Integrin αvβ3 in the Mediating Effects of Dihydrotestosterone and Resveratrol on Breast Cancer Cell Proliferation
Abstract
1. Introduction
2. Androgen and Other Non-Peptide Hormones Act via Different Receptors to Induce Proliferation of Human Breast Cancer Cells
3. Membrane Androgen Receptors
4. Integrin αvβ3 as a Receptor for DHT
5. Androgens and Breast Cancer Cell Proliferation
6. Integrin αvβ3 as a Receptor for Resveratrol
6.1. Resveratrol-Induced Apoptosis Signal Transduction Pathways: ERK1/2 and AMPK
6.2. Resveratrol-Induced Nuclear COX-2
6.3. Other Mechanisms Involved in Resveratrol-Induced Anti-Proliferation in Breast Cancers
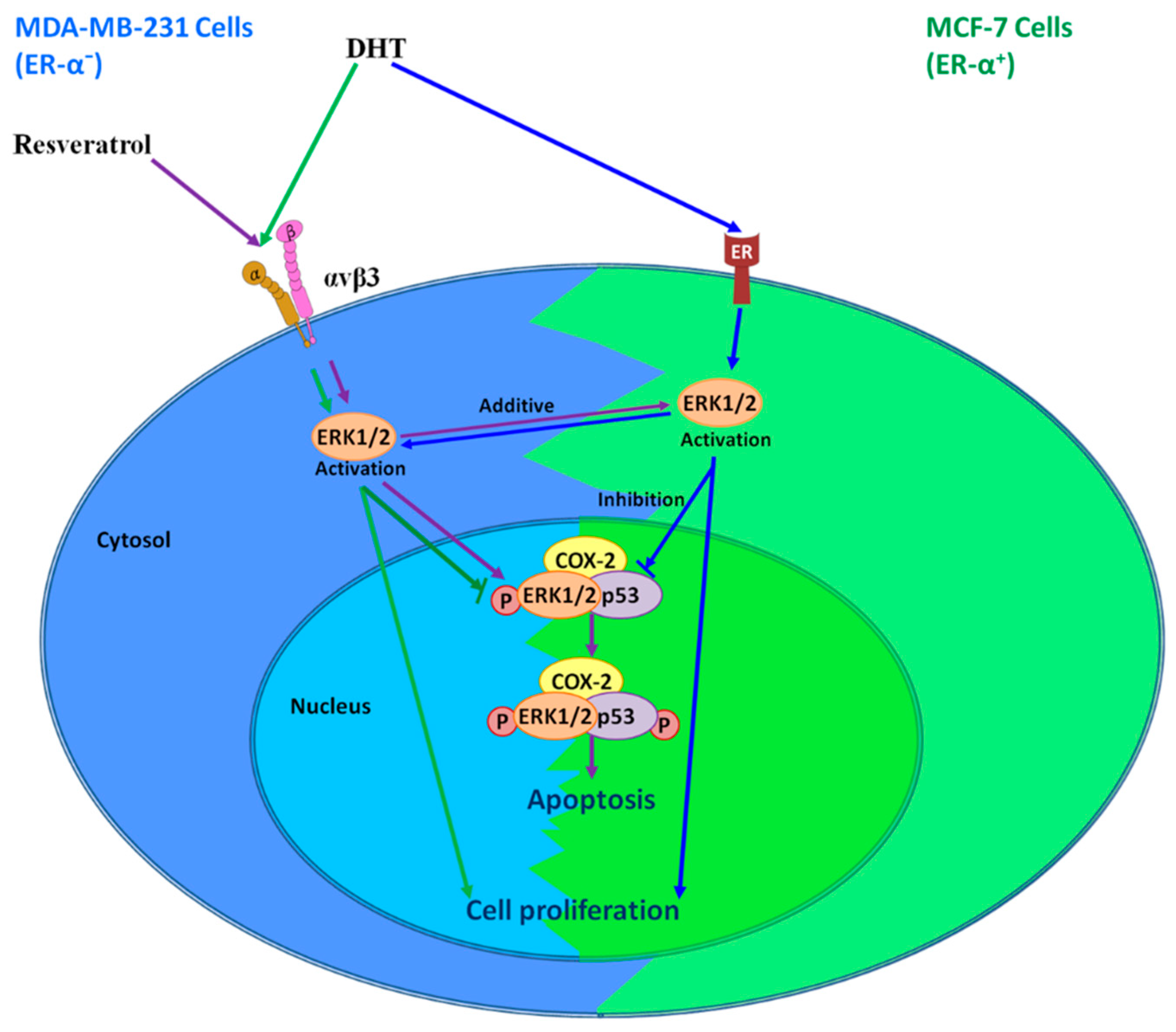
7. Interaction of Resveratrol and DHT
8. Effect of DHT and Resveratrol on Metastasis
9. Integrin αvβ3 and Metastasis
10. VEGF and Metastasis
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTN4 | Actin-binding protein, actinin α 4 |
| AMPK | AMP-activated protein kinase |
| AMPKK | AMP-activated protein kinase kinase |
| 7α-APTADD | 7α (4′-amino) phenylthio-1,4-androstadiene-3,17-dione |
| AR | Androgen receptor |
| BRCA1 | Breast cancer 1 gene |
| Cav-1 | Caveolin-1 |
| COX-2 | Cyclooxygenase-2 |
| CYP1A1 | Cytochrome P450-1A1 |
| DHEAS | Dehydroepiandrosterone sulfate |
| DHT | Dihydrotestosterone |
| E1 | Estrone |
| E1S | Estrone sulfate |
| EGFR | Epidermal growth factor receptor |
| ER | Estrogen receptor |
| ERK1/2 | Extracellular signal-regulated kinases |
| FAK | Focal adhesion kinase |
| HER2 | Human epidermal growth factor receptor 2 |
| 17β-HSD1 | 17β-hydroxysteroid hydrogenase type 1 |
| iARs | Classical intracellular androgen receptors |
| LKB1 | Liver kinase B1 |
| MAPK | Mitogen-activated protein kinase |
| mARs | Membrane androgen receptors |
| MMP-2 | Matrix metalloproteinase-2 |
| PI-3K | Phosphatidylinositol-3-kinase |
| PKC δ | Protein kinase C δ |
| PR | Progesterone receptor |
| PTEN | Phosphatase and tensin homolog |
| RGD | Arg-Gly-Asp |
| TSP1 | Thrombospondin-1 |
| VEGF | Vascular endothelial growth factor |
References
- Levin, E.R. Membrane oestrogen receptor alpha signalling to cell functions. J. Physiol. 2009, 587, 5019–5023. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.W.; Chien, S.T.; Chen, P.S.; Lee, J.H.; Wu, S.H.; Yin, L.T. Alpha-mangostin suppresses phorbol 12-myristate 13-acetate-induced MMP-2/MMP-9 expressions via alphavbeta3 integrin/FAK/ERK and NF-kappaB signaling pathway in human lung adenocarcinoma A549 cells. Cell Biochem. Biophys. 2010, 58, 31–44. [Google Scholar] [CrossRef]
- Fujita, M.; Ieguchi, K.; Cedano-Prieto, D.M.; Fong, A.; Wilkerson, C.; Chen, J.Q.; Wu, M.; Lo, S.H.; Cheung, A.T.; Wilson, M.D.; et al. An integrin binding-defective mutant of insulin-like growth factor-1 (R36E/R37E IGF1) acts as a dominant-negative antagonist of the IGF1 receptor (IGF1R) and suppresses tumorigenesis but still binds to IGF1R. J. Biol. Chem. 2013, 288, 19593–19603. [Google Scholar] [CrossRef] [PubMed]
- Rapraeger, A.C. Synstatin: A selective inhibitor of the syndecan-1-coupled IGF1R-alphavbeta3 integrin complex in tumorigenesis and angiogenesis. FEBS J. 2013, 280, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Medici, N.; Bilancio, A.; Migliaccio, A.; Castoria, G. Breast cancer stem cells: The role of sex steroid receptors. World J. Stem Cells 2019, 11, 594–603. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Bilancio, A.; Migliaccio, A. The Androgen Receptor in Breast Cancer. Front. Endocrinol. 2018, 9, 492. [Google Scholar] [CrossRef]
