Neonatal Clonazepam Administration Induced Long-Lasting Changes in GABAA and GABAB Receptors
Abstract
1. Introduction
2. Results
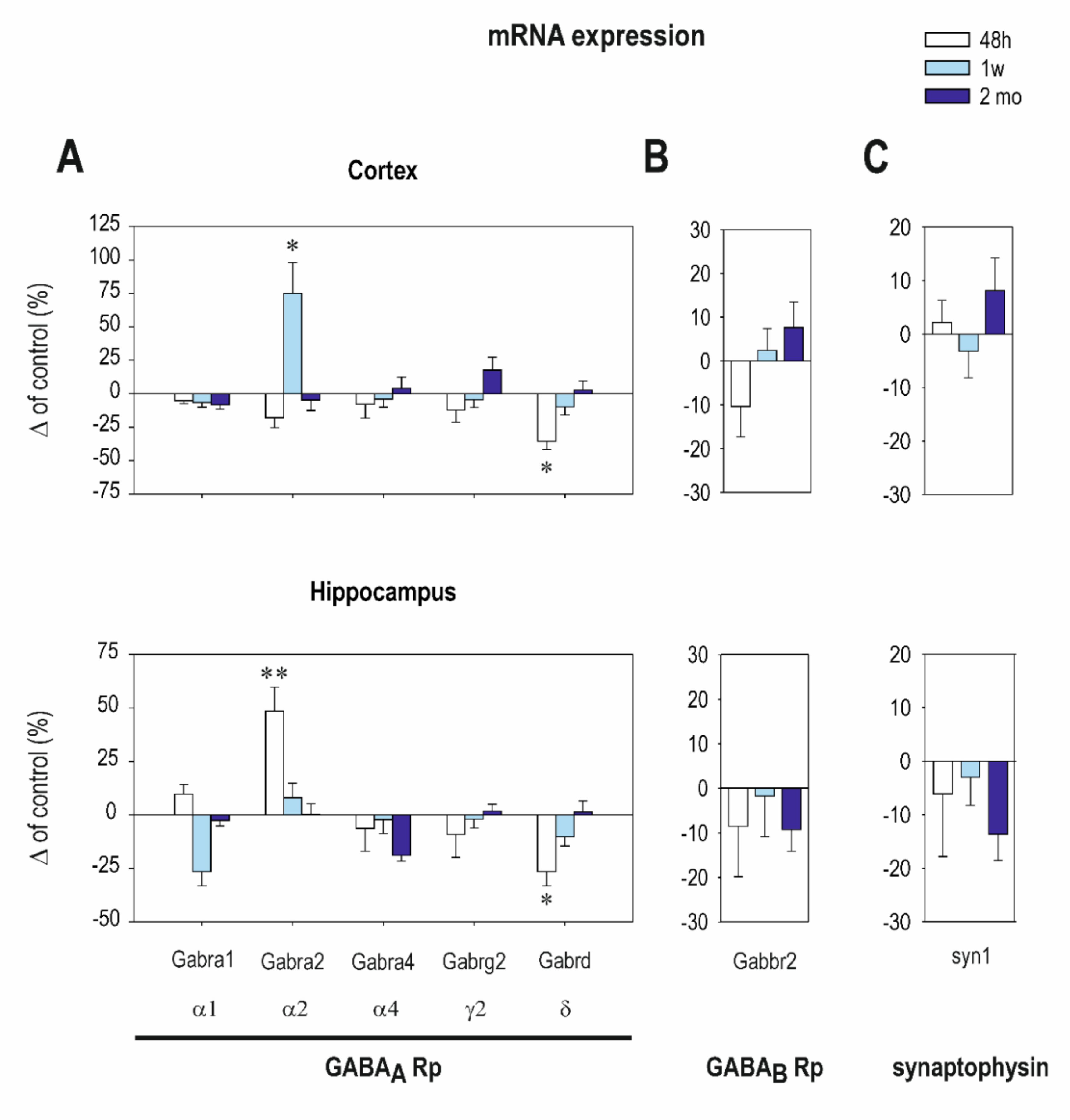
2.1. The Effect of CZP Administration on GABAA and GABAB R2 Receptor (Rp) Subunit mRNA Expression
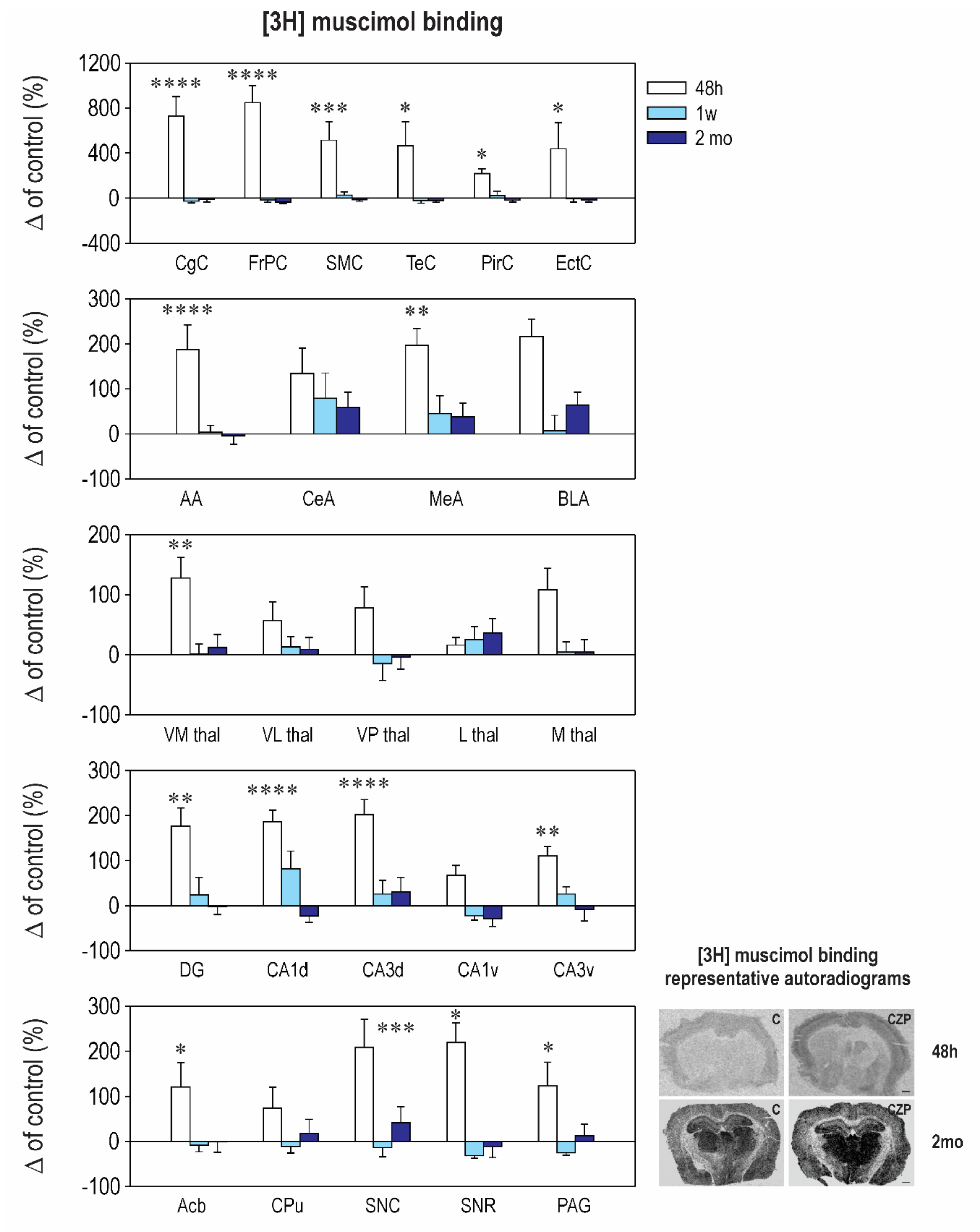
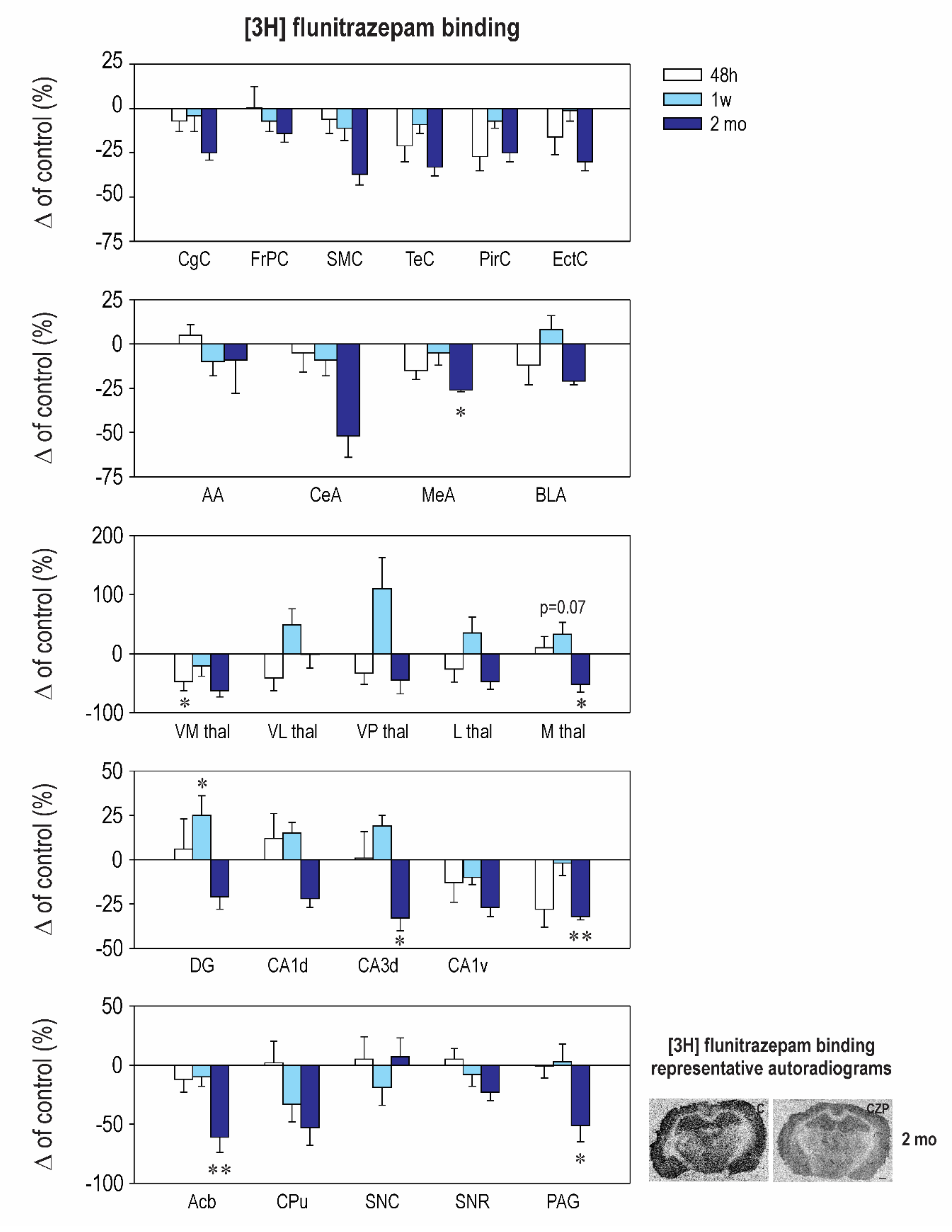
2.2. Effect of CZP Administration on [3H] Muscimol Binding and [3H] Flunitrazepam Binding
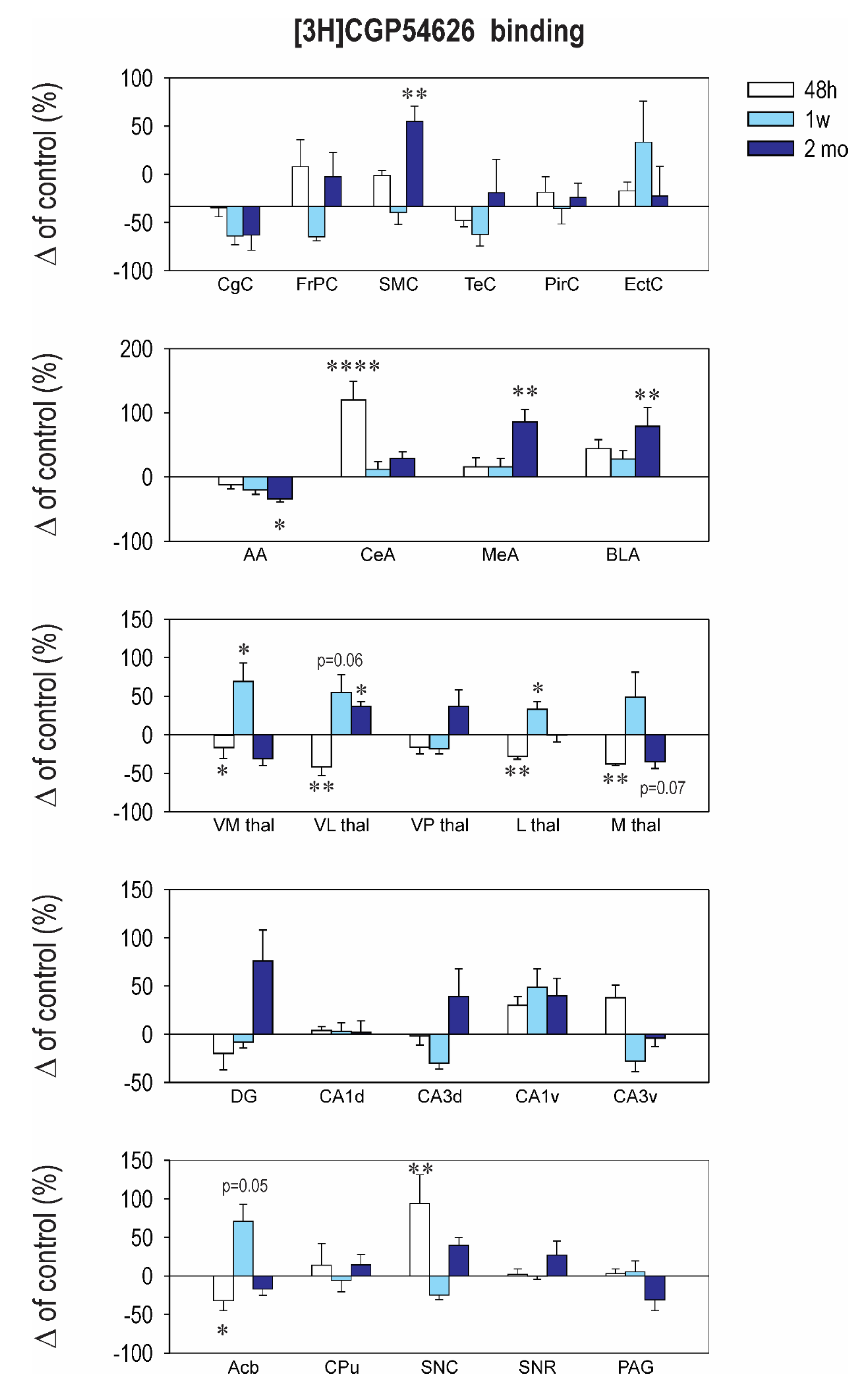
2.3. Effect of CZP Administration on [3H] CGP54626 Binding
3. Discussion
4. Materials and Methods
4.1. Pharmacological Treatment
4.2. Quantitative Real-Time RT-PCR
4.3. Receptor Binding
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DOAJ | Directory of open access journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
Appendix
| Cortex | Interval 48 h Mean ± SEM Control CZP | Interval 1 Week Mean ± SEM Control CZP | Interval 2 Months Mean ± SEM Control CZP | |||
|---|---|---|---|---|---|---|
| Gabra1 (α1) | 16.3 ± 0.7 | 15.3 ± 0.6 | 24.2 ± 1.7 | 22.7 ± 0.9 | 27.3 ± 2.4 | 26.6 ± 1.6 |
| Gabra2 (α2) | 11.7 ± 1.3 | 10.7 ± 1.1 | 6.6 ± 1.2 | ⬆ 11.1 ± 1.1 t = 2.705, df= 13, p = 0.018 | 14.5 ± 1.8 | 13.8 ± 1.3 |
| Gabra4 (α4) | 2.3 ± 0.3 | 2.2 ± 0.2 | 2.8 ± 0.3 | 2.7 ± 0.3 | 3.2 ± 0.3 | 3.3 ± 0.2 |
| Gabrg2 (γ2) | 3.4 ± 0.4 | 3.08 ± 0.32 | 3.5 ± 0.4 | 3.4 ± 0.3 | 5.5 ± 0.506 | 6.2 ± 0.3 |
| Gabrd (δ) | 0.14 ± 0.01 | ⬇ 0.06 ± 0.0 t = 2.196, df = 17, p = 0.0423 | 0.14 ± 0.02 | 0.13 ± 0.01 | 0.24 ± 0.02 | 0.25 ± 0.01 |
| Synaptophysin | 3.03 ± 0.27 | 3.400 ± 0.1 | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.7 ± 0.27 | 4.0 ± 0.1 |
| Gabbr2 | 2.1 ± 0.1 | 1.8 ± 0.1 | 1.9 ± 0.2 | 2.02 ± 0.2 | 2.1 ± 0.2 | 2.3 ± 0.1 |
| Hippocampus | ||||||
| Gabra1 (α1) | 7.8 ± 0.9 | 8.6 ± 0.9 | 10.0 ± 1.1 | 9.6 ± 0.6 | 12.2 ± 1.0 | 11.2 ± 0.9 |
| Gabra2 (α2) | 17.2 ± 1.3 | ⬆ 26.1 ± 2.6 t = 2.956, df = 15, p = 0.0098 | 29.7 ± 4.0 | 27.4 ± 3.9 | 36.3 ± 4.7 | 37.8 ± 5.7 |
| Gabra4 (α4) | 2.1 ± 0.5 | 1.7 ± 0.4 | 3.2 ± 0.8 | 3.2 ± 0.8 | 9.2 ± 1.42 | 4.267 ± 1.1 |
| Gabrg2 (γ2) | 9.9 ± 1.1 | 7.8 ± 0.6 | 13.5 ± 1.6 | 12.6 ± 1.6 | 19.0 ± 2.2 | 19.50 ± 2.3 |
| Gabrd (δ) | 0.1 ± 0.02 | ⬇ 0.064 ± 0.01 t = 2.129, df = 16, p = 0.0435 | 0.2 ± 0.03 | 0.13 ± 0.03 | 0.3 ± 0.06 | 0.28 ± 0.06 |
| Synaptophysin | 45.0 ± 9.7 | 31.4 ± 5.1 | 69.0 ± 15.2 | 65.0 ± 16.9 | 103.2 ± 3.0 | 86.8 ± 6.2 |
| Gabbr2 | 3.3 ± 0.2 | 3.04 ± 0.4 | 3.9 ± 0.2 | 3.9 ± 0.4 | 4.7 ± 0.1 | 4.2 ± 0.2 |
| Interval 48 h Mean ± SEM | Interval 1 week Mean ± SEM | Interval 2 months Mean ± SEM | ||||
|---|---|---|---|---|---|---|
| Control | CZP | Control | CZP | Control | CZP | |
| Cingulate Cx. | 50.1 ± 8.6 | ⬆ 416.0 ± 86.8 t = 6.921, df = 35, p < 0.0001 | 120.0 ± 35.3 | 88.3 ± 18.8 | 67.8 ± 14.8 | 60.0 ± 14.1 |
| Frontoparital Cx. | 55.8 ± 10.4 | ⬆ 529.0 ± 84.3 t = 8.006, df = 35, p < 0.0001 | 152.0 ± 49.6 | 127.3 ± 32.9 | 93.8 ± 15.7 | 59.8 ± 12.3 |
| Sensorimotor Cx. | 52.8 ± 12.0 | ⬆ 324.0 ± 88.0 t = 4.289, df = 35, p = 0.0004 | 151.1 ± 49.0 | 189.0 ± 42.4 | 72.1 ± 14.6 | 62.4 ± 9.9 |
| Temporal Cx. | 50.0 ± 10.5 | ⬆ 283.2 ± 105.0 t = 2.901, df = 35, p = 0.0191 | 211.7 ± 75.3 | 165.9 ± 48.7 | 76.5 ± 23.7 | 59.4 ± 11.3 |
| Piriform Cx. | 34.2 ± 5.5 | ⬆ 109.0 ± 14.2 t = 3.036, df = 35, p = 0.0135 | 66.4 ± 18.5 | 81.8 ± 26.5 | 52.3 ± 12 | 42.2 ± 8.3 |
| Enthorinal Cx. | 34.5 ± 2.8 | ⬆ 104 ± 7.4 t = 3.036, df = 35, p = 0.0134 | 51.7 ± 19.6 | 37 ± 6.1 | 52.3 ± 16.1 | 43.2 ± 10.0 |
| Anterior AMG | 28.8 ± 4.6 | ⬆ 82.8 ± 15.8 t = 4.756, df = 36, p < 0.0001 | 33.2 ± 5.3 | 34.5 ± 5.0 | 43.6 ± 9.1 | 42.1 ± 8.1 |
