MfPIF1 of Resurrection Plant Myrothamnus flabellifolia Plays a Positive Regulatory Role in Responding to Drought and Salinity Stresses in Arabidopsis
Abstract
1. Introduction
2. Results
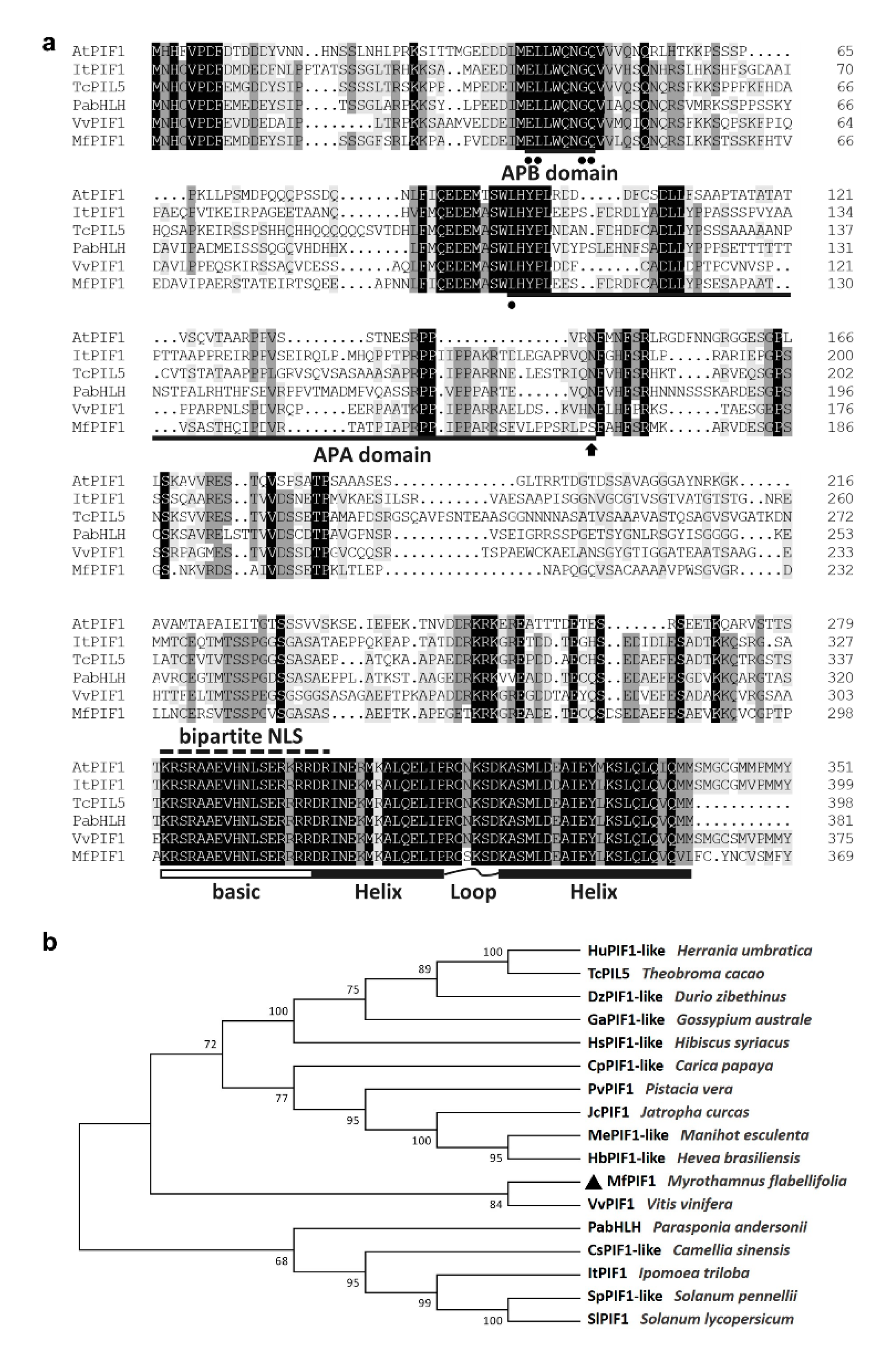
2.1. Isolation and Characterization of MfPIF1
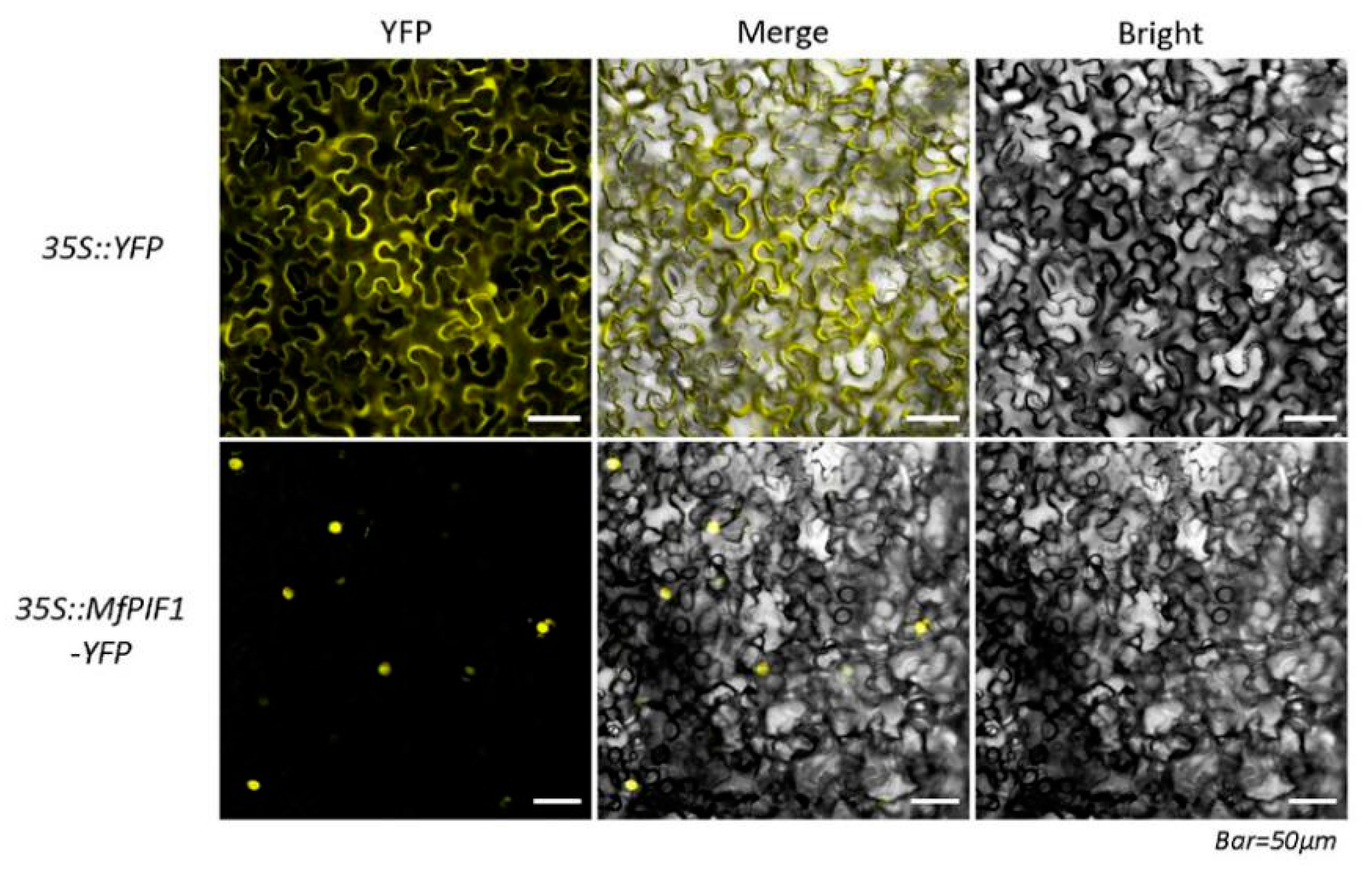
2.2. MfPIF1 is Localized in the Nucleus of Cells
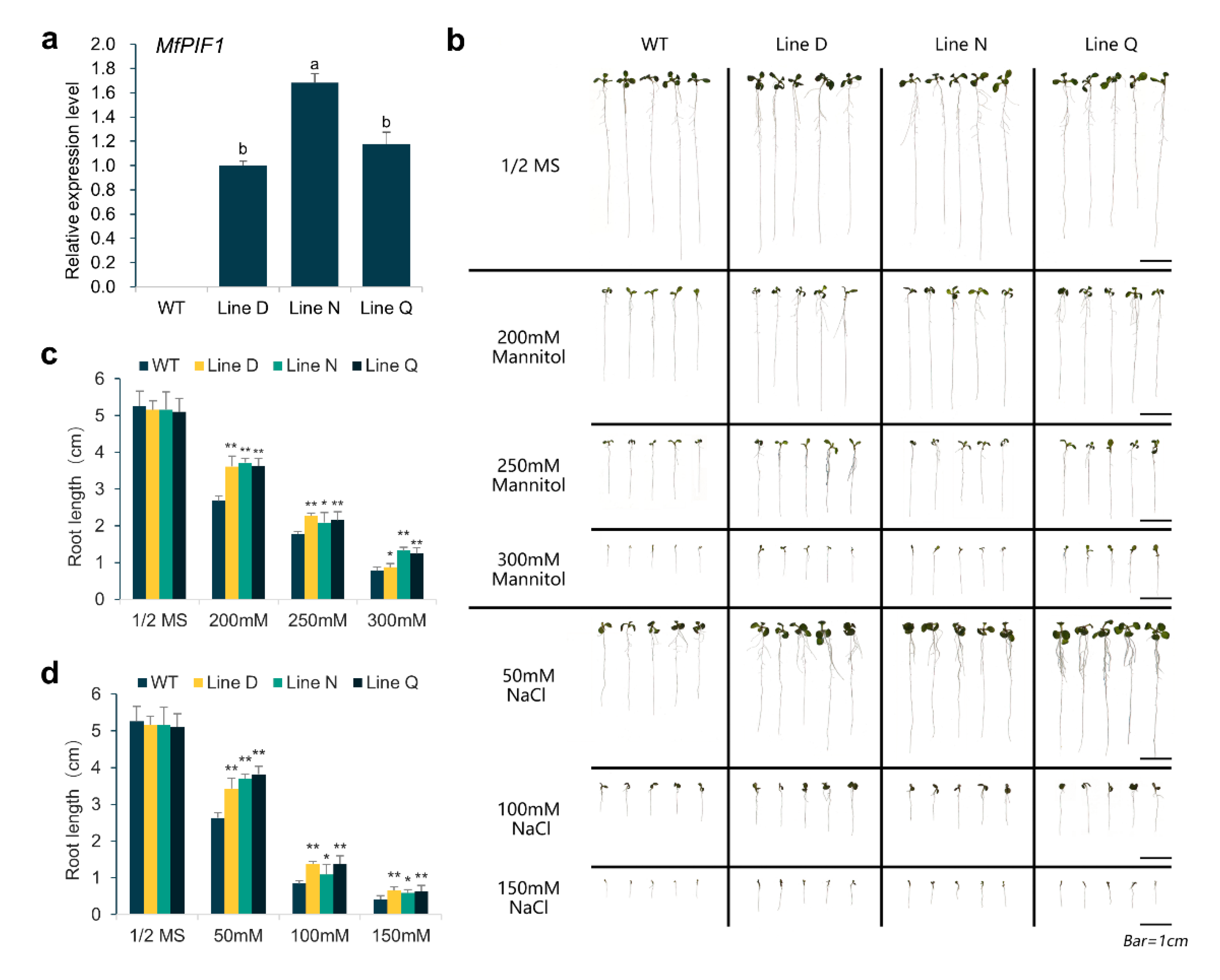
2.3. Overexpressing MfPIF1 Enhanced Tolerance to Drought and Salt
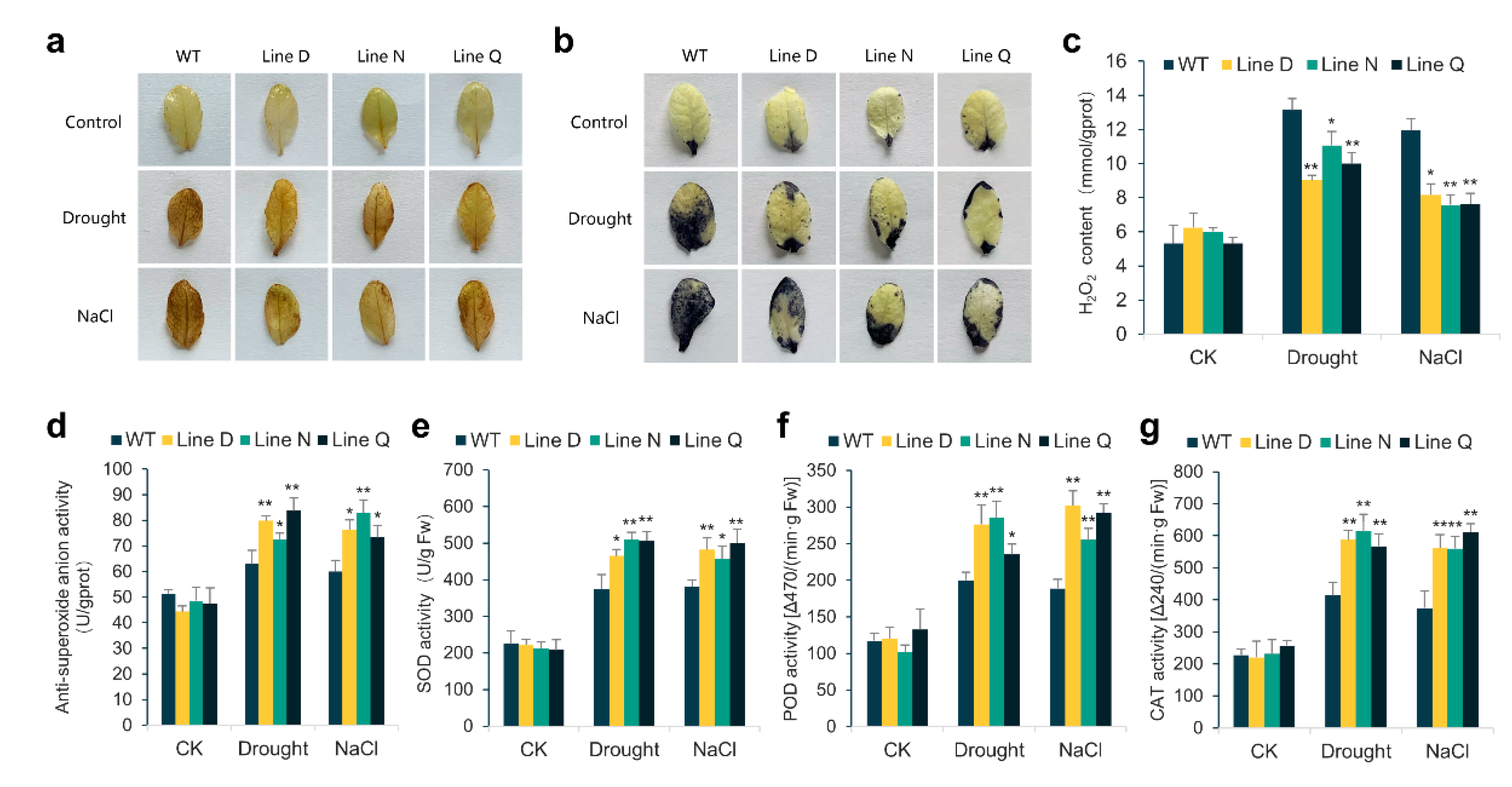
2.4. Effect of MfPIF1 Overexpression on Antioxidant Metabolism in Arabidopsis under Drought and Salinity Stresses
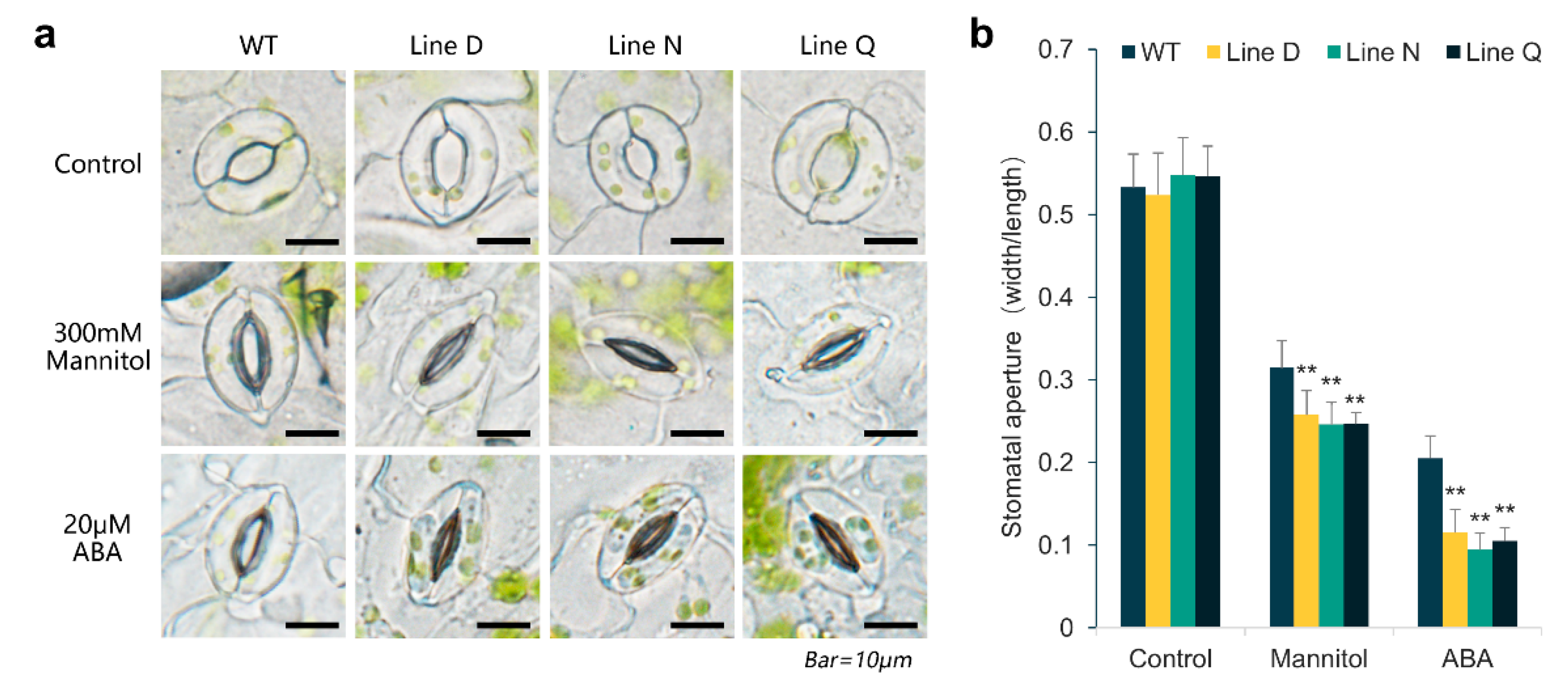
2.5. MfPIF1 Overexpression Promoted Stomatal Closure Induced by Drought and ABA
2.6. Overexpression of MfPIF1 Up-Regulates Expression Levels of ABA-Responsive Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Cloning and Bioinformatic Analysis of MfPIF1
4.3. Subcellular Localization of MfPIF1
4.4. Vector Construction and Generation of Transgenic Lines
4.5. Expression Analysis of MfPIF1 and ABA-Responsive Genes
4.6. Assays of Drought and Salinity Stress Tolerance
4.7. Estimation of Water Loss Rate
4.8. Physiological Measurements
4.9. Analysis of Stomatal Aperture Responsive to Drought and ABA Treatment
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PIFs | phytochrome-interacting factors |
| bHLH | basic helix-loop-helix |
| TFs | transcription factors |
| DT | desiccation tolerance |
| APB | active phytochrome B-binding |
| APA | active phytochrome A-binding |
| ORF | open reading frame |
| NLS | nuclear localization signal |
| WT | wild type |
| P5CS | Δ-1-pyrroline-5-carboxylate synthetase |
References
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, U.; Field, B. Molecular mechanisms controlling plant growth during abiotic stress. J. Exp. Bot. 2018, 69, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, I.H.; Nou, I.S.; Lee, K.D.; Rashotte, A.M.; Kang, K.K. BrRZFP1 a Brassica rapa C3HC4-type RING zinc finger protein involved in cold, salt and dehydration stress. Plant Biol. 2013, 15, 274–283. [Google Scholar] [CrossRef]
