Endocannabinoid-Mediated Neuromodulation in the Olfactory Bulb: Functional and Therapeutic Significance
Abstract
1. Introduction
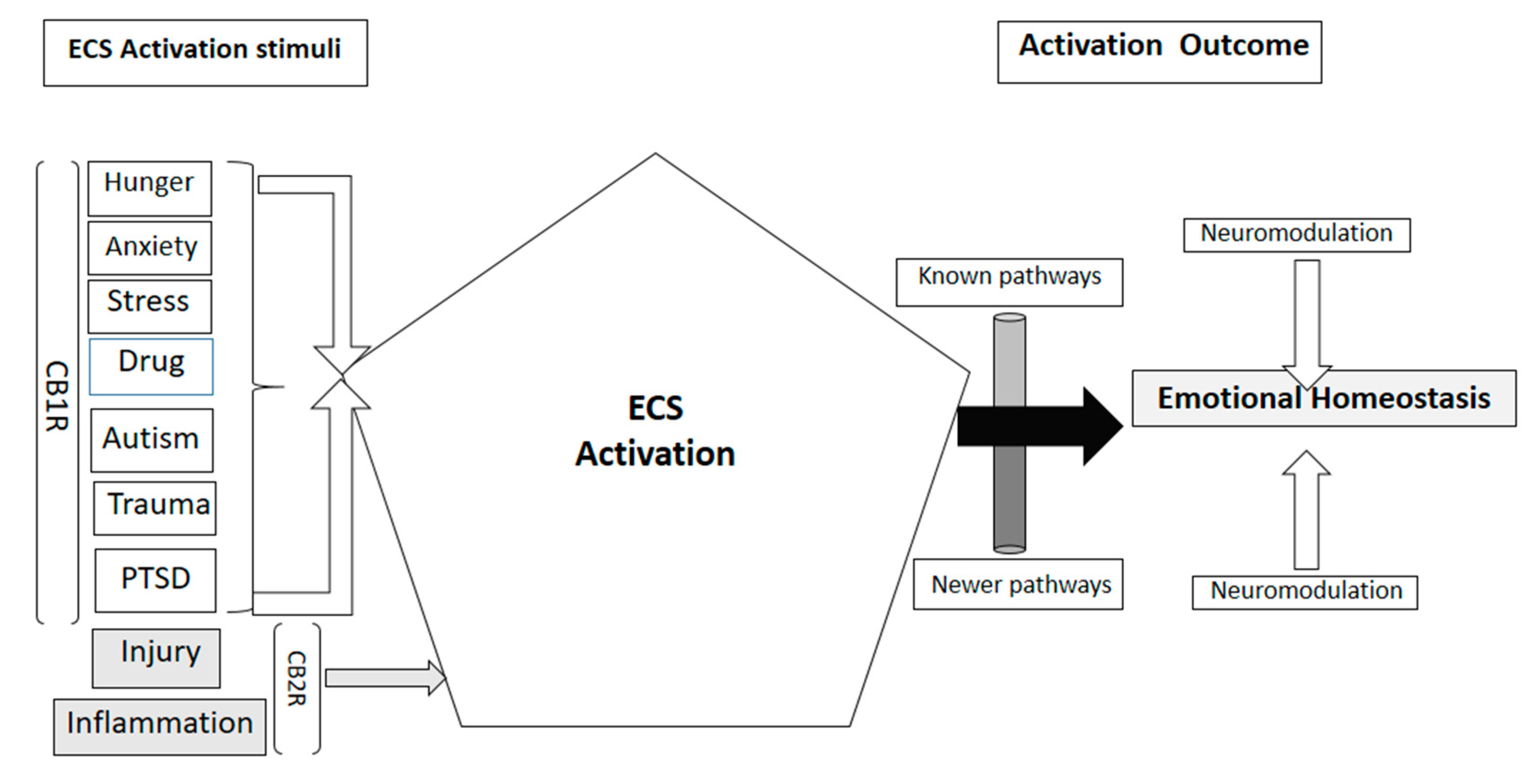
2. Endocannabinoid Signaling System Components in Biological Functions
3. Endocannabinoid Participation in Neurodegenerative Pathology
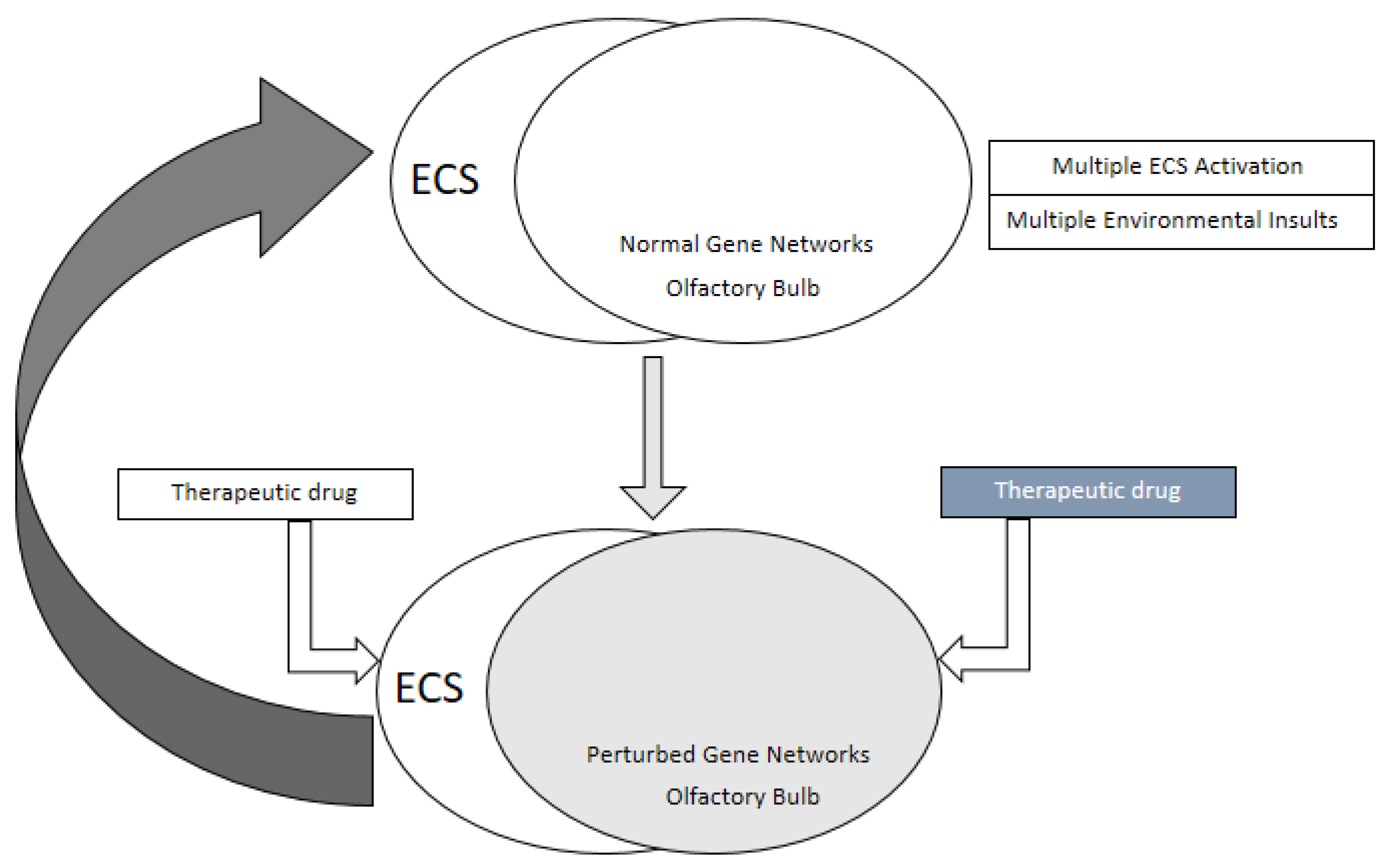
4. Endocannabinoid Mediated Signaling in the Olfactory Bulb
5. Olfactory Dysfunction and Neurodegenerative Pathology
6. Olfactory Capacity and Cellular Changes in Neurodegeneration
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Marzo, V.D.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating endocannabinoids: From whence do they come and where are they going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.R.; Watts, J.J.; Boileau, I.; Tong, J.; Mizrahi, R. A systematic review of phytocannabinoid exposure on the endocannabinoid system: Implications for psychosis. Neuropsychopharmacology 2019, 29, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.I.; Nicoll, R.A. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature 2001, 410, 588–592. [Google Scholar] [CrossRef]
- Heinbockel, T.; Wang, Z. Cellular Mechanisms of Action of Drug Abuse on Olfactory Neurons. Int. J. Environ. Res. Public Health 2016, 13, 5. [Google Scholar] [CrossRef]
- Wang, Z.J.; Hu, S.S.; Bradshaw, H.B.; Sun, L.; Mackie, K.; Straiker, A.; Heinbockel, T. Cannabinoid receptor-mediated modulation of inhibitory inputs to mitral cells in the main olfactory bulb. J. Neurophysiol. 2019, 122, 749–759. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Regehr, W.G. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J. Neurosci. 2001, 21, RC174. [Google Scholar] [CrossRef]
- Bisogno, T.; Oddi, S.; Piccoli, A.; Fazio, D.; Maccarrone, M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol. Res. 2016, 111, 721–730. [Google Scholar] [CrossRef]
- Ibarra-Lecue, I.; Pilar-Cuéllar, F.; Muguruza, C.; Florensa-Zanuy, E.; Diaz, A.; Urigüen, L.; Castro, E.; Pazos, A.; Callado, L.F. The endocannabinoid system in mental disorders: Evidence from human brain studies. Biochem. Pharmacol. 2018, 157, 97–107. [Google Scholar] [CrossRef]
- Stumm, C.; Hiebel, C.; Hanstein, R.; Purrio, M.; Nagel, H.; Conrad, A.; Lutz, B.; Behl, C.; Clement, A.B. Cannabinoid receptor 1 deficiency in a mouse model of Alzheimer’s disease leads to enhanced cognitive impairment despite of a reduction in amyloid deposition. Neurobiol. Aging 2013, 34, 2574–2584. [Google Scholar] [CrossRef]