| Central AMG | 25.2 ± 2.5 | 79.0 ± 19.0 | 32.7 ± 6.6 | 58.5 ± 18.2 | 27.0 ± 8.4 | 43.0 ± 8.8 |
| Medial AMG | 34.0 ± 3.1 | ⬆ 101.0 ± 12.0 t = 3.688, df = 35, p = 0.0023 | 49.2 ± 10.3 | 71.3 ± 19.0 | 35.5 ± 5.8 | 49.1 ± 10.8 |
| Basolateral AMG | 40.1 ± 11.0 | 126.8 ± 15.0 | 110.3 ± 37.6 | 119.6 ± 37.6 | 36.3 ± 7.5 | 59.4 ± 10 |
| N. Accumbens | 25.7 ± 3.9 | ⬆ 56.8 ± 13.0 t = 2.791, df = 35, p = 0.0252 | 22.0 ± 2.7 | 20.2 ± 3.3 | 30.1 ± 12.0 | 30.0 ± 7.0 |
| CPU | 31.0 ± 5.3 | 53.8 ± 14.0 | 20.4 ± 2.5 | 18.2 ± 3.0 | 29.3 ± 11.0 | 34.7 ± 9.0 |
| Ventromedial TH | 28.0 ± 4.1 | ⬆ 63.8 ± 9.0 t = 3.09, df = 35, p = 0.0117 | 37.4 ± 8.8 | 37.8 ± 6.0 | 44.5 ± 8.5 | 49.7 ± 9.6 |
| Ventrolateral TH | 24.1 ± 4.2 | 37.8 ± 7.0 | 27.0 ± 5.7 | 30.6 ± 4.5 | 46.3 ± 6.6 | 50.5 ± 9.4 |
| Ventralpost. TH | 34.7 ± 4.7 | 61.6 ± 12.0 | 137.4 ± 56.1 | 118.3 ± 39.0 | 63.6 ± 4.2 | 61.4 ± 13 |
| Lateral TH | 32.4 ± 5.9 | 37.6 ± 4.0 | 27.8 ± 4.9 | 34.8 ± 6.2 | 43.5 ± 10.6 | 59.0 ± 10.0 |
| Medial TH | 19.7 ± 2.8 | 41.0 ± 7.1 | 27.5 ± 4.8 | 29.0 ± 4.7 | 46.1 ± 7.9 | 48.4 ± 9.4 |
| Dentate Gyrus | 31.8 ± 2.8 | ⬆ 106.0 ± 15.9 t = 3.671, df = 35, p = 0.0024 | 47.1 ± 7.0 | 58.2 ± 18 | 49.6 ± 11.0 | 48.7 ± 8.7 |
| CA1 Dorsal | 29.4 ± 2.5 | ⬆ 84.1 ± 7.7 t = 5.424, df = 35, p < 0.0001 | 23.8 ± 2.6 | 43.3 ± 9.3 | 68.3 ± 2.9 | 52.7 ± 9.7 |
| CA2 Dorsal | 29.2 ± 5.1 | ⬆ 79.0 ± 14.0 t = 3.941, df = 35, p = 0.0011 | 26.2 ± 5.2 | 35.8 ± 6.8 | 36.1 ± 10.6 | 39.1 ± 9.3 |
| CA3 Dorsal | 31.2 ± 4.3 | ⬆ 94.5 ± 10.0 t = 5.175, df = 35, p < 0.0001 | 31.0 ± 5.9 | 39.0 ± 9.3 | 30.0 ± 9.0 | 39.0 ± 9.9 |
| CA1 Ventral | 44.1 ± 12.3 | 73.6 ± 9.6 | 40.5 ± 15.5 | 31.7 ± 4.0 | 55.8 ± 10.7 | 39.7 ± 9.7 |
| CA2 Ventral | 34.8 ± 8.4 | ⬆ 83.6 ± 10.0 t = 4.228, df = 35, p = 0.0005 | 24.8 ± 5.5 | 32.8 ± 3.2 | 56.8 ± 6.7 | 43.1 ± 11.2 |
| CA3 Ventral | 35.5 ± 7.3 | ⬆ 74.8 ± 7.6 t = 3.175, df = 35, p = 0.0093 | 25.5 ± 6.1 | 32.2 ± 4.1 | 45.1 ± 12.6 | 41.5 ± 11.9 |
| SN Compacta | 26.8 ± 4.0 | ⬆ 83.0 ± 16.5 t = 4.591, df = 35, p = 0.0002 | 35.7 ± 6.1 | 30.8 ± 7.2 | 24.3 ± 3.3 | 34.5 ± 8.5 |
| SN Reticulata | 31.5 ± 4.9 | ⬆ 101.0 ± 13.0 t = 2.883, df = 35, p = 0.0199 | 88.2 ± 23.5 | 60.6 ± 15.4 | 64.0 ± 20.3 | 56.1 ± 15.4 |
| PAG | 27.7 ± 3.3 | ⬆ 61.6 ± 14.0 t = 2.764, df = 35, p = 0.0269 | 41.2 ± 6.7 | 30.8 ± 4.9 | 35.6 ± 10.7 | 40.4 ± 8.7 |
| Interval 48 h Mean ± SEM | Interval 1 Week Mean ± SEM | Interval 2 Months Mean ± SEM | ||||
|---|---|---|---|---|---|---|
| Structure | Control | CZP | Control | CZP | Control | CZP |
| Cingulate Cx. | 388.0 ± 25.5 | 361.0 ± 17.2 | 387.4 ± 15.5 | 373.0 ± 36.0 | 370.2 ± 35.1 | 276.0 ± 15.0 |
| Frontoparital Cx. | 380.8 ± 23.9 | 382.4 ± 45.2 | 396.6 ± 26.9 | 368.9 ± 22.0 | 306.0 ± 34.0 | 263.1 ± 15.0 |
| Sensorimotor Cx. | 363.9 ± 48.2 | 342.1 ± 28.0 | 410.4 ± 37.6 | 364.1 ± 30.6 | 314.8 ± 25.2 | 198.4 ±19.0 |
| Temporal Cx. | 389.8 ± 55.2 | 270.3 ± 25.5 | 389.7 ± 19.0 | 355.5 ± 19.8 | 374.3 ± 49.6 | 250.7 ± 18.0 |
| Piriform Cx. | 336.0 ± 42.9 | 245.7 ± 25.5 | 281.0 ± 24.6 | 260.4 ± 11.0 | 282.3 ± 18.1 | 212.7 ± 14.0 |
| Enthorinal Cx. | 367.6 ± 54.0 | 271.3 ± 7.8 | 305.9 ± 22.0 | 301.6 ± 19.7 | 318.8 ± 52.2 | 223.0 ± 15.8 |
| Anterior AMG | 215.9 ± 21.8 | 226.1 ± 12.0 | 233.6 ± 7.8 | 211.1 ± 19.3 | 126.8 ± 44.4 | 115.0 ± 24.5 |
| Central AMG | 200.9 ± 40.0 | 190.9 ± 21.0 | 210.5 ± 7.2 | 210.6 ± 20.0 | 160.7 ± 39.0 | 77.2 ± 20.0 |
| Medial AMG | 317.3 ± 32.8 | 269.3 ± 15.9 | 295.0 ± 33.4 | 280.4 ± 19.0 | 304.0 ± 30.3 | ⬇ 224.7 ± 4.2 t = 2.572, df = 37, p = 0.0422 |
| Basolateral AMG | 343.6 ± 38.0 | 301.1 ± 37.1 | 318.0 ± 30.3 | 343.0 ± 24.0 | 319.3 ± 43.9 | 252.9 ± 5.5 |
