Induction of CEMIP in Chondrocytes by Inflammatory Cytokines: Underlying Mechanisms and Potential Involvement in Osteoarthritis
Abstract
1. Introduction
2. Results
2.1. Immunolocalization of CEMIP in OA Articular Cartilage
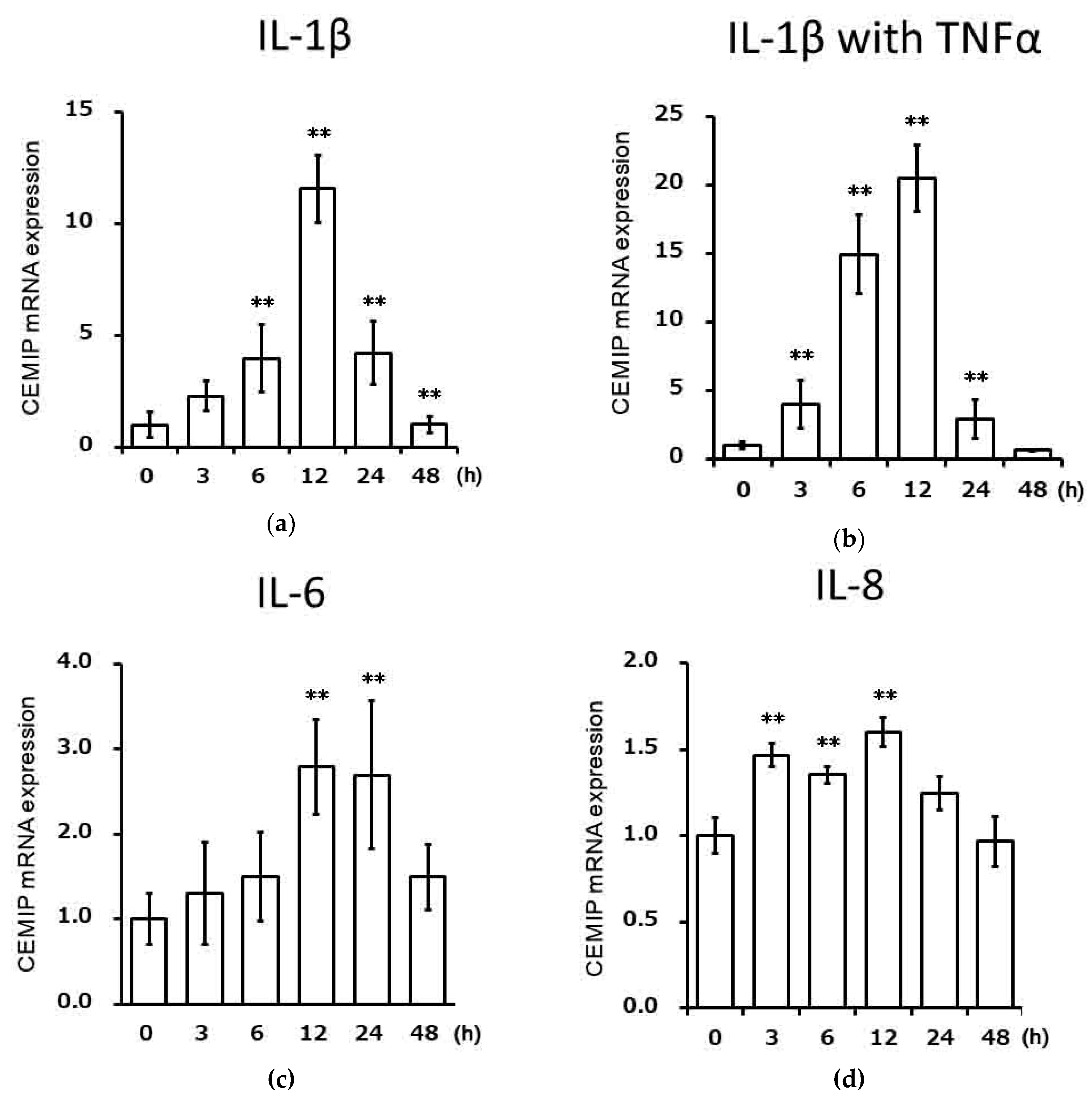
2.2. Effects of Inflammatory Cytokines on CEMIP mRNA Expression in Chondrocytic Cells
2.3. IL-1β Induces CEMIP Protein Expression
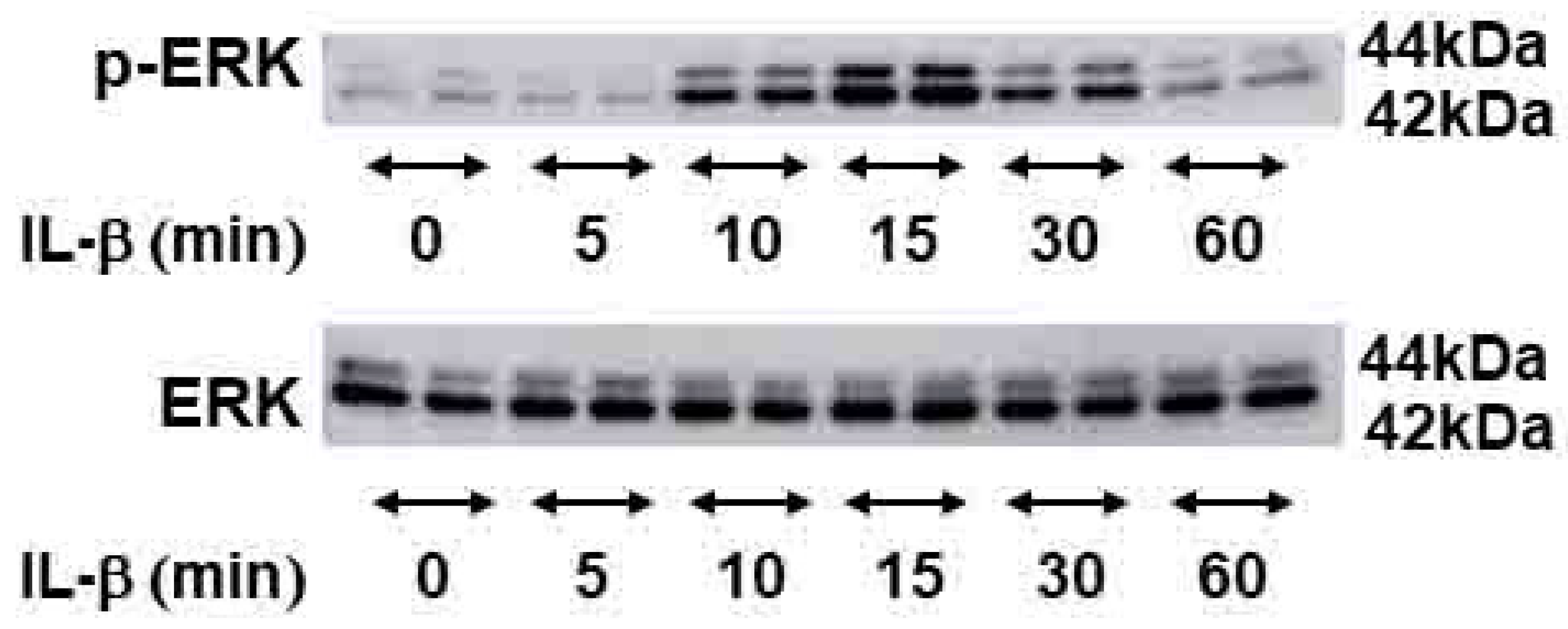
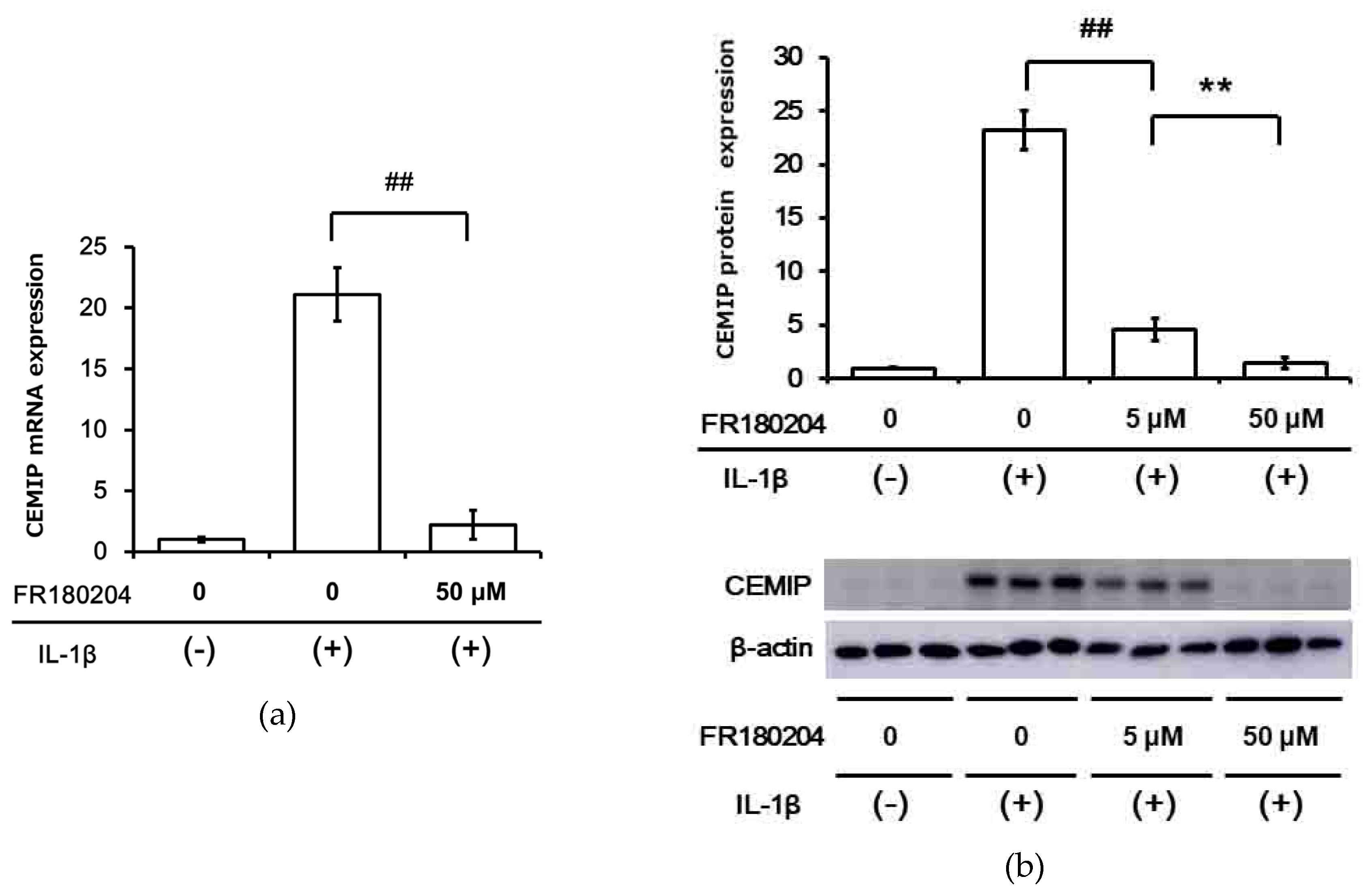
2.4. Signal Transduction Pathway Involved in CEMIP Induction
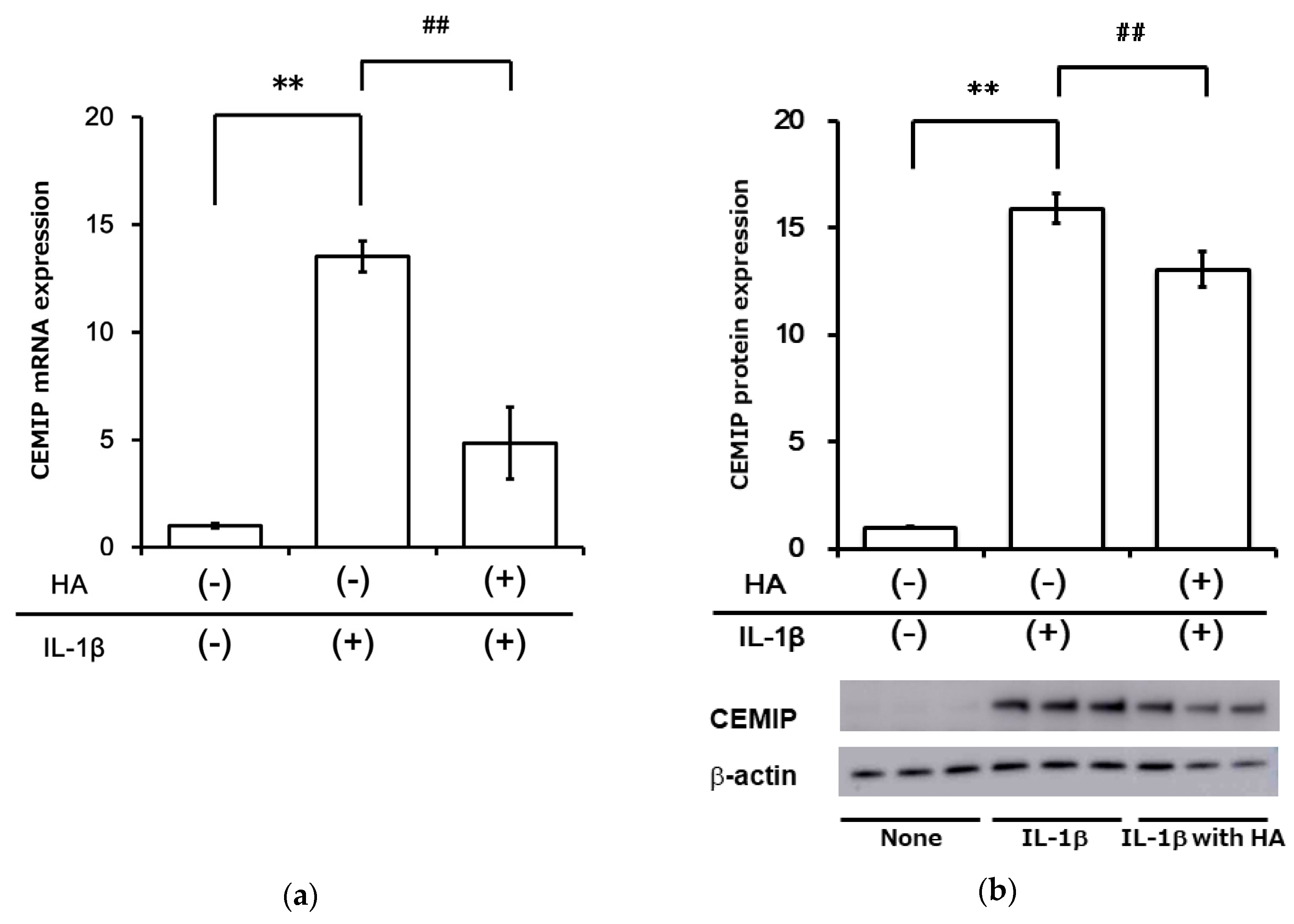
2.5. HA Inhibits Inflammatory Cytokine-Induced CEMIP at mRNA and Protein Levels
2.6. Mechanical Strain (Cycle Tensile Strain) Attenuated Inflammatory Cytokine-Induced Expression of CEMIP
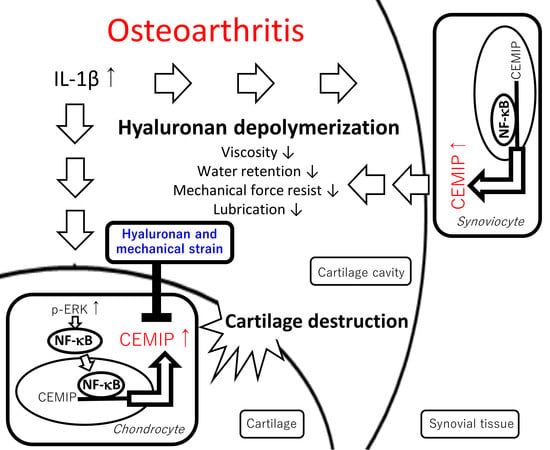
3. Discussion
4. Materials and Methods
4.1. OA Cartilage Tissue Samples
4.2. Reagents
4.3. Immunohistochemistry
4.4. Cell Culture and Treatments
4.5. RNA Extraction and Real-Time Quantitative Reverse Transcription PCR (qRT)-PCR
4.6. Protein Extraction, SDS-PAGE, and Western Blotting
4.7. Evaluation of NF-κB Translocation
4.8. Mechanical Strain
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| BAPTA-AM | (1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetra acetic acid tetrakis (acetoxymethyl ester) |
| ECM | extracellular matrix |
| HA | hyaluronan |
| MMP | matrix metalloproteinase |
| NF-κB | nuclear factor κB |
| OA | osteoarthritis |
References
- Kiani, C.; Chen, L.; Wu, Y.J.; Yee, A.J.; Yang, B.B. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, T.E.; Muir, H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem. J. 1974, 139, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Uebelhart, D.; Williams, J.M. Effects of hyaluronic acid on cartilage degradation. Curr. Opin. Rheumatol. 1999, 11, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Balazs, E.A.; Watson, D.; Duff, I.F.; Roseman, S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum. 1967, 10, 357–376. [Google Scholar] [CrossRef]
- Cowman, M.K.; Matsuoka, S. Experimental approaches to hyaluronan structure. Carbohydr. Res. 2005, 340, 791–809. [Google Scholar] [CrossRef]