- Poleszak, E.; Wosko, S.; Slawinska, K.; Szopa, A.; Wrobel, A.; Serefko, A. Cannabinoids in depressive disorders. Life Sci. 2018, 213, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.; Xi, Z.X. Progress in brain cannabinoid CB2 receptors: From gene to behavior. Neurosci. Biobehav. Rev. 2019, 98, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, R.; Dawe, G.S. OBscure but not OBsolete: Perturbations of the frontal cortex in common between rodent olfactory bulbectomy model and major depression. J. Chem. Neuroanat. 2018, 91, 63–100. [Google Scholar] [CrossRef] [PubMed]
- Wachowiak, M. All in a Sniff: Olfaction as a Model for Active Sensing. Neuron 2011, 71, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Brunert, D.; Tsuno, Y.; Rothermel, M.; Shipley, M.T.; Wachowiak, M. Cell-type-specific modulation of sensory responses in olfactory bulb circuits by serotonergic projections from the raphe nuclei. J. Neurosci. 2016, 36, 6820–6835. [Google Scholar] [CrossRef] [PubMed]
- Mouro, F.M.; Köfalvi, A.; André, L.A.Y.; Baqi, Y.; Müller, C.E.; Ribeiro, J.A.; Sebastião, A.M. Memory deficits induced by chronic cannabinoid exposure are prevented by adenosine A2AR receptor antagonism. Neuropharmacology 2019, 155, 10–21. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Shang, C.; Yan, F.; Shi, Y.; Zhang, J.; Qu, B.; Han, H.; Wang, Y.; Li, D.; et al. IGF1-dependent synaptic plasticity of mitral cells in olfactory memory during social learning. Neuron 2017, 95, 106–122. [Google Scholar] [CrossRef]
- Shang, M.; Xing, J. Blocking of Dendrodendritic Inhibition Unleashes Widely Spread Lateral Propagation of Odor-evoked Activity in the Mouse Olfactory Bulb. Neuroscience 2018, 391, 50–59. [Google Scholar] [CrossRef]
- Hutch, C.R.; Hillard, C.J.; Jia, C.; Hegg, C.C. An endocannabinoid system is present in the mouse olfactory epithelium but does not modulate olfaction. Neuroscience 2015, 300, 539–553. [Google Scholar] [CrossRef]
- Hawkes, C. Olfaction in neurodegenerative disorders. Mov. Disord. 2003, 18, 364–372. [Google Scholar] [CrossRef]
- Kovacs, T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res. 2004, 3, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C. Olfaction in neurodegenerative disorder. Adv. Otorhinolaryngol. 2006, 63, 133–151. [Google Scholar] [PubMed]
- Alves, J. Olfactory dysfunction in dementia. World J. Clin. Case 2014, 2, 661. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kuan, W.L.; Spillantini, M.G. Progressive tauopathy in P301S tau transgenic mice is associated with a functional deficit of the olfactory system. Eur. J. Neurosci. 2016, 44, 2396–2403. [Google Scholar] [CrossRef]
- Rey, N.L.; Wesson, D.W.; Brundin, P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol. Dis. 2018, 109, 226–248. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Wu, X.; Li, J.; Yang, J.; Tu, C.; Ye, X.; Ling, S. Olfactory deficit is associated with mitral cell dysfunction in the olfactory bulb of P301S tau transgenic mice. Brain Res. Bull. 2019, 148, 34–45. [Google Scholar] [CrossRef]