| N. Accumbens | 223.3 ± 29.4 | 197.6 ± 24.0 | 216.7 ± 22.4 | 196.0 ± 17.4 | 233.8 ± 33.1 | ⬇ 91.3 ± 3.0 t = 3.637, df = 37, p = 0.0025 |
| CPU | 145.1 ± 30.1 | 148.7 ± 25.6 | 121.6 ± 26.1 | 81.1 ± 17.9 | 109.3 ± 21.4 | 51.1 ± 16.1 |
| Ventromedial TH | 230.1 ± 5.3 | ⬇ 122.9 ± 37.2 t = 2.859, df = 37, p = 0.0207 | 222.4 ± 17.2 | 175.1 ± 37.2 | 92 ± 37.0 | 33.9 ± 9.0 |
| Ventrolateral TH | 146.4 ± 28.6 | 86.1 ± 31.6 | 102.4 ± 27.4 | 153.0 ± 28.0 | 50.3 ± 19.1 | 49.8 ± 11.5 |
| Ventralpost. TH | 116.8 ± 26.7 | 78.2 ± 21.8 | 57.2 ± 12.9 | 120.5 ± 30.4 | 24.0 ± 11.4 | 13.1 ± 5.4 |
| Lateral TH | 157.0 ± 31.6 | 115.9 ± 33.9 | 106.6 ± 30.3 | 143.8 ± 28.5 | 74.0 ± 19.6 | 39.0 ± 9.7 |
| Medial TH | 193.3 ± 37.1 | 212.1 ± 36.5 | 158.1 ± 34.0 | 235.7 ± 19.7 | 178.0 ± 29.8 | 85.0 ± 23.4 |
| Dentate Gyrus | 313.4 ± 26.8 | 333.3 ± 53.6 | 311.1 ± 19.0 | 390.1 ± 33.6 | 337.8 ± 56.7 | 267.3 ± 23.2 |
| CA1 Dorsal | 261.5 ± 32.9 | 292.4 ± 35.7 | 288.9 ± 24.2 | 331.6 ± 17 | 249.7 ± 34.8 | 195.4 ± 16.4 |
| CA2 Dorsal | 228.9 ± 33.0 | 231.0 ± 41.4 | 230.6 ± 25.3 | 272.9 ± 15.1 | 206.8 ± 29.2 | 120.1 ± 23.2 |
| CA3 Dorsal | 294.8 ± 25.4 | 297.0 ± 45.5 | 261.6 ± 26.5 | 311.3 ± 14.9 | 267.8 ± 20.9 | ⬇ 179.0 ± 18.3 t = 3.795, df = 37, p = 0.0016 |
| CA1 Ventral | 373.1 ± 63.0 | 251.5 ± 10.9 | 289.1 ± 12.2 | 261.6 ± 12.9 | 280.0 ± 31.8 | 205.0 ± 14.8 |
| CA2 Ventral | 316.9 ± 60.8 | 248.9 ± 19.4 | 294.0 ± 20.5 | 280.9 ± 12.9 | 304.2 ± 41.4 | 218.0 ± 15.1 |
| CA3 Ventral | 356.9 ± 72.9 | 256.3 ± 35.0 | 294.4 ± 24.6 | 289.8 ± 19.8 | 320.8 ± 31.2 | 218.6 ± 7.3 |
| SN Compacta | 168.3 ± 49.4 | 177.3 ± 31.8 | 206.7 ± 11.9 | 168.1 ± 31.0 | 132.3 ± 49.2 | 141.0 ± 20.7 |
| SN Reticulata | 228.0 ± 60.4 | 238.4 ± 21.6 | 252.0 ± 21.6 | 232.1 ± 26.3 | 249.8 ± 27.6 | 192.0 ± 16.7 |
| PAG | 223.8 ± 35.9 | 220.9 ± 22.8 | 227.0 ± 22.7 | 233.3 ± 33.8 | 197.0 ± 22.0 | ⬇ 97.0 ± 28.1 t = 2.522, df = 37, p = 0.0492 |
| Structure | Control 48 h | CZP 48 h | Control 1 week | CZP 1 week | Control 50 days | CZP 50 days |
|---|---|---|---|---|---|---|
| Cingulate Cx. | 274.1 ± 25.1 | 271.0 ± 19.0 | 311.1 ± 18.9 | 239.3 ± 20.9 | 404.7 ± 75.1 | 314.9 ± 49.3 |
| Frontoparital Cx. | 266.4 ± 9.3 | 349.8 ± 55.0 | 377.1 ± 39.6 | 287.6 ± 11.6 | 206.0 ± 26.3 | 253.6 ± 39.3 |
| Sensorimotor Cx. | 227.4 ± 15.0 | 283.0 ± 10.0 | 242.6 ± 14.7 | 230.6 ± 21.4 | 141.5 ± 16.5 | ⬆ 235.0 ± 17.7 t = 2.416, df = 37, p = 0.0017 |
| Temporal Cx. | 292.3 ± 27.4 | 260.1 ± 15.5 | 327.9 ± 38.6 | 256.0 ± 25.2 | 195.0 ± 26.9 | 215.7 ± 50.5 |
| Piriform Cx. | 210.1 ± 21.2 | 233.1 ± 25.6 | 237.1 ± 28.3 | 233.3 ± 27.5 | 269.0 ± 48.7 | 287.7 ± 30.6 |
| Enthorinal Cx. | 233.5 ± 24.7 | 262.3 ± 15.7 | 276.6 ± 24.0 | 414.9 ± 88.4 | 427.5 ± 87.3 | 462.6 ± 97.1 |
| Anterior AMG | 260.6 ± 20.0 | 228.7 ± 16.9 | 315.7 ± 31.2 | 253.9 ± 21.6 | 229.3 ± 49.3 | ⬇152.3 ± 10.7 t = 2.72, df = 37, p = 0.0293 |
| Central AMG | 231.1 ± 12.8 | ⬆ 507.7 ± 66.0 t = 5.706, df = 37, p < 0.0001 | 198.4 ± 19.4 | 221.5 ± 23.9 | 210.7 ± 37.8 | 271.9 ± 21.3 |
| Medial AMG | 439.9 ± 47.3 | 511.4 ± 63.3 | 298.1 ± 23.2 | 347.3 ± 40.0 | 365.5 ± 92.5 | ⬆ 678.6 ± 70.0 t = 3.679, df = 39, p = 0.0023 |
| Basolateral AMG | 251.9 ± 14.9 | 362.1 ± 34.0 | 218.7 ± 23.5 | 279.9 ± 27.0 | 268.0 ± 48.3 | ⬆ 479.1 ± 77 t = 3.422, df = 36, p = 0.0047 |
| N. Accumbens | 1028.0 ± 76.0 | ⬇ 697.6 ± 134.0 t = 2.857, df = 36, p = 0.021 | 384.6 ± 33.4 | 657.0 ± 85.0 | 369.5 ± 78.6 | 306.7 ± 29.6 |
| CPU | 673.1 ± 65.7 | 767.1 ± 191.0 | 454.3 ± 52.1 | 428.4 ± 68.0 | 174.8 ± 17.8 | 200.3 ± 22.8 |