- Lin, H.Y.; Sun, M.; Lin, C.; Tang, H.Y.; London, D.; Shih, A.; Davis, F.B.; Davis, P.J. Androgen-induced human breast cancer cell proliferation is mediated by discrete mechanisms in estrogen receptor-alpha-positive and -negative breast cancer cells. J. Steroid Biochem. Mol. Biol. 2009, 113, 182–188. [Google Scholar] [CrossRef]
- Lin, H.Y.; Hsieh, M.T.; Cheng, G.Y.; Lai, H.Y.; Chin, Y.T.; Shih, Y.J.; Nana, A.W.; Lin, S.Y.; Yang, Y.S.H.; Tang, H.Y.; et al. Mechanisms of action of nonpeptide hormones on resveratrol-induced antiproliferation of cancer cells. Ann. N. Y. Acad. Sci. 2017, 1403, 92–100. [Google Scholar] [CrossRef]
- Jang, Y.G.; Go, R.E.; Hwang, K.A.; Choi, K.C. Resveratrol inhibits DHT-induced progression of prostate cancer cell line through interfering with the AR and CXCR4 pathway. J. Steroid Biochem. Mol. Biol. 2019, 192, 105406. [Google Scholar] [CrossRef]
- Chin, Y.T.; Yang, S.H.; Chang, T.C.; Changou, C.A.; Lai, H.Y.; Fu, E.; HuangFu, W.C.; Davis, P.J.; Lin, H.Y.; Liu, L.F. Mechanisms of dihydrotestosterone action on resveratrol-induced anti-proliferation in breast cancer cells with different ERalpha status. Oncotarget 2015, 6, 35866–35879. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chin, Y.T.; Yang, Y.C.; Lai, H.Y.; Wang-Peng, J.; Liu, L.F.; Tang, H.Y.; Davis, P.J. Thyroid Hormone, Cancer, and Apoptosis. Compr. Physiol. 2016, 6, 1221–1237. [Google Scholar] [PubMed]
- Chen, Y.; Li, Z.; Shih, Y.; Davis, P.; Whang-Peng, J.; Lin, H.; Wang, C.C. Thyroid hormone, PD-L1, and cancer. J. Cancer Res. Pract. 2019, 6, 162. [Google Scholar]
- Davis, P.J.; Tang, H.Y.; Hercbergs, A.; Lin, H.Y.; Keating, K.A.; Mousa, S.A. Bioactivity of Thyroid Hormone Analogs at Cancer Cells. Front. Endocrinol. 2018, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Toth-Fejel, S.; Cheek, J.; Calhoun, K.; Muller, P.; Pommier, R.F. Estrogen and androgen receptors as comediators of breast cancer cell proliferation: Providing a new therapeutic tool. Arch. Surg. 2004, 139, 50–54. [Google Scholar] [CrossRef]
- Schover, L.R. Androgen therapy for loss of desire in women: Is the benefit worth the breast cancer risk? Fertil. Steril. 2008, 90, 129–140. [Google Scholar] [CrossRef]
- Yeh, S.; Hu, Y.C.; Wang, P.H.; Xie, C.; Xu, Q.; Tsai, M.Y.; Dong, Z.; Wang, R.S.; Lee, T.H.; Chang, C. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J. Exp. Med. 2003, 198, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Bayer-Garner, I.B.; Smoller, B. Androgen receptors: A marker to increase sensitivity for identifying breast cancer in skin metastasis of unknown primary site. Mod. Pathol. 2000, 13, 119–122. [Google Scholar] [CrossRef]
- Agoff, S.N.; Swanson, P.E.; Linden, H.; Hawes, S.E.; Lawton, T.J. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am. J. Clin. Pathol. 2003, 120, 725–731. [Google Scholar] [CrossRef]
- Niemeier, L.A.; Dabbs, D.J.; Beriwal, S.; Striebel, J.M.; Bhargava, R. Androgen receptor in breast cancer: Expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod. Pathol. 2010, 23, 205–212. [Google Scholar] [CrossRef]
- Pristauz, G.; Petru, E.; Stacher, E.; Geigl, J.B.; Schwarzbraun, T.; Tsybrovskyy, O.; Winter, R.; Moinfar, F. Androgen receptor expression in breast cancer patients tested for BRCA1 and BRCA2 mutations. Histopathology 2010, 57, 877–884. [Google Scholar] [CrossRef]
- Henderson, B.E.; Feigelson, H.S. Hormonal carcinogenesis. Carcinogenesis 2000, 21, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; Gellert, L.L.; Zou, X.; Wang, J.; Singh, B.; Xu, R.; Chiriboga, L.; Daniels, G.; Pan, R.; et al. Androgen receptor coactivator p44/Mep50 in breast cancer growth and invasion. J. Cell Mol. Med. 2010, 14, 2780–2789. [Google Scholar] [CrossRef] [PubMed]