- Průšová, A.; Vergeldt, F.J.; Kučerík, J. Influence of water content and drying on the physical structure of native hyaluronan. Carbohydr. Polym. 2013, 95, 515–521. [Google Scholar] [CrossRef]
- Gigante, A.; Callegari, L. The role of intra-articular hyaluronan (Sinovial®) in the treatment of osteoarthritis. Rheumatol. Int. 2011, 31, 427–444. [Google Scholar] [CrossRef]
- Vuorio, E.; Einola, S.; Hakkaramen, S.; Penttinen, R. Synthesis of underpolymerized hyaluronic acid by fibroblasts cultured from rheumatoid and non-rheumatoid synovitis. Rheumatol. Int. 1982, 2, 97–102. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Asano, K.; Inagaki, J.; Shinaoka, A.; Kumagishi-Shinaoka, K.; Cilek, M.Z.; Hatipoglu, O.F.; Oohashi, T.; Nishida, K.; Komatsubara, I.; et al. High molecular weight hyaluronan protects cartilage from degradation by inhibiting aggrecanase expression. J. Orthop. Res. 2018, 36, 3247–3255. [Google Scholar] [CrossRef]
- Yoshida, M.; Sai, S.; Marumo, K.; Tanaka, T.; Itano, N.; Kimata, K.; Fujii, K. Expression analysis of three isoforms of hyaluronan synthase and hyaluronidase in the synovium of knees in osteoarthritis and rheumatoid arthritis by quantitative real-time reverse transcriptase polymerase chain reaction. Arthritis Res. Ther. 2004, 6, R514–R520. [Google Scholar] [CrossRef] [PubMed]
- Csóka, A.B.; Scherer, S.W.; Stern, R. Expression Analysis of Six Paralogous Human Hyaluronidase Genes Clustered on Chromosomes 3p21 and 7q31. Genomics 1999, 60, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, J.; De Vega, S.; Cilek, M.Z.; Yoshinaga, C.; Nakamura, T.; Kasamatsu, S.; Yoshida, H.; Kaneko, H.; Ishijima, M.; Kaneko, K.; et al. Implication of cell migration inducing hyaluronidase 1 (CEMIP) in hyaluronan degradation by synovial fibroblasts in patients with knee osteoarthritis. Am. J. Pathol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Yoshida, H.; Mizuno, S.; Hirozane, T.; Horiuchi, K.; Yoshino, Y.; Hara, H.; Kanai, Y.; Inoue, S.; Ishijima, M.; et al. Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization Controls Endochondral Ossification through Hyaluronan Metabolism. Am. J. Pathol. 