Gender Predilection in Sporadic Parathyroid Adenomas
Abstract
1. Introduction
2. Epidemiology
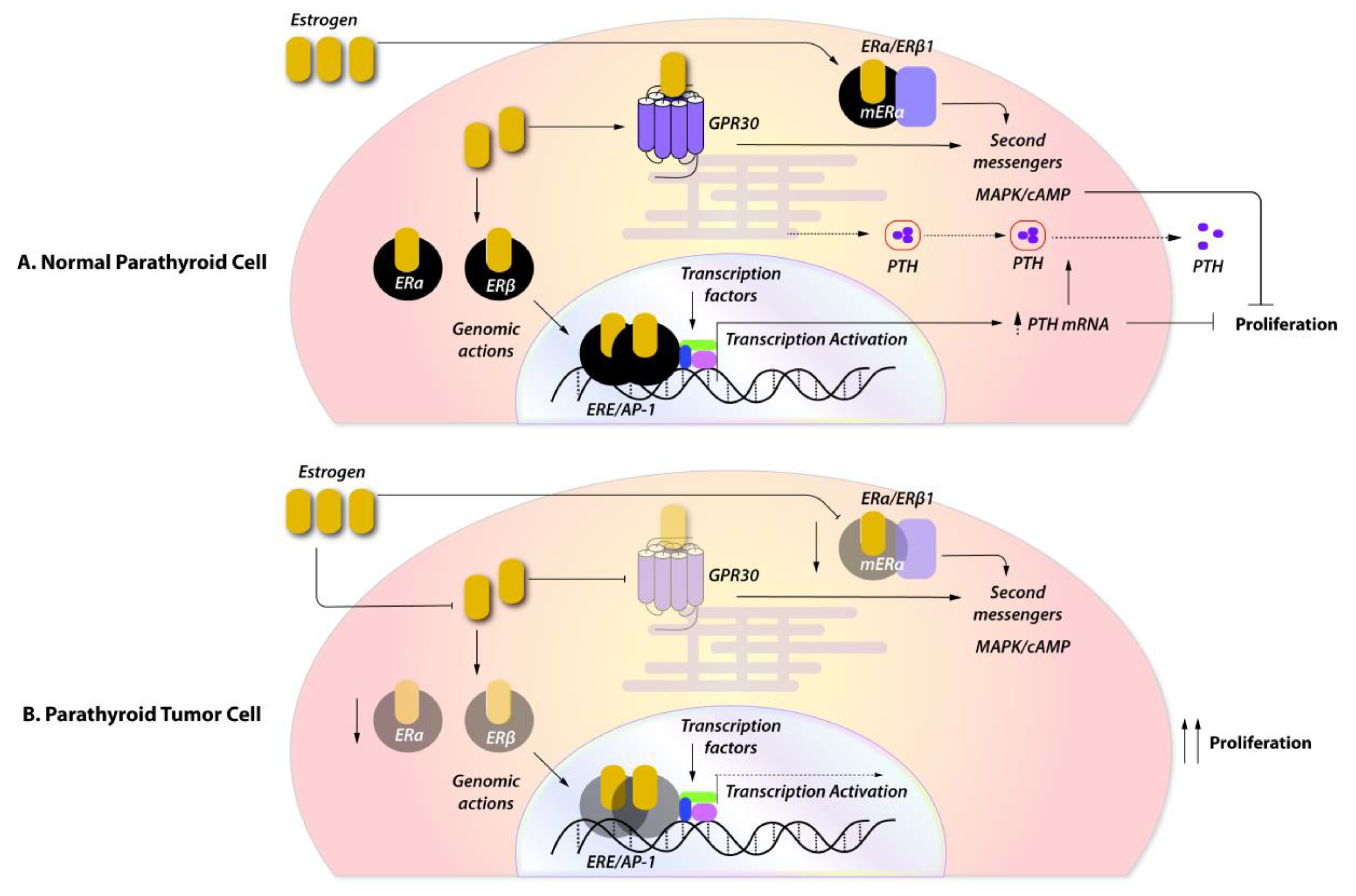
3. The Role of Female Sex Steroids in the Physiology of Parathyroid Hormone Secretion
4. Expression of Female Sex Steroids in Sporadic Parathyroid Tumors
5. Gender-Based Genetic and Epigenetic Mechanisms in the Pathogenesis of Sporadic Parathyroid Adenomas
6. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| pHPT | primary hyperparathyroidism |
| E2 | estradiol |
| ER | estrogen receptors |
| Pg | progesterone |
| MEN1 | multiple endocrine neoplasia type 1 |
| MEN2a | multiple endocrine neoplasia type 2a |
| MEN4 | multiple endocrine neoplasia type 4 |
| FHH | familial hypocalciuric hypercalcemia |
| NSHPT | neonatal severe hyperparathyroidism |
| HPT-JT syndrome | hyperparathyroidism-jaw tumor syndrome |
| FIPH | familial isolated primary hyperparathyroidism |
| PR | progesterone receptor |
| FGF23 | fibroblast growth factor 23 |
| ERK | extracellular-signal-regulated kinase |
| cAMP | cyclic adenosine monophosphate |
| PKA | protein kinase A |
| PI3 K | phosphatidylinositol 3-kinases |
| Akt | protein kinase B |
| circRNA | circular RNA |
| miRNA | microRNA |
| lnc-RNA | long non-coding RNA |
| CDKI | cyclin-dependent kinase inhibitors |
| MAPK | mitogen activated protein kinase |
References
- Castellano, E.; Attanasio, R.; Boriano, A.; Borretta, G. The Clinical Presentation of Primary Hyperparathyroidism: A Southern European Perspective over the Last 2 Decades. Endocr Pract. 2018, 24, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, D.A.; Makras, P.; Polyzos, S.A.; Anastasilakis, A.D.; Part of the, C.E.T. Asymptomatic and normocalcemic hyperparathyroidism, the silent attack: a combo-endocrinology overview. Horm. (Athens) 2019, 18, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Makras, P.; Anastasilakis, A.D. Bone disease in primary hyperparathyroidism. Metab. Clin. Exp. 2018, 80, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Cianferotti, L.; Giusti, F.; Brandi, M.L. Molecular genetics in primary hyperparathyroidism: the role of genetic tests in differential diagnosis, disease prevention strategy, and therapeutic planning. A 2017 update. Clin. Cases Min. Bone Metab 2017, 14, 60–70. [Google Scholar] [CrossRef]
- Eller-Vainicher, C.; Chiodini, I.; Battista, C.; Viti, R.; Mascia, M.L.; Massironi, S.; Peracchi, M.; D’Agruma, L.; Minisola, S.; Corbetta, S.; et al. Sporadic and MEN1-related primary hyperparathyroidism: differences in clinical expression and severity. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2009, 24, 1404–1410. [Google Scholar] [CrossRef]
- Castellano, E.; Attanasio, R.; Boriano, A.; Pellegrino, M.; Garino, F.; Gianotti, L.; Borretta, G. Sex Difference in the Clinical Presentation of Primary Hyperparathyroidism: Influence of Menopausal Status. J. Clin. Endocrinol. Metab. 2017, 102, 4148–4152. [Google Scholar] [CrossRef]