| Ventromedial TH | 1151.0 ± 56.5 | 892.5 ± 68.7 | 734.3 ± 113 | ⬆ 1238.0 ± 175.0 t = 2.88, df = 36, p = 0.0199 | 601.0 ± 131 | 415.1 ± 51.4 |
| Ventrolateral TH | 1085.0 ± 66.5 | ⬇ 632.0 ± 120.0 t = 2.961, df = 36, p = 0.0161 | 735.6 ± 79.0 | ⬆ 1139.0 ± 166.0 t = 2.961, df = 36, p = 0.0293 | 606.5 ± 94.2 | 380.7 ± 37 |
| Ventralpost. TH | 1898.0 ± 321.0 | 1592.0 ± 178.0 | 1409.0 ± 77.8 | 1161.0 ± 99.0 | 249.7 ± 36.9 | ⬆ 342.4 ± 52.6 t = 3.738, df = 35, p = 0.0020 |
| Lateral TH | 2187.0 ± 125.0 | ⬇ 1566 ± 83.0 t = 3.608, df = 36, p = 0.0028 | 1532.0 ± 162.2 | ⬆ 2033.0 ± 156.0 t = 3.006, df = 39, p = 0.0143 | 718.7 ± 66.2 | 718.3 ± 63.8 |
| Medial TH | 1585.0 ± 119.0 | 1081 ± 16.5 | 1558.0 ± 190.3 | 2318.0 ± 501.0 | 1076 ± 179.6 | 694.0 ± 95 |
| Dentate Gyrus | 552.3 ± 66.9 | 441.9 ± 96.0 | 272.3 ± 17.3 | 250.8 ± 15.6 | 200.8 ± 24.5 | 353.1 ± 64 |
| CA1 Dorsal | 257.0 ± 18.8 | 267.0 ± 9.8 | 205.6 ± 19.6 | 211.8 ± 19.3 | 154.2 ± 10.3 | 138.7 ± 6.1 |
| CA2 Dorsal | 546.9 ± 61 | 611.6 ± 123.0 | 256.3 ± 13.0 | 281.8 ± 27.5 | 391.3 ± 87.2 | 324.9 ± 56.5 |
| CA3 Dorsal | 1087.0 ± 53.1 | 1060.0 ± 93.0 | 500.4 ± 76.0 | 351.8 ± 29.0 | 361.2 ± 34.5 | 500.9 ± 104 |
| CA1 Ventral | 257.7 ± 21.9 | 335.1 ± 22.0 | 352.1 ± 46.2 | 523.0 ± 69.0 | 503.8 ± 83.8 | 708.4 ± 92 |
| CA2 Ventral | 264.3 ± 35.5 | 312.3 ± 21.0 | 381.3 ± 40.0 | 442.0 ± 53.9 | 385.0 ± 68.2 | 292.7 ± 49.6 |
| CA3 Ventral | 693.0 ± 72.0 | 954.1 ± 88.0 | 714.3 ± 102.0 | 511.0 ± 78.0 | 772.7 ± 35.2 | 742.9 ± 71 |
| SN Compacta | 276.1 ± 15.0 | ⬆ 536.7 ± 102.0 t = 4.026, df = 36, p = 0.0008 | 352.4 ± 31.4 | 265.8 ± 22.0 | 113.5 ± 4.4 | 158.7 ± 11 |
| SN Reticulata | 264.4 ± 20.8 | 269.1 ± 17.3 | 290.0 ± 22.0 | 288.8 ± 12.0 | 124.5 ± 6.5 | 158.6 ± 22 |
| PAG | 967.6 ± 60.0 | 999.7 ± 54.0 | 564.1 ± 69.2 | 594.0 ± 76.0 | 154.0 ± 9.5 | 107.0 ± 22 |
References
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Fritschy, J.M.; Panzanelli, P. GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur. J. Neurosci. 2014, 39, 1845–1865. [Google Scholar] [CrossRef] [PubMed]
- Terunuma, M. Diversity of structure and function of GABAB receptors: A complexity of GABAB-mediated signaling. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2018, 94, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Fritschy, J.M. Significance of GABA(A) receptor heterogeneity: Clues from developing neurons. Adv. Pharmacol. 2015, 73, 13–39. [Google Scholar]
- Wang, D.D.; Kriegstein, A.R. Defining the role of GABA in cortical development. J. Physiol. 2009, 1873–1879. [Google Scholar] [CrossRef]
- Rennie, J.M.; Boylan, G.B. Neonatal seizures and their treatment. Curr. Opin. Neurol. 2003, 16, 177–181. [Google Scholar] [CrossRef]
- Gai, N.; Grimm, V.E. The effect of prenatal exposure to diazepam on aspects of postnatal development and behavior in rats. Psychopharmacology (Berl) 1982, 78, 225–229. [Google Scholar] [CrossRef]
- Tucker, J.C. Benzodiazepines and the developing rat:a critical review. Neurosci. Biobehav. Rev. 1985, 9, 101–111. [Google Scholar] [CrossRef]
- Kellogg, C.K. Benzodiazepines: Influence on the developing brain. Prog. Brain Res. 1988, 73, 207–228. [Google Scholar]
- Ikonomidoou, C.; Turski, L. Antiepileptic drugs and brain development. Epilepsy Res. 2010, 88, 11–22. [Google Scholar] [CrossRef]
- Sundbakk, L.M.; Wood, M.; Gran, J.M.; Nordeng, H. Impact of prenatal exposure to benzodiazepines and z-hypnotics on behavioral problems at 5 years of age: A study from the Norwegian Mother and Child Cohort Study. PLoS ONE 2019, 14, e0217830. [Google Scholar] [CrossRef] [PubMed]