- Tran, L.-S.P.; Nakashima, K.; Sakuma, Y.; Osakabe, Y.; Qin, F.; Simpson, S.D.; Maruyama, K.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Co-expression of the stress-inducible zinc finger homeodomain ZFHD1 and NAC transcription factors enhances expression of the ERD1 gene in Arabidopsis. Plant J. 2007, 49, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Xianjun, P.; Xingyong, M.; Weihong, F.; Man, S.; Liqin, C.; Alam, I.; Lee, B.-H.; Dongmei, Q.; Shihua, S.; Gongshe, L. Improved drought and salt tolerance of Arabidopsis thaliana by transgenic expression of a novel DREB gene from Leymus chinensis. Plant Cell Rep. 2011, 30, 1493–1502. [Google Scholar] [CrossRef]
- Pires, N.; Dolan, L. Origin and diversification of basic-helix-loop-helix proteins in plants. Mol. Biol. Evol. 2010, 27, 862–874. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Quail, P.H. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 2003, 15, 1749–1770. [Google Scholar] [CrossRef]
- Leivar, P.; Monte, E.; Oka, Y.; Liu, T.; Carle, C.; Castillon, A.; Huq, E.; Quail, P.H. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 2008, 18, 1815–1823. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Mao, K.; Qiao, Y.; Jiang, H.; Li, Y.-Y.; Hao, Y.-J. Functional identification of MdPIF1 as a phytochrome interacting factor in apple. Plant Physiol. Biochem. 2017, 119, 178–188. [Google Scholar] [CrossRef]
- Atchley, W.R.; Terhalle, W.; Dress, A. Positional dependence, cliques, and predictive motifs in the bHLH protein domain. J. Mol. Evol. 1999, 48, 501–516. [Google Scholar] [CrossRef]
- Nesi, N.; Debeaujon, I.; Jond, C.; Pelletier, G.; Caboche, M.; Lepiniec, L. The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 2000, 12, 1863–1878. [Google Scholar] [CrossRef]
- Park, E.; Park, J.; Kim, J.; Nagatani, A.; Lagarias, J.C.; Choi, G. Phytochrome B inhibits binding of phytochrome-interacting factors to their target promoters. Plant J. 2012, 72, 537–546. [Google Scholar] [CrossRef]
- Al-Sady, B.; Ni, W.; Kircher, S.; Schäfer, E.; Quail, P.H. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 2006, 23, 439–446. [Google Scholar] [CrossRef]
- Shen, H.; Zhu, L.; Castillon, A.; Majee, M.; Downie, B.; Huq, E. Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 2008, 20, 1586–1602. [Google Scholar] [CrossRef] [PubMed]
