Higher Accumulation of Docosahexaenoic Acid in the Vermilion of the Human Lip than in the Skin
Abstract
1. Introduction
2. Results
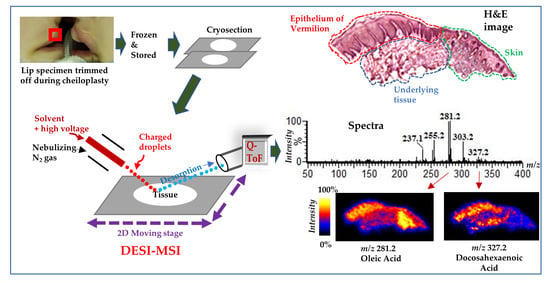
2.1. DESI–MSI of Human Lip
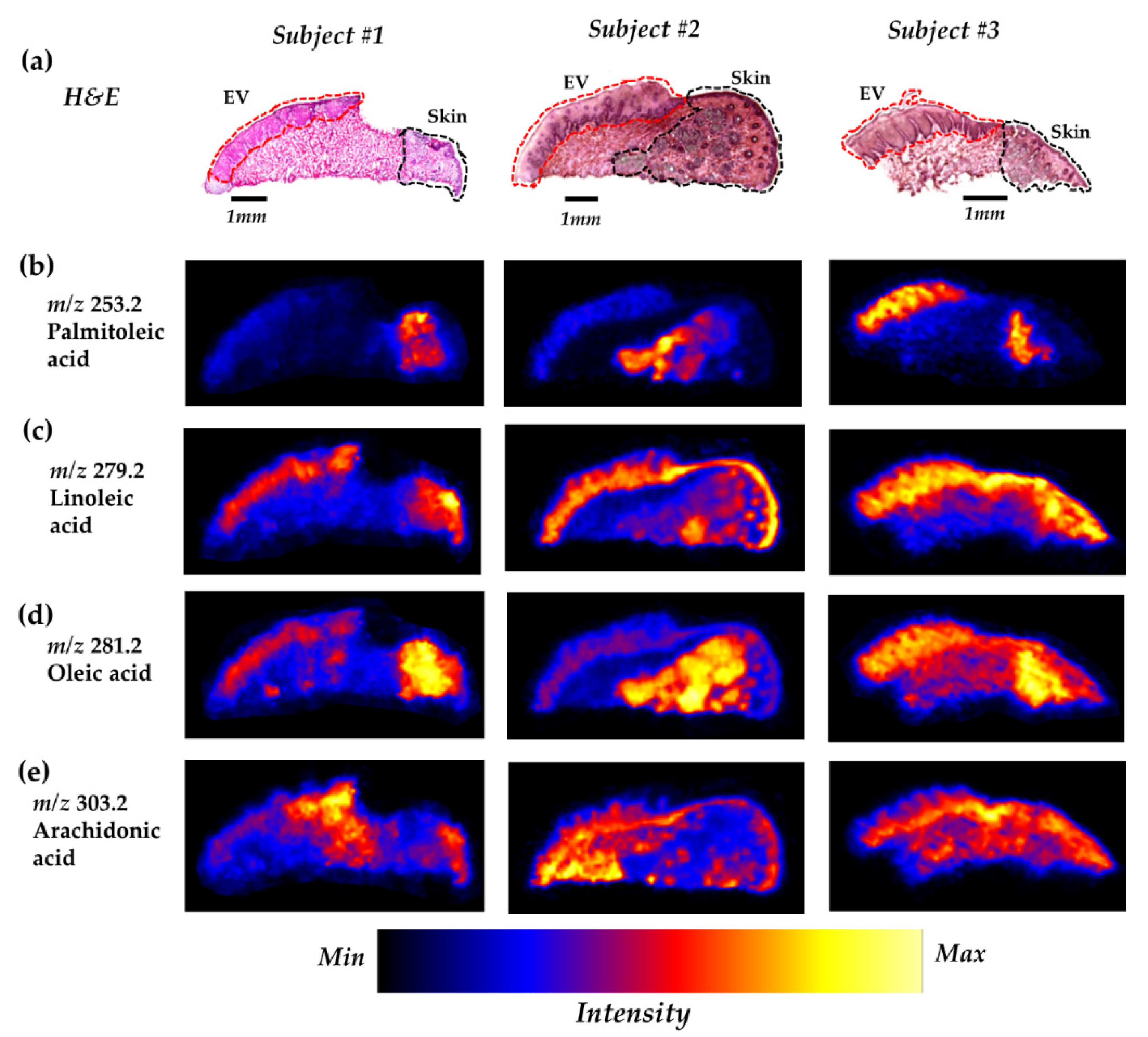
2.2. Several FFAs are Differentially Distributed Across the Vermilion and Skin
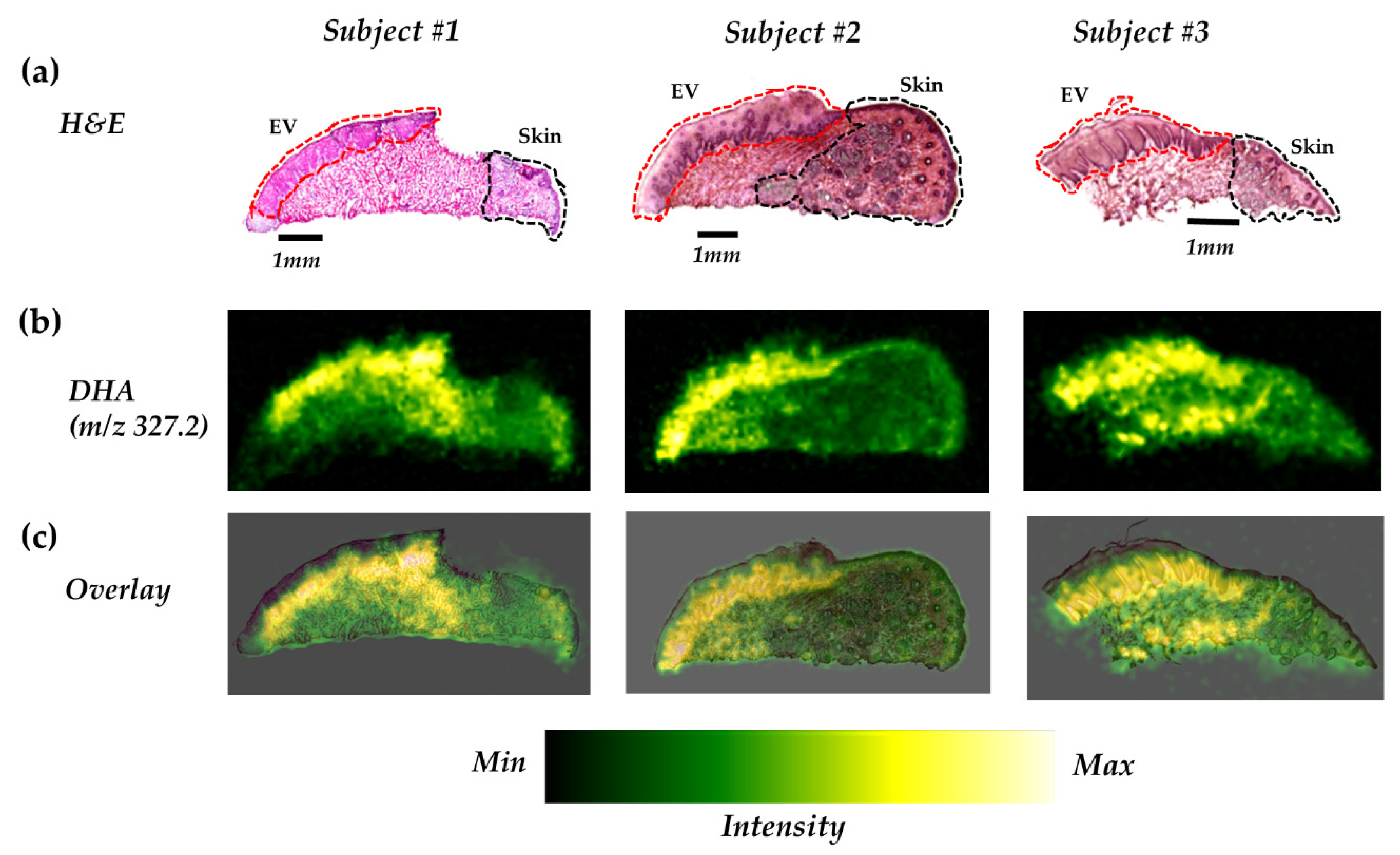
2.3. DHA is Highly Distributed in the Epithelium of the Vermilion
3. Discussion
4. Materials and Methods
4.1. Tissue Sample
4.2. Sample Preparation for DESI–MSI Analysis
4.3. DESI–MSI Analysis
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piccinin, M.A.; Zito, P.M. Anatomy, Head and Neck, Lips. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Mescher, A.L.; Junqueira, L.C.U. Junqueira’s Basic Histology: Text and Atlas; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Kobayashi, H.; Tagami, H. Functional properties of the surface of the vermilion border of the lips are distinct from those of the facial skin. Br. J. Dermatol. 2004, 150, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Bielfeldt, S.; Laing, S.; Sadowski, T.; Gunt, H.; Wilhelm, K. Characterization and validation of an in vivo confocal Raman spectroscopy led tri-method approach in the evaluation of the lip barrier. Ski. Res. Technol. 2019, srt.12814. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.H.; Pawlina, W. Histology: A Text and Atlas: With Correlated Cell and Molecular Biology; LWW: Philadelphia, PA, USA, 2010. [Google Scholar]
- McCusker, M.M.; Grant-Kels, J.M. Healing fats of the skin: The structural and immunologic roles of the ω-6 and ω-3 fatty acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, M.; Ren, J.; Djuric, Z.; Fisher, G.J.; Wang, X.; Li, Y. Gas chromatography-mass spectrometry analysis of effects of dietary fish oil on total fatty acid composition in mouse skin. Sci. Rep. 2017, 7, 42641. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A. Epidermal surface lipids. Dermatoendocrinol 2009, 1, 72. [Google Scholar] [CrossRef] [PubMed]