- Marcocci, C.; Saponaro, F. Epidemiology, pathogenesis of primary hyperparathyroidism: Current data. Ann. Endocrinol (Paris) 2015, 76, 113–115. [Google Scholar] [CrossRef]
- Melton, L.J., 3rd. The epidemiology of primary hyperparathyroidism in North America. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2002, 17 (Suppl. 2), N12-17. [Google Scholar]
- Mazeh, H.; Sippel, R.S.; Chen, H. The role of gender in primary hyperparathyroidism: same disease, different presentation. Ann. Surg. Oncol. 2012, 19, 2958–2962. [Google Scholar] [CrossRef]
- Shah, V.N.; Bhadada, S.K.; Bhansali, A.; Behera, A.; Mittal, B.R.; Bhavin, V. Influence of age and gender on presentation of symptomatic primary hyperparathyroidism. J. Postgrad. Med. 2012, 58, 107–111. [Google Scholar] [CrossRef]
- Twigt, B.A.; Scholten, A.; Valk, G.D.; Rinkes, I.H.; Vriens, M.R. Differences between sporadic and MEN related primary hyperparathyroidism; clinical expression, preoperative workup, operative strategy and follow-up. Orphanet J. Rare Dis. 2013, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.S.; Dimick, J.; Wainess, R.; Burney, R.E. Age- and sex-related incidence of surgically treated primary hyperparathyroidism. World J. Surg 2008, 32, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.; Trombetti, A.; Herrmann, F.R.; Triponez, F.; Meier, C.; Robert, J.H.; Rizzoli, R. Age and gender distribution of primary hyperparathyroidism and incidence of surgical treatment in a European country with a particularly high life expectancy. Swiss Med. Wkly. 2009, 139, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Vodopivec, D.M.; Silva, A.M.; Garcia-Banigan, D.C.; Christakis, I.; Stewart, A.; Schwarz, K.; Hussey, C.S.; Bassett, R.; Hu, M.I.; Perrier, N.D. Gender differences in bone mineral density in patients with sporadic primary hyperparathyroidism. Endocrinol. Diabetes Metab. 2018, 1, e00037. [Google Scholar] [CrossRef]
- Strope, S.A.; Wolf, J.S.; Hollenbeck, B.K. Changes in gender distribution of urinary stone disease. Urology 2010, 75, 543–546, 546 e541. [Google Scholar] [CrossRef]
- Alswat, K.A. Gender Disparities in Osteoporosis. J. Clin. Med. Res. 2017, 9, 382–387. [Google Scholar] [CrossRef]
- Burns, K.A.; Korach, K.S. Estrogen receptors and human disease: an update. Arch. Toxicol. 2012, 86, 1491–1504. [Google Scholar] [CrossRef]
- Huang, P.; Chandra, V.; Rastinejad, F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu. Rev. Physiol 2010, 72, 247–272. [Google Scholar] [CrossRef]
- Kumar, R.; Zakharov, M.N.; Khan, S.H.; Miki, R.; Jang, H.; Toraldo, G.; Singh, R.; Bhasin, S.; Jasuja, R. The dynamic structure of the estrogen receptor. J. Amino Acids 2011, 2011, 812540. [Google Scholar] [CrossRef]
- Kim, C.K.; Torcaso, A.; Asimes, A.; Chung, W.C.J.; Pak, T.R. Structural and functional characteristics of oestrogen receptor beta splice variants: Implications for the ageing brain. J. Neuroendocr. 2018, 30. [Google Scholar] [CrossRef]
- Kelly, M.J.; Levin, E.R. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol. Metab. 2001, 12, 152–156. [Google Scholar] [CrossRef]