2017, 187, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Nagaoka, A.; Kusaka-Kikushima, A.; Tobiishi, M.; Kawabata, K.; Sayo, T.; Sakai, S.; Sugiyama, Y.; Enomoto, H.; Okada, Y.; et al. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc. Natl. Acad. Sci. USA 2013, 110, 5612–5617. [Google Scholar] [CrossRef]
- Jia, S.; Qu, T.; Wang, X.; Feng, M.; Yang, Y.; Feng, X.; Ma, R.; Li, W.; Hu, Y.; Feng, Y.; et al. KIAA1199 promotes migration and invasion by Wnt/β-catenin pathway and MMPs mediated EMT progression and serves as a poor prognosis marker in gastric cancer. PLoS ONE 2017, 12, e0175058. [Google Scholar] [CrossRef]
- Yoshida, H.; Okada, Y. Role of HYBID (Hyaluronan Binding Protein Involved in Hyaluronan Depolymerization), Alias KIAA1199/CEMIP, in Hyaluronan Degradation in Normal and Photoaged Skin. Int. J. Mol. Sci. 2019, 20, 5804. [Google Scholar] [CrossRef]
- Nishida, Y.; D’Souza, A.L.; Thonar, E.J.-M.A.; Knudson, W. Stimulation of hyaluronan metabolism by interleukin-1α in human articular cartilage. Arthritis Rheum. 2000, 43, 1315–1326. [Google Scholar] [CrossRef]
- Chan, D.D.; Xiao, W.F.; Li, J.; de la Motte, C.A.; Sandy, J.D.; Plaas, A. Deficiency of hyaluronan synthase 1 (Has1) results in chronic joint inflammation and widespread intra-articular fibrosis in a murine model of knee joint cartilage damage. Osteoarthr. Cartil. 2015, 23, 1879–1889. [Google Scholar] [CrossRef]
- Shimizu, H.; Shimoda, M.; Mochizuki, S.; Miyamae, Y.; Abe, H.; Chijiiwa, M.; Yoshida, H.; Shiozawa, J.; Ishijima, M.; Kaneko, K.; et al. Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization Is Up-Regulated and Involved in Hyaluronan Degradation in Human Osteoarthritic Cartilage. Am. J. Pathol. 2018, 188, 2109–2119. [Google Scholar] [CrossRef]
- Stylianou, E.; O’Neill, L.A.J.; Rawlinson, L.; Edbrooke, M.R.; Woo, P.; Saklatvala, J. Interleukin 1 induces NF-κB through its type I but not its type II receptor in lymphocytes. J. Biol. Chem. 1992, 267, 15836–15841. [Google Scholar] [PubMed]