- Ligr, M.; Patwa, R.R.; Daniels, G.; Pan, L.; Wu, X.; Li, Y.; Tian, L.; Wang, Z.; Xu, R.; Wu, J.; et al. Expression and function of androgen receptor coactivator p44/Mep50/WDR77 in ovarian cancer. PLoS ONE 2011, 6, e26250. [Google Scholar] [CrossRef]
- Khurana, S.; Chakraborty, S.; Cheng, X.; Su, Y.T.; Kao, H.Y. The actin-binding protein, actinin alpha 4 (ACTN4), is a nuclear receptor coactivator that promotes proliferation of MCF-7 breast cancer cells. J. Biol. Chem. 2011, 286, 1850–1859. [Google Scholar] [CrossRef]
- Jasavala, R.; Martinez, H.; Thumar, J.; Andaya, A.; Gingras, A.C.; Eng, J.K.; Aebersold, R.; Han, D.K.; Wright, M.E. Identification of putative androgen receptor interaction protein modules: Cytoskeleton and endosomes modulate androgen receptor signaling in prostate cancer cells. Mol. Cell Proteomics 2007, 6, 252–271. [Google Scholar] [CrossRef]
- Gu, S.; Papadopoulou, N.; Gehring, E.M.; Nasir, O.; Dimas, K.; Bhavsar, S.K.; Foller, M.; Alevizopoulos, K.; Lang, F.; Stournaras, C. Functional membrane androgen receptors in colon tumors trigger pro-apoptotic responses in vitro and reduce drastically tumor incidence in vivo. Mol. Cancer 2009, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, N.; Papakonstanti, E.A.; Kallergi, G.; Alevizopoulos, K.; Stournaras, C. Membrane androgen receptor activation in prostate and breast tumor cells: Molecular signaling and clinical impact. IUBMB Life 2009, 61, 56–61. [Google Scholar] [CrossRef]
- Kampa, M.; Theodoropoulou, K.; Mavromati, F.; Pelekanou, V.; Notas, G.; Lagoudaki, E.D.; Nifli, A.P.; Morel-Salmi, C.; Stathopoulos, E.N.; Vercauteren, J.; et al. Novel oligomeric proanthocyanidin derivatives interact with membrane androgen sites and induce regression of hormone-independent prostate cancer. J. Pharmacol. Exp. Ther. 2011, 337, 24–32. [Google Scholar] [CrossRef]
- Pelekanou, V.; Notas, G.; Sanidas, E.; Tsapis, A.; Castanas, E.; Kampa, M. Testosterone membrane-initiated action in breast cancer cells: Interaction with the androgen signaling pathway and EPOR. Mol. Oncol. 2010, 4, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Quinn, A.L.; Burak, W.E., Jr.; Brueggemeier, R.W. Effects of matrix components on aromatase activity in breast stromal cells in culture. J. Steroid Biochem. Mol. Biol. 1999, 70, 249–256. [Google Scholar] [CrossRef]
- Birrell, S.N.; Bentel, J.M.; Hickey, T.E.; Ricciardelli, C.; Weger, M.A.; Horsfall, D.J.; Tilley, W.D. Androgens induce divergent proliferative responses in human breast cancer cell lines. J. Steroid Biochem. Mol. Biol. 1995, 52, 459–467. [Google Scholar] [CrossRef]
- Szelei, J.; Jimenez, J.; Soto, A.M.; Luizzi, M.F.; Sonnenschein, C. Androgen-induced inhibition of proliferation in human breast cancer MCF7 cells transfected with androgen receptor. Endocrinology 1997, 138, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- Greeve, M.A.; Allan, R.K.; Harvey, J.M.; Bentel, J.M. Inhibition of MCF-7 breast cancer cell proliferation by 5alpha-dihydrotestosterone; a role for p21(Cip1/Waf1). J. Mol. Endocrinol. 2004, 32, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Kallergi, G.; Mavroudis, D.; Georgoulias, V.; Stournaras, C. Phosphorylation of FAK, PI-3K, and impaired actin organization in CK-positive micrometastatic breast cancer cells. Mol. Med. 2007, 13, 79–88. [Google Scholar] [CrossRef]
- Hecker, T.P.; Grammer, J.R.; Gillespie, G.Y.; Stewart, J., Jr.; Gladson, C.L. Focal adhesion kinase enhances signaling through the Shc/extracellular signal-regulated kinase pathway in anaplastic astrocytoma tumor biopsy samples. Cancer Res. 2002, 62, 2699–2707. [Google Scholar]