- Kubová, H.; Mareš, P. Time course of the anticonvulsant action of clonazepam in the developing rats. Arch. Int. Pharmacodyn. 1989, 298, 15–24. [Google Scholar] [PubMed]
- Kubová, H.; Mareš, P.; Vorlíček, J. Stable anticonvulsant action of benzodiazepines during development in rats. J. Pharm. Pharmacol. 1993, 45, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Mikulecká, A.; Mareš, P.; Kubová, H. Rebound increase in seizure susceptibility but not isolation-induced calls after single administration of clonazepam and Ro 19-8022 in infant rats. Epilepsy Behav. 2011, 20, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Mikulecká, A.; Šubrt, M.; Stuchlík, A.; Kubová, H. Consequences of early postnatal benzodiazepines exposure in rats. I. Cognitive-like behavior. Front. Behav. Neurosci. 2014, 8, 101, eCollection 2014. [Google Scholar] [CrossRef]
- Mikulecká, A.; Šubrt, M.; Pařízková, M.; Mareš, P.; Kubová, H. Consequences of early postnatal benzodiazepines exposure in rats. II. Social behavior. Front. Behav. Neurosci. 2014, 8, 169, eCollection 2014. [Google Scholar] [CrossRef]
- File, S.E. Behavioral changes persisting in to adulthood after neonatal benzodiazepine administration in the rat. Neurobehav. Toxicol. Teratol. 1986, 8, 453–461. [Google Scholar]
- File, S.E. Effects of neonatal administration of diazepam and lorazepam on performance of adolescent rats in tests of anxiety, aggression, learning and convulsions. Neurobehav. Toxicol. Teratol. 1986, 8, 301–306. [Google Scholar]
- File, S.E. The effects of neonatal administration of clonazepam on passive avoidance and on social, aggressive and exploratory behavior of adolescent male rats. Neurobehav. Toxicol. Teratol. 1986, 8, 447–452. [Google Scholar]
- File, S.E. Diazepam and caffeine administration during the first week of life: Changes in neonatal and adolescent behavior. Neurotoxicol. Teratol. 1987, 9, 9–16. [Google Scholar] [CrossRef]
- Kubová, H.; Mareš, P. Partial agonist of benzodiazepine receptors Ro 19-8022 elicits withdrawal symptoms after short-term administration in immature rats. Physiol. Res. 2012, 61, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Kubová, H.; Bendová, Z.; Moravcová, S.; Pačesová, D.; Rocha, L.; Mareš, P. Neonatal clonazepam administration induced long-lasting changes in glutamate receptors. Front. Mol. Neurosci. 2018, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Whissell, P.D.; Rosenzweig, S.; Lecker, I.; Wang, D.S.; Wojtowicz, J.M.; Orser, B.A. γ-aminobutyric acid type A receptors that contain the δ subunit promote memory and neurogenesis in the dentate gyrus. Ann. Neurol. 2013, 74, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Whissell, P.D.; Lecker, I.; Wang, D.S.; Yu, J.; Orser, B.A. Altered expression of δGABAA receptors in health and disease. Neuropharmacology 2015, 88, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Laurie, D.J.; Wisden, W.; Seeburg, P.H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992, 12, 4151–4172. [Google Scholar] [CrossRef]
- Martini, C.; Rigacci, T.; Lucacchini, A. [3H]muscimol binding site on purified benzodiazepine receptor. J. Neurochem. 1983, 41, 1183–1185. [Google Scholar] [CrossRef]
- Benkherouf, A.Y.; Taina, K.R.; Meera, P.; Aalto, A.J.; Li, X.G.; Soini, S.L.; Wallner, M.; Uusi-Oukari, M.J. Extrasynaptic δ-GABAA receptors are high-affinity muscimol receptors. J. Neurochem. 2019, 149, 41–53. [Google Scholar] [CrossRef]
- Brett, R.R.; Pratt, J.A. Changes in benzodiazepine-GABA receptor coupling in an accumbens-habenula circuit after chronic diazepam treatment. Br. J. Pharmacol. 1995, 116, 2375–2384. [Google Scholar] [CrossRef]