- Santoro, A.; Conde, J.; Scotece, M.; Abella, V.; Lois, A.; Lopez, V.; Pino, J.; Gomez, R.; Gomez-Reino, J.J.; Gualillo, O. SERPINE2 Inhibits IL-1α-Induced MMP-13 Expression in Human Chondrocytes: Involvement of ERK/NF-κB/AP-1 Pathways. PLoS ONE 2015, 10, e0135979. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Lin, C.Y.; Tsai, C.H.; Huang, Y.L.; Tang, C.H. Glucose suppresses IL-1β-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environ. Toxicol. 2018, 33, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Hirohata, S.; Miyoshi, T.; Takahashi, K.; Ogawa, H.; Shinohata, R.; Demircan, K.; Kusachi, S.; Yamamoto, K.; Ninomiya, Y. Hyaluronan receptors involved in cytokine induction in monocytes. Glycobiology 2009, 19, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, T.; Mochizuki, S.; Takizawa, M.; Chijiiwa, M.; Okada, A.; Kimura, T.; Fujita, Y.; Matsumoto, H.; Toyama, Y.; Okada, Y. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann. Rheum. Dis. 2009, 68, 1051–1058. [Google Scholar] [CrossRef]
- Suzuki, M.; Takahashi, N.; Sobue, Y.; Ohashi, Y.; Kishimoto, K.; Hattori, K.; Ishiguro, N.; Kojima, T. Hyaluronan suppresses enhanced cathepsin K expression via activation of NF-κB with mechanical stress loading in a human chondrocytic HCS-2/8 cells. Sci. Rep. 2020, 10, 216. [Google Scholar] [CrossRef]
- Shostak, K.; Zhang, X.; Hubert, P.; Göktuna, S.I.; Jiang, Z.; Klevernic, I.; Hildebrand, J.; Roncarati, P.; Hennuy, B.; Ladang, A.; et al. NF-κB-induced KIAA1199 promotes survival through EGFR signalling. Nat. Commun. 2014, 5, 5232. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. Ther. 2001, 4, 157. [Google Scholar] [CrossRef]
- Ozawa, M.; Nishida, K.; Yoshida, A.; Saito, T.; Harada, R.; Machida, T.; Ozaki, T. Hyaluronan suppresses mechanical stress-induced expression of catabolic enzymes by human chondrocytes via inhibition of IL-1β production and subsequent NF-κB activation. Inflamm. Res. 2015, 64, 243–252. [Google Scholar] [CrossRef][Green Version]
- Tian, Y.; Yuan, W.; Fujita, N.; Wang, J.; Wang, H.; Shapiro, I.M.; Risbud, M.V. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-κB. Am. J. Pathol. 2013, 182, 2310–2321. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hirata, M.; Saito, T.; Itoh, S.; Chung, U.; Kawaguchi, H. Transcriptional induction of ADAMTS5 protein by nuclear factor-κB (NF-κB) family member RelA/p65 in chondrocytes during osteoarthritis development. J. Biol. Chem. 2013, 288, 28620–28629. [Google Scholar] [CrossRef] [PubMed]