- Lampe, M.A.; Burlingame, A.L.; Whitney, J.; Williams, M.L.; Brown, B.E.; Roitman, E.; Elias, P.M.; Burlingame, L.; Whitney, J.; Williams, M.L.; et al. Human stratum corneum lipids: Characterization and regional variations. J. Lipid Res. 1983, 24, 120–130. [Google Scholar]
- Sjövall, P.; Skedung, L.; Gregoire, S.; Biganska, O.; Clément, F.; Luengo, G.S. Imaging the distribution of skin lipids and topically applied compounds in human skin using mass spectrometry. Sci. Rep. 2018, 8, 16683. [Google Scholar] [CrossRef]
- Goto-Inoue, N.; Hayasaka, T.; Zaima, N.; Setou, M. Imaging mass spectrometry for lipidomics. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 961–969. [Google Scholar] [CrossRef]
- Ide, Y.; Waki, M.; Hayasaka, T.; Nishio, T.; Morita, Y.; Tanaka, H.; Sasaki, T.; Koizumi, K.; Matsunuma, R.; Hosokawa, Y.; et al. Human Breast Cancer Tissues Contain Abundant Phosphatidylcholine(36∶1) with High Stearoyl-CoA Desaturase-1 Expression. PLoS ONE 2013, 8, e61204. [Google Scholar] [CrossRef]
- Kurabe, N.; Hayasaka, T.; Ogawa, M.; Masaki, N.; Ide, Y.; Waki, M.; Nakamura, T.; Kurachi, K.; Kahyo, T.; Shinmura, K.; et al. Accumulated phosphatidylcholine (16:0/16:1) in human colorectal cancer; possible involvement of LPCAT4. Cancer Sci. 2013, 104, 1295–1302. [Google Scholar] [CrossRef]
- Parrot, D.; Papazian, S.; Foil, D.; Tasdemir, D. Imaging the Unimaginable: Desorption Electrospray Ionization—Imaging Mass Spectrometry (DESI-IMS) in Natural Product Research. Planta Med. 2018, 84, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Takats, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, J.M.; Ifa, D.R.; Song, Q.; Cooks, R.G. Tissue Imaging at Atmospheric Pressure Using Desorption Electrospray Ionization (DESI) Mass Spectrometry. Angew Chemie Int. Ed. 2006, 45, 7188–7192. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Horikawa, M.; Sato, S.; Miyake, H.; Setou, M. Discovery of lipid biomarkers correlated with disease progression in clear cell renal cell carcinoma using desorption electrospray ionization imaging mass spectrometry. Oncotarget 2019, 10, 1688–1703. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Horikawa, M.; Takei, S.; Yamazaki, F.; Ito, T.K.; Kondo, T.; Sakurai, T.; Kahyo, T.; Ikegami, K.; Sato, S.; et al. Preferential Incorporation of Administered Eicosapentaenoic Acid Into Thin-Cap Atherosclerotic Plaques. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1802–1816. [Google Scholar] [CrossRef]
