The Functions of Mitochondrial 2′,3′-Cyclic Nucleotide-3′-Phosphodiesterase and Prospects for Its Future
Abstract
1. Introduction
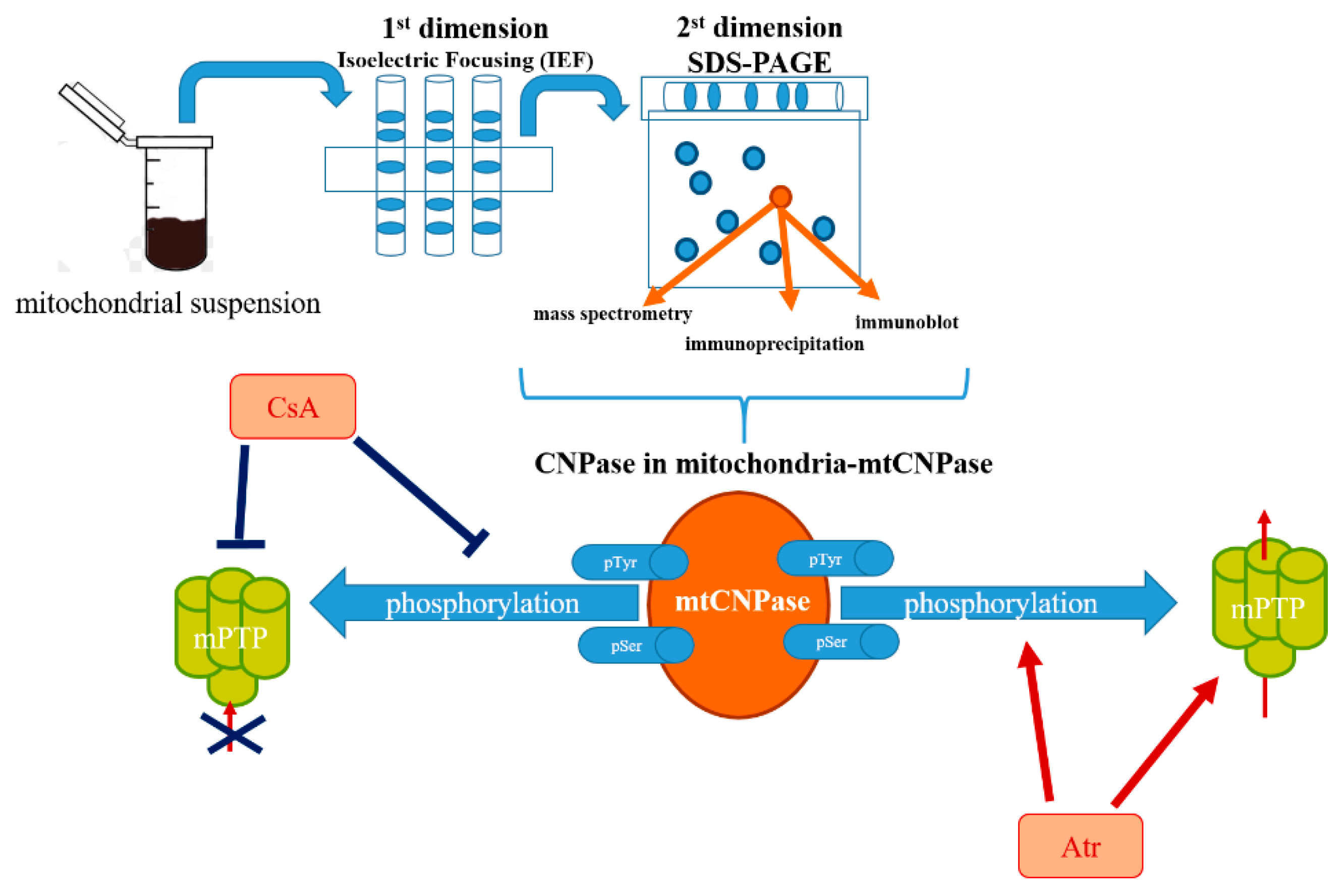
2. Detection of mtCNPase
3. Interaction Partners of mtCNPase
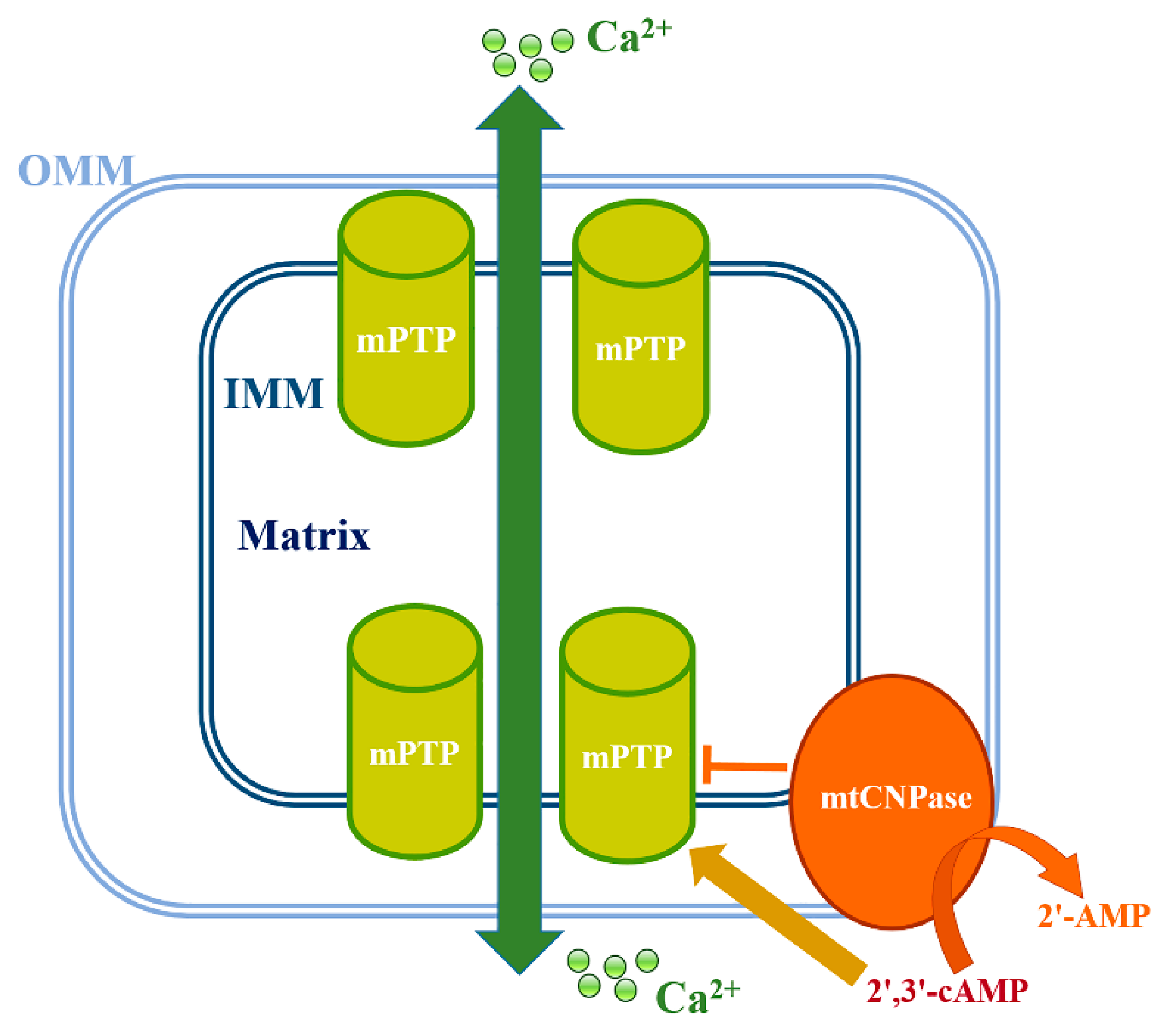
4. Involvement of mtCNPase in the Regulation of Ca2+-Induced mPTP Opening
5. CNPase in Pathology and Aging
5.1. CNPase in Pathology and Aging
5.2. mtCNPase in Aging
5.3. CNPase and Cancer
5.4. mtCNPase and Acute Heart Failure
5.5. mtCNPase and Alcohol
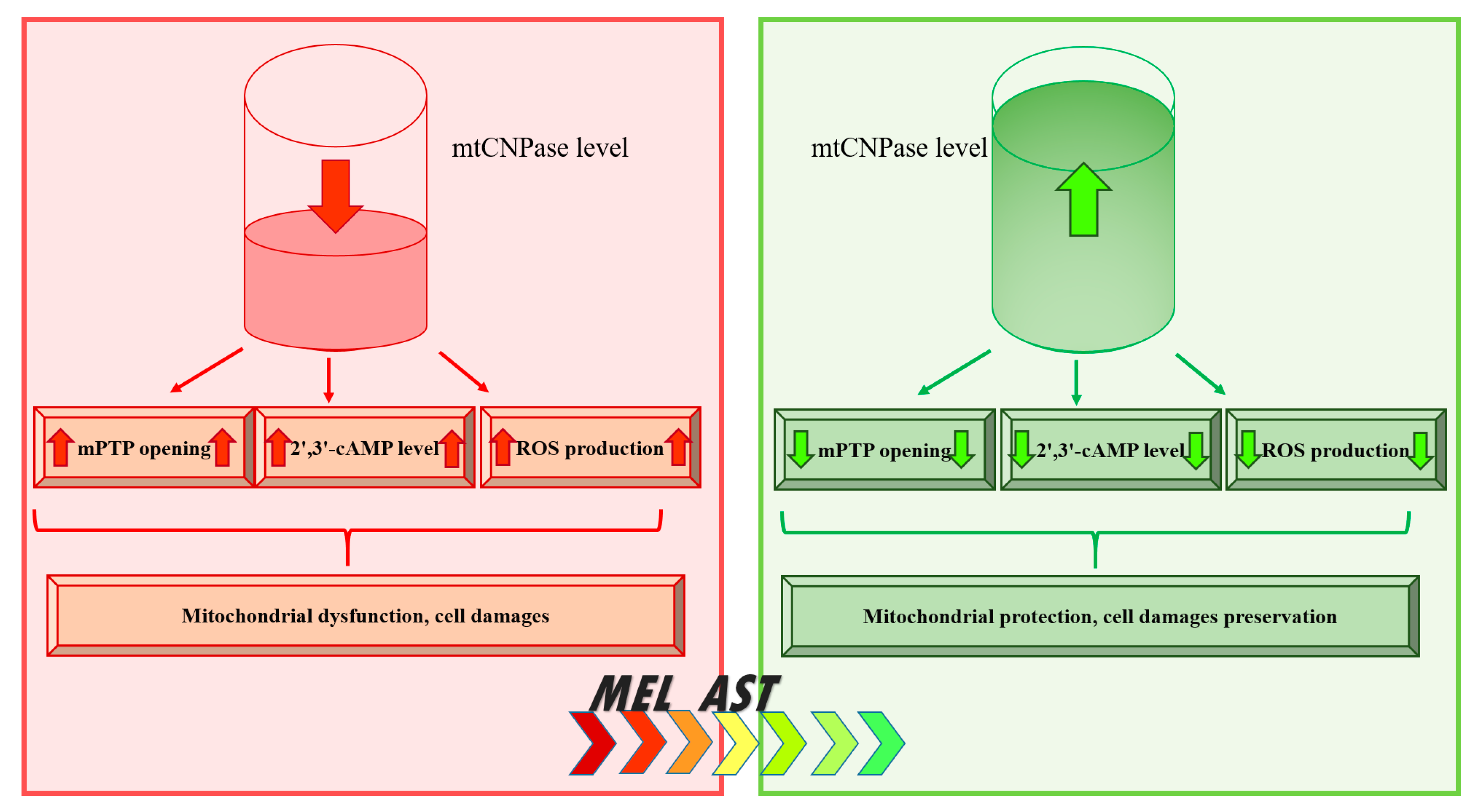
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allen, F.W.; Davis, F.F. A specific phosphodiesterase from beef pancreas. Biochim. Biophys. Acta 1956, 21, 14–17. [Google Scholar] [CrossRef]
- Sprinkle, T.J. 2’,3’-cyclic nucleotide 3’-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit. Rev. Neurobiol. 1989, 4, 235–301. [Google Scholar] [PubMed]
- Vogel, U.S.; Thompson, R.J. Molecular structure, localization, and possible functions of the myelin-associated enzyme 2’,3’-cyclic nucleotide 3’-phosphodiesterase. J. Neurochem. 1988, 50, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Dreiling, C.E.; Schilling, R.J.; Reitz, R.C. 2’,3’-cyclic nucleotide 3’-phosphohydrolase in rat liver mitochondrial membranes. Biochim. Biophys. Acta 1981, 640, 114–120. [Google Scholar] [CrossRef]
- Giulian, D.; Moore, S. Identification of 2’:3’-cyclic nucleotide 3’-phosphodiesterase in the vertebrate retina. J. Biol. Chem. 1980, 255, 5993–5995. [Google Scholar]
- Weissbarth, S.; Maker, H.S.; Raes, I.; Brannan, T.S.; Lapin, E.P.; Lehrer, G.M. The activity of 2’,3’-cyclic nucleotide 3’-phosphodiesterase in rat tissues. J. Neurochem. 1981, 37, 677–680. [Google Scholar] [CrossRef] [PubMed]
- McFerran, B.; Burgoyne, R. 2’,3’-Cyclic nucleotide 3’-phosphodiesterase is associated with mitochondria in diverse adrenal cell types. J. Cell Sci. 1997, 110, 2979–2985. [Google Scholar] [PubMed]
- Gravel, M.; DeAngelis, D.; Braun, P.E. Molecular cloning and characterization of rat brain 2’,3’-cyclic nucleotide 3’-phosphodiesterase isoform 2. J. Neurosci. Res. 1994, 38, 243–247. [Google Scholar] [CrossRef]
- Kurihara, T.; Tohyama, Y.; Yamamoto, J.; Kanamatsu, T.; Watanabe, R.; Kitajima, S. Origin of brain 2’,3’-cyclic-nucleotide 3’-phosphodiesterase doublet. Neurosci. Lett. 1992, 138, 49–52. [Google Scholar] [CrossRef]
- Monoh, K.; Kurihara, T.; Takahashi, Y.; Ichikawa, T.; Kumanishi, T.; Hayashi, S.; Minoshima, S.; Shimizu, N. Structure, expression and chromosomal localization of the gene encoding human 2’,3’-cyclic-nucleotide 3’-phosphodiesterase. Gene 1993, 129, 297–301. [Google Scholar] [CrossRef]
- Gillespie, C.S.; Bernier, L.; Brophy, P.J.; Colman, D.R. Biosynthesis of the myelin 2’,3’-cyclic nucleotide 3’-phosphodiesterases. J. Neurochem. 1990, 54, 656–661. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.C.; Minuk, J.; Cox, M.E.; Braun, P.E.; Gravel, M. CNP2 mRNA directs synthesis of both CNP1 and CNP2 polypeptides. J. Neurosci. Res. 1997, 50, 248–257. [Google Scholar] [CrossRef]
- Stricker, R.; Lottspeich, F.; Reiser, G. The myelin protein CNP (2’,3’-cyclic nucleotide 3’-phosphodiesterase): Immunoaffinity purification of CNP from pig and rat brain using a monoclonal antibody and phosphorylation of CNP by cyclic nucleotide-dependent protein kinases. Biol. Chem. Hoppe. Seyler. 1994, 375, 205–209. [Google Scholar] [PubMed]