- Soroosh, A.; Albeiroti, S.; West, G.A.; Willard, B.; Fiocchi, C.; de la Motte, C.A. Crohn’s Disease Fibroblasts Overproduce the Novel Protein KIAA1199 to Create Proinflammatory Hyaluronan Fragments. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 358–368.e4. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hayashi, S.; Miyajima, M.; Omori, M.; Wang, J.; Kaihara, K.; Morimatsu, M.; Wang, C.; Chen, J.; Iribe, G.; et al. L-type calcium channel modulates mechanosensitivity of the cardiomyocyte cell line H9c2. Cell Calcium 2019, 79, 68–74. [Google Scholar] [CrossRef]
- Tokuyama, E.; Nagai, Y.; Takahashi, K.; Kimata, Y.; Naruse, K. Mechanical stretch on human skin equivalents increases the epidermal thickness and develops the basement membrane. PLoS ONE 2015, 10, e0141989. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Shinaoka, A.; Kumagishi-Shinaoka, K.; Asano, K.; Hatipoglu, O.F.; Inagaki, J.; Takahashi, K.; Oohashi, T.; Nishida, K.; Naruse, K.; et al. Mechanical strain attenuates cytokine-induced ADAMTS9 expression via transient receptor potential vanilloid type 1. Exp. Cell Res. 2019, 383, 111556. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Ünal, Z.N.; Erdoğan, S.; Akyol, S.; Ramazan Yiğitoğlu, M.; Hirohata, S.; Işık, B.; Demircan, K. Augmentation of ADAMTS9 gene expression by IL-1β is reversed by NFκB and MAPK inhibitors, but not PI3 kinase inhibitors. Cell Biochem. Funct. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Altuntas, A.; Halacli, S.O.; Cakmak, O.; Erden, G.; Akyol, S.; Ugurcu, V.; Hirohata, S.; Demircan, K. Interleukin-1β induced nuclear factor-κB binds to a disintegrin-like and metalloproteinase with thrombospondin type 1 motif 9 promoter in human chondrosarcoma cells. Mol. Med. Rep. 2015, 12, 595–600. [Google Scholar] [CrossRef]
