Gold Nanocomplex Strongly Modulates the PI3K/Akt Pathway and Other Pathways in MCF-7 Breast Cancer Cell Line
Abstract
1. Introduction
2. Results and Discussion
2.1. Transcription Factors
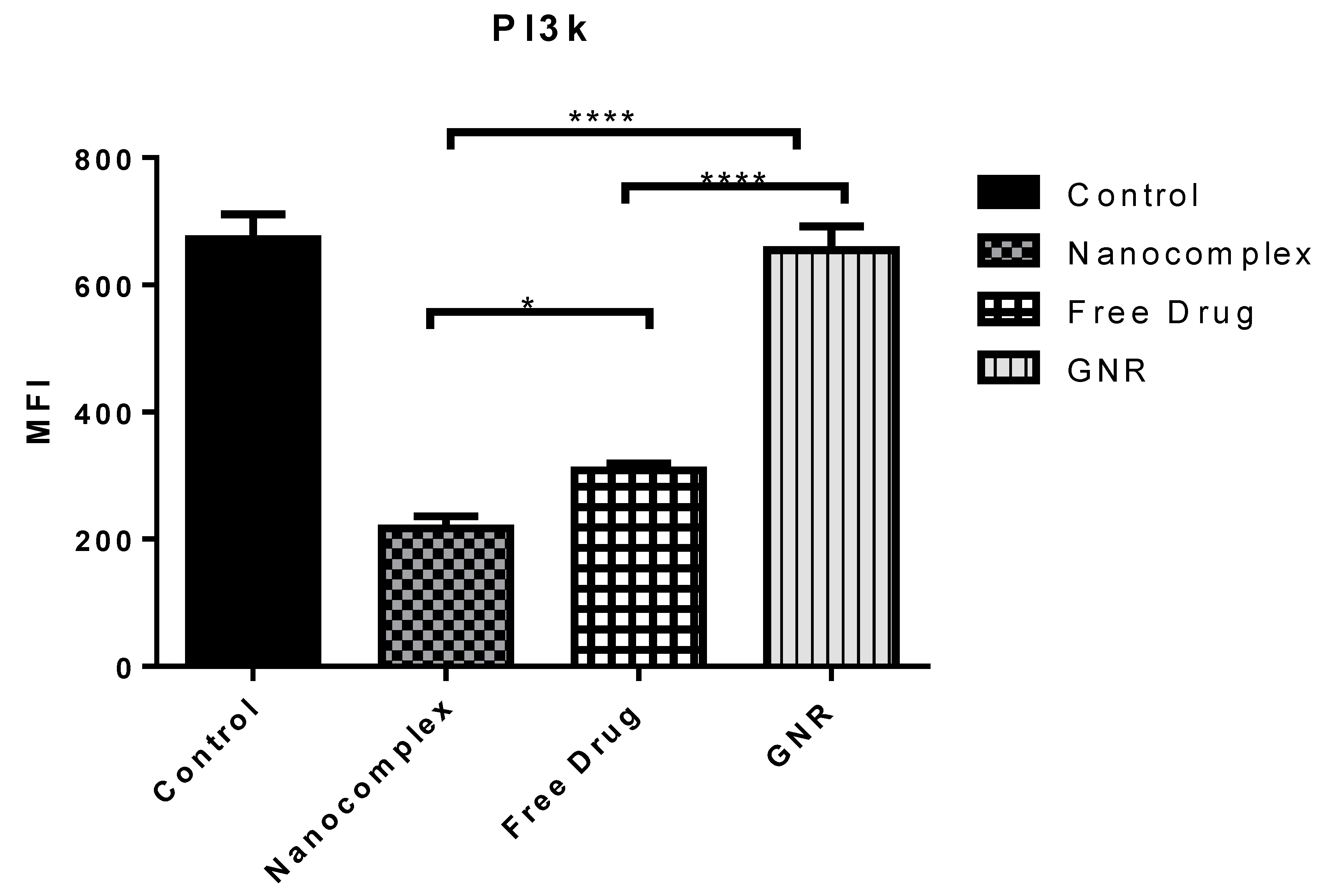
2.2. Regulatory Proteins
3. Material and Methods
3.1. Synthesis and Characterization of Cholesterol-GNR Coupled with PI3K∝ Inhibitor
3.2. Polymerase Chain Reaction (PCR) Array
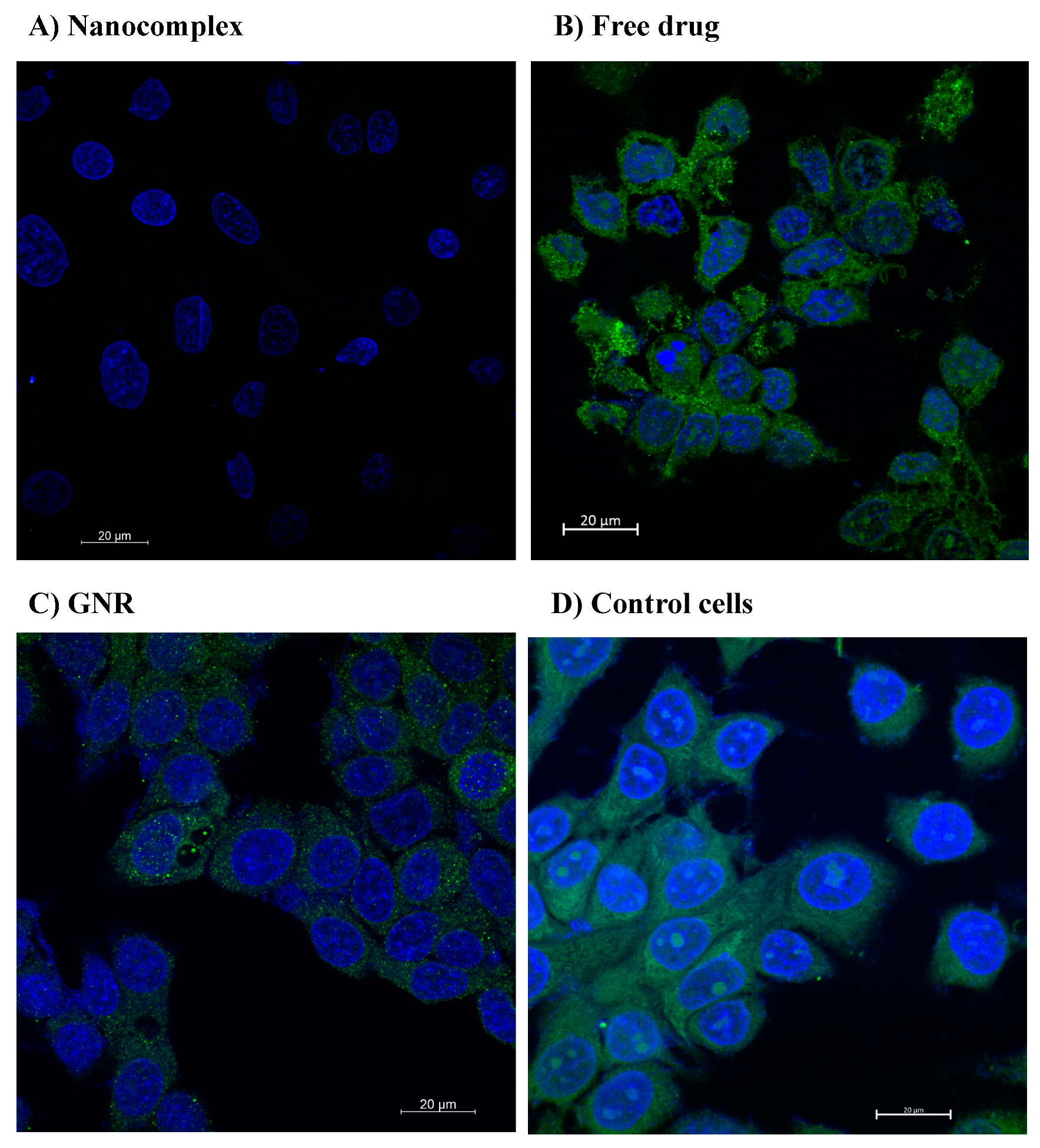
3.3. Confocal Microscopy
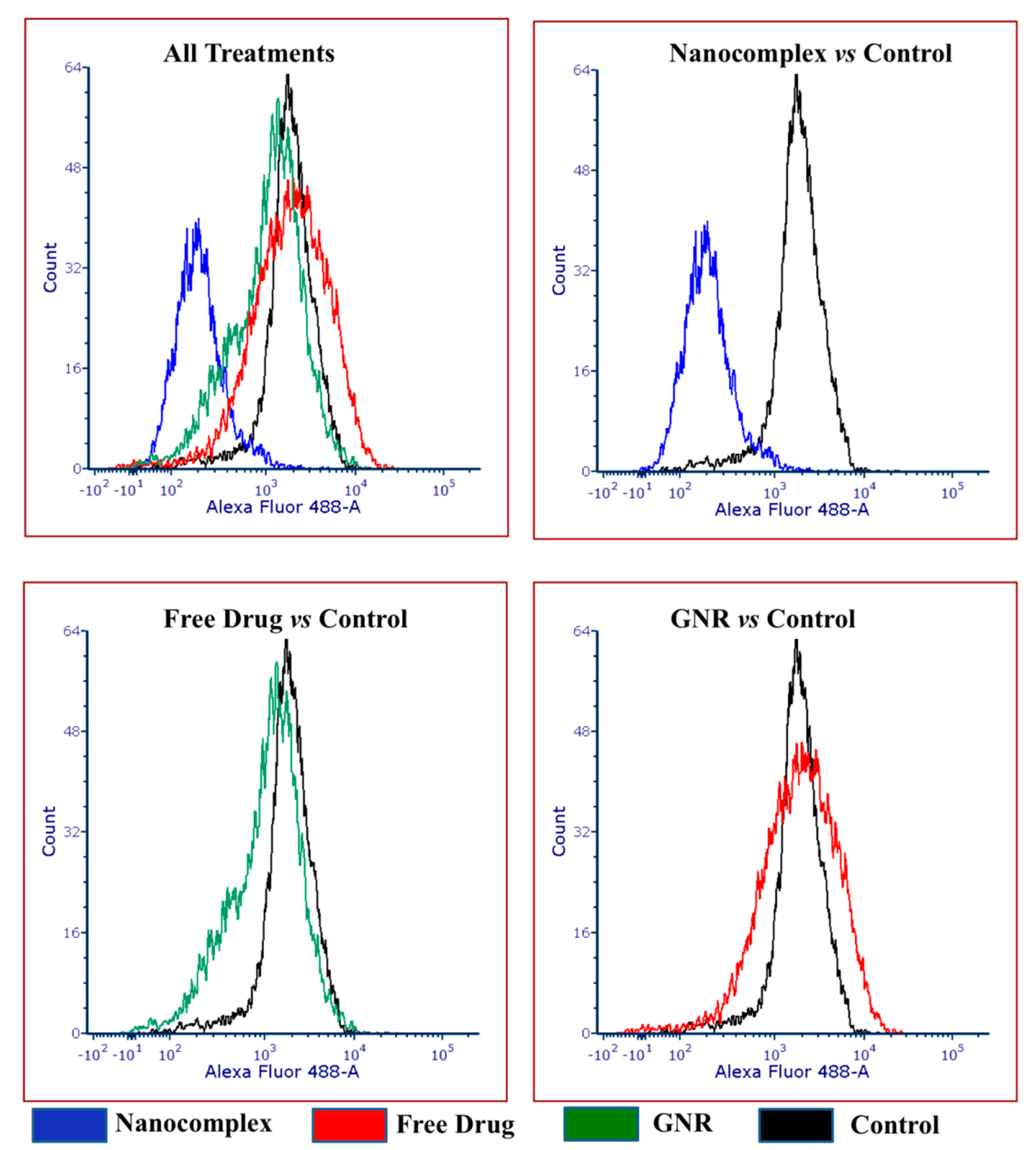
3.4. Flow Cytometry
3.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. (Int. Ed. Engl.) 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Nano-Photothermal ablation effect of Hydrophilic and Hydrophobic Functionalized Gold Nanorods on Staphylococcus aureus and Propionibacterium acnes. Sci. Rep. 2018, 8, 6881. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, M.H.; Amani, H.; Pourfatollah, A.A.; Pazoki-Toroudi, H.; Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sens. Res. 2016, 9, 17–22. [Google Scholar] [CrossRef]
- Farooq, M.U.; Novosad, V.; Rozhkova, E.A.; Wali, H.; Ali, A.; Fateh, A.A.; Neogi, P.B.; Neogi, A.; Wang, Z. Gold Nanoparticles-enabled Efficient Dual Delivery of Anticancer Therapeutics to HeLa Cells. Sci. Rep. 2018, 8, 2907. [Google Scholar] [CrossRef] [PubMed]
- Capek, I. Polymer decorated gold nanoparticles in nanomedicine conjugates. Adv. Colloid Interface Sci. 2017, 249, 386–399. [Google Scholar] [CrossRef]