- Noyola-Martinez, N.; Halhali, A.; Barrera, D. Steroid hormones and pregnancy. Gynecol. Endocrinol. 2019, 35, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Taraborrelli, S. Physiology, production and action of progesterone. Acta Obs. Gynecol. Scand. 2015, 94 (Suppl. 161), 8–16. [Google Scholar] [CrossRef]
- Greenberg, C.; Kukreja, S.C.; Bowser, E.N.; Hargis, G.K.; Henderson, W.J.; Williams, G.A. Parathyroid hormone secretion: effect of estradiol and progesterone. Metab. Clin. Exp. 1987, 36, 151–154. [Google Scholar] [CrossRef]
- Duarte, B.; Hargis, G.K.; Kukreja, S.C. Effects of estradiol and progesterone on parathyroid hormone secretion from human parathyroid tissue. J. Clin. Endocrinol. Metab. 1988, 66, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E.; Silver, J.; Almogi, G.; Livni, N.; Naveh-Many, T. Parathyroid hormone mRNA levels are increased by progestins and vary during rat estrous cycle. Am. J. Physiol. 1996, 270, E158–E163. [Google Scholar] [CrossRef] [PubMed]
- Emura, S.; Hayakawa, D.; Yamahira, T.; Terasawa, K.; Tamada, A.; Arakawa, M.; Isono, H.; Shoumura, S. Effects of progesterone on the ultrastructure of the golden hamster parathyroid gland. Histol. Histopathol. 1995, 10, 907–911. [Google Scholar]
- Carrillo-Lopez, N.; Roman-Garcia, P.; Rodriguez-Rebollar, A.; Fernandez-Martin, J.L.; Naves-Diaz, M.; Cannata-Andia, J.B. Indirect regulation of PTH by estrogens may require FGF23. J. Am. Soc. Nephrol. Jasn 2009, 20, 2009–2017. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Riggs, B.L.; DeLuca, H.F. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 1980, 51, 1359–1364. [Google Scholar] [CrossRef]
- Scharla, S.H.; Minne, H.W.; Waibel-Treber, S.; Schaible, A.; Lempert, U.G.; Wuster, C.; Leyendecker, G.; Ziegler, R. Bone mass reduction after estrogen deprivation by long-acting gonadotropin-releasing hormone agonists and its relation to pretreatment serum concentrations of 1,25-dihydroxyvitamin D3. J. Clin. Endocrinol. Metab. 1990, 70, 1055–1061. [Google Scholar] [CrossRef]
- Prince, R.L.; Dick, I.; Devine, A.; Price, R.I.; Gutteridge, D.H.; Kerr, D.; Criddle, A.; Garcia-Webb, P.; St John, A. The effects of menopause and age on calcitropic hormones: a cross-sectional study of 655 healthy women aged 35 to 90. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1995, 10, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Gennari, C.; Agnusdei, D.; Nardi, P.; Civitelli, R. Estrogen preserves a normal intestinal responsiveness to 1,25-dihydroxyvitamin D3 in oophorectomized women. J. Clin. Endocrinol. Metab. 1990, 71, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- McKane, W.R.; Khosla, S.; Burritt, M.F.; Kao, P.C.; Wilson, D.M.; Ory, S.J.; Riggs, B.L. Mechanism of renal calcium conservation with estrogen replacement therapy in women in early postmenopause--a clinical research center study. J. Clin. Endocrinol. Metab. 1995, 80, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Prince, R.L.; Schiff, I.; Neer, R.M. Effects of transdermal estrogen replacement on parathyroid hormone secretion. J. Clin. Endocrinol. Metab. 1990, 71, 1284–1287. [Google Scholar] [CrossRef]