- Lachén-Montes, M.; González-Morales, A.; Schvartz, D.A.; Zelaya, M.V.; Ausin, K.; Fernández-Irigoyen, J.; Sánchez, J.C.; Santamaria, E. The olfactory bulb prototype differs across frontotemporal dementia spectrum. J. Proteom. 2019, 201, 37–47. [Google Scholar] [CrossRef]
- Tan, L.; Li, Q.; Xie, X.S. Olfactory sensory neurons transiently express multiple olfactory receptors during development. Mol. Syst. Biol. 2015, 11, 844. [Google Scholar] [CrossRef]
- Hanchate, N.K.; Kondoh, K.; Lu, Z.; Kuang, D.; Ye, X.; Qiu, X.; Pachter, L.; Trapnell, C.; Buck, L.B. Single-cell transcriptomics reveals receptor transformations during olfactory neurogenesis. Science 2015, 350, 1251–1255. [Google Scholar] [CrossRef]
- Lötsch, J.; Schaeffeler, E.; Mittelbronn, M.; Winter, S.; Gudziol, V.; Schwarzacher, S.W.; Hummel, T.; Doehring, A.; Schwab, M.; Ultsch, A. Functional genomics suggest neurogenesis in the adult human olfactory bulb. Brain Struct. Funct. 2014, 219, 1991. [Google Scholar] [CrossRef]
- Simon, V.; Cota, D. Mechanisms in endocrinology: Endocannabinoids and metabolism: Past, present and future. Eur. J. Endocrinal. 2017, 176, R309–R324. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Guzman, M.; Mackie, K.; Doherty, P.; Harkany, T. Programming of neural cells by (endo) cannabinoids: From physiological rules to emerging therapies. Nat. Rev. Neurosci. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Muzik, O.; Diwadkar, V.A. Hierarchical control systems for the regulation of physiological homeostasis and affect: Can their interactions modulate mood and anhedonia? Neurosci. Biobehav. Rev. 2019, 105, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar]
- Xi, Z.X.; Peng, X.Q.; Li, X.; Song, R.; Zhang, H.Y.; Liu, Q.R.; Yang, H.J.; Bi, G.H.; Li, J.; Gardner, E.L. Brain cannabinoid CB receptors modulate cocaine’s actions in mice. Nat. Neurosci. 2011, 14, 1160–1166. [Google Scholar] [CrossRef]
- Pistis, M.; Melis, M. From surface to nuclear receptors: The endocannabinoid family extends its assets. Curr. Med. Chem. 2010, 17, 1450–1467. [Google Scholar] [CrossRef]
- Moriconi, A.; Cerbara, I.; Maccarrone, M.; Topai, A. GPR55: Current knowledge and future perspectives of a purported “Type-3” cannabinoid receptor. Curr. Med. Chem. 2010, 17, 1411–1429. [Google Scholar] [CrossRef]
- Piazza, P.V.; Cota, D.; Marsicano, G. The CB1 receptor as the cornerstone of exostasis. Neuron 2017, 93, 1252–1274. [Google Scholar] [CrossRef]