- Lee, J.; O’Neill, R.C.; Park, M.W.; Gravel, M.; Braun, P.E. Mitochondrial localization of CNP2 is regulated by phosphorylation of the N-terminal targeting signal by PKC: Implications of a mitochondrial function for CNP2 in glial and non-glial cells. Mol. Cell Neurosci. 2006, 31, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Lu, J.; Cao, Q.; Guo, C.H.; Gao, Q.; Ling, E.A. Expression of 2’,3’-cyclic nucleotide 3’-phosphodiesterase in the amoeboid microglial cells in the developing rat brain. Neuroscience 2006, 142, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Krestinina, O.; Galvita, A.; Grachev, D.; Baburina, Y.; Stricker, R.; Evtodienko, Y.; Reiser, G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2’,3’-cyclic nucleotides and 2’,3’-cyclic nucleotide 3’-phosphodiesterase. Am. J. Physiol. Cell Physiol. 2009, 296, C1428–C1439. [Google Scholar] [CrossRef]
- Azarashvili, T.S.; Tyynela, J.; Odinokova, I.V.; Grigorjev, P.A.; Baumann, M.; Evtodienko, Y.V.; Saris, N.E.L. Phosphorylation of a peptide related to subunit c of the F0F1-ATPase/ATP synthase and relationship to permeability transition pore opening in mitochondria. J. Bioenerg. Biomembr. 2002, 34, 279–284. [Google Scholar] [CrossRef]
- Azarashvili, T.S.; Odinokova, I.V.; Evtodienko, Y.V. Phosphorylation of a low-molecular-weight polypeptide in rat liver mitochondria and dependence of its phosphorylation on mitochondrial functional state. Biochem. Biokhimiia 1999, 64, 556–560. [Google Scholar]
- Azarashvili, T.; Krestinina, O.; Yurkov, I.; Evtodienko, Y.; Reiser, G. High-affinity peripheral benzodiazepine receptor ligand, PK11195, regulates protein phosphorylation in rat brain mitochondria under control of Ca(2+). J. Neurochem. 2005, 94, 1054–1062. [Google Scholar] [CrossRef]
- Azarashvili, T.; Krestinina, O.; Odinokova, I.; Evtodienko, Y.; Reiser, G. Physiological Ca2+ level and Ca2+-induced Permeability Transition Pore control protein phosphorylation in rat brain mitochondria. Cell Calcium. 2003, 34, 253–259. [Google Scholar] [CrossRef]
- Brustovetsky, N.; Klingenberg, M. Mitochondrial ADP/ATP carrier can be reversibly converted into a large channel by Ca2+. Biochemistry 1996, 35, 8483–8488. [Google Scholar] [CrossRef] [PubMed]
- Azarashvili, T.; Krestinina, O.; Galvita, A.; Grachev, D.; Baburina, Y.; Stricker, R.; Reiser, G. Identification of phosphorylated form of 2’, 3’-cyclic nucleotide 3’-phosphodiesterase (CNPase) as 46 kDa phosphoprotein in brain non-synaptic mitochondria overloaded by calcium. J. Bioenerg. Biomembr. 2014, 46, 135–145. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, D.A.; Braun, P.E. 2’,3’-Cyclic nucleotide 3’-phosphodiesterase binds to actin-based cytoskeletal elements in an isoprenylation-independent manner. J. Neurochem. 1996, 67, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Wolff, J.; Bifulco, M. Identification of a 48-kDa prenylated protein that associates with microtubules as 2’,3’-cyclic nucleotide 3’-phosphodiesterase in FRTL-5 cells. Febs. Lett. 1997, 413, 260–264. [Google Scholar] [CrossRef]
- Bifulco, M.; Laezza, C.; Stingo, S.; Wolff, J. 2’,3’-Cyclic nucleotide 3’-phosphodiesterase: A membrane-bound, microtubule-associated protein and membrane anchor for tubulin. Proc. Natl. Acad. Sci. USA 2002, 99, 1807–1812. [Google Scholar] [CrossRef]
- Galvita, A.; Grachev, D.; Azarashvili, T.; Baburina, Y.; Krestinina, O.; Stricker, R.; Reiser, G. The brain-specific protein, p42(IP4) (ADAP 1) is localized in mitochondria and involved in regulation of mitochondrial Ca2+. J. Neurochem. 2009, 109, 1701–1713. [Google Scholar] [CrossRef]
- Kokoszka, J.E.; Waymire, K.G.; Levy, S.E.; Sligh, J.E.; Cai, J.; Jones, D.P.; MacGregor, G.R.; Wallace, D.C. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 2004, 427, 461–465. [Google Scholar] [CrossRef]
