties. Prep. Biochem. Biotechnol. 2014, 44, 545–557. [Google Scholar] [CrossRef]
- Wang, S.; Lin, J.; Ye, M.; Ng, T.B.; Rao, P.; Ye, X. Isolation and characterization of a novel mung bean protease inhibitor with antipathogenic and anti-proliferative activities. Peptides 2006, 27, 3129–3136. [Google Scholar] [CrossRef]
- Urwin, P.E.; Lilley, C.J.; McPherson, M.J.; Atkinson, H.J. Resistance to both cyst and root-knot nematodes conferred by transgenic Arabidopsis expressing a modified plant cystatin. Plant J. 1997, 12, 455–461. [Google Scholar] [CrossRef]
- Alkharouf, N.W.; Klink, V.P.; Chouikha, I.B.; Beard, H.S.; MacDonald, M.H.; Meyer, S.; Knap, H.T.; Khan, R.; Matthews, B.F. The Timecourse microarray analyses reveal global changes in gene expression of susceptible Glycine max (soybean) roots during infection by Heterodera glycines (soybean cyst nematode). Planta 2006, 224, 838–852. [Google Scholar] [CrossRef] [PubMed]
- De Souza Cândido, E.; Pinto, M.F.S.; Pelegrini, P.B.; Lima, T.B.; Silva, O.N.; Pogue, R.; Grossi-De-Sá, M.F.; Franco, O.L. Plant storage proteins with antimicrobial activity: Novel insights into plant defense mechanisms. FASEB J. 2011, 25, 3290–3305. [Google Scholar] [CrossRef] [PubMed]
- Migliolo, L.; de Oliveira, A.S.; Santos, E.A.; Franco, O.L.; de Sales, M.P. Structural and mechanistic insights into a novel non-competitive Kunitz trypsin inhibitor from Adenanthera pavonina L. seeds with double activity toward serine- and cysteine-proteinases. J. Mol. Graph. Model. 2010, 29, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yuan, S.S.; Jiang, L.L.; Ye, X.J.; Ng, T.B.; Wu, Z.J. Plant antifungal proteins and their applications in agriculture. Appl. Microbiol. Biotechnol. 2015, 99, 4961–4981. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, S.C.; Kim, J.Y.; Lee, S.Y.; Lim, H.T.; Cheong, H.; Hahm, K.S.; Park, Y. Purification and characterization of a heat-stable serine protease inhibitor from the tubers of new potato variety “Golden Valley”. Biochem. Biophys. Res. Commun. 2006, 346, 681–686. [Google Scholar] [CrossRef]
- Da Silva Bezerra, C.; De Oliveira, C.F.R.; Machado, O.L.T.; De Mello, G.S.V.; Da Rocha Pitta, M.G.; De Melo Rêgo, M.J.B.; Napoleão, T.H.; Paiva, P.M.G.; De Fátima Ferreira Ribeiro, S.; Gomes, V.M.; et al. Exploiting the biological roles of the trypsin inhibitor from Inga vera seeds: A multifunctional Kunitz inhibitor. Process Biochem. 2016, 51, 792–803. [Google Scholar] [CrossRef]
- Brito, M.S.d.; Melo, M.B.; Alves, J.P.d.A.; Fontenelle, R.O.d.S.; Mata, M.F.; Andrade, L.B.d.S. Partial purification of trypsin/papain inhibitors from Hymenaea courbaril L. seeds and antibacterial effect of protein fractions. Hoehnea 2016, 43, 11–18. [Google Scholar] [CrossRef]
- Shamsi, T.N.; Parveen, R.; Afreen, S.; Azam, M.; Sen, P.; Sharma, Y.; Haque, Q.M.R.; Fatma, T.; Manzoor, N.; Fatima, S. Trypsin inhibitors from Cajanus cajan and Phaseolus limensis possess antioxidant, anti-inflammatory, and antibacterial activity. J. Diet. Suppl. 2018, 15, 939–950. [Google Scholar] [CrossRef]
- Dona, A.; Arvanitoyannis, I.S. Health risks of genetically modified foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 164–175. [Google Scholar] [CrossRef]