- Laurikainen, H.; Tuominen, L.; Tikka, M.; Merisaari, H.; Armio, R.L.; Sormunen, E.; Borgan, F.; Veronese, M.; Howes, O.; Haaparanta-Solin, M.; et al. Sex difference in brain CB1 receptor availability in man. NeuroImage 2019, 184, 834–842. [Google Scholar] [CrossRef]
- Buckley, N.E. The peripheral cannabinoid receptor knockout mice: An update. Br. J. Pharmacol. 2008, 153, 309–318. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.E.; McCoy, K.L.; Mezey, E.; Bonner, T.; Zimmer, A.; Felder, C.C.; Glass, M.; Zimmer, A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur. J. Pharmacol. 2000, 396, 141–149. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, M.S.; Navarrete, F.; Navarro, G.; Reyes-Resin, I.; Franco, R.; Lanciego, J.L.; Giner, S.; Manzanares, J. Alterations in gene and protein expression of cannabinoid CB2 and GPR55 receptors in the dorsolateral prefrontal cortex of suicide victims. Neurotherapeutics 2018, 15, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, J.; Cabañero, D.; Puente, N.; García-Gutiérrez, M.S.; Grandes, P.; Maldonado, R. Role of the endocannabinoid system in drug addiction. Biochem. Pharmacol. 2018, 157, 108–121. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, M.S.; Manzanares, J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J. Psychopharmacol. 2011, 25, 111–120. [Google Scholar] [CrossRef]
- Smaga, I.; Jastrzębska, J.; Zaniewska, M.; Bystrowska, B.; Gawliński, D.; Faron-Górecka, A.; Broniowska, Ż.; Miszkiel, J.; Filip, M. Changes in the Brain Endocannabinoid System in Rat Models of Depression. Neurotox. Res. 2017, 31, 421–435. [Google Scholar] [CrossRef]
- Wilker, S.; Pfeiffer, A.; Elbert, T.; Ovuga, E.; Karabatsiakis, A.; Krumbholz, A.; Kolassa, I.T. Endocannabinoid concentrations in hair are associated with PTSD symptom severity. Psychoneuroendocrinology 2016, 67, 198–206. [Google Scholar] [CrossRef]
- Szűcs, E.; Dvorácskó, S.; Tömböly, C.; Büki, A.; Kékesi, G.; Horváth, G.; Benyhe, S. Decreased CB receptor binding and cannabinoid signaling in three brain regions of a rat model of schizophrenia. Neurosci. Lett. 2016, 633, 87–93. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabala, M.; Parolaro, D. The endocannabinoid system and autism spectrum disorders: Insights from animal models. Int. J. Mol. Sci. 2017, 18, 1916. [Google Scholar] [CrossRef]
- Cabranes, A.; Venderova, K.; de Lago, E.; Fezza, F.; Sánchez, A.; Mestre, L.; Valenti, M.; García-Merino, A.; Ramos, J.A.; Di Marzo, V.; et al. Decreased endocannabinoid levels in the brain and beneficial effects of agents activating cannabinoid and/or vanilloid receptors in a rat model of multiple sclerosis. Neurobiol. Dis. 2005, 20, 207–217. [Google Scholar] [CrossRef]