- Morciano, G.; Giorgi, C.; Bonora, M.; Punzetti, S.; Pavasini, R.; Wieckowski, M.R.; Campo, G.; Pinton, P. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J. Mol. Cell Cardiol. 2015, 78, 142–153. [Google Scholar] [CrossRef]
- Da Cruz, S.; Xenarios, I.; Langridge, J.; Vilbois, F.; Parone, P.A.; Martinou, J.C. Proteomic analysis of the mouse liver mitochondrial inner membrane. J. Biol. Chem. 2003, 278, 41566–41571. [Google Scholar] [CrossRef]
- Halestrap, A.P. Mitochondrial permeability: Dual role for the ADP/ATP translocator? Nature 2004, 430, 983. [Google Scholar] [CrossRef]
- Hurst, S.; Hoek, J.; Sheu, S.S. Mitochondrial Ca(2+) and regulation of the permeability transition pore. J. Bioenerg. Biomembr. 2017, 49, 27–47. [Google Scholar] [CrossRef]
- Elrod, J.W.; Molkentin, J.D. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ. J. 2013, 77, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Baburina, Y.; Azarashvili, T.; Grachev, D.; Krestinina, O.; Galvita, A.; Stricker, R.; Reiser, G. Mitochondrial 2’, 3’-cyclic nucleotide 3’-phosphodiesterase (CNP) interacts with mPTP modulators and functional complexes (I-V) coupled with release of apoptotic factors. Neurochem. Int. 2015, 90, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Colombini, M. VDAC closure increases calcium ion flux. Biochim. Biophys. Acta 2007, 1768, 2510–2515. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, T.K.; Sheldon, K.L.; Hassanzadeh, E.; Monge, C.; Saks, V.; Bezrukov, S.M.; Sackett, D.L. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 2008, 105, 18746–18751. [Google Scholar] [CrossRef]
- Le, Q.K.; Le, Q.D. Involvement of the ADP/ATP carrier in calcium-induced perturbations of the mitochondrial inner membrane permeability: Importance of the orientation of the nucleotide binding site. Arch. Biochem. Biophys. 1988, 265, 249–257. [Google Scholar]
- Winkler, H.H. Localization of the atractyloside-sensitive nucleotide binding sites in rat liver mitochondria. Biochim. Biophys. Acta 1969, 189, 152–161. [Google Scholar] [CrossRef]
- Bradbury, J.M.; Campbell, R.S.; Thompson, R.J. Endogenous cyclic AMP-stimulated phosphorylation of a Wolfgram protein component in rabbit central-nervous-system myelin. Biochem. J. 1984, 221, 351–359. [Google Scholar] [CrossRef]
- Schlaepfer, W.W. Structural alterations of peripheral nerve induced by the calcium ionophore A23187. Brain Res 1977, 136, 1–9. [Google Scholar] [CrossRef]
- Yin, X.; Peterson, J.; Gravel, M.; Braun, P.E.; Trapp, B.D. CNP overexpression induces aberrant oligodendrocyte membranes and inhibits MBP accumulation and myelin compaction. J. Neurosci. Res. 1997, 50, 238–247. [Google Scholar] [CrossRef]
- Rasband, M.N.; Tayler, J.; Kaga, Y.; Yang, Y.; Lappe-Siefke, C.; Nave, K.A.; Bansal, R. CNP is required for maintenance of axon-glia interactions at nodes of Ranvier in the CNS. Glia 2005, 50, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Lappe-Siefke, C.; Goebbels, S.; Gravel, M.; Nicksch, E.; Lee, J.; Braun, P.E.; Griffiths, I.R.; Nave, K.A. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 2003, 33, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Gravel, M.; Peterson, J.; Yong, V.W.; Kottis, V.; Trapp, B.; Braun, P.E. Overexpression of 2’,3’-cyclic nucleotide 3’-phosphodiesterase in transgenic mice alters oligodendrocyte development and produces aberrant myelination. Mol. Cell Neurosci. 1996, 7, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Sloane, J.A.; Hinman, J.D.; Lubonia, M.; Hollander, W.; Abraham, C.R. Age-dependent myelin degeneration and proteolysis of oligodendrocyte proteins is associated with the activation of calpain-1 in the rhesus monkey. J. Neurochem. 