- Andersen, S.L.; Navalta, C.P. Altering the course of neurodevelopment: A framework for understanding the enduring effects of psychotropic drugs. Int. J. Dev. Neurosci. 2004, 22, 423–440. [Google Scholar] [CrossRef]
- Dobbing, J.; Smart, J.L. Vulnerability of developing brain and behaviour. Br. Med. Bull. 1974, 30, 164–168. [Google Scholar] [CrossRef]
- Lohmann, C.; Kessels, H.W. The developmental stages of synaptic plasticity. J. Physiol. 2014, 592, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Forcelli, P.A.; Janssen, M.J.; Vicini, S.; Gale, K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann. Neurol. 2012, 72, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Oukari, M.; Korpi, E.R. Regulation of GABA(A) receptor subunit expression by pharmacological agents. Pharmacol. Rev. 2010, 62, 97–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rosenberg, H.C.; Chiu, T.H.; Zhao, T.J. Subunit- and brain region-specific reduction of GABAA receptor subunit mRNAs during chronic treatment of rats with diazepam. J. Mol. Neurosci. 1994, 5, 105–120. [Google Scholar] [CrossRef]
- Holt, R.A.; Bateson, A.N.; Martin, I.L. Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology 1996, 35, 1457–1463. [Google Scholar] [CrossRef]
- Tietz, E.I.; Huang, X.; Chen, S.; Ferencak, W.F., 3rd. Temporal and regional regulation of alpha1, beta2 and beta3, but not alpha2, alpha4, alpha5, alpha6, beta1 or gamma2 GABA(A) receptor subunit messenger RNAs following one-week oral flurazepam administration. Neuroscience 1999, 91, 327–341. [Google Scholar] [CrossRef]
- Tietz, E.I.; Huang, X.; Weng, X.; Rosenberg, H.C.; Chiu, T.H. Expression of alpha 1, alpha 5, and gamma 2 GABAA receptor subunit mRNAs measured in situ in rat hippocampus and cortex following chronic flurazepam administration. J. Mol. Neurosci. 1993, 4, 277–292. [Google Scholar] [CrossRef]
- Chen, S.; Huang, X.; Zeng, X.J.; Sieghart, W.; Tietz, E.I. Benzodiazepine-mediated regulation of alpha1, alpha2, beta1-3 and gamma2 GABA(A) receptor subunit proteins in the rat brain hippocampus and cortex. Neuroscience 1999, 93, 33–44. [Google Scholar] [CrossRef]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Sato, T.N.; Neale, J.H. Type I and type II gamma-aminobutyric acid/benzodiazepine receptors: Purification and analysis of novel receptor complex from neonatal cortex. J. Neurochem. 1989, 52, 1114–1122. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sato, M.; Tohyama, M. Different postnatal development profiles of neurons containing distinct GABAA receptor beta subunit mRNAs (beta 1, beta 2, and beta 3) in the rat forebrain. J. Comp. Neurol. 1991, 308, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Sato, M.; Tohyama, M. Different postnatal ontogenic profiles of neurons containing beta (beta 1, beta 2 and beta 3) subunit mRNAs of GABAA receptor in the rat thalamus. Brain Res. Dev. Brain Res. 1991, 58, 289–292. [Google Scholar] [CrossRef]
- Zhang, J.H.; Sato, M.; Araki, T.; Tohyama, M. Postnatal ontogenesis of neurons containing GABAA alpha 1 subunit mRNA in the rat forebrain. Brain Res. Mol. Brain Res. 1992, 16, 193–203. [Google Scholar] [CrossRef]
- Hornung, J.P.; Fritschy, J.M. Developmental profile of GABAA-receptors in the marmoset monkey: Expression of distinct subtypes in pre- and postnatal brain. J. Comp. Neurol. 1996, 367, 413–430. [Google Scholar] [CrossRef]
- Stell, B.M.; Brickley, S.G.; Tang, C.Y.; Farrant, M.; Mody, I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 14439–14444. [Google Scholar] [CrossRef]
