RX-3117 (Fluorocyclopentenyl-Cytosine)-Mediated Down-Regulation of DNA Methyltransferase 1 Leads to Protein Expression of Tumor-Suppressor Genes and Increased Functionality of the Proton-Coupled Folate Carrier
Abstract
1. Introduction
2. Results
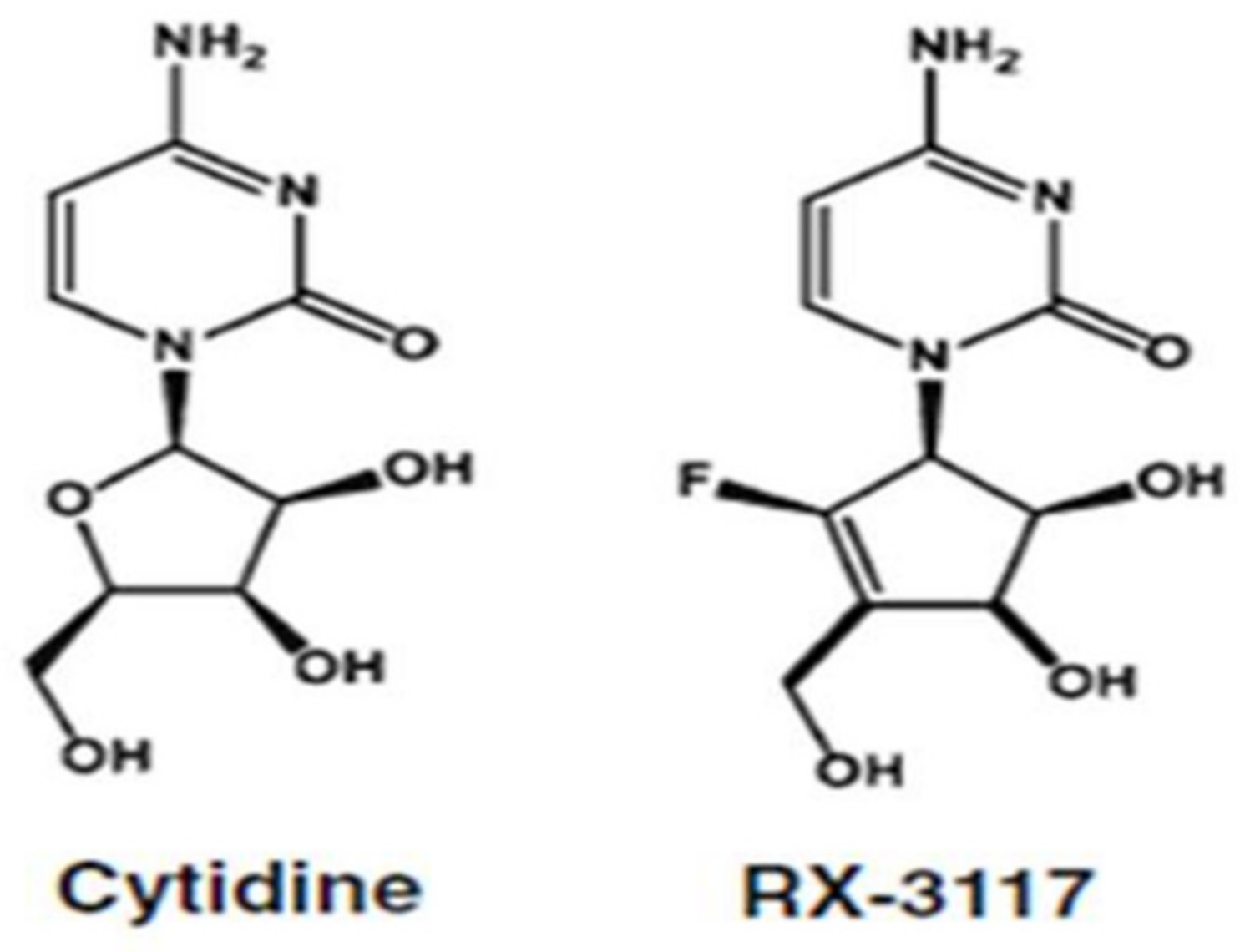
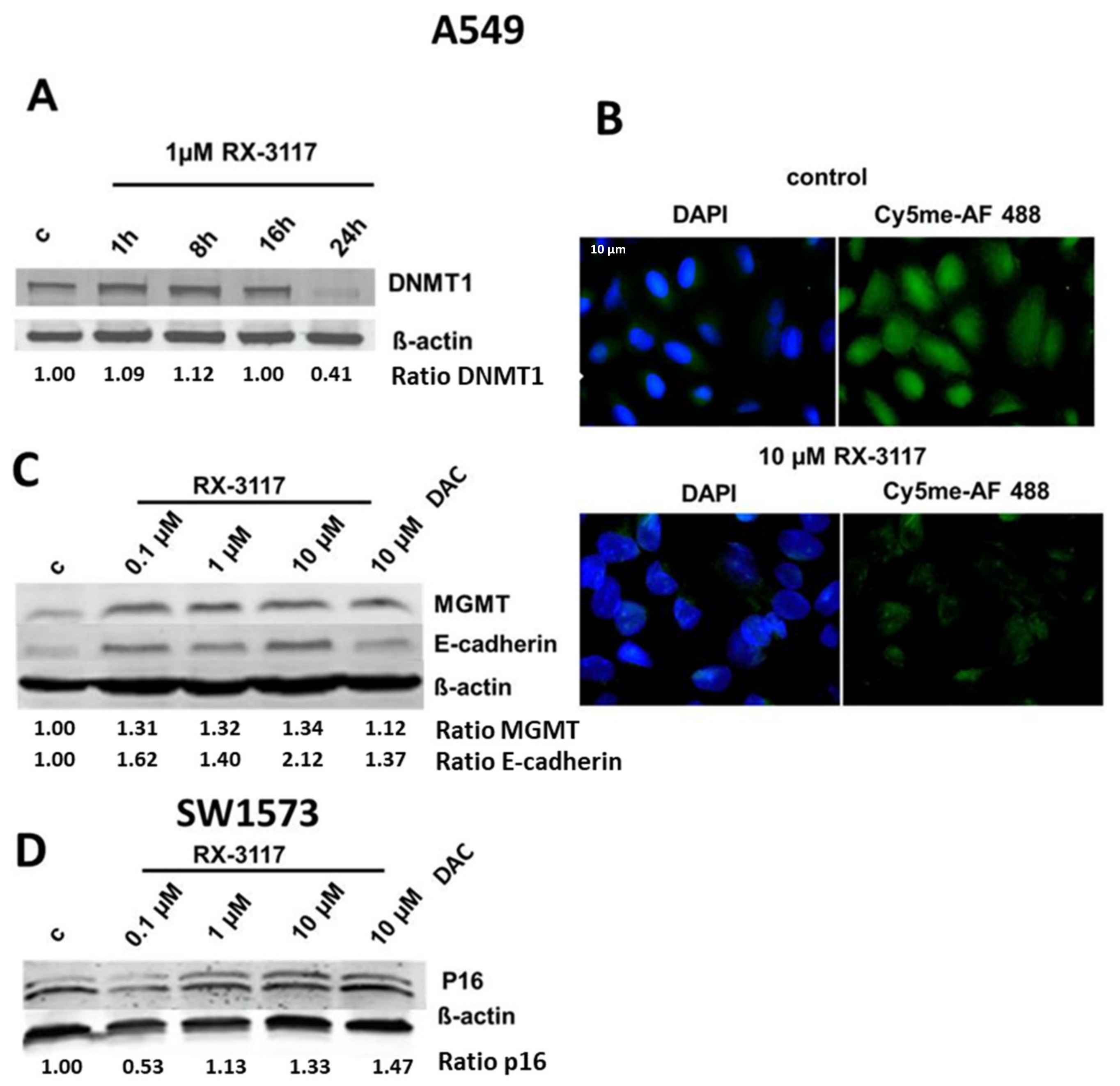
2.1. Targeting DNA Methylation
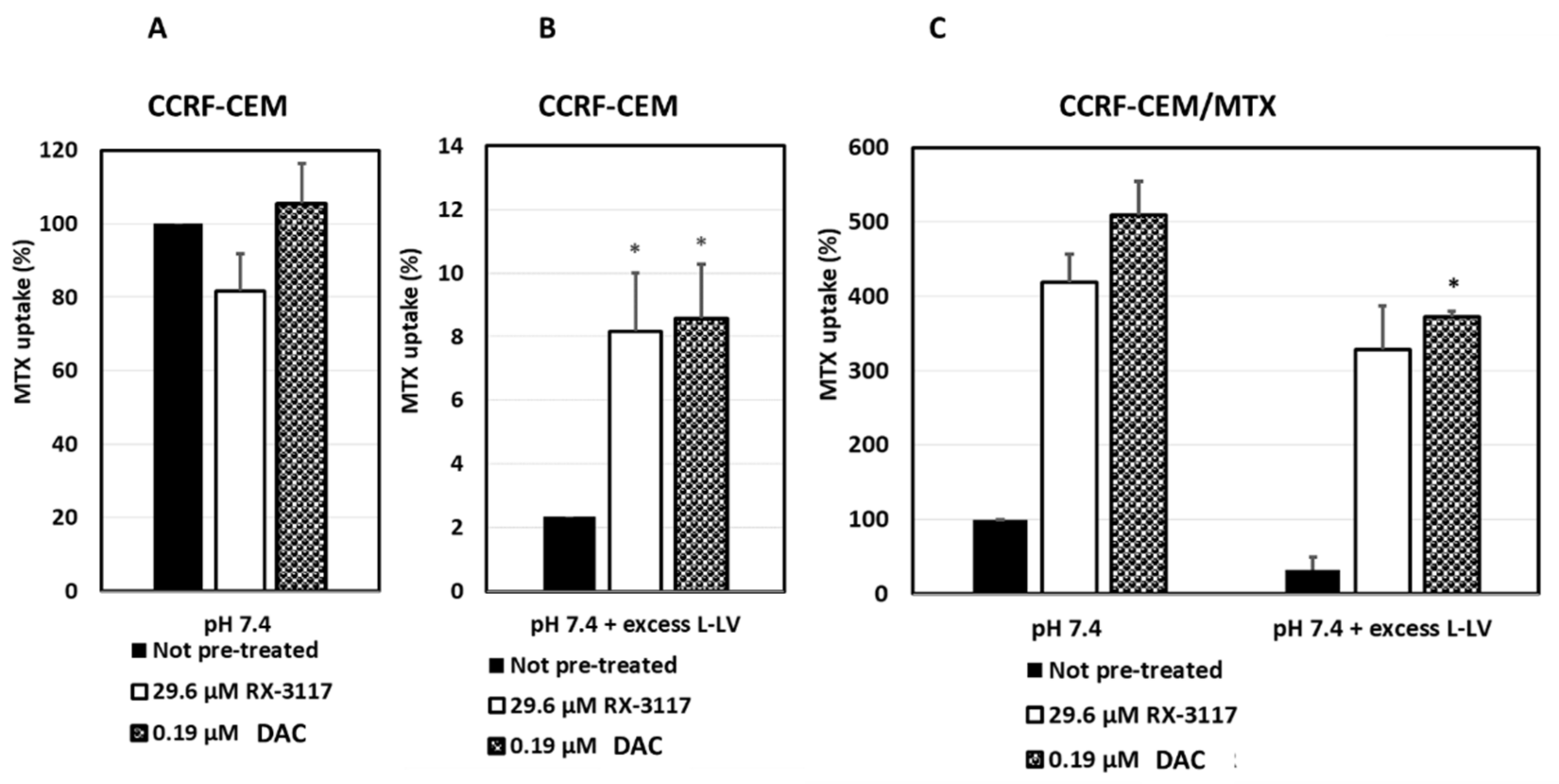
2.2. Modulation of Methotrexate Uptake by RX-3117
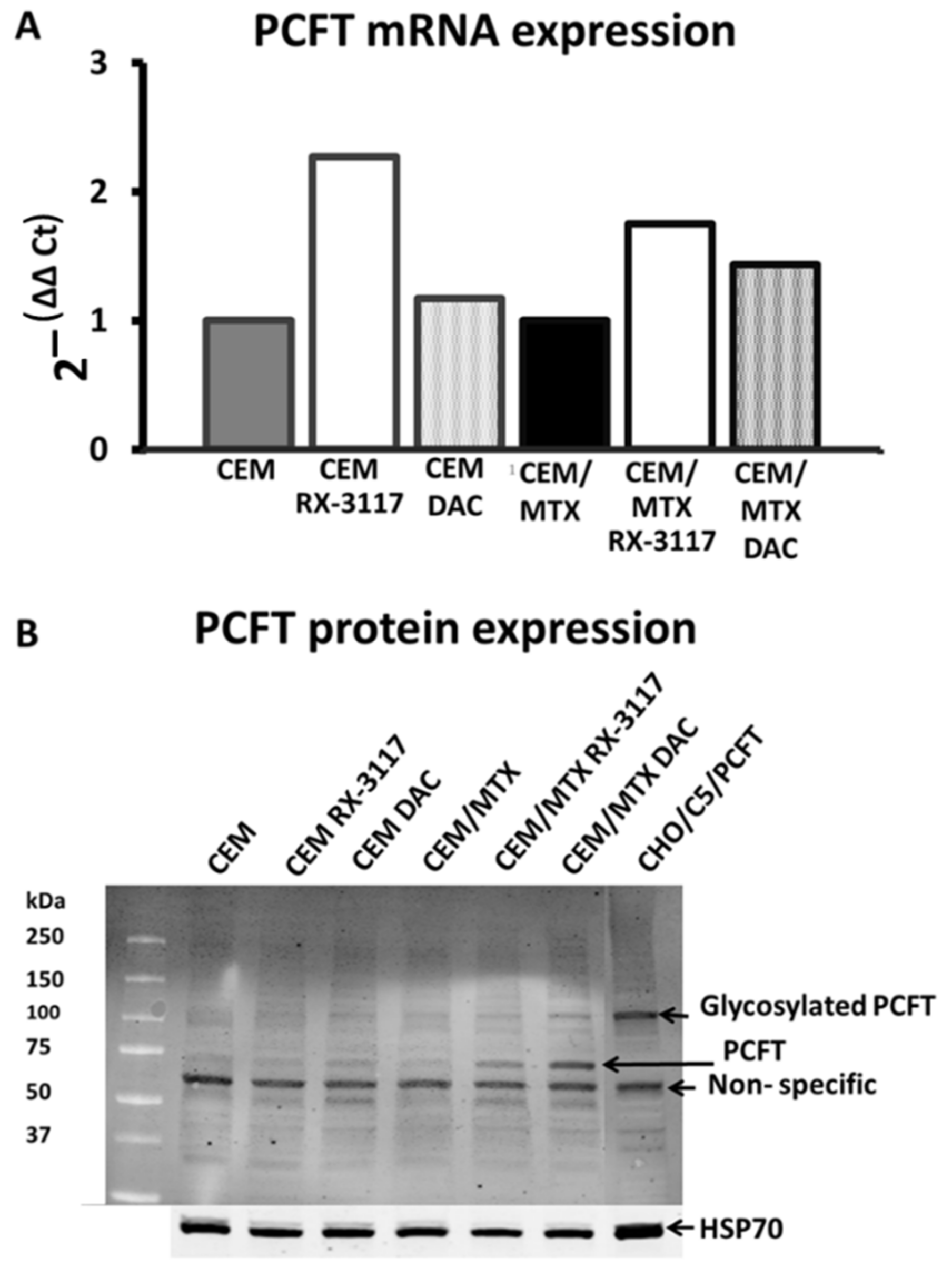
2.3. Re-Activation of PCFT by RX-3117
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Confocal Microscopy
4.3. Protein Expression
4.4. Modulation of Transport
4.5. PCFT Gene Expression
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, W.J.; Chung, H.-J.; Chandra, G.; Alexander, V.; Zhao, L.X.; Lee, H.W.; Nayak, A.; Majik, M.S.; Kim, H.O.; Kim, J.; et al. Fluorocyclopentenyl-cytosine with broad spectrum and potent antitumor activity. J. Med. Chem. 2012, 55, 4521–4525. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Lee, Y.B.; Ahn, C.-H.; Kaye, J.; Fine, T.; Kashi, R.; Ohne, O.; Smid, K.; Peters, G.J.; Kim, D.J. A novel cytidine analog, RX-3117, shows potent efficacy in xenograft models, even in tumors that are resistant to gemcitabine. Anticancer Res. 2014, 34, 6951–6959. [Google Scholar] [PubMed]
- Balboni, B.; El Hassouni, B.; Honeywell, R.J.; Sarkisjan, D.; Giovannetti, E.; Poore, J.; Heaton, C.; Peterson, C.; Benaim, E.; Lee, Y.B.; et al. RX-3117 (fluorocyclopentenyl cytosine): A novel specific antimetabolite for selective cancer treatment. Expert Opin. Investig. Drugs 2019, 28, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.J.; Smid, K.; Vecchi, L.; Kathmann, I.; Sarkisjan, D.; Honeywell, R.; Losekoot, N.; Ohne, O.; Orbach, A.; Blaugrund, E.; et al. Metabolism, mechanism of action and sensitivity profile of fluorocyclopentenylcytosine (RX-3117; TV-1360). Investig. New Drugs 2013, 31, 1444–1457. [Google Scholar] [CrossRef]
- Sarkisjan, D.; Steenbergen, R.D.M.; Cloos, J.; Peters, G.J. Re-emerging antimetabolites with novel mechanism of action with respect to epigenetic regulation: Basic aspects. In Chemotherapy for Leukemia: Novel Drugs and Treatment; Ueda, T., Ed.; Springer Nature, Singapore Pte Ltd: Singapore, 2017; Chapter 18; pp. 311–326. [Google Scholar]
- Sarkisjan, D.; Julsing, J.R.; Smid, K.; De Klerk, D.; Van Kuilenburg, A.B.P.; Meinsma, R.; Lee, Y.B.; Kim, D.J.; Peters, G.J. The Cytidine Analog Fluorocyclopentenylcytosine (RX-3117) Is Activated by Uridine-Cytidine Kinase 2. PLoS ONE 2016, 11, e0162901. [Google Scholar] [CrossRef]
- Bestor, T.H.; Ingram, V.M. Two DNA methyltransferases from murine erythroleukemia cells: Purification, sequence specificity, and mode of interaction with DNA. Proc. Natl. Acad. Sci. USA 1983, 80, 5559–5563. [Google Scholar] [CrossRef]
- Saxonov, S.; Berg, P.; Brutlag, D.L. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc. Natl. Acad. Sci. USA 2006, 103, 1412–1417. [Google Scholar] [CrossRef]
- Christman, J.K. 5-Azacytidine and 5-aza-2’-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene 2002, 21, 5483–5495. [Google Scholar] [CrossRef]
- Šorm, F.; Piskala, A.; Čihák, A.; Veselý, J. 5-Azacytidine, a new, highly effective cancerostatic. Experientia 1964, 20, 202–203. [Google Scholar] [CrossRef]