- Al Mamun, M.; Gonzalez, T.V.; Islam, A.; Sato, T.; Sato, S.; Ito, T.K.; Horikawa, M.; Yamazaki, F.; Alarcon, R.C.; Ido, T.; et al. Analysis of potential anti-aging beverage Pru, a traditional Cuban refreshment, by desorption electrospray ionization-mass spectrometry and FTICR tandem mass spectrometry. J. Food Drug Anal. 2019. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, E.; Islam, A.; Watanabe, N.; Tsubaki, H.; Fukushima, M.; Al Mamun, M.; Sato, S.; Sato, T.; Eto, F.; Yao, I.; et al. Dietary Intake of Green Nut Oil or DHA Ameliorates DHA Distribution in the Brain of a Mouse Model of Dementia Accompanied by Memory Recovery. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Margulis, K.; Chiou, A.S.; Aasi, S.Z.; Tibshirani, R.J.; Tang, J.Y.; Zare, R.N. Distinguishing malignant from benign microscopic skin lesions using desorption electrospray ionization mass spectrometry imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 6347–6352. [Google Scholar] [CrossRef]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of antiinflammatory and antiproliferative metabolites. Am. J. Clin. Nutr. 2000, 71, 361s–366s. [Google Scholar] [CrossRef]
- Huang, T.-H.; Wang, P.-W.; Yang, S.-C.; Chou, W.-L.; Fang, J.-Y. Cosmetic and Therapeutic Applications of Fish Oil’s Fatty Acids on the Skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Ho, P.-C.; Tu, Y.-K.; Jou, I.-M.; Tsai, K.-J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Kiezel-Tsugunova, M.; Brownbridge, L.C.; Harwood, J.L.; Nicolaou, A. Lipid functions in skin: Differential effects of n-3 polyunsaturated fatty acids on cutaneous ceramides, in a human skin organ culture model. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.C.; Drosatos, K.; Goldberg, I.J. Lipid Use and Misuse by the Heart. Circ. Res. 2016, 118, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Kundu, J.K.; Shin, J.-W.; Na, H.-K.; Surh, Y.-J. Docosahexaenoic Acid Inhibits UVB-Induced Activation of NF-κB and Expression of COX-2 and NOX-4 in HR-1 Hairless Mouse Skin by Blocking MSK1 Signaling. PLoS ONE 2011, 6, e28065. [Google Scholar] [CrossRef]