- Spartz, A.K.; Gray, W.M. Plant hormone receptors: New perceptions. Genes Dev. 2008, 22, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. A new look at stress: Abscisic acid patterns and dynamics at high-resolution. New Phytol. 2016, 210, 38–44. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; Zhou, J.; Chen, F.; Wang, B.; Xie, X. Phytochrome B control of total leaf area and stomatal density affects drought tolerance in rice. Plant Mol. Biol. 2012, 78, 289–300. [Google Scholar] [CrossRef] [PubMed]
- González, C.V.; Ibarra, S.E.; Piccoli, P.N.; Botto, J.F.; Boccalandro, H.E. Phytochrome B increases drought tolerance by enhancing ABA sensitivity in Arabidopsis thaliana. Plant Cell Environ. 2012, 35, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lv, R.; Li, J.; Lin, H.; Xi, D. Phytochrome A and B negatively regulate salt stress tolerance of Nicotiana tobacum via ABA–jasmonic acid synergistic cross-talk. Plant Cell Physiol. 2018, 59, 2381–2393. [Google Scholar] [CrossRef] [PubMed]
- Casson, S.A.; Franklin, K.A.; Gray, J.E.; Grierson, C.S.; Whitelam, G.C.; Hetherington, A.M. phytochrome B and PIF4 regulate stomatal development in response to light quantity. Curr. Biol. 2009, 19, 229–234. [Google Scholar] [CrossRef]
- Kudo, M.; Kidokoro, S.; Yoshida, T.; Mizoi, J.; Todaka, D.; Fernie, A.R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Double overexpression of DREB and PIF transcription factors improves drought stress tolerance and cell elongation in transgenic plants. Plant Biotechnol. J. 2017, 15, 458–471. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.; Zhang, M.; Jiang, W.; Ren, X.; Liang, E.; Zhang, D.; Zhang, C.; Xiao, N.; Li, Y.; et al. A maize phytochrome-interacting factors protein ZmPIF1 enhances drought tolerance by inducing stomatal closure and improves grain yield in Oryza sativa. Plant Biotechnol. J. 2018, 16, 1375–1387. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, W.; Dai, Y.; Xiao, N.; Zhang, C.; Li, H.; Lu, Y.; Wu, M.; Tao, X.; Deng, D.; et al. A maize phytochrome-interacting factor 3 improves drought and salt stress tolerance in rice. Plant Mol. Biol. 2015, 87, 413–428. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.; Zhang, M.; Jiang, W.; Liang, E.; Zhang, D.; Zhang, C.; Xiao, N.; Chen, J. Roles of a maize phytochrome-interacting factors protein ZmPIF3 in regulation of drought stress responses by controlling stomatal closure in transgenic rice without yield penalty. Plant Mol. Biol. 2018, 97, 311–323. [Google Scholar] [CrossRef]