- Calcagno, D.Q.; de Arruda Cardoso Smith, M.; Burbano, R.R. Cancer type-specific epigenetic changes: Gastric cancer. Methods Mol. Biol. 2015, 1238, 79–101. [Google Scholar]
- Rodríguez-Paredes, M.; Esteller, M. Cancer epigenetics reaches mainstream oncology. Nat. Med. 2011, 17, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Matherly, L.H.; Hou, Z.; Gangjee, A. The promise and challenges of exploiting the proton-coupled folate transporter for selective therapeutic targeting of cancer. Cancer Chemother. Pharmacol. 2018, 81, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gonen, N.; Bram, E.; Assaraf, Y.G. PCFT/SLC46A1 promoter methylation and restoration of gene expression in human leukemia cells. Biochem. Biophys. Res. Commun. 2008, 376, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Rahat, B.; Hamid, A.; Najar, R.A.; Kaur, J. Identification of regulatory mechanisms of intestinal folate transport in condition of folate deficiency. J. Nutr. Biochem. 2015, 26, 1084–1094. [Google Scholar] [CrossRef]
- Diop-Bove, N.K.; Wu, J.; Zhao, R.; Locker, J.; Goldman, I.D. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther. 2009, 8, 2424–2431. [Google Scholar] [CrossRef]
- Farkas, S.; Befekadu, R.; Hahn-Strömberg, V.; Nilsson, T.K. DNA methylation and expression of the folate transporter genes in colorectal cancer. Tumour Biol. 2015, 36, 5581–5590. [Google Scholar] [CrossRef]
- Giovannetti, E.; Zucali, P.; Assaraf, Y.G.; Funel, N.; Gemelli, M.; Stark, M.; Thunnissen, E.; Hou, Z.; Muller, I.; Struys, E.; et al. Role of proton-coupled folate transporter in pemetrexed resistance of mesothelioma: Clinical evidence and new pharmacological tools. Ann. Oncol. 2017, 28, 2725–2732. [Google Scholar] [CrossRef]