- Yum, H.W.; Park, J.; Park, H.J.; Shin, J.W.; Cho, Y.Y.; Kim, S.J.; Kang, J.X.; Surh, Y.J. Endogenous ω-3 Fatty Acid Production by fat-1 Transgene and Topically Applied Docosahexaenoic Acid Protect against UVB-induced Mouse Skin Carcinogenesis. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Arantes, E.L.; Dragano, N.; Ramalho, A.; Vitorino, D.; de-Souza, G.F.; Lima, M.H.M.; Velloso, L.A.; Araújo, E.P. Topical Docosahexaenoic Acid (DHA) Accelerates Skin Wound Healing in Rats and Activates GPR120. Biol. Res. Nurs. 2016, 18, 411–419. [Google Scholar] [CrossRef]
- Balcos, M.C.; Kim, S.Y.; Jeong, H.S.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Park, K.C.; Kim, D.S. Docosahexaenoic acid inhibits melanin synthesis in murine melanoma cells in vitro through increasing tyrosinase degradation. Acta Pharmacol. Sin. 2014, 35, 489–495. [Google Scholar] [CrossRef]
- Chiu, L.C.M.; Tong, K.F.; Ooi, V.E.C. Cytostatic and cytotoxic effects of cyclooxygenase inhibitors and their synergy with docosahexaenoic acid on the growth of human skin melanoma A-375 cells. Biomed. Pharmacother. 2005, 59. [Google Scholar] [CrossRef]
- Hansen, H.S.; Jensen, B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and α-linolenate. Biochim. Biophys. Acta Lipids Lipid Metab. 1985, 834, 357–363. [Google Scholar] [CrossRef]
- Weimann, E.; Silva, M.B.B.; Murata, G.M.; Bortolon, J.R.; Dermargos, A.; Curi, R.; Hatanaka, E. Topical anti-inflammatory activity of palmitoleic acid improves wound healing. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.R.B.; Souza, M.A.; Ferro, E.A.V.; Favoreto, S.; Pena, J.D.O. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen 2004, 12, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The human metabolome database. Nucleic Acids Res. 2007, 35. [Google Scholar] [CrossRef] [PubMed]


| Subject ID | Age | Sex | Diagnosis | Sample Collection Period |