- Brown, S.D.; Nativo, P.; Smith, J.-A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold Nanoparticles for the Improved Anticancer Drug Delivery of the Active Component of Oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef]
- Yahyaei, B.; Pourali, P. One step conjugation of some chemotherapeutic drugs to the biologically produced gold nanoparticles and assessment of their anticancer effects. Sci. Rep. 2019, 9, 10242. [Google Scholar] [CrossRef]
- Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Doxorubicin and Varlitinib Delivery by Functionalized Gold Nanoparticles Against Human Pancreatic Adenocarcinoma. Pharmaceutics 2019, 11, 551. [Google Scholar] [CrossRef]
- Thambiraj, S.; Shruthi, S.; Vijayalakshmi, R.; Ravi Shankaran, D. Evaluation of cytotoxic activity of docetaxel loaded gold nanoparticles for lung cancer drug delivery. Cancer Treat. Res. Commun. 2019, 21, 100157. [Google Scholar] [CrossRef]
- Gad El-Rab, S.M.F.; Halawani, E.M.; Hassan, A. Formulation of Ceftriaxone Conjugated Gold Nanoparticles and Their Medical Applications against Extended-Spectrum beta-Lactamase Producing Bacteria and Breast Cancer. J. Microbiol. Biotechnol. 2018, 28, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Khutale, G.V.; Casey, A. Synthesis and characterization of a multifunctional gold-doxorubicin nanoparticle system for pH triggered intracellular anticancer drug release. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. e.V 2017, 119, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Tomuleasa, C.; Bojan, A.; Berindan-Neagoe, I.; Boca, S.; Astilean, S. Design of FLT3 Inhibitor—Gold Nanoparticle Conjugates as Potential Therapeutic Agents for the Treatment