- Bertino, J.R. Karnofsky memorial lecture. Ode to methotrexate. J. Clin. Oncol. 1993, 11, 5–14. [Google Scholar] [CrossRef]
- Goldman, I.D.; Lichtenstein, N.S.; Oliverio, V.T. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell. J. Biol. Chem. 1968, 243, 5007–5017. [Google Scholar]
- Jansen, G.; Mauritz, R.; Drori, S.; Sprecher, H.; Kathmann, I.; Bunni, M.; Priest, D.G.; Noordhuis, P.; Schornagel, J.H.; Pinedo, H.M.; et al. A structurally altered human reduced folate carrier with increased folic acid transport mediates a novel mechanism of antifolate resistance. J. Biol. Chem. 1998, 273, 30189–30198. [Google Scholar] [CrossRef]
- Gonen, N.; Assaraf, Y.G. Antifolates in cancer therapy: Structure, activity and mechanisms of drug resistance. Drug Resist Updat. 2012, 15, 183–210. [Google Scholar] [CrossRef] [PubMed]
- Adema, A.D.; van der Born, K.; Honeywell, R.J.; Peters, G.J. Cell cycle effects and increased adduct formation by temozolomide enhance the effect of cytotoxic and targeted agents in lung cancer cell lines. J. Chemother. 2009, 21, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Jansen, G.; Westerhof, G.R.; Kathmann, I.; Rademaker, B.C.; Rijksen, G.; Schornagel, J.H. Identification of a membrane-associated folate-binding protein in human leukemic CCRF-CEM cells with transport-related methotrexate resistance. Cancer Res. 1989, 49, 2455–2459. [Google Scholar] [PubMed]
- Rekers, N.H.; Sminia, P.; Peters, G.J. Towards tailored therapy of glioblastoma multiforme. J. Chemother. 2011, 23, 187–199. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Adams, C.L.; Nelson, W.J. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr. Opin. Cell Biol. 1998, 10, 572–577. [Google Scholar] [CrossRef]