2003, 84, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kan, E.M.; Lu, J.; Wu, C.; Ling, E.A. Expression of 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNPase) and its roles in activated microglia in vivo and in vitro. J. Neuroinflammation 2014, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Dheen, S.T.; Jun, Y.; Yan, Z.; Tay, S.S.; Ling, E.A. Retinoic acid inhibits expression of TNF-alpha and iNOS in activated rat microglia. Glia 2005, 50, 21–31. [Google Scholar] [CrossRef]
- Minagar, A.; Shapshak, P.; Fujimura, R.; Ownby, R.; Heyes, M.; Eisdorfer, C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 2002, 202, 13–23. [Google Scholar] [CrossRef]
- Tai, Y.F.; Pavese, N.; Gerhard, A.; Tabrizi, S.J.; Barker, R.A.; Brooks, D.J.; Piccini, P. Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain 2007, 130, 1759–1766. [Google Scholar] [CrossRef]
- Kim, Y.S.; Joh, T.H. Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006, 38, 333–347. [Google Scholar] [CrossRef]
- Peters, A.; Rosene, D.L.; Moss, M.B.; Kemper, T.L.; Abraham, C.R.; Tigges, J.; Albert, M.S. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J. Neuropathol. Exp. Neurol. 1996, 55, 861–874. [Google Scholar] [CrossRef]
- Hinman, J.D.; Chen, C.D.; Oh, S.Y.; Hollander, W.; Abraham, C.R. Age-dependent accumulation of ubiquitinated 2’,3’-cyclic nucleotide 3’-phosphodiesterase in myelin lipid rafts. Glia 2008, 56, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Krestinina, O.; Azarashvili, T.; Baburina, Y.; Galvita, A.; Grachev, D.; Stricker, R.; Reiser, G. In aging, the vulnerability of rat brain mitochondria is enhanced due to reduced level of 2 ‘,3 ‘-cyclic nucleotide-3 ‘-phosphodiesterase (CNP) and subsequently increased permeability transition in brain mitochondria in old animals. Neurochem. Int. 2015, 80, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Verrier, J.D.; Jackson, T.C.; Bansal, R.; Kochanek, P.M.; Puccio, A.M.; Okonkwo, D.O.; Jackson, E.K. The brain in vivo expresses the 2’,3’-cAMP-adenosine pathway. J. Neurochem. 2012, 122, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.E.; Venegas, F.D.; Raines, R.T. Energetics of catalysis by ribonucleases: Fate of the 2’,3’-cyclic phosphodiester intermediate. Biochemistry 1994, 33, 7408–7414. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.K.; Ren, J.; Mi, Z. Extracellular 2’,3’-cAMP is a source of adenosine. J. Biol. Chem. 2009, 284, 33097–33106. [Google Scholar] [CrossRef]
- Verrier, J.D.; Exo, J.L.; Jackson, T.C.; Ren, J.; Gillespie, D.G.; Dubey, R.K.; Kochanek, P.M.; Jackson, E.K. Expression of the 2’,3’-cAMP-adenosine pathway in astrocytes and microglia. J. Neurochem. 2011, 118, 979–987. [Google Scholar] [CrossRef]
- Ohshima, H.; Bartsch, H. Chronic infections and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutat. Res. 1994, 305, 253–264. [Google Scholar] [CrossRef]
- Dohan, F.C., Jr.; Kornblith, P.L.; Wellum, G.R.; Pfeiffer, S.E.; Levine, L. S-100 protein and 2’,3’-cyclic nucleotide 3’-phosphohydrolase in human brain tumors. Acta Neuropathol. 1977, 40, 123–128. [Google Scholar] [CrossRef]
- Kurihara, T.; Kawakami, S.; Ueki, K.; Takahashi, Y. 2’,3’-Cyclic nucleotide 3’-phosphohydrolase activity in human brain tumours. J. Neurochem. 1974, 22, 1143–1144. [Google Scholar] [CrossRef]
- Krestinina, O.V.; Myakisheva, S.N.; Baburina, Y.L.; Fadeev, R.S.; Azarashvili, T.S.; Akatov, V.S. The Effects of Isoquinoline Carboxamide and Melatonin on the Differentiation of N1E-115 Mouse Neuroblastoma Cells (Clone C-1300) and on the Expression of the TSPO Translocation Protein and 2 ‘,3 ‘-Cyclonucleotide-3 ‘-Phosphodiesterase in These Cells. Neurochem. J.+ 2017, 11, 31–37. [Google Scholar] [CrossRef]