- De, S.; Razorenova, O.; McCabe, N.P.; O’Toole, T.; Qin, J.; Byzova, T.V. VEGF-integrin interplay controls tumor growth and vascularization. Proc. Natl. Acad. Sci. USA 2005, 102, 7589–7594. [Google Scholar] [CrossRef]
- Rajendran, M.; Thomes, P.; Zhang, L.; Veeramani, S.; Lin, M.F. p66Shc—A longevity redox protein in human prostate cancer progression and metastasis: p66Shc in cancer progression and metastasis. Cancer Metastasis Rev. 2010, 29, 207–222. [Google Scholar] [CrossRef]
- Veeramani, S.; Yuan, T.C.; Lin, F.F.; Lin, M.F. Mitochondrial redox signaling by p66Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells. Oncogene 2008, 27, 5057–5068. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Rajendran, M.; Alam, S.M.; Lin, F.F.; Cheng, P.W.; Lin, M.F. Steroids up-regulate p66Shc longevity protein in growth regulation by inhibiting its ubiquitination. PLoS ONE 2011, 6, e15942. [Google Scholar] [CrossRef]
- Sonne-Hansen, K.; Lykkesfeldt, A.E. Endogenous aromatization of testosterone results in growth stimulation of the human MCF-7 breast cancer cell line. J. Steroid Biochem. Mol. Biol. 2005, 93, 25–34. [Google Scholar] [CrossRef]
- Nativelle-Serpentini, C.; Lambard, S.; Seralini, G.E.; Sourdaine, P. Aromatase and breast cancer: W39R, an inactive protein. Eur. J. Endocrinol. 2002, 146, 583–589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burak, W.E., Jr.; Quinn, A.L.; Farrar, W.B.; Brueggemeier, R.W. Androgens influence estrogen-induced responses in human breast carcinoma cells through cytochrome P450 aromatase. Breast Cancer Res. Treat. 1997, 44, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Aka, J.A.; Mazumdar, M.; Chen, C.Q.; Poirier, D.; Lin, S.X. 17beta-hydroxysteroid dehydrogenase type 1 stimulates breast cancer by dihydrotestosterone inactivation in addition to estradiol production. Mol. Endocrinol. 2010, 24, 832–845. [Google Scholar] [CrossRef]
- Das, S.; Mitrovsky, G.; Vasanthi, H.R.; Das, D.K. Antiaging properties of a grape-derived antioxidant are regulated by mitochondrial balance of fusion and fission leading to mitophagy triggered by a signaling network of Sirt1-Sirt3-Foxo3-PINK1-PARKIN. Oxid. Med. Cell Longev. 2014, 2014, 345105. [Google Scholar] [CrossRef]
- Lin, H.Y.; Davis, P.J.; Tang, H.Y.; Mousa, S.A.; Luidens, M.K.; Hercbergs, A.H.; Davis, F.B. The pro-apoptotic action of stilbene-induced COX-2 in cancer cells: Convergence with the anti-apoptotic effect of thyroid hormone. Cell Cycle 2009, 8, 1877–1882. [Google Scholar] [CrossRef]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lansing, L.; Merillon, J.M.; Davis, F.B.; Tang, H.Y.; Shih, A.; Vitrac, X.; Krisa, S.; Keating, T.; Cao, H.J.; et al. Integrin alphaVbeta3 contains a receptor site for resveratrol. FASEB J. 2006, 20, 1742–1744. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.N.; Lin, V.C.; Rau, K.M.; Shieh, P.C.; Kuo, D.H.; Shieh, J.C.; Chen, W.J.; Tsai, S.C.; Way, T.D. Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J. Agric. Food Chem. 2010, 58, 1584–1592. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tang, H.Y.; Keating, T.; Wu, Y.H.; Shih, A.; Hammond, D.; Sun, M.; Hercbergs, A.; Davis, F.B.; Davis, P.J. Resveratrol is pro-apoptotic and thyroid hormone is anti-apoptotic in glioma cells: Both actions are integrin and ERK mediated. Carcinogenesis 2008, 29, 62–69. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tang, H.Y.; Shih, A.; Keating, T.; Cao, G.; Davis, P.J.; Davis, F.B. Thyroid hormone is a MAPK-dependent growth factor for thyroid cancer cells and is anti-apoptotic. Steroids 2007, 72, 180–187. [Google Scholar] [CrossRef]