- Glykys, J.; Peng, Z.; Chandra, D.; Homanics, G.E.; Houser, C.R.; Mody, I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat. Neurosci. 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Holter, N.I.; Zylla, M.M.; Zuber, N.; Bruehl, C.; Draguhn, A. Tonic GABAergic control of mouse dentate granule cells during postnatal development. Eur. J. Neurosci. 2010, 32, 1300–1309. [Google Scholar] [CrossRef]
- Korpi, E.R.; Mihalek, R.M.; Sinkkonen, S.T.; Hauer, B.; Hevers, W.; Homanics, G.E.; Sieghart, W.; Lüddens, H. Altered receptor subtypes in the forebrain of GABA(A) receptor delta subunit-deficient mice: Recruitment of gamma 2 subunits. Neuroscience 2002, 109, 733–743. [Google Scholar] [CrossRef]
- Allison, C.; Pratt, J.A. Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacol. Ther. 2003, 98, 171–195. [Google Scholar] [CrossRef]
- Tietz, E.I.; Rosenberg, H.C.; Chiu, T.H. Autoradiographic localization of benzodiazepine receptor downregulation. J. Pharmacol. Exp. Ther. 1986, 236, 284–292. [Google Scholar]
- Percic, D.; Svob Strac, D.; Jazvincak Jemberek, M.; Vlainic, J. Allosteric uncoupling and up-regulation of benzodiazepine and GABA recognition sites following chronic diazepam treatment of HEK 293 cells stably transfected with α1β2γ2S subunits of GABAA receptors. Naunyn. Schmiedebergs Arch. Pharmacol. 2007, 375, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Fyhn, M.; Molden, S.; Witter, M.P.; Moser, E.I.; Moser, M.B. Spatial representation in the entorhinal cortex. Science 2004, 305, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- White, N.M. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behav. Brain Res. 2009, 199, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J. The basic emotional circuits of mammalian brains: Do animals have affective lives? Neurosci. Biobehav. Rev. 2011, 35, 1791–1804. [Google Scholar] [CrossRef]
- Benarroch, E.E. Periaqueductal gray: An interface for behavioral control. Neurology 2012, 78, 210–217. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Schroeder, H.; Humbert, A.C.; Desor, D.; Nehlig, A. Long-term consequences of neonatal exposure to diazepam on cerebral glucose utilization, learning, memory and anxiety. Brain Res. 1997, 766, 142–152. [Google Scholar] [CrossRef]
- Chalifoux, J.R.; Carter, A.G. GABAB receptor modulation of synaptic function. Curr. Opin. Neurobiol. 2011, 21, 339–344. [Google Scholar] [CrossRef]
- Gaiarsa, J.L.; Porcher, C. Emerging neurotrophic role of GABAB receptors in neuronal circuit development. Front. Cell Neurosci. 2013, 7, 206. [Google Scholar] [CrossRef]
- Prosser, H.M.; Gill, C.H.; Hirst, W.D.; Grau, E.; Robbins, M.; Calver, A.; Soffin, E.M.; Farmer, C.E.; Lanneau, C.; Gray, J.; et al. Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol. Cell Neurosci. 2001, 17, 1059–1070. [Google Scholar] [CrossRef]
- Haller, C.; Casanova, E.; Müller, M.; Vacher, C.M.; Vigot, R.; Doll, T.; Barbieri, S.; Gassmann, M.; Bettler, B. Floxed allele for conditional inactivation of the GABAB(1) gene. Genesis 2004, 40, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Bony, G.; Szczurkowska, J.; Tamagno, I.; Shelly, M.; Contestabile, A.; Cancedda, L. Non-hyperpolarizing GABAB receptor activation regulates neuronal migration and neurite growth and specification by cAMP/LKB1. Nat. Commun. 2013, 4, 1800. [Google Scholar] [CrossRef] [PubMed]
- Heaney, C.F.; Kinney, J.W. Role of GABA(B) receptors in learning and memory and neurological disorders. Neurosci. Biobehav. Rev. 2016, 63, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef]