- Vincent, A.; Riggs, B.L.; Atkinson, E.J.; Oberg, A.L.; Khosla, S. Effect of estrogen replacement therapy on parathyroid hormone secretion in elderly postmenopausal women. Menopause 2003, 10, 165–171. [Google Scholar] [CrossRef]
- Baran, D.T.; Whyte, M.P.; Haussler, M.R.; Deftos, L.J.; Slatopolsky, E.; Avioli, L.V. Effect of the menstrual cycle on calcium-regulating hormones in the normal young woman. J. Clin. Endocrinol. Metab. 1980, 50, 377–379. [Google Scholar] [CrossRef]
- Thys-Jacobs, S.; Alvir, M.J. Calcium-regulating hormones across the menstrual cycle: evidence of a secondary hyperparathyroidism in women with PMS. J. Clin. Endocrinol. Metab. 1995, 80, 2227–2232. [Google Scholar] [CrossRef]
- Muse, K.N.; Manolagas, S.C.; Deftos, L.J.; Alexander, N.; Yen, S.S. Calcium-regulating hormones across the menstrual cycle. J. Clin. Endocrinol. Metab. 1986, 62, 1313–1316. [Google Scholar] [CrossRef]
- Buchanan, J.R.; Santen, R.J.; Cavaliere, A.; Cauffman, S.W.; Greer, R.B.; Demers, L.M. Interaction between parathyroid hormone and endogenous estrogen in normal women. Metab. Clin. Exp. 1986, 35, 489–494. [Google Scholar] [CrossRef]
- Nielsen, H.K.; Brixen, K.; Bouillon, R.; Mosekilde, L. Changes in biochemical markers of osteoblastic activity during the menstrual cycle. J. Clin. Endocrinol. Metab. 1990, 70, 1431–1437. [Google Scholar] [CrossRef]
- Pitkin, R.M.; Reynolds, W.A.; Williams, G.A.; Hargis, G.K. Calcium-regulating hormones during the menstrual cycle. J. Clin. Endocrinol. Metab. 1978, 47, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Schwarz, I.; Scheld, K.; Sudhop, T.; Berthold, H.K.; von Bergmann, K.; van der Ven, H.; Stehle, P. Physiologic fluctuations of serum estradiol levels influence biochemical markers of bone resorption in young women. J. Clin. Endocrinol. Metab. 2000, 85, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Calcium and bone metabolism disorders during pregnancy and lactation. Endocrinol Metab Clin. North. Am. 2011, 40, 795–826. [Google Scholar] [CrossRef] [PubMed]
- Wermers, R.A.; Khosla, S.; Atkinson, E.J.; Achenbach, S.J.; Oberg, A.L.; Grant, C.S.; Melton, L.J., 3rd. Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993-2001: an update on the changing epidemiology of the disease. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2006, 21, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Saxe, A.W.; Gibson, G.W.; Russo, I.H.; Gimotty, P. Measurement of estrogen and progesterone receptors in abnormal human parathyroid tissue. Calcif. Tissue Int. 1992, 51, 344–347. [Google Scholar] [CrossRef]
- Wong, C.; Lai, T.; Hilly, J.M.; Stewart, C.E.; Farndon, J.R. Selective estrogen receptor modulators inhibit the effects of insulin-like growth factors in hyperparathyroidism. Surgery 2002, 132, 998–1006, discussion 1006-1007. [Google Scholar] [CrossRef]