- Huang, S.W.; Wu, C.Y.; Wang, Y.T.; Kao, J.K.; Lin, C.C.; Chang, C.C.; Mu, S.W.; Chen, Y.Y.; Chiu, H.W.; Chang, C.H.; et al. p53 modulates the AMPK inhibitor compound C induced apoptosis in human skin cancer cells. Toxicol. Appl. Pharmacol. 2013, 267, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Potter, D.A. CYP1A1 regulates breast cancer proliferation and survival. Mol. Cancer Res. 2013, 11, 780–792. [Google Scholar] [CrossRef]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Hurst, D.; Taylor, E.B.; Cline, T.D.; Greenwood, L.J.; Compton, C.L.; Lamb, J.D.; Winder, W.W. AMP-activated protein kinase kinase activity and phosphorylation of AMP-activated protein kinase in contracting muscle of sedentary and endurance-trained rats. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E710–E715. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Connors, K.E.; Yang, D.Q. AICAR induces phosphorylation of AMPK in an ATM-dependent, LKB1-independent manner. Mol. Cell Biochem. 2007, 306, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Sun, M.; Tang, H.Y.; Simone, T.M.; Wu, Y.H.; Grandis, J.R.; Cao, H.J.; Davis, P.J.; Davis, F.B. Resveratrol causes COX-2- and p53-dependent apoptosis in head and neck squamous cell cancer cells. J. Cell Biochem. 2008, 104, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Crawford, D.R.; Lin, S.; Hwang, J.; Sebuyira, A.; Meng, R.; Westfall, J.E.; Tang, H.Y.; Lin, S.; Yu, P.Y.; et al. Inducible COX-2-dependent apoptosis in human ovarian cancer cells. Carcinogenesis 2011, 32, 19–26. [Google Scholar] [CrossRef]
- Lin, H.Y.; Delmas, D.; Vang, O.; Hsieh, T.C.; Lin, S.; Cheng, G.Y.; Chiang, H.L.; Chen, C.E.; Tang, H.Y.; Crawford, D.R.; et al. Mechanisms of ceramide-induced COX-2-dependent apoptosis in human ovarian cancer OVCAR-3 cells partially overlapped with resveratrol. J. Cell Biochem. 2013, 114, 1940–1954. [Google Scholar] [CrossRef]
- Schmitz, K.J.; Callies, R.; Wohlschlaeger, J.; Kimmig, R.; Otterbach, F.; Bohr, J.; Lee, H.S.; Takeda, A.; Schmid, K.W.; Baba, H.A. Overexpression of cyclo-oxygenase-2 is an independent predictor of unfavourable outcome in node-negative breast cancer, but is not associated with protein kinase B (Akt) and mitogen-activated protein kinase (ERK1/2, p38) activation or with Her-2/neu signalling pathways. J. Clin. Pathol. 2006, 59, 685–691. [Google Scholar] [PubMed]
- Perdiki, M.; Korkolopoulou, P.; Thymara, I.; Agrogiannis, G.; Piperi, C.; Boviatsis, E.; Kotsiakis, X.; Angelidakis, D.; Diamantopoulou, K.; Thomas-Tsagli, E.; et al. Cyclooxygenase-2 expression in astrocytomas. Relationship with microvascular parameters, angiogenic factors expression and survival. Mol. Cell Biochem. 2007, 295, 75–83. [Google Scholar] [CrossRef]
- Harris, R.E. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology 2009, 17, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Gao, M.; Dong, W.; Hu, M.; Li, J.; Shi, X.; Hao, Y.; Li, Y.; Huang, C. p85alpha mediates p53 K370 acetylation by p300 and regulates its promoter-specific transactivity in the cellular UVB response. Oncogene 2011, 30, 1360–1371. [Google Scholar] [CrossRef]
- Kalkhoven, E. CBP and p300: HATs for different occasions. Biochem. Pharmacol. 2004, 68, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Shih, A.; Cao, H.J.; Davis, F.B.; Davis, P.J.; Lin, H.Y. Resveratrol-induced cyclooxygenase-2 facilitates p53-dependent apoptosis in human breast cancer cells. Mol. Cancer Ther. 2006, 5, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Bobermin, L.D.; Latini, A.; Wajner, M.; Souza, D.O.; Goncalves, C.A.; Gottfried, C. Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS ONE 2013, 8, e64372. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Guisado, E.; Merino, J.M.; Mulero-Navarro, S.; Lorenzo-Benayas, M.J.; Centeno, F.; Alvarez-Barrientos, A.; Fernandez-Salguero, P.M. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int. J. Cancer 2005, 115, 74–84. [Google Scholar] [CrossRef]