- Loría, F.; Petrosino, S.; Mestre, L.; Spagnolo, A.; Correa, F.; Hernangómez, M.; Guaza, C.; Di Marzo, V.; Docagne, F. Study of the regulation of the endocannabinoid system in a virus model of multiple sclerosis reveals a therapeutic effect of palmitoylethanolamide. Eur. J. Neurosci. 2008, 28, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Cencioni, M.T.; Bisicchia, E.; De Bardi, M.; Gasperini, C.; Borsellino, G.; Centonze, D.; Battistini, L.; Maccarrone, M. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann. Neurol. 2013, 73, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Sánchez López, A.J.; Román-Vega, L.; Ramil Tojeiro, E.; Giuffrida, A.; García-Merino, A. Regulation of cannabinoid receptor gene expression and endocannabinoid levels in lymphocyte subsets by interferon-β: A longitudinal study in multiple sclerosis patients. Clin. Exp. Immunol. 2015, 179, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Maldonado, R.; Casas, M.; Henze, T.; Centonze, D. Cannabinoids therapeutic use: What is our current understanding following the introduction of THC, THC: CBD oromucosal spray and others? Exp. Rev. Clin. Pharmacol. 2017, 10, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; van der Stelt, M.; Centonze, D.; Maccarrone, M. The endocannabinoid system and its therapeutic exploitation in multiple sclerosis: Clues for other neuroinflammatory diseases. Prog. Neurobiol. 2018, 160, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.G.S.; Neuhofer, D.; Manzoni, O.J.J. The Endocannabinoid System in Fragile X Syndrome; Syndrome, F.X., Rob Willemsen, R., Kooy, F., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 241–259. [Google Scholar]
- Bloomfield, M.A.P.; Hindocha, C.; Green, S.F.; Wall, M.B.; Lees, R.; Petrilli, K.; Costello, H.; Olabisi Ogunbiyi, M.; Bossong, M.G.; Freeman, T.P. The Neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol. Ther. 2019, 195, 132–161. [Google Scholar] [CrossRef]
- Eisenstein, S.A.; Clapper, J.R.; Holmes, P.V.; Piomelli, D.; Hohmann, A.G. A role for 2-arachidonoylglycerol and endocannabinoid signaling in the locomotor response to novelty induced by olfactory bulbectomy. Pharmacol. Res. 2010, 61, 419–429. [Google Scholar] [CrossRef]
- Albeanu, D.F.; Provost, A.C.; Agarwal, P.; Soucy, E.R.; Zak, J.D.; Murthy, V.N. Olfactory marker protein (omp) regulates formation and refinement of the olfactory glomerular map. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Breunig, E.; Czesnik, D.; Piscitelli, F.; Di Marzo, V.; Manzini, I.; Schild, D. Endocannabinoid modulation in the olfactory epithelium. Results. Probl. Cell. Differ. 2010, 52, 139–145. [Google Scholar]
- Soria-Gómez, E.; Bellocchio, L.; Reguero, L.; Lepousez, G.; Martin, C.; Bendahmane, M.; Ruehle, S.; Remmers, F.; Desprez, T.; Matias, I.; et al. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 2014, 17, 407–415. [Google Scholar] [CrossRef]
