Karyopherin α-2 Mediates MDC1 Nuclear Import through a Functional Nuclear Localization Signal in the tBRCT Domain of MDC1
Abstract
1. Introduction
2. Results
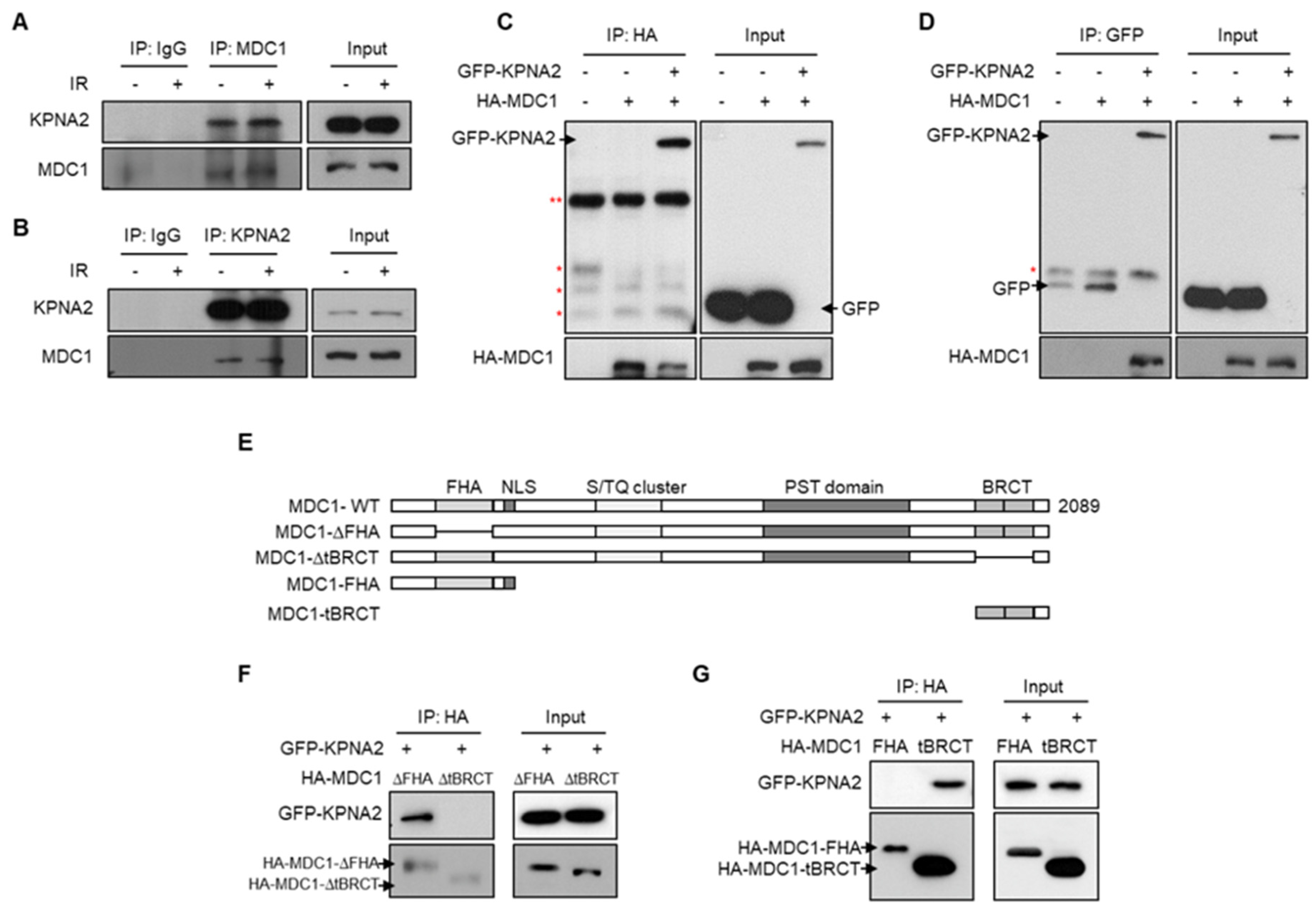
2.1. KPNA2 Binds to the tBRCT Domain of MDC1
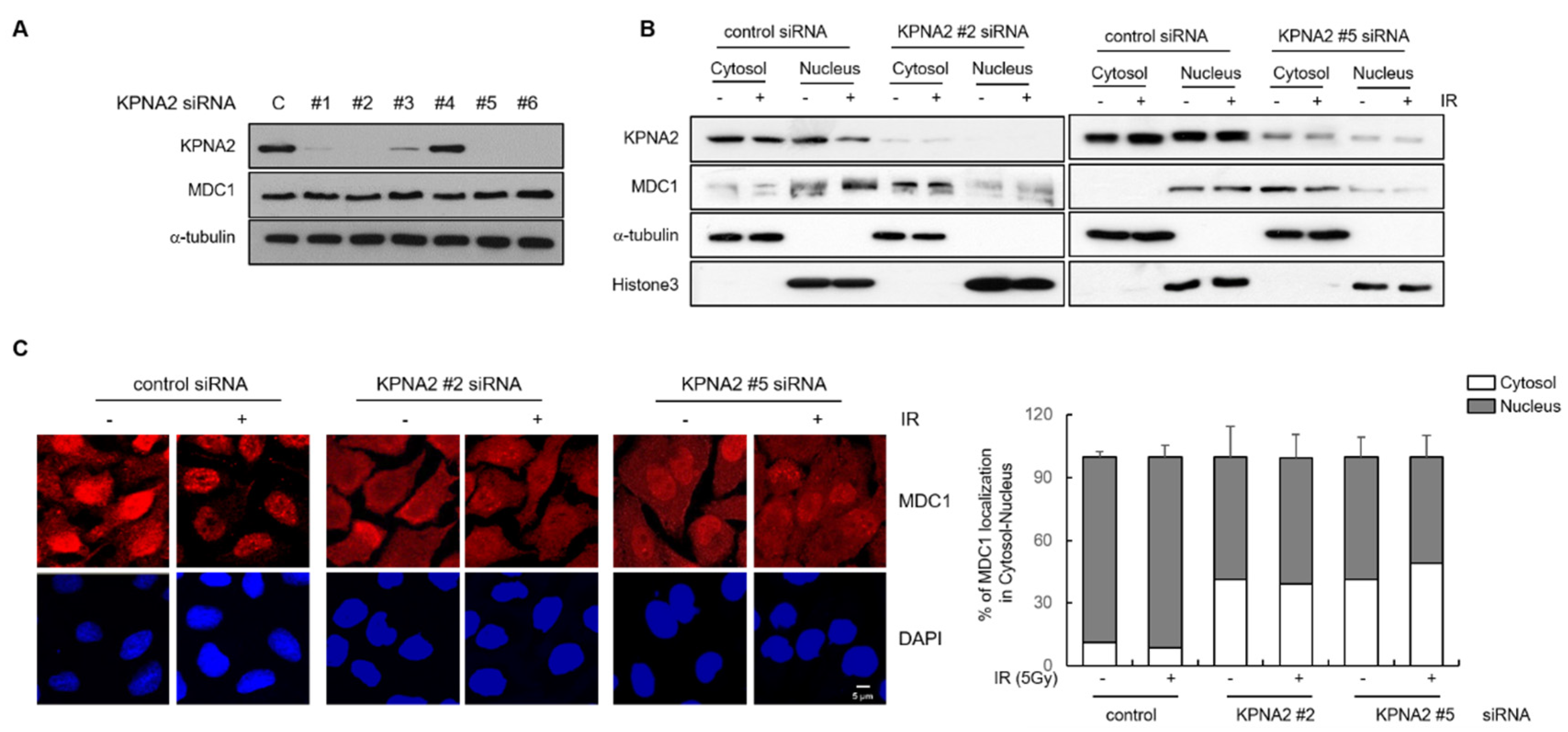