- Paparini, A.; Romano-Spica, V. Public health issues related with the consumption of food obtained from genetically modified organisms. Biotechnol. Annu. Rev. 2004, 10, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Rosa, C.; Scully, E.D.; Peiffer, M.; Tooker, J.F.; Hoover, K.; Luthe, D.S.; Felton, G.W. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 15728–15733. [Google Scholar] [CrossRef] [PubMed]
- Wielkopolan, B.; Krawczyk, K.; Obrępalska-Stęplowska, A. Gene expression of serine and cysteine proteinase inhibitors during cereal leaf beetle larvae feeding on wheat: The role of insect-associated microorganisms. Arthropod. Plant. Interact. 2018, 12, 601–612. [Google Scholar] [CrossRef]
- Wielkopolan, B.; Obrępalska-Stęplowska, A. Three-way interaction among plants, bacteria, and coleopteran insects. Planta 2016, 244, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Emani, C. Gossypium. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berling/Heidelberg, Germany, 2011; pp. 109–122. ISBN 978-3-642-20449-4. [Google Scholar]
- Mohanraj, S.S.; Tetali, S.D.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Biochemical properties of a bacterially expressed Bowman-Birk inhibitor from Rhynchosia sublobata (Schumach.) Meikle seeds and its activity against gut proteases of Achaea janata. Phytochemistry 2018, 151, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Júnior, J.E.; Valadares, N.F.; Pereira, H.D.M.; Dyszy, F.H.; da Costa Filho, A.J.; Uchôa, A.F.; de Oliveira, A.S.; da Silveira Carvalho, C.P.; Grangeiro, T.B. Expression in Escherichia coli of cysteine protease inhibitors from cowpea (Vigna unguiculata): The crystal structure of a single-domain cystatin gives insights on its thermal and pH stability. Int. J. Biol. Macromol. 2017, 102, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.D.; Ahn, J.E.; Salzman, R.A.; Moon, J.; Chi, Y.H.; Yun, D.J.; Lee, S.Y.; Koiwa, H.; Zhu-Salzman, K. Functional expression of an insect cathepsin B-like counter-defence protein. Insect Mol. Biol. 2008, 17, 235–245. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Koiwa, H.; Salzman, R.A.; Shade, R.E.; Ahn, J.-E. Cowpea bruchid Callosobruchus maculatus uses a three-component strategy to overcome a plant defensive cysteine protease inhibitor. Insect Mol. Biol. 2003, 12, 135–145. [Google Scholar] [CrossRef]
- Luo, X.M.; Xie, C.J.; Wang, D.; Wei, Y.M.; Cai, J.; Cheng, S.S.; Yang, X.Y.; Sui, A.P. Psc-AFP from Psoralea corylifolia L. overexpressed in Pichia pastoris increases antimicrobial activity and enhances disease resistance of transgenic tobacco. Appl. Microbiol. Biotechnol. 2017, 101, 1073–1084. [Google Scholar] [CrossRef]
| Legume Seed | Protein | Fat | Minerals | Crude Fiber | Carbohydrates |
|---|---|---|---|---|---|
| Chickpea (Cicer arietinum) | 17.1 | 5.3 | 3.0 | 3.9 | 60.9 |
| Soybean (Glycine max) | 43.2 | 19.5 | 19.5 | 3.7 | 20.9 |
| Lentil (Lens esculenta) | 25.1 | 0.7 | 2.1 | 0.7 | 59.0 |
| Cowpea (Vigna catjang) | 24.1 | 1.0 | 3.2 | 3.8 | 54.5 |
| Peas dry (Pisum sativum) | 19.7 | 1.1 | 2.2 | 4.5 | 56.5 |
| Pigeon pea (Cajanus cajan) | 22.3 | 1.7 | 3.5 | 1.5 | 57.6 |
| Kidney bean (Phaseolus vulgaris) | 22.9 | 1.3 | 3.2 | 4.8 | 60.6 |
| Seed Legumes | Protease Target | Insect Pest Target | Molecular Mass (kDa) | N-terminal Amino Acid Sequence | Reference |
|---|---|---|---|---|---|
| Soybean (Glycine max) | Trypsin and chymotrypsin | Spodoptera littoralis | 17.9 | ND | [43] |
| Pigeonpea (Cajanus cajan, Cajanus platycarpus) | Trypsin and chymotrypsin | Helicoverpa armigera | ND | ND | [44] |