- Sareen, D.; Darjatmoko, S.R.; Albert, D.M.; Polans, A.S. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol. Pharmacol. 2007, 72, 1466–1475. [Google Scholar] [CrossRef]
- Wang, Y.; Romigh, T.; He, X.; Orloff, M.S.; Silverman, R.H.; Heston, W.D.; Eng, C. Resveratrol regulates the PTEN/AKT pathway through androgen receptor-dependent and -independent mechanisms in prostate cancer cell lines. Hum. Mol. Genet. 2010, 19, 4319–4329. [Google Scholar] [CrossRef]
- Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Kempf, R.C.; Long, J.; Laidler, P.; Mijatovic, S.; Maksimovic-Ivanic, D.; Stivala, F.; Mazzarino, M.C.; et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging 2011, 3, 192–222. [Google Scholar] [CrossRef]
- Bose, S.; Crane, A.; Hibshoosh, H.; Mansukhani, M.; Sandweis, L.; Parsons, R. Reduced expression of PTEN correlates with breast cancer progression. Hum. Pathol. 2002, 33, 405–409. [Google Scholar] [CrossRef]
- Kappes, H.; Goemann, C.; Bamberger, A.M.; Loning, T.; Milde-Langosch, K. PTEN expression in breast and endometrial cancer: Correlations with steroid hormone receptor status. Pathobiology 2001, 69, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.A.; Sinden, M.R.; Eng, C. Phytoestrogen exposure elevates PTEN levels. Hum. Mol. Genet. 2005, 14, 1457–1463. [Google Scholar] [CrossRef]
- Harada, N.; Atarashi, K.; Murata, Y.; Yamaji, R.; Nakano, Y.; Inui, H. Inhibitory mechanisms of the transcriptional activity of androgen receptor by resveratrol: Implication of DNA binding and acetylation of the receptor. J. Steroid Biochem. Mol. Biol. 2011, 123, 65–70. [Google Scholar] [CrossRef]
- Shi, W.F.; Leong, M.; Cho, E.; Farrell, J.; Chen, H.C.; Tian, J.; Zhang, D. Repressive effects of resveratrol on androgen receptor transcriptional activity. PLoS ONE 2009, 4, e7398. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, Y.H.; Choi, K.C.; Seong, A.R.; Choi, H.K.; Lee, O.H.; Hwang, H.J.; Yoon, H.G. Grape seed extract regulates androgen receptor-mediated transcription in prostate cancer cells through potent anti-histone acetyltransferase activity. J. Med. Food 2011, 14, 9–16. [Google Scholar] [CrossRef]
- Brass, A.L.; Barnard, J.; Patai, B.L.; Salvi, D.; Rukstalis, D.B. Androgen up-regulates epidermal growth factor receptor expression and binding affinity in PC3 cell lines expressing the human androgen receptor. Cancer Res. 1995, 55, 3197–3203. [Google Scholar] [PubMed]
- Zheng, Y.; Izumi, K.; Yao, J.L.; Miyamoto, H. Dihydrotestosterone upregulates the expression of epidermal growth factor receptor and ERBB2 in androgen receptor-positive bladder cancer cells. Endocr. Relat. Cancer 2011, 18, 451–464. [Google Scholar] [CrossRef]
- Davidson, N.E.; Gelmann, E.P.; Lippman, M.E.; Dickson, R.B. Epidermal growth factor receptor gene expression in estrogen receptor-positive and negative human breast cancer cell lines. Mol. Endocrinol. 1987, 1, 216–223. [Google Scholar] [CrossRef]
- Kao, R.T.; Hall, J.; Stern, R. Collagen and elastin synthesis in human stroma and breast carcinoma cell lines: Modulation by the extracellular matrix. Connect. Tissue Res. 1986, 14, 245–255. [Google Scholar] [CrossRef]