- Pouille, F.; Schoppa, N.E. Cannabinoid receptors modulate excitation of an olfactory bulb local circuit by cortical feedback. Front. Cell. Neurosci. 2018, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, M.T.; Vázquez, M.; Lomazzo, E.; Hofmann, C.; Blanco, R.N.; Alén, F.; Antón, M.; Decara, J.; Arco, R.; Orio, L.; et al. A moderate diet restriction during pregnancy alters the levels of endocannabinoids and endocannabinoid-related lipids in the hypothalamus, hippocampus and olfactory bulb of rat offspring in a sex-specific manner. PLoS ONE 2017, 12, e0174307. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Oertel, B.G.; Felden, L.; Nöth, U.; Deichmann, R.; Lötsch, J. The effects of delta-9-tetrahydrocannabinol on nasal chemosensitivity: A pharmacological fMRI study in healthy volunteers. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 75. [Google Scholar]
- Lotsch, J.; Geisslinger, G.; Hummel, T. Sniffing out pharmacology: Interactions of drugs with human olfaction. Trends Pharmacol. Sci. 2012, 33, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Elbatsh, M.M.; Moklas, M.A.; Marsden, C.A.; Kendall, D.A. Antidepressant-like effects of Δ9-tetrahydrocannabinol and rimonabant in the olfactory bulbectomised rat model of depression. Pharmacol. Biochem. Behav. 2012, 102, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Albers, M.W.; Tabert, M.H.; Devanand, D.P. Olfactory dysfunction as a predictor of neurodegenerative disease. Curr. Neurol. Neurosci. Rep. 2006, 6, 379–386. [Google Scholar] [CrossRef]
- Doty, R.L.; Kamath, V. The influences of age on olfaction: A review. Front. Psychol. 2014, 5, 20. [Google Scholar] [CrossRef]
- Mobley, A.S.; Rodriguez-Gil, D.J.; Imamura, F.; Greer, C.A. Aging in the olfactory system. Trends Neurosci. 2013, 37, 77–84. [Google Scholar] [CrossRef]
- Huttenbrink, K.-B.; Hummel, T.; Berg, D.; Gasser, T.; Hähner, A. Olfactory dysfunction: Common in later life and early warning of neurodegenerative disease. Dtsch. Arztebl. Int. 2013, 110, 1. [Google Scholar]
- Doty, R.L.; Hawkes, C.H.; Good, K.P.; Duda, J.E. Handbook of Olfaction and Gustation; Doty, R.L., Ed.; John Wiley and sons: Hoboken, NJ, USA, 2015; p. 403. [Google Scholar]
- Marin, C.; Vilas, D.; Langdon, C.; Alobid, I.; López-Chacón, M.; Haehner, A.; Hummel, T.; Mullol, J. Olfactory dysfunction in neurodegenerative diseases. Curr. Allergy Asthma Rep. 2018, 18, 42. [Google Scholar] [CrossRef]
- Doty, R.L. Olfactory dysfunction in neurodegenerative diseases: Is there a common pathological substrate? Lancet Neurol. 2017, 16, 478–488. [Google Scholar] [CrossRef]