of Acute Myeloid Leukemia. Nanoscale Res. Lett. 2015, 10, 466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, B.; Hu, N.; Han, L.; Pi, Y.; Gao, Y.; Chen, K. Anticancer activity of green synthesised gold nanoparticles from Marsdenia tenacissima inhibits A549 cell proliferation through the apoptotic pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4012–4019. [Google Scholar] [CrossRef]
- Lee, D.G.; Go, E.B.; Lee, M.; Pak, P.J.; Kim, J.-S.; Chung, N. Gold nanoparticles conjugated with resveratrol induce cell cycle arrest in MCF-7 cell lines. Appl. Biol. Chem. 2019, 62, 33. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Hishmah, B.; Sweidan, K.; Bardaweel, S.; AlDamen, M.; Zhong, H.A.; Abu Khalaf, R.; Hasan Ibrahim, A.; Al-Qirim, T.; Abu Sheikha, G.; et al. Structure-Based Design: Synthesis, X-ray Crystallography, and Biological Evaluation of N-Substituted-4-Hydroxy-2-Quinolone-3-Carboxamides as Potential Cytotoxic Agents. Anti-Cancer Agents Med. Chem. 2018, 18, 263–276. [Google Scholar] [CrossRef]
- Mahmoud Nouf, N.; Sabbah, D.A.; Abu-Dahab, R.; Abuarqoub, D.; Abdallah, M.; Ameerah, M.; Khalil, E.A. Cholesterol-coated gold nanorods as an efficient nano-carrier for chemotherapeutic delivery and potential treatment of breast cancer: In vitro studies using the MCF-7 cell line. RSC Adv. 2019, 9, 12718–12731. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- van der Vos, K.E.; Coffer, P.J. The extending network of FOXO transcriptional target genes. Antioxid. Redox Signal. 2011, 14, 579–592. [Google Scholar] [CrossRef]