- Shekhar, M.P.; Werdell, J.; Santner, S.J.; Pauley, R.J.; Tait, L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: Implications for tumor development and progression. Cancer Res. 2001, 61, 1320–1326. [Google Scholar]
- Evangelou, A.; Letarte, M.; Marks, A.; Brown, T.J. Androgen modulation of adhesion and antiadhesion molecules in PC-3 prostate cancer cells expressing androgen receptor. Endocrinology 2002, 143, 3897–3904. [Google Scholar] [CrossRef]
- Liegibel, U.M.; Sommer, U.; Tomakidi, P.; Hilscher, U.; Van Den Heuvel, L.; Pirzer, R.; Hillmeier, J.; Nawroth, P.; Kasperk, C. Concerted action of androgens and mechanical strain shifts bone metabolism from high turnover into an osteoanabolic mode. J. Exp. Med. 2002, 196, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Engebraaten, O.; Trikha, M.; Juell, S.; Garman-Vik, S.; Fodstad, O. Inhibition of in vivo tumour growth by the blocking of host alpha(v)beta3 and alphaII(b)beta3 integrins. Anticancer Res. 2009, 29, 131–137. [Google Scholar] [PubMed]
- Takayama, S.; Ishii, S.; Ikeda, T.; Masamura, S.; Doi, M.; Kitajima, M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res. 2005, 25, 79–83. [Google Scholar] [PubMed]
- Weis, S.M.; Cheresh, D.A. alphaV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 2011, 1, a006478. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; O’Connell, M.J.; Miyamoto, H.; Huang, J.; Yao, J.L.; Messing, E.M.; Reeder, J.E. Androgenic dependence of exophytic tumor growth in a transgenic mouse model of bladder cancer: A role for thrombospondin-1. BMC Urol. 2008, 8, 7. [Google Scholar] [CrossRef]
- Belleri, M.; Ribatti, D.; Savio, M.; Stivala, L.A.; Forti, L.; Tanghetti, E.; Alessi, P.; Coltrini, D.; Bugatti, A.; Mitola, S.; et al. alphavbeta3 Integrin-dependent antiangiogenic activity of resveratrol stereoisomers. Mol. Cancer Ther. 2008, 7, 3761–3770. [Google Scholar] [CrossRef]
- Klinge, C.M.; Wickramasinghe, N.S.; Ivanova, M.M.; Dougherty, S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008, 22, 2185–2197. [Google Scholar] [CrossRef]
- Shibata, Y.; Kashiwagi, B.; Arai, S.; Fukabori, Y.; Suzuki, K.; Honma, S.; Yamanaka, H. Direct regulation of prostate blood flow by vascular endothelial growth factor and its participation in the androgenic regulation of prostate blood flow in vivo. Endocrinology 2004, 145, 4507–4512. [Google Scholar] [CrossRef]
- Sordello, S.; Bertrand, N.; Plouet, J. Vascular endothelial growth factor is up-regulated in vitro and in vivo by androgens. Biochem. Biophys. Res. Commun. 1998, 251, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.G.; Yoneda, T.; Clark, G.M.; Yee, D. Elevated levels of p66 Shc are found in breast cancer cell lines and primary tumors with high metastatic potential. Clin. Cancer Res. 2000, 6, 1135–1139. [Google Scholar] [PubMed]
- Lin, M.T.; Yen, M.L.; Lin, C.Y.; Kuo, M.L. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol. Pharmacol. 2003, 64, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, T.; Lopez, E.; Wu, W.; Wang, X.; Cao, J.; Teng, L. The therapeutic target of estrogen receptor-alpha36 in estrogen-dependent tumors. J. Transl. Med. 2014, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Davis, F.B.; Luidens, M.K.; Hercbergs, A.; Mousa, S.A.; Bharali, D.J.; Davis, P.J. Newly-Recognized Small Molecule Receptors on Human Breast Cancer Cell Integrin αvβ3 that Affect Tumor Cell Behavior, Targeting New Pathways and Cell Death in Breast Cancer; InTech: Rijeka, Croatia, 2012; Available online: https://www.intechopen.com/books/targeting-new-pathways-and-cell-death-in-breast-cancer/newly-recognized-small-molecule-receptors-on-human-breast-cancer-cell-integrin-alphavbeta3-that-affe (accessed on 18 April 2020).