- Haglund, F.; Ma, R.; Huss, M.; Sulaiman, L.; Lu, M.; Nilsson, I.L.; Hoog, A.; Juhlin, C.C.; Hartman, J.; Larsson, C. Evidence of a functional estrogen receptor in parathyroid adenomas. J. Clin. Endocrinol. Metab. 2012, 97, 4631–4639. [Google Scholar] [CrossRef]
- Haglund, F.; Rosin, G.; Nilsson, I.L.; Juhlin, C.C.; Pernow, Y.; Norenstedt, S.; Dinets, A.; Larsson, C.; Hartman, J.; Hoog, A. Tumour nuclear oestrogen receptor beta 1 correlates inversely with parathyroid tumour weight. Endocr Connect. 2015, 4, 76–85. [Google Scholar] [CrossRef]
- Carling, T.; Rastad, J.; Kindmark, A.; Lundgren, E.; Ljunghall, S.; Akerstrom, G. Estrogen receptor gene polymorphism in postmenopausal primary hyperparathyroidism. Surgery 1997, 122, 1101–1105. [Google Scholar] [CrossRef]
- Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen signaling via estrogen receptor {beta}. J. Biol. Chem. 2010, 285, 39575–39579. [Google Scholar] [CrossRef]
- Newey, P.J.; Nesbit, M.A.; Rimmer, A.J.; Attar, M.; Head, R.T.; Christie, P.T.; Gorvin, C.M.; Stechman, M.; Gregory, L.; Mihai, R.; et al. Whole-exome sequencing studies of nonhereditary (sporadic) parathyroid adenomas. J. Clin. Endocrinol. Metab. 2012, 97, E1995–E2005. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; McPherson, J.R.; Stevenson, M.; van Eijk, R.; Heng, H.L.; Newey, P.; Gan, A.; Ruano, D.; Huang, D.; Poon, S.L.; et al. Whole-exome sequencing studies of parathyroid carcinomas reveal novel PRUNE2 mutations, distinctive mutational spectra related to APOBEC-catalyzed DNA mutagenesis and mutational enrichment in kinases associated with cell migration and invasion. J. Clin. Endocrinol. Metab. 2015, 100, E360–E364. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Cancer Epigenetics for the 21st Century: What’s Next? Genes Cancer 2011, 2, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Yavropoulou, M.P.; Yovos, J.G. The “dark matter” of DNA and the regulation of bone metabolism: The role of non-coding RNAs. J. Musculoskelet Neuronal Interact. 2018, 18, 18–31. [Google Scholar]
- Yavropoulou, M.P.; Poulios, C.; Michalopoulos, N.; Gatzou, A.; Chrisafi, S.; Mantalovas, S.; Papavramidis, T.; Daskalaki, E.; Sofou, E.; Kotsa, K.; et al. A Role for Circular Non-Coding RNAs in the Pathogenesis of Sporadic Parathyroid Adenomas and the Impact of Gender-Specific Epigenetic Regulation. Cells 2018, 8, 15. [Google Scholar] [CrossRef]
- Verdelli, C.; Forno, I.; Vaira, V.; Corbetta, S. Epigenetic alterations in human parathyroid tumors. Endocrine 2015, 49, 324–332. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Tan, C.; Liu, X. Circular RNAs: a new frontier in the study of human diseases. J. Med. Genet. 2016, 53, 359–365. [Google Scholar] [CrossRef]
- Sharma, S.; Eghbali, M. Influence of sex differences on microRNA gene regulation in disease. Biol Sex. Differ. 2014, 5, 3. [Google Scholar] [CrossRef]
- Klinge, C.M. miRNAs and estrogen action. Trends Endocrinol. Metab. 2012, 23, 223–233. [Google Scholar] [CrossRef]