- Waldton, S. Clinical observations of impaired cranial nerve function in senile dementia. Acta Psychiatr. Scand. 1974, 50, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.H.; Raji, C.A.; Maceachern, M.P.; Burke, J.F. Olfactory identification testing as a predictor of the development of Alzheimer’s dementia: A systematic review. Laryngoscope 2012, 122, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.M.; Lu, D.; Liu, L.P.; Zhang, H.H.; Zhou, Y.Y. Olfactory dysfunction in Alzheimer’s disease. Neuropsychiatry Dis. Treat. 2016, 12, 869–875. [Google Scholar] [CrossRef]
- Doty, R.L.; Ferguson-Segall, M.; Lucki, I.; Kreider, M. Effects of intrabulbar injections of hydroxydopamine on ethyl acetate odor detection in castrate and non-castrate male rats. Brain Res. 1988, 444, 95–103. [Google Scholar] [CrossRef]
- Haener, A.; Hummel, T.; Reichmann, H. A clinical approach towards smell loss in Parkinson’s disease. J. Parkinsons Dis. 2014, 4, 189–195. [Google Scholar] [CrossRef]
- Camargo, C.H.F.; Jobbins, V.A.; Serpa, R.A.; Berbetz, F.A.; Sabatini, J.S.; Teive, H.A.G. Association between olfactory loss and cognitive deficits in Parkinson’s disease. Clin. Neurol. Neurosurg. 2018, 173, 120–123. [Google Scholar] [CrossRef]
- Luzzi, S.; Snowden, J.S.; Neary, D.; Coccia, M.; Provinciali, L.; Ralph, M.A.L. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 2007, 45, 1823–1831. [Google Scholar] [CrossRef]
- McLaughlin, N.C.; Westervelt, H.J. Odor identification deficits in frontotemporal dementia: A preliminary study. Arch. Clin. Neuropsychol. 2008, 23, 119–123. [Google Scholar] [CrossRef]
- Roalf, D.R.; Moberg, M.J.; Turetsky, B.I.; Brennan, L.; Kabadi, S.; Wolk, D.A.; Moberg, P.J. A quantitative meta-analysis of olfactory dysfunction in mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2017, 88, 226–232. [Google Scholar] [CrossRef]
- Doty, R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012, 8, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Tepe, B.; Hill, M.C.; Pekarek, B.T.; Hunt, P.J.; Martin, T.J.; Martin, J.F.; Arenkiel, B.R. Single-cell RNA-Seq of mouse olfactory bulb reveals cellular heterogeneity and activity-dependent molecular census of adult-born neurons. Cell Rep. 2018, 25, 2689–2703. [Google Scholar] [CrossRef] [PubMed]
- Muir, E.R.; Biju, K.C.; Cong, L.; Rogers, W.E.; Hernandez, E.T.; Duong, T.Q.; Clark, R.A. Functional MRI of the mouse olfactory system. Neurosci. Lett. 2019, 704, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.L.; Postma, E.M.; Smeets, P.A.M.; Boek, W.M.; de Graaf, K.; Schöpf, V.; Boesveldt, S. Severity of olfactory deficits is reflected in functional brain networks. An fMRI study. Hum. Brain Mapp. 2018, 39, 3166–3177. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Goedert, M. Tau pathology and neurodegeneration. Lancet Neurol. 2013, 123, 609–622. [Google Scholar] [CrossRef]