- Benarroch, E.E. GABAB receptors: Structure, functions, and clinical implications. Neurology 2012, 78, 578–584. [Google Scholar] [CrossRef]
- Bittigau, P.; Sifringer, M.; Genz, K.; Reith, E.; Pospischil, D.; Govindarajalu, S.; Dzietko, M.; Pesditschek, S.; Mai, I.; Dikranian, K.; et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl. Acad. Sci. USA 2002, 99, 15089–15094. [Google Scholar] [CrossRef]
- Stefovska, V.G.; Uckeremann, O.; Czuczwar, M.; Smitka, M.; Czuczwar, P.; Kis, J.; Kaindl, A.M.; Turski, L.; Turski, W.A.; Ikonomidou, C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann. Neurol. 2008, 64, 434–445. [Google Scholar] [CrossRef]
- Conklin, P.; Heggeness, F.W. Maturation of temperature homeostasis in the rat. Am. J. Physiol. 1971, 220, 333–336. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.; Alonso-Vanegas, M.; Martínez-Juárez, I.E.; Orozco-Suárez, S.; Escalante-Santiago, D.; Feria-Romero, I.A.; Zavala-Tecuapetla, C.; Cisneros-Franco, J.M.; Buentello-García, R.M.; Cienfuegos, J. GABAergic alterations in neocortex of patients with pharmacoresistant temporal lobe epilepsy can explain the comorbidity of anxiety and depression: The potential impact of clinical factors. Front. Cell Neurosci. 2015, 8, 442. [Google Scholar] [CrossRef]
| Ref. No | Gene Symbol | Gene Name | Gene Aliases |
|---|---|---|---|
| Rn00690933_m1 | Ppia | peptidylprolyl isomerase A, cyclophilin A | CYCA, CyP-A |
| Rn00788315_m1 | Gabra1 | gamma-aminobutyric acid (GABA) A receptor, alpha 1 | - |
| Rn01413643_m1 | Gabra2 | gamma-aminobutyric acid (GABA) A receptor, alpha 2 | - |
| Rn00589846_m1 | Gabra4 | gamma-aminobutyric acid (GABA) A receptor, alpha 4 | - |
| Rn01464079_m1 | Gabrg2 | gamma-aminobutyric acid (GABA) A receptor, gamma 2 | - |
| Rn00568740_m1 | Gabrd | gamma-aminobutyric acid (GABA) A receptor, delta | GABAA-RD |
| Rn00561986_m1 | Syp | synaptophysin | Syp1 |
| Rn00582550_m1 | Gabbr2 | gamma-aminobutyric acid (GABA) B receptor 2 | Gpr51 |
| Binding | Ligand (nM) and S.A. | Buffer pH 7.4 | Incubation | Exposition (RT) | Non-Labeled Ligand |
|---|---|---|---|---|---|
| GABAA | [3H] Muscimol (20 nM); 20 Ci/mmol | Tris citrate (50 mM) | 45 min at 4 °C | 8 weeks | GABA (10 µM) |
| GABAB | [3H] CGP54626 (4 nM); 30 Ci/mmol | Tris HCl (50 mM) and CaCl2 (10 mM) | 90 min at 22 °C | 12 weeks | CGP 55845 (100 µM) |
| BDZ | [3H] Flunirazepam (2 nM); 85.2 Ci/mmol | Tris HCl (170 mM) | 45 min at 4 °C | 3 weeks | Clonazepam (1µM) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubová, H.; Bendová, Z.; Moravcová, S.; Pačesová, D.; Rocha, L.; Mareš, P. Neonatal Clonazepam Administration Induced Long-Lasting Changes in GABAA and GABAB Receptors. Int. J. Mol. Sci. 2020, 21, 3184. https://doi.org/10.3390/ijms21093184
Kubová H, Bendová Z, Moravcová S, Pačesová D, Rocha L, Mareš P. Neonatal Clonazepam Administration Induced Long-Lasting Changes in GABAA and GABAB Receptors. International Journal of Molecular Sciences. 2020; 21(9):3184. https://doi.org/10.3390/ijms21093184
Chicago/Turabian StyleKubová, Hana, Zdeňka Bendová, Simona Moravcová, Dominika Pačesová, Luisa Rocha, and Pavel Mareš. 2020. "Neonatal Clonazepam Administration Induced Long-Lasting Changes in GABAA and GABAB Receptors" International Journal of Molecular Sciences 21, no. 9: 3184. https://doi.org/10.3390/ijms21093184
APA StyleKubová, H., Bendová, Z., Moravcová, S., Pačesová, D., Rocha, L., & Mareš, P. (2020). Neonatal Clonazepam Administration Induced Long-Lasting Changes in GABAA and GABAB Receptors. International Journal of Molecular Sciences, 21(9), 3184. https://doi.org/10.3390/ijms21093184