- Krestinina, O.; Fadeev, R.; Lomovsky, A.; Baburina, Y.; Kobyakova, M.; Akatov, V. Melatonin Can Strengthen the Effect of Retinoic Acid in HL-60 Cells. Int. J. Mol. Sci. 2018, 19, 2873. [Google Scholar] [CrossRef] [PubMed]
- Zorniak, M.; Clark, P.A.; Leeper, H.E.; Tipping, M.D.; Francis, D.M.; Kozak, K.R.; Salamat, M.S.; Kuo, J.S. Differential expression of 2’,3’-cyclic-nucleotide 3’-phosphodiesterase and neural lineage markers correlate with glioblastoma xenograft infiltration and patient survival. Clin. Cancer Res. 2012, 18, 3628–3636. [Google Scholar] [CrossRef] [PubMed]
- Black, K.L.; Ikezaki, K.; Santori, E.; Becker, D.P.; Vinters, H.V. Specific High-Affinity Binding of Peripheral Benzodiazepine Receptor Ligands to Brain-Tumors in Rat and Man. Cancer 1990, 65, 93–97. [Google Scholar] [CrossRef]
- Ferrarese, C.; Appollonio, I.; Frigo, M.; Gaini, S.M.; Piolti, R.; Frattola, L. Benzodiazepine Receptors and Diazepam-Binding Inhibitor in Human Cerebral-Tumors. Ann. Neurol. 1989, 26, 564–568. [Google Scholar] [CrossRef]
- Veenman, L.; Gavish, M. Peripheral-type benzodiazepine receptors: Their implication in brain disease. Drug Dev. Res. 2000, 50, 355–370. [Google Scholar] [CrossRef]
- Beinlich, A.; Strohmeier, R.; Kaufmann, M.; Kuhl, H. Relation of cell proliferation to expression of peripheral benzodiazepine receptors in human breast cancer cell lines. Biochem. Pharm. 2000, 60, 397–402. [Google Scholar] [CrossRef]
- Landau, M.; Weizman, A.; Zoref-Shani, E.; Beery, E.; Wasseman, L.; Landau, O.; Gavish, M.; Brenner, S.; Nordenberg, J. Antiproliferative and differentiating effects of benzodiazepine receptor ligands on B16 melanoma cells. Biochem. Pharm. 1998, 56, 1029–1034. [Google Scholar] [CrossRef]
- Wu, X.; Gallo, K.A. The 18-kDa translocator protein (TSPO) disrupts mammary epithelial morphogenesis and promotes breast cancer cell migration. PLoS ONE 2013, 8, e71258. [Google Scholar] [CrossRef]
- Wang, Y.; Bogenhagen, D.F. Human mitochondrial DNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane. J. Biol. Chem. 2006, 281, 25791–25802. [Google Scholar] [CrossRef]
- Gravel, M.; Robert, F.; Kottis, V.; Gallouzi, I.E.; Pelletier, J.; Braun, P.E. 2’,3’-Cyclic nucleotide 3’-phosphodiesterase: A novel RNA-binding protein that inhibits protein synthesis. J. Neurosci. Res. 2009, 87, 1069–1079. [Google Scholar] [CrossRef]
- Lopez, A.D.; Murray, C.C. The global burden of disease, 1990-2020. Nat. Med. 1998, 4, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Luptak, I.; Sverdlov, A.L.; Panagia, M.; Qin, F.; Pimentel, D.R.; Croteau, D.; Siwik, D.A.; Ingwall, J.S.; Bachschmid, M.M.; Balschi, J.A.; et al. Decreased ATP production and myocardial contractile reserve in metabolic heart disease. J. Mol. Cell Cardiol. 2018, 116, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Szalai, G.; Krishnamurthy, R.; Hajnoczky, G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. Embo J. 1999, 18, 6349–6361. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. What is the mitochondrial permeability transition pore? J. Mol. Cell Cardiol. 2009, 46, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.J. Mitochondria and heart disease. Adv. Exp. Med. Biol. 2012, 942, 249–267. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Moghaddas, S.; Tandler, B.; Kerner, J.; Hoppel, C.L. Mitochondrial dysfunction in cardiac disease: Ischemia--reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 2001, 33, 1065–1089. [Google Scholar] [CrossRef]