|---|---|---|---|---|
| 1 | 6 months | Female | Cleft lip, alveolus and palate, right side | April, 2018 |
| 2 | 8 months | Male | Cleft lip, alveolus and palate, right side | March, 2019 |
| 3 | 5 months | Female | Cleft lip and alveolus, Left side | May, 2019 |
| m/z Observed | Monoisotopic Mass (M-H)- | Molecular Assignments | Mass Error (Δppm) | Reference(s) |
|---|---|---|---|---|
| 253.2171 | 253.2173 | Palmitoleic acid(C16:1) | 0.8 | [17] |
| 279.2328 | 279.2330 | Linoleic acid (C18:2) | 0.7 | [17,20] |
| 281.2485 | 281.2486 | Oleic acid (C18:1) | 0.4 | [16,17,20,21] |
| 303.2329 | 303.2330 | Arachidonic acid (C20:4) | 0.3 | [16,20,21] |
| 327.2328 | 327.2330 | Docosahexaenoic acid (C22:6) | 0.6 | [16,20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamun, M.A.; Sato, S.; Naru, E.; Sakata, O.; Hoshikawa, E.; Suzuki, A.; Islam, A.; Kahyo, T.; Sato, T.; Ito, T.K.; et al. Higher Accumulation of Docosahexaenoic Acid in the Vermilion of the Human Lip than in the Skin. Int. J. Mol. Sci. 2020, 21, 2807. https://doi.org/10.3390/ijms21082807
Mamun MA, Sato S, Naru E, Sakata O, Hoshikawa E, Suzuki A, Islam A, Kahyo T, Sato T, Ito TK, et al. Higher Accumulation of Docosahexaenoic Acid in the Vermilion of the Human Lip than in the Skin. International Journal of Molecular Sciences. 2020; 21(8):2807. https://doi.org/10.3390/ijms21082807
Chicago/Turabian StyleMamun, Md. Al, Shumpei Sato, Eiji Naru, Osamu Sakata, Emi Hoshikawa, Ayako Suzuki, Ariful Islam, Tomoaki Kahyo, Tomohito Sato, Takashi K. Ito, and et al. 2020. "Higher Accumulation of Docosahexaenoic Acid in the Vermilion of the Human Lip than in the Skin" International Journal of Molecular Sciences 21, no. 8: 2807. https://doi.org/10.3390/ijms21082807
APA StyleMamun, M. A., Sato, S., Naru, E., Sakata, O., Hoshikawa, E., Suzuki, A., Islam, A., Kahyo, T., Sato, T., Ito, T. K., Horikawa, M., Fukui, R., Izumi, K., & Setou, M. (2020). Higher Accumulation of Docosahexaenoic Acid in the Vermilion of the Human Lip than in the Skin. International Journal of Molecular Sciences, 21(8), 2807. https://doi.org/10.3390/ijms21082807