- Demircan, K.; Hirohata, S.; Nishida, K.; Hatipoglu, O.F.; Oohashi, T.; Yonezawa, T.; Apte, S.S.S.; Ninomiya, Y. ADAMTS-9 is synergistically induced by interleukin-1β and tumor necrosis factor α in OUMS-27 chondrosarcoma cells and in human chondrocytes. Arthritis Rheum. 2005, 52, 1451–1460. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tobisawa, Y.; Inubushi, T.; Irie, F.; Ohyama, C.; Yamaguchi, Y. A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J. Biol. Chem. 2017, 292, 7304–7313. [Google Scholar] [CrossRef]
- Komatsubara, I.; Murakami, T.; Kusachi, S.; Nakamura, K.; Hirohata, S.; Hayashi, J.; Takemoto, S.; Suezawa, C.; Ninomiya, Y.; Shiratori, Y. Spatially and temporally different expression of osteonectin and osteopontin in the infarct zone of experimentally induced myocardial infarction in rats. Cardiovasc. Pathol. 2003, 12, 186–194. [Google Scholar] [CrossRef]
- Yaykasli, K.O.; Oohashi, T.; Hirohata, S.; Hatipoglu, O.F.; Inagawa, K.; Demircan, K.; Ninomiya, Y. ADAMTS9 activation by interleukin 1 beta via NFATc1 in OUMS-27 chondrosarcoma cells and in human chondrocytes. Mol. Cell. Biochem. 2009, 323, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.N.; Nishida, K.; Doi, H.; Oohashi, T.; Hirohata, S.; Ozaki, T.; Yoshida, A.; Ninomiya, Y.; Inoue, H. Suppression of chondrosarcoma cells by 15-deoxy-Δ12,14- prostaglandin J2 is associated with altered expression of Bax/Bcl-xL and p21. Biochem. Biophys. Res. Commun. 2005, 328, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Toeda, K.; Nakamura, K.; Hirohata, S.; Hatipoglu, O.F.; Demircan, K.; Yamawaki, H.; Ogawa, H.; Kusachi, S.; Shiratori, Y.; Ninomiya, Y. Versican is induced in infiltrating monocytes in myocardial infarction. Mol. Cell. Biochem. 2005, 280, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Komata, T.; Kondo, Y.; Kanzawa, T.; Ito, H.; Hirohata, S.; Koga, S.; Sumiyoshi, H.; Takakura, M.; Inoue, M.; Barna, B.P.; et al. Caspase-8 gene therapy using the human telomerase reverse transcriptase promoter for malignant glioma cells. Hum. Gene Ther. 2002, 13, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Okano, H.; Miyagawa, W.; Visse, R.; Shitomi, Y.; Santamaria, S.; Dudhia, J.; Troeberg, L.; Strickland, D.K.; Hirohata, S.; et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016, 56, 57–73. [Google Scholar] [CrossRef]