- Guo, X.; Yang, C.; Qian, X.; Lei, T.; Li, Y.; Shen, H.; Fu, L.; Xu, B. Estrogen receptor alpha regulates ATM Expression through miRNAs in breast cancer. Clin. Cancer Res. 2013, 19, 4994–5002. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, C.; Wang, J.; Xiao, J.; Gatalica, Z.; Recker, R.R.; Xiao, G.G. Let-7 family miRNAs regulate estrogen receptor alpha signaling in estrogen receptor positive breast cancer. Breast Cancer Res. Treat. 2011, 127, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D. Parathyroid function: Key role for dicer-dependent miRNAs. Nat. Rev. Endocrinol. 2015, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Vaira, V.; Verdelli, C.; Forno, I.; Corbetta, S. MicroRNAs in parathyroid physiopathology. Mol. Cell Endocrinol. 2017, 456, 9–15. [Google Scholar] [CrossRef]
- Feng, F.; Wu, J.; Gao, Z.; Yu, S.; Cui, Y. Screening the key microRNAs and transcription factors in prostate cancer based on microRNA functional synergistic relationships. Med. (Baltim.) 2017, 96, e5679. [Google Scholar] [CrossRef] [PubMed]
- Farina, N.H.; Ramsey, J.E.; Cuke, M.E.; Ahern, T.P.; Shirley, D.J.; Stein, J.L.; Stein, G.S.; Lian, J.B.; Wood, M.E. Development of a predictive miRNA signature for breast cancer risk among high-risk women. Oncotarget 2017, 8, 112170–112183. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Cairns, M.J. Circular RNAs are temporospatially regulated throughout development and ageing in the rat. Sci Rep. 2019, 9, 2564. [Google Scholar] [CrossRef]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008, 173, 600–609. [Google Scholar] [CrossRef]
- Lockshin, M.D. Sex differences in autoimmune disease. Lupus 2006, 15, 753–756. [Google Scholar] [CrossRef]
- Kim, H.I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef]
- Barrett-Connor, E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation 1997, 95, 252–264. [Google Scholar] [CrossRef]
- Sandelin, K.; Skoog, L.; Humla, S.; Farnebo, L.O. Oestrogen, progesterone, and glucocorticoid receptors in normal and neoplastic parathyroid glands. Eur. J. Surg. Acta Chir. 1992, 158, 467–472. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yavropoulou, M.P.; Anastasilakis, A.D.; Panagiotakou, A.; Kassi, E.; Makras, P. Gender Predilection in Sporadic Parathyroid Adenomas. Int. J. Mol. Sci. 2020, 21, 2964. https://doi.org/10.3390/ijms21082964
Yavropoulou MP, Anastasilakis AD, Panagiotakou A, Kassi E, Makras P. Gender Predilection in Sporadic Parathyroid Adenomas. International Journal of Molecular Sciences. 2020; 21(8):2964. https://doi.org/10.3390/ijms21082964
Chicago/Turabian StyleYavropoulou, Maria P., Athanasios D. Anastasilakis, Argyro Panagiotakou, Evanthia Kassi, and Polyzois Makras. 2020. "Gender Predilection in Sporadic Parathyroid Adenomas" International Journal of Molecular Sciences 21, no. 8: 2964. https://doi.org/10.3390/ijms21082964
APA StyleYavropoulou, M. P., Anastasilakis, A. D., Panagiotakou, A., Kassi, E., & Makras, P. (2020). Gender Predilection in Sporadic Parathyroid Adenomas. International Journal of Molecular Sciences, 21(8), 2964. https://doi.org/10.3390/ijms21082964