- Morin, D.; Assaly, R.; Paradis, S.; Berdeaux, A. Inhibition of mitochondrial membrane permeability as a putative pharmacological target for cardioprotection. Curr. Med. Chem. 2009, 16, 4382–4398. [Google Scholar] [CrossRef][Green Version]
- Ahmad, F.; Singh, A.P.; Tomar, D.; Rahmani, M.; Zhang, Q.; Woodgett, J.R.; Tilley, D.G.; Lal, H.; Force, T. Cardiomyocyte-GSK-3alpha promotes mPTP opening and heart failure in mice with chronic pressure overload. J. Mol. Cell Cardiol. 2019, 130, 65–75. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, H.; Xu, A.; Ross, T.; Bowler, E.; Hu, Y.; Lesnefsky, E.J. Inhibition of Bcl-2 sensitizes mitochondrial permeability transition pore (MPTP) opening in ischemia-damaged mitochondria. PLoS ONE 2015, 10, e0118834. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Pasdois, P. The role of the mitochondrial permeability transition pore in heart disease. Biochim. Biophys. Acta 2009, 1787, 1402–1415. [Google Scholar] [CrossRef]
- Dongworth, R.K.; Hall, A.R.; Burke, N.; Hausenloy, D.J. Targeting mitochondria for cardioprotection: Examining the benefit for patients. Future Cardiol. 2014, 10, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Samangouei, P.; Kalkhoran, S.B.; Hausenloy, D.J. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell Cardiol. 2015, 78, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R., 3rd; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef]
- Asin-Cayuela, J.; Manas, A.R.; James, A.M.; Smith, R.A.; Murphy, M.P. Fine-tuning the hydrophobicity of a mitochondria-targeted antioxidant. Febs Lett. 2004, 571, 9–16. [Google Scholar] [CrossRef]
- Smith, R.A.; Porteous, C.M.; Coulter, C.V.; Murphy, M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999, 263, 709–716. [Google Scholar] [CrossRef]
- Menendez-Pelaez, A.; Reiter, R.J. Distribution of melatonin in mammalian tissues: The relative importance of nuclear versus cytosolic localization. J. Pineal. Res. 1993, 15, 59–69. [Google Scholar] [CrossRef]
- Krestinina, O.V.; Baburina, Y.L.; Azarashvili, T.S. Effect of Melatonin on Stress-Induced PTP Opening in Mitochondria from Brain of Young and Old Rats. Biol. Membr. 2014, 31, 95–103. [Google Scholar] [CrossRef]
- Krestinina, O.V.; Baburina, Y.L.; Azarashvili, T.S. Effect of Melatonin on Stress-Induced Opening of Non-Selective Pore in Mitochondria from Brain of Young and Old Rats. Biochem. Mosc. Suppl. S 2015, 9, 116–123. [Google Scholar] [CrossRef]
- Baburina, Y.; Odinokova, I.; Azarashvili, T.; Akatov, V.; Lemasters, J.J.; Krestinina, O. 2 ‘,3 ‘-Cyclic nucleotide 3 ‘-phosphodiesterase as a messenger of protection of the mitochondrial function during melatonin treatment in aging. Bba Biomembr. 2017, 1859, 94–103. [Google Scholar] [CrossRef]
- Odinokova, I.; Baburina, Y.; Kruglov, A.; Fadeeva, I.; Zvyagina, A.; Sotnikova, L.; Akatov, V.; Krestinina, O. Effect of Melatonin on Rat Heart Mitochondria in Acute Heart Failure in Aged Rats. Int. J. Mol. Sci. 2018, 19, 1555. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Carotenoid biosynthesis in microorganisms and plants. Eur. J. Biochem. 1994, 223, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends. Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Fan, C.D.; Sun, J.Y.; Fu, X.T.; Hou, Y.J.; Li, Y.; Yang, M.F.; Fu, X.Y.; Sun, B.L. Astaxanthin Attenuates Homocysteine-Induced Cardiotoxicity in Vitro and in Vivo by Inhibiting Mitochondrial Dysfunction and Oxidative Damage. Front. Physiol. 2017, 8, 1041. [Google Scholar] [CrossRef] [PubMed]