- Koga, S.; Hirohata, S.; Kondo, Y.; Komata, T.; Takakura, M.; Inoue, M.; Kyo, S.; Kondo, S. FADD gene therapy using the human telomerase catalytic subunit (hTERT) gene promoter to restrict induction of apoptosis to tumors in vitro and in vivo. Anticancer Res. 2001, 21, 1937–1943. [Google Scholar]
- Nakamura, K.; Hirohata, S.; Murakami, T.; Miyoshi, T.; Demircan, K.; Oohashi, T.; Ogawa, H.; Koten, K.; Toeda, K.; Kusachi, S.; et al. Dynamic induction of ADAMTS1 gene in the early phase of acute myocardial infarction. J. Biochem. 2004, 136, 439–446. [Google Scholar] [CrossRef]
- Iwamoto, M.; Hirohata, S.; Ogawa, H.; Ohtsuki, T.; Shinohata, R.; Miyoshi, T.; Hatipoglu, F.O.; Kusachi, S.; Yamamoto, K.; Ninomiya, Y. Connective tissue growth factor induction in a pressure-overloaded heart ameliorated by the angiotensin II type 1 receptor blocker olmesartan. Hypertens. Res. 2010, 33, 1305–1311. [Google Scholar] [CrossRef]
- Sezaki, S.; Hirohata, S.; Iwabu, A.; Nakamura, K.; Toeda, K.; Miyoshi, T.; Yamawaki, H.; Demircan, K.; Kusachi, S.; Shiratori, Y.; et al. Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp. Biol. Med. (Maywood) 2005, 230, 621–630. [Google Scholar] [CrossRef]
- Hatipoglu, O.F.; Hirohata, S.; Cilek, M.Z.; Ogawa, H.; Miyoshi, T.; Obika, M.; Demircan, K.; Shinohata, R.; Kusachi, S.; Ninomiya, Y. ADAMTS1 Is a Unique Hypoxic Early Response Gene Expressed by Endothelial Cells. J. Biol. Chem. 2009, 284, 16325–16333. [Google Scholar] [CrossRef]
- Inagaki, J.; Takahashi, K.; Ogawa, H.; Asano, K.; Faruk Hatipoglu, O.; Cilek, M.Z.; Obika, M.; Ohtsuki, T.; Hofmann, M.; Kusachi, S.; et al. ADAMTS1 inhibits lymphangiogenesis by attenuating phosphorylation of the lymphatic endothelial cell-specific VEGF receptor. Exp. Cell Res. 2014, 323, 263–275. [Google Scholar] [CrossRef]