- Richter, J.D.; Bassell, G.J.; Klann, E. Dysregulation and restoration of translational homeostasis in fragile X syndrome. Nat. Rev. Neurosci. 2015, 16, 595–605. [Google Scholar] [CrossRef]
- Tan, L. Three-dimensional genome structure of a single cell. Science 2019, 366, 964–965. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Lim, D.A. For the long run: Maintaining germinal niches in the adult brain. Neuron 2004, 41, 683–686. [Google Scholar] [CrossRef]
- Lledo, P.M.; Merkle, F.T.; Alvarez-Buylla, A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008, 31, 392–400. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Lledo, P.M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Lepousez, G.; Sebastien, W.; Bardy, C.; Gabellec, M.M.; Torquet, N.; Lledo, P.M. Activation of adult-born neurons facilitates learning and memory. Nat. Neurosci. 2012, 15, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Mori, K. Critical period for sensory experience dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc. Natl. Acad. Sci. USA 2005, 102, 9697–9702. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Cunha, M.P.; Neis, V.B. Fluoxetine reverses depressive-like behaviors and increases hippocampal acetylcholinesterase activity induced by olfactory bulbectomy. Pharmacol. Biochem. Behav. 2012, 103, 220–229. [Google Scholar] [CrossRef]
- Rodriguez-Gaztelumendi, A.; Rojo, M.L.; Pazos, A.; Diaz, A. Altered CB receptor-signaling in prefrontal cortex from an animal model of depression is reversed by chronic fluoxetine. J. Neurochem. 2009, 108, 1423–1433. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatia-Dey, N.; Heinbockel, T. Endocannabinoid-Mediated Neuromodulation in the Olfactory Bulb: Functional and Therapeutic Significance. Int. J. Mol. Sci. 2020, 21, 2850. https://doi.org/10.3390/ijms21082850
Bhatia-Dey N, Heinbockel T. Endocannabinoid-Mediated Neuromodulation in the Olfactory Bulb: Functional and Therapeutic Significance. International Journal of Molecular Sciences. 2020; 21(8):2850. https://doi.org/10.3390/ijms21082850
Chicago/Turabian StyleBhatia-Dey, Naina, and Thomas Heinbockel. 2020. "Endocannabinoid-Mediated Neuromodulation in the Olfactory Bulb: Functional and Therapeutic Significance" International Journal of Molecular Sciences 21, no. 8: 2850. https://doi.org/10.3390/ijms21082850
APA StyleBhatia-Dey, N., & Heinbockel, T. (2020). Endocannabinoid-Mediated Neuromodulation in the Olfactory Bulb: Functional and Therapeutic Significance. International Journal of Molecular Sciences, 21(8), 2850. https://doi.org/10.3390/ijms21082850