- Pongkan, W.; Takatori, O.; Ni, Y.; Xu, L.; Nagata, N.; Chattipakorn, S.C.; Usui, S.; Kaneko, S.; Takamura, M.; Sugiura, M.; et al. beta-Cryptoxanthin exerts greater cardioprotective effects on cardiac ischemia-reperfusion injury than astaxanthin by attenuating mitochondrial dysfunction in mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Baburina, Y.; Krestinin, R.; Odinokova, I.; Sotnikova, L.; Kruglov, A.; Krestinina, O. Astaxanthin Inhibits Mitochondrial Permeability Transition Pore Opening in Rat Heart Mitochondria. Antioxidants 2019, 8, 576. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhu, X.L.; Sun, M.H.; Dang, Y.K. Effects of astaxanthin onaxonal regeneration via cAMP/PKA signaling pathway in mice with focal cerebral infarction. Eur. Rev. Med. Pharm. Sci. 2019, 23, 135–143. [Google Scholar] [CrossRef]
- Krestinina, O.V.; Odinokova, I.V.; Baburina, Y.L.; Azarashvili, T.S. Detection of Protein Kinase A and C Target Proteins in Rat Brain Mitochondria. Biochem. Mosc. Suppl. S 2018, 12, 70–73. [Google Scholar] [CrossRef]
- Vankoningsloo, S.; De Pauw, A.; Houbion, A.; Tejerina, S.; Demazy, C.; de Longueville, F.; Bertholet, V.; Renard, P.; Remacle, J.; Holvoet, P.; et al. CREB activation induced by mitochondrial dysfunction triggers triglyceride accumulation in 3T3-L1 preadipocytes. J. Cell Sci. 2006, 119, 1266–1282. [Google Scholar] [CrossRef]
- Krestinina, O.; Baburina, Y.; Krestinin, R.; Odinokova, I.; Fadeeva, I.; Sotnikova, L. Astaxanthin Prevents Mitochondrial Impairment Induced by Isoproterenol in Isolated Rat Heart Mitochondria. Antioxidants 2020, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and mitochondria: A dysfunctional relationship. Gastroenterology 2002, 122, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Manzo-Avalos, S.; Saavedra-Molina, A. Cellular and mitochondrial effects of alcohol consumption. Int. J. Envir. Res. Public Health 2010, 7, 4281–4304. [Google Scholar] [CrossRef] [PubMed]
- Mantena, S.K.; King, A.L.; Andringa, K.K.; Eccleston, H.B.; Bailey, S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 2008, 44, 1259–1272. [Google Scholar] [CrossRef]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef]
- Mann, R.E.; Smart, R.G.; Govoni, R. The epidemiology of alcoholic liver disease. Alcohol Res. Health 2003, 27, 209–219. [Google Scholar]
- Miranda-Mendez, A.; Lugo-Baruqui, A.; Armendariz-Borunda, J. Molecular basis and current treatment for alcoholic liver disease. Int. J. Env. Res. Public Health 2010, 7, 1872–1888. [Google Scholar] [CrossRef]
- Bergheim, I.; McClain, C.J.; Arteel, G.E. Treatment of alcoholic liver disease. Dig. Dis. 2005, 23, 275–284. [Google Scholar] [CrossRef]
- Bailey, S.M.; Cunningham, C.C. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic. Biol. Med. 2002, 32, 11–16. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Lu, S.C. Current concepts in the pathogenesis of alcoholic liver injury. Faseb. J. 2001, 15, 1335–1349. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Introduction-serial review: Alcohol, oxidative stress and cell injury. Free Radic Biol. Med. 2001, 31, 1524–1526. [Google Scholar] [CrossRef]
- French, S.W. Mechanisms of alcoholic liver injury. Can. J. Gastroenterol. 2000, 14, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Preedy, V.R. Free radicals as mediators of alcohol toxicity. Advers. Drug React Toxicol. Rev. 1999, 18, 235–252. [Google Scholar]
- Cunningham, C.C.; Spach, P.I. The effect of chronic ethanol consumption on the lipids in liver mitochondria. Ann. N. Y. Acad. Sci. 1987, 492, 181–192. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Coleman, W.B.; Spach, P.I. The effects of chronic ethanol consumption on hepatic mitochondrial energy metabolism. Alcohol Alcohol. 1990, 25, 127–136. [Google Scholar] [CrossRef]