| Soybean (Glycine max) | Trypsin | Spodoptera litura | ND | ND | [45] |
| Soybean (Glycine max) | Trypsin | B. cucurbitae | ND | ND | [46] |
| Chickpea (Cicer arietinum, Cicer cuneatum, Cicer bijugum, Cicer chrossanicum, Cicer reticulatum) | Trypsin and chymotrypsin | Helicoverpa armigera | ND | ND | [47] |
| Common bean (Phaseolus vulgaris) | Trypsin | Spodoptera litura | ND | ND | [48] |
| Faba bean (Vicia faba) | Cysteine protease | Halyomorpha halys | ND | ND | [49] |
| Uruvalheira (Platypodium elegans) | Trypsin and chymotrypsin | Spodoptera frugiperda | 19.7 | FVVDTDGDPLRNGGSYYILPVFRGRGGGIEQAAIGTETCPLTVVQSPSEVSKGLPLR | [50] |
| Soybean (Glycine max) | Cysteine protease | Nezara viridula L. | ND | ND | [30] |
| Seed Legume | Protease Target | Nematode Pest Target | Molecular Mass (kDa) | N-terminal Amino Acid Sequence | Reference |
| Soybean (Glycine max) | Trypsin and papain | Meloidogyne incognita | 4.53–22.546 | ND | [51] |
| Crotalaria pallida | Papain | Meloidogyne incognita | 15 | FAFEDENTSPVAPAKLFKALTKDADVIIPKVIEPDQ | [52] |
| Soybean (Glycine max) | Trypsin | Heterodera glycines | ND | ND | [53] |
| Seed Legume | Protease Target | Fungus Pest Target | Molecular Mass (kDa) | N-terminal Amino Acid Sequence | Reference |
| Psoralea corylifolia | Trypsin | Alternaria brassicae, Aspergillus niger, Fusarium oxyxporum, Rhizoctonia cerealis | 18 | EWEPVQNGGSSYYMVPRIWA | [54] |
| Acacia plumosa | Trypsin and chymotrypsin | Aspergillus niger, Thielaviopsis paradoxa, Colletotrichum sp. | 20 | KELLVDNEGEI | [55] |
| Chickpea (Cicer arietinum) | Trypsin | Fusarium oxysporum | 20 | ND | [56] |
| Lupin (Lupinus albus) | Cysteine protease | Fusarium oxysporum, Botrytis cinerea, Alternaria solani,Aspergillus niger, Penicillium expansum | 10.7–11.8 | ND | [57] |
| Peanut (Arachis hypogaea) | Trypsin and chymotrypsin | Aspergillus parasiticus | 16.82 | ND | [58] |
| Black soybean (Glycine max L. merr) | Trypsin and chymotrypsin | Alternaria alternata, Fusarium oxysporum, Pythium aphanidermatum, Physalospora piricola, Botrytis cinereal, Fusarium solani | 20 | DEYSKPCCDLCMCTRRCPPQ | [59] |
| Mung bean (Phaseolus mungo) | Trypsin and chymotrypsin | Physalospora piricola, Mycosphaerella arachidicola, Botrytis cinerea, Pythium aphanidermatum, Sclerotium rolfsii, and Fusarium oxysporum | 10 | EMPGKPACLDTDDFCYKP | [60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Sifuentes, L.; Marszalek, J.E.; Chuck-Hernández, C.; Serna-Saldívar, S.O. Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors. Int. J. Mol. Sci. 2020, 21, 3322. https://doi.org/10.3390/ijms21093322
Rodríguez-Sifuentes L, Marszalek JE, Chuck-Hernández C, Serna-Saldívar SO. Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors. International Journal of Molecular Sciences. 2020; 21(9):3322. https://doi.org/10.3390/ijms21093322
Chicago/Turabian StyleRodríguez-Sifuentes, Lucio, Jolanta Elzbieta Marszalek, Cristina Chuck-Hernández, and Sergio O. Serna-Saldívar. 2020. "Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors" International Journal of Molecular Sciences 21, no. 9: 3322. https://doi.org/10.3390/ijms21093322
APA StyleRodríguez-Sifuentes, L., Marszalek, J. E., Chuck-Hernández, C., & Serna-Saldívar, S. O. (2020). Legumes Protease Inhibitors as Biopesticides and Their Defense Mechanisms against Biotic Factors. International Journal of Molecular Sciences, 21(9), 3322. https://doi.org/10.3390/ijms21093322