- Oh, E.; Kim, J.; Park, E.; Kim, J.-I.; Kang, C.; Choi, G. PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 2004, 16, 3045–3058. [Google Scholar] [CrossRef]
- Oh, E.; Yamaguchi, S.; Hu, J.; Yusuke, J.; Jung, B.; Paik, I.; Lee, H.-S.; Sun, T.; Kamiya, Y.; Choi, G. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 2007, 19, 1192–1208. [Google Scholar] [CrossRef]
- Kim, D.H.; Yamaguchi, S.; Lim, S.; Oh, E.; Park, J.; Hanada, A.; Kamiya, Y.; Choi, G. SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, Negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 2008, 20, 1260–1277. [Google Scholar] [CrossRef]
- Moon, J.; Zhu, L.; Shen, H.; Huq, E. PIF1 directly and indirectly regulates chlorophyll biosynthesis to optimize the greening process in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 9433–9438. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, J.; Kim, J.; Lee, N.; Kim, M.E.; Lee, S.; Chang Kim, S.; Choi, G. PIF1 regulates plastid development by repressing photosynthetic genes in the endodermis. Mol. Plant 2016, 9, 1415–1427. [Google Scholar] [CrossRef]
- Oh, E.; Kang, H.; Yamaguchi, S.; Park, J.; Lee, D.; Kamiya, Y.; Choi, G. Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell 2009, 21, 403–419. [Google Scholar] [CrossRef]
- Park, J.; Lee, N.; Kim, W.; Lim, S.; Choi, G. ABI3 and PIL5 collaboratively activate the expression of SOMNUS by directly binding to its promoter in imbibed Arabidopsis seeds. Plant Cell 2011, 23, 1404–1415. [Google Scholar] [CrossRef]
- Shi, H.; Zhong, S.; Mo, X.; Liu, N.; Nezames, C.D.; Deng, X.W. HFR1 sequesters PIF1 to govern the transcriptional network underlying light-initiated seed germination in Arabidopsis. Plant Cell 2013, 25, 3770–3784. [Google Scholar] [CrossRef]
- Lee, N.; Park, J.; Kim, K.; Choi, G. The transcriptional coregulator LEUNIG_HOMOLOG inhibits light-dependent seed germination in Arabidopsis. Plant Cell 2015, 27, 2301–2313. [Google Scholar] [CrossRef]
- Farrant, J.M. A comparison of mechanisms of desiccation tolerance among three angiosperm resurrection plant species. Plant Ecol. 2000, 151, 29–39. [Google Scholar] [CrossRef]