- Kim, T.H.; Shin, Y.J.; Won, A.J.; Lee, B.M.; Choi, W.S.; Jung, J.H.; Chung, H.Y.; Kim, H.S. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim. Biophys. Acta 2014, 1840, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Leon-Galicia, I.; Diaz-Chavez, J.; Garcia-Villa, E.; Uribe-Figueroa, L.; Hidalgo-Miranda, A.; Herrera, L.A.; Alvarez-Rios, E.; Garcia-Mena, J.; Gariglio, P. Resveratrol induces downregulation of DNA repair genes in MCF-7 human breast cancer cells. Eur. J. Cancer Prev. 2013, 22, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Nucci, D.; Vannini, S.; Villarini, M.; Moretti, M. In vitro Biological Effects of Sulforaphane (SFN), Epigallocatechin-3-gallate (EGCG), and Curcumin on Breast Cancer Cells: A Systematic Review of the Literature. Nutr. Cancer 2017, 69, 969–978. [Google Scholar] [CrossRef]
- Gianfredi, V.; Vannini, S.; Moretti, M.; Villarini, M.; Bragazzi, N.L.; Izzotti, A.; Nucci, D. Sulforaphane and Epigallocatechin Gallate Restore Estrogen Receptor Expression by Modulating Epigenetic Events in the Breast Cancer Cell Line MDA-MB-231: A Systematic Review and Meta-Analysis. J. Nutrigenet. Nutrigenom. 2017, 10, 126–135. [Google Scholar] [CrossRef]
| Hormone | Receptor | Functions | References |
|---|---|---|---|
| Estrogen | Estrogen Receptor-α (ER-α) | To form ligand-ER complex and controlling gene expression. To stimulate proliferation of breast cancer cells | [7] |
| Integrin αvβ3 | NA | [8] | |
| DHT | Androgen Receptor (AR) | To form ligand-AR complex and controlling gene expression To stimulate proliferation of prostate cancer cells. | [9] |
| Estrogen Receptor-α (ER-α) | To stimulate proliferation of ER-positive breast cancer cells | [7] | |
| Integrin αvβ3 | To stimulate proliferation of ER-negative breast cancer cells | [10] | |
| Thyroid hormone | Thyroid hormone Receptor-α (TR-α) | To stimulate cancer cell growth | [11] |
| Thyroid hormone Receptor-β (TR-β) | To inhibit cancer cell growth, however, mutant TR-β may activate cancer cell growth | [12] | |
| Integrin αvβ3 | To stimulate cancer cell growth | [13] |
| Resveratrol | DHT | |
|---|---|---|
| Binding Site | Integrin αvβ3 | Integrin αvβ3/ER-α/AR |
| ERK1/2 | ↑ | ↑ |
| PI-3K | ↓ | ↑ |
| AKT | ↓ | ↑ |
| PTEN | ↑ | ↓ |
| Resveratrol | DHT | |
|---|---|---|
| Integrin | β3 ↑ | α2β1 ↓ |
| EGFR | ↓ | ↑ |
| VEGFR | ─ | ↑ |
| VEGF | ↓ | ↑ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, Y.; Li, Z.-L.; Shih, Y.-J.; Chen, Y.-R.; Wang, K.; Whang-Peng, J.; Lin, H.-Y.; Davis, P.J. Integrin αvβ3 in the Mediating Effects of Dihydrotestosterone and Resveratrol on Breast Cancer Cell Proliferation. Int. J. Mol. Sci. 2020, 21, 2906. https://doi.org/10.3390/ijms21082906
Ho Y, Li Z-L, Shih Y-J, Chen Y-R, Wang K, Whang-Peng J, Lin H-Y, Davis PJ. Integrin αvβ3 in the Mediating Effects of Dihydrotestosterone and Resveratrol on Breast Cancer Cell Proliferation. International Journal of Molecular Sciences. 2020; 21(8):2906. https://doi.org/10.3390/ijms21082906
Chicago/Turabian StyleHo, Yih, Zi-Lin Li, Ya-Jung Shih, Yi-Ru Chen, Kuan Wang, Jacqueline Whang-Peng, Hung-Yun Lin, and Paul J. Davis. 2020. "Integrin αvβ3 in the Mediating Effects of Dihydrotestosterone and Resveratrol on Breast Cancer Cell Proliferation" International Journal of Molecular Sciences 21, no. 8: 2906. https://doi.org/10.3390/ijms21082906
APA StyleHo, Y., Li, Z.-L., Shih, Y.-J., Chen, Y.-R., Wang, K., Whang-Peng, J., Lin, H.-Y., & Davis, P. J. (2020). Integrin αvβ3 in the Mediating Effects of Dihydrotestosterone and Resveratrol on Breast Cancer Cell Proliferation. International Journal of Molecular Sciences, 21(8), 2906. https://doi.org/10.3390/ijms21082906