- Das, S.; Hajnoczky, N.; Antony, A.N.; Csordas, G.; Gaspers, L.D.; Clemens, D.L.; Hoek, J.B.; Hajnoczky, G. Mitochondrial morphology and dynamics in hepatocytes from normal and ethanol-fed rats. Pflug. Arch. 2012, 464, 101–109. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Jacobson, J.; Duchen, M.R. Interplay between mitochondria and cellular calcium signalling. Mol. Cell Biochem. 2004, 256–257, 209–218. [Google Scholar] [CrossRef]
- Rimessi, A.; Giorgi, C.; Pinton, P.; Rizzuto, R. The versatility of mitochondrial calcium signals: From stimulation of cell metabolism to induction of cell death. Biochim. Biophys. Acta 2008, 1777, 808–816. [Google Scholar] [CrossRef]
- Shalbueva, N.; Mareninova, O.A.; Gerloff, A.; Yuan, J.; Waldron, R.T.; Pandol, S.J.; Gukovskaya, A.S. Effects of oxidative alcohol metabolism on the mitochondrial permeability transition pore and necrosis in a mouse model of alcoholic pancreatitis. Gastroenterology 2013, 144, 437–446. [Google Scholar] [CrossRef]
- King, A.L.; Swain, T.M.; Mao, Z.; Udoh, U.S.; Oliva, C.R.; Betancourt, A.M.; Griguer, C.E.; Crowe, D.R.; Lesort, M.; Bailey, S.M. Involvement of the mitochondrial permeability transition pore in chronic ethanol-mediated liver injury in mice. Am. J. Physiol. Gastrointest Liver Physiol. 2014, 306, G265–G277. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, J.G.; Hoek, J.B. Ethanol potentiates tumor necrosis factor-alpha cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology 2000, 31, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Juhaszova, M.; Wang, S.; Zorov, D.B.; Nuss, H.B.; Gleichmann, M.; Mattson, M.P.; Sollott, S.J. The identity and regulation of the mitochondrial permeability transition pore: Where the known meets the unknown. Ann. N. Y. Acad. Sci. 2008, 1123, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef]
- Logrip, M.L. Phosphodiesterase regulation of alcohol drinking in rodents. Alcohol 2015, 49, 795–802. [Google Scholar] [CrossRef]
- Olsen, C.M.; Liu, Q.S. Phosphodiesterase 4 inhibitors and drugs of abuse: Current knowledge and therapeutic opportunities. Front. Biol. 2016, 11, 376–386. [Google Scholar] [CrossRef]


| Relative Values of Ca2+ Influx Rate | Relative Values of Lag Time | Relative Values of Ca2+ Capacity | |
|---|---|---|---|
| Wild type | 1.00 ± 0.11 | 1.00 ± 0.06 | 1.00 ± 0.14 |
| Scrambled siRNA | 0.97 ± 0.10 | 0.85 ± 0.08 | 0.98 ± 0.05 |
| CNPase siRNA | 0.87 ± 0.13 | 0.66 ± 0.09 ** | 0.68 ± 0.11 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olga, K.; Yulia, B.; Vassilios, P. The Functions of Mitochondrial 2′,3′-Cyclic Nucleotide-3′-Phosphodiesterase and Prospects for Its Future. Int. J. Mol. Sci. 2020, 21, 3217. https://doi.org/10.3390/ijms21093217
Olga K, Yulia B, Vassilios P. The Functions of Mitochondrial 2′,3′-Cyclic Nucleotide-3′-Phosphodiesterase and Prospects for Its Future. International Journal of Molecular Sciences. 2020; 21(9):3217. https://doi.org/10.3390/ijms21093217
Chicago/Turabian StyleOlga, Krestinina, Baburina Yulia, and Papadopoulos Vassilios. 2020. "The Functions of Mitochondrial 2′,3′-Cyclic Nucleotide-3′-Phosphodiesterase and Prospects for Its Future" International Journal of Molecular Sciences 21, no. 9: 3217. https://doi.org/10.3390/ijms21093217
APA StyleOlga, K., Yulia, B., & Vassilios, P. (2020). The Functions of Mitochondrial 2′,3′-Cyclic Nucleotide-3′-Phosphodiesterase and Prospects for Its Future. International Journal of Molecular Sciences, 21(9), 3217. https://doi.org/10.3390/ijms21093217