- Moore, J.P.; Lindsey, G.G.; Farrant, J.M.; Brandt, W.F. An overview of the biology of the desiccation-tolerant resurrection plant Myrothamnus flabellifolia. Ann. Bot. 2007, 99, 211–217. [Google Scholar] [CrossRef]
- Drennan, P.M.; Goldsworthy, D.; Buswell, A. Marginal and laminar hydathode-like structures in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius Welw. Flora Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 210–219. [Google Scholar] [CrossRef]
- Rascio, N.; La Rocca, N. Resurrection plants: The puzzle of surviving extreme vegetative desiccation. Crit. Rev. Plant Sci. 2005, 24, 209–225. [Google Scholar] [CrossRef]
- Challabathula, D.; Zhang, Q.; Bartels, D. Protection of photosynthesis in desiccation-tolerant resurrection plants. J. Plant Physiol. 2018, 227, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Farrant, J.M.; Willigen, C.V.; Loffell, D.A.; Bartsch, S.; Whittaker, A. An investigation into the role of light during desiccation of three angiosperm resurrection plants. Plant Cell Environ. 2003, 26, 1275–1286. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Macnish, A.J.; Estrada-Melo, A.C.; Lin, J.; Chang, Y.; Reid, M.S.; Jiang, C.-Z. Transcriptomic analysis reveals numerous diverse protein kinases and transcription factors involved in desiccation tolerance in the resurrection plant Myrothamnus flabellifolia. Hortic. Res. 2015, 2, 15034. [Google Scholar] [CrossRef]
- Leivar, P.; Quail, P.H. PIFs: Pivotal components in a cellular signaling hub. Trends Plant Sci. 2011, 16, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Manzi, M.; Zandalinas, S.I.; Vives-Peris, V.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Physiological, metabolic, and molecular responses of plants to abiotic stress. In Stress Signaling in Plants: Genomics and Proteomics Perspective; Springer: Cham, Switzerland, 2017; Volume 2, pp. 1–35. [Google Scholar] [CrossRef]
- Fenta, B.A.; Beebe, S.E.; Kunert, K.J.; Burridge, J.D.; Barlow, K.M.; Lynch, J.P.; Foyer, C.H. Field phenotyping of soybean roots for drought stress tolerance. Agronomy 2014, 4, 418–435. [Google Scholar] [CrossRef]
- Awad, W.; Byrne, P.; Reid, S.; Comas, L.; Haley, S. Great plains winter wheat varies for root length and diameter under drought stress. Agron. J. 2017, 110, 226–235. [Google Scholar] [CrossRef]