- Webb, A.E.; Brunet, A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 2014, 39, 159–169. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Shen, W.H. PTEN: A new guardian of the genome. Oncogene 2008, 27, 5443–5453. [Google Scholar] [CrossRef] [PubMed]
- Castellano, E.; Downward, J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. Genes Cancer 2011, 2, 261–274. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, N.N.; Abuarqoub, D.; Zaza, R.; Sabbah, D.A.; Khalil, E.A.; Abu-Dahab, R. Gold Nanocomplex Strongly Modulates the PI3K/Akt Pathway and Other Pathways in MCF-7 Breast Cancer Cell Line. Int. J. Mol. Sci. 2020, 21, 3320. https://doi.org/10.3390/ijms21093320
Mahmoud NN, Abuarqoub D, Zaza R, Sabbah DA, Khalil EA, Abu-Dahab R. Gold Nanocomplex Strongly Modulates the PI3K/Akt Pathway and Other Pathways in MCF-7 Breast Cancer Cell Line. International Journal of Molecular Sciences. 2020; 21(9):3320. https://doi.org/10.3390/ijms21093320
Chicago/Turabian StyleMahmoud, Nouf N., Duaa Abuarqoub, Rand Zaza, Dima A. Sabbah, Enam A. Khalil, and Rana Abu-Dahab. 2020. "Gold Nanocomplex Strongly Modulates the PI3K/Akt Pathway and Other Pathways in MCF-7 Breast Cancer Cell Line" International Journal of Molecular Sciences 21, no. 9: 3320. https://doi.org/10.3390/ijms21093320
APA StyleMahmoud, N. N., Abuarqoub, D., Zaza, R., Sabbah, D. A., Khalil, E. A., & Abu-Dahab, R. (2020). Gold Nanocomplex Strongly Modulates the PI3K/Akt Pathway and Other Pathways in MCF-7 Breast Cancer Cell Line. International Journal of Molecular Sciences, 21(9), 3320. https://doi.org/10.3390/ijms21093320