- Batlle, E.; Sancho, E.; Francí, C.; Domínguez, D.; Monfar, M.; Baulida, J.; De Herreros, A.G. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000, 2, 84–89. [Google Scholar] [CrossRef]
- Semb, H.; Christofori, G. The tumor-suppressor function of E-cadherin. Am. J. Hum. Genet. 1998, 63, 1588–1593. [Google Scholar] [CrossRef]
- Perl, A.K.; Wilgenbus, P.; Dahl, U.; Semb, H.; Christofori, G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 1998, 392, 190–193. [Google Scholar] [CrossRef]
- Nobori, T.; Miura, K.; Wu, D.J.; Lois, A.; Takabayashi, K.; Carson, D.A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 1994, 368, 753–756. [Google Scholar] [CrossRef]
- Nuovo, G.J.; Plaia, T.W.; Belinsky, S.A.; Baylin, S.B.; Herman, J.G. In situ detection of the hypermethylation-induced inactivation of the p16 gene as an early event in oncogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 12754–12759. [Google Scholar] [CrossRef]
- Sarkisjan, D.; Van den Berg, J.; Smit, E.; Lee, Y.B.; Kim, D.J.; Peters, G.J. The radiosensitizing effect of fluorocyclopentenyl-cytosine (RX-3117) in ovarian and lung cancer cell lines. Nucleosides Nucleotides Nucleic Acids 2016, 35, 619–630. [Google Scholar] [CrossRef]
- Galvani, E.; Toffalorio, F.; Peters, G.J.; De Pas, T.; Giovannetti, E. Pharmacogenetics of non-small cell lung cancer (NSCLC): Time to “work it out”? Curr. Pharm. Des. 2014, 20, 3863–3874. [Google Scholar] [CrossRef]
- Westerhof, G.R.; Schornagel, J.H.; Kathmann, I.; Jackman, A.L.; Rosowsky, A.; Forsch, R.; Hynes, J.B.; Boyle, F.T.; Peters, G.J.; Pinedo, H.M.; et al. Carrier- and receptor-mediated transport of folate antagonists targeting folate-dependent enzymes: Correlates of molecular-structure and biological activity. Mol. Pharmacol. 1995, 48, 459–471. [Google Scholar]