- Mahlagha, G.; Maryam, G.; Tannaz, A.; Bahareh, A.M. Investigation of proline, total protein, chlorophyll, ascorbate and dehydroascorbate changes under drought stress in Akria and Mobil tomato cultivars. Iran. J. Plant Physiol. 2013, 3, 651–658. [Google Scholar]
- Taïbi, K.; Taïbi, F.; Ait Abderrahim, L.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Labudda, M. Lipid Peroxidation as a Biochemical Marker for Oxidative Stress during Drought. An Effective Tool for Plant Breeding; E-wydawnictwo: Warsaw, Poland, 2013; pp. 1–12. [Google Scholar]
- Watanabe, S.; Kojima, K.; Ide, Y.; Sasaki, S. Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue Organ Cult. 2000, 63, 199. [Google Scholar] [CrossRef]
- Gandonou, C.B.; Bada, F.; Abrini, J.; Skali-Senhaji, N. Free proline, soluble sugars and soluble proteins concentration as affected by salt stress in two sugarcane (Saccharum sp.) cultivars differing in their salt tolerance. Int. J. Biol. Chem. Sci. 2011, 5, 2441–2453. [Google Scholar] [CrossRef]
- Javadipour, Z.; Movahhedi Dehnavi, M.; Balouchi, H. Changes in leaf proline, soluble sugars, glycinebetaine and protein content in six spring safflower under salinity stress. J. Plant Process Funct. 2013, 1, 13–23. [Google Scholar]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Luan, S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Schiller, P.; Heilmeier, H.; Hartung, W. Abscisic acid (ABA) relations in the aquatic resurrection plant Chamaegigas intrepidus under naturally fluctuating environmental conditions. New Phytol. 1997, 136, 603–611. [Google Scholar] [CrossRef]
- Todaka, D.; Nakashima, K.; Maruyama, K.; Kidokoro, S.; Osakabe, Y.; Ito, Y.; Matsukura, S.; Fujita, Y.; Yoshiwara, K.; Ohme-Takagi, M.; et al. Rice phytochrome-interacting factor-like protein OsPIL1 functions as a key regulator of internode elongation and induces a morphological response to drought stress. Proc. Natl. Acad. Sci. USA 2012, 109, 15947–15952. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Beckett, R.P.; Wornik, S.; Zorn, M.; Pfeifhofer, H.W. Revival of a resurrection plant correlates with its antioxidant status. Plant J. 2002, 31, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Drennan, P.M.; Smith, M.T.; Goldsworthy, D.; Van Staden, J. The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius welw. J. Plant Physiol. 1993, 142, 493–496. [Google Scholar] [CrossRef]