- Obika, M.; Ogawa, H.; Takahashi, K.; Li, J.; Hatipoglu, O.F.; Cilek, M.Z.; Miyoshi, T.; Inagaki, J.; Ohtsuki, T.; Kusachi, S.; et al. Tumor growth inhibitory effect of ADAMTS1 is accompanied by the inhibition of tumor angiogenesis. Cancer Sci. 2012, 103, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Cilek, M.Z.; Hirohata, S.; Faruk Hatipoglu, O.; Ogawa, H.; Miyoshi, T.; Inagaki, J.; Ohtsuki, T.; Harada, H.; Kamikawa, S.; Kusachi, S.; et al. AHR, a novel acute hypoxia-response sequence, drives reporter gene expression under hypoxia in vitro and in vivo. Cell Biol. Int. 2011, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Nelson, C.M.; Nandadasa, S.; Aramaki-Hattori, N.; Lindner, D.J.; Alban, T.; Inagaki, J.; Ohtsuki, T.; Oohashi, T.; Apte, S.S.; et al. Stromal Versican Regulates Tumor Growth by Promoting Angiogenesis. Sci. Rep. 2017, 7, 17225. [Google Scholar] [CrossRef] [PubMed]
- Tetsunaga, T.; Nishida, K.; Furumatsu, T.; Naruse, K.; Hirohata, S.; Yoshida, A.; Saito, T.; Ozaki, T. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthr. Cartil. 2011, 19, 222–232. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohtsuki, T.; Hatipoglu, O.F.; Asano, K.; Inagaki, J.; Nishida, K.; Hirohata, S. Induction of CEMIP in Chondrocytes by Inflammatory Cytokines: Underlying Mechanisms and Potential Involvement in Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3140. https://doi.org/10.3390/ijms21093140
Ohtsuki T, Hatipoglu OF, Asano K, Inagaki J, Nishida K, Hirohata S. Induction of CEMIP in Chondrocytes by Inflammatory Cytokines: Underlying Mechanisms and Potential Involvement in Osteoarthritis. International Journal of Molecular Sciences. 2020; 21(9):3140. https://doi.org/10.3390/ijms21093140
Chicago/Turabian StyleOhtsuki, Takashi, Omer F. Hatipoglu, Keiichi Asano, Junko Inagaki, Keiichiro Nishida, and Satoshi Hirohata. 2020. "Induction of CEMIP in Chondrocytes by Inflammatory Cytokines: Underlying Mechanisms and Potential Involvement in Osteoarthritis" International Journal of Molecular Sciences 21, no. 9: 3140. https://doi.org/10.3390/ijms21093140
APA StyleOhtsuki, T., Hatipoglu, O. F., Asano, K., Inagaki, J., Nishida, K., & Hirohata, S. (2020). Induction of CEMIP in Chondrocytes by Inflammatory Cytokines: Underlying Mechanisms and Potential Involvement in Osteoarthritis. International Journal of Molecular Sciences, 21(9), 3140. https://doi.org/10.3390/ijms21093140