- Aparicio, A.; North, B.; Barske, L.; Wang, X.; Bollati, V.; Weisenberger, D.; Yoo, C.; Tannir, N.M.; Horne, E.; Groshen, S.; et al. LINE-1 methylation in plasma DNA as a biomarker of activity of DNA methylation inhibitors in patients with solid tumors. Epigenetics 2009, 4, 176–184. [Google Scholar] [CrossRef]
- Cree, I.; For the UK Early Cancer Detection Consortium; Uttley, L.; Woods, H.B.; Kikuchi, H.; Reiman, A.; Harnan, S.; Whiteman, B.; Taylor-Phillips, S.; Messenger, M.; et al. The evidence base for circulating tumour DNA blood-based biomarkers for the early detection of cancer: A systematic mapping review. BMC Cancer 2017, 17, 697. [Google Scholar] [CrossRef]
- Kim, D.J.; Yang, J.; Wu, W.; Davis, D.W.; Eisen, A. Phenotyping Pancreatic Cancer CTCs as Biomarkers for RX-3117 Clinical Trials. In Proceedings of the AACR 110th Annual Meeting Atlanta, (Abstract #428), Atlanta, GA, USA, 29 March–3 April 2019. [Google Scholar]
- Varga, J.; De Oliveira, T.; Greten, F.R. The architect who never sleeps: Tumor-induced plasticity. FEBS Lett. 2014, 588, 2422–2427. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
| External pH Level | CEM | CEM/MTX |
|---|---|---|
| Addition | MTX uptake (pmol/min/1 × 107 cells) | |
| pH 5.5 | 1.11 ± 0.14 | 0.49 ± 0.13 |
| pH 5.5 + 1 μM FA | 0.66 ± 0.11 | 0.51 ± 0.21 |
| pH 7.4 | 2.80 ± 0.52 | 0.03 ± 0.02 |
| pH 7.4 + 1 μM L-LV | 0.046 ± 0.01 | 0.01 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkisjan, D.; Julsing, J.R.; El Hassouni, B.; Honeywell, R.J.; Kathmann, I.; Matherly, L.H.; Lee, Y.B.; Kim, D.J.; Peters, G.J. RX-3117 (Fluorocyclopentenyl-Cytosine)-Mediated Down-Regulation of DNA Methyltransferase 1 Leads to Protein Expression of Tumor-Suppressor Genes and Increased Functionality of the Proton-Coupled Folate Carrier. Int. J. Mol. Sci. 2020, 21, 2717. https://doi.org/10.3390/ijms21082717
Sarkisjan D, Julsing JR, El Hassouni B, Honeywell RJ, Kathmann I, Matherly LH, Lee YB, Kim DJ, Peters GJ. RX-3117 (Fluorocyclopentenyl-Cytosine)-Mediated Down-Regulation of DNA Methyltransferase 1 Leads to Protein Expression of Tumor-Suppressor Genes and Increased Functionality of the Proton-Coupled Folate Carrier. International Journal of Molecular Sciences. 2020; 21(8):2717. https://doi.org/10.3390/ijms21082717
Chicago/Turabian StyleSarkisjan, Dzjemma, Joris R. Julsing, Btissame El Hassouni, Richard J. Honeywell, Ietje Kathmann, Larry H. Matherly, Young B. Lee, Deog J. Kim, and Godefridus J. Peters. 2020. "RX-3117 (Fluorocyclopentenyl-Cytosine)-Mediated Down-Regulation of DNA Methyltransferase 1 Leads to Protein Expression of Tumor-Suppressor Genes and Increased Functionality of the Proton-Coupled Folate Carrier" International Journal of Molecular Sciences 21, no. 8: 2717. https://doi.org/10.3390/ijms21082717
APA StyleSarkisjan, D., Julsing, J. R., El Hassouni, B., Honeywell, R. J., Kathmann, I., Matherly, L. H., Lee, Y. B., Kim, D. J., & Peters, G. J. (2020). RX-3117 (Fluorocyclopentenyl-Cytosine)-Mediated Down-Regulation of DNA Methyltransferase 1 Leads to Protein Expression of Tumor-Suppressor Genes and Increased Functionality of the Proton-Coupled Folate Carrier. International Journal of Molecular Sciences, 21(8), 2717. https://doi.org/10.3390/ijms21082717