- Zia, A.; Walker, B.J.; Oung, H.M.O.; Charuvi, D.; Jahns, P.; Cousins, A.B.; Farrant, J.M.; Reich, Z.; Kirchhoff, H. Protection of the photosynthetic apparatus against dehydration stress in the resurrection plant Craterostigma pumilum. Plant J. 2016, 87, 664–680. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Wang, Q.; Wu, H.; Wan, Y. The effects of environmental light on the reorganization of chloroplasts in the resurrection of Selaginella tamariscina. Plant Signal. Behav. 2019, 14, 1621089. [Google Scholar] [CrossRef]
- Tan, T.; Sun, Y.; Luo, S.; Zhang, C.; Zhou, H.; Lin, H. Efficient modulation of photosynthetic apparatus confers desiccation tolerance in the resurrection plant Boea hygrometrica. Plant Cell Physiol. 2017, 58, 1976–1990. [Google Scholar] [CrossRef]
- Charuvi, D.; Nevo, R.; Aviv-Sharon, E.; Gal, A.; Kiss, V.; Shimoni, E.; Farrant, J.M.; Kirchhoff, H.; Reich, Z. Chloroplast breakdown during dehydration of a homoiochlorophyllous resurrection plant proceeds via senescence-like processes. Environ. Exp. Bot. 2019, 157, 100–111. [Google Scholar] [CrossRef]
- Jensen, M.K.; Lindemose, S.; De Masi, F.; Reimer, J.J.; Nielsen, M.; Perera, V.; Workman, C.T.; Turck, F.; Grant, M.R.; Mundy, J.; et al. ATAF1 transcription factor directly regulates abscisic acid biosynthetic gene NCED3 in Arabidopsis thaliana. FEBS Open Bio 2013, 3, 321–327. [Google Scholar] [CrossRef]
- Su, M.; Li, X.-F.; Ma, X.-Y.; Peng, X.-J.; Zhao, A.-G.; Cheng, L.-Q.; Chen, S.-Y.; Liu, G.-S. Cloning two P5CS genes from bioenergy sorghum and their expression profiles under abiotic stresses and MeJA treatment. Plant Sci. 2011, 181, 652–659. [Google Scholar] [CrossRef]
- Bihmidine, S.; Lin, J.; Stone, J.M.; Awada, T.; Specht, J.E.; Clemente, T.E. Activity of the Arabidopsis RD29A and RD29B promoter elements in soybean under water stress. Planta 2013, 237, 55–64. [Google Scholar] [CrossRef]
- Székely, G.; Ábrahám, E.; Cséplő, Á.; Rigó, G.; Zsigmond, L.; Csiszár, J.; Ayaydin, F.; Strizhov, N.; Jásik, J.; Schmelzer, E.; et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008, 53, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Msanne, J.; Lin, J.; Stone, J.M.; Awada, T. Characterization of abiotic stress-responsive Arabidopsis thaliana RD29A and RD29B genes and evaluation of transgenes. Planta 2011, 234, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Lyall, R.; Schlebusch, S.A.; Proctor, J.; Prag, M.; Hussey, S.G.; Ingle, R.A.; Illing, N. Vegetative desiccation tolerance in the resurrection plant Xerophyta humilis has not evolved through reactivation of the seed canonical LAFL regulatory network. Plant J. 2020, 101, 1349–1367. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Kj, L.; Td, S. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Fryer, M.J.; Oxborough, K.; Mullineaux, P.M.; Baker, N.R. Imaging of photo-oxidative stress responses in leaves. J. Exp. Bot. 2002, 53, 1249–1254. [Google Scholar] [CrossRef]
- Zheng, X.; Tian, S.; Meng, X.; Li, B. Physiological and biochemical responses in peach fruit to oxalic acid treatment during storage at room temperature. Food Chem. 2007, 104, 156–162. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.-R.; Xiang, X.-Y.; Wang, J.-T.; Xu, W.-X.; Chen, J.; Xiao, Y.; Jiang, C.-Z.; Huang, Z. MfPIF1 of Resurrection Plant Myrothamnus flabellifolia Plays a Positive Regulatory Role in Responding to Drought and Salinity Stresses in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 3011. https://doi.org/10.3390/ijms21083011
Qiu J-R, Xiang X-Y, Wang J-T, Xu W-X, Chen J, Xiao Y, Jiang C-Z, Huang Z. MfPIF1 of Resurrection Plant Myrothamnus flabellifolia Plays a Positive Regulatory Role in Responding to Drought and Salinity Stresses in Arabidopsis. International Journal of Molecular Sciences. 2020; 21(8):3011. https://doi.org/10.3390/ijms21083011
Chicago/Turabian StyleQiu, Jia-Rui, Xiang-Ying Xiang, Jia-Tong Wang, Wen-Xin Xu, Jia Chen, Yao Xiao, Cai-Zhong Jiang, and Zhuo Huang. 2020. "MfPIF1 of Resurrection Plant Myrothamnus flabellifolia Plays a Positive Regulatory Role in Responding to Drought and Salinity Stresses in Arabidopsis" International Journal of Molecular Sciences 21, no. 8: 3011. https://doi.org/10.3390/ijms21083011
APA StyleQiu, J.-R., Xiang, X.-Y., Wang, J.-T., Xu, W.-X., Chen, J., Xiao, Y., Jiang, C.-Z., & Huang, Z. (2020). MfPIF1 of Resurrection Plant Myrothamnus flabellifolia Plays a Positive Regulatory Role in Responding to Drought and Salinity Stresses in Arabidopsis. International Journal of Molecular Sciences, 21(8), 3011. https://doi.org/10.3390/ijms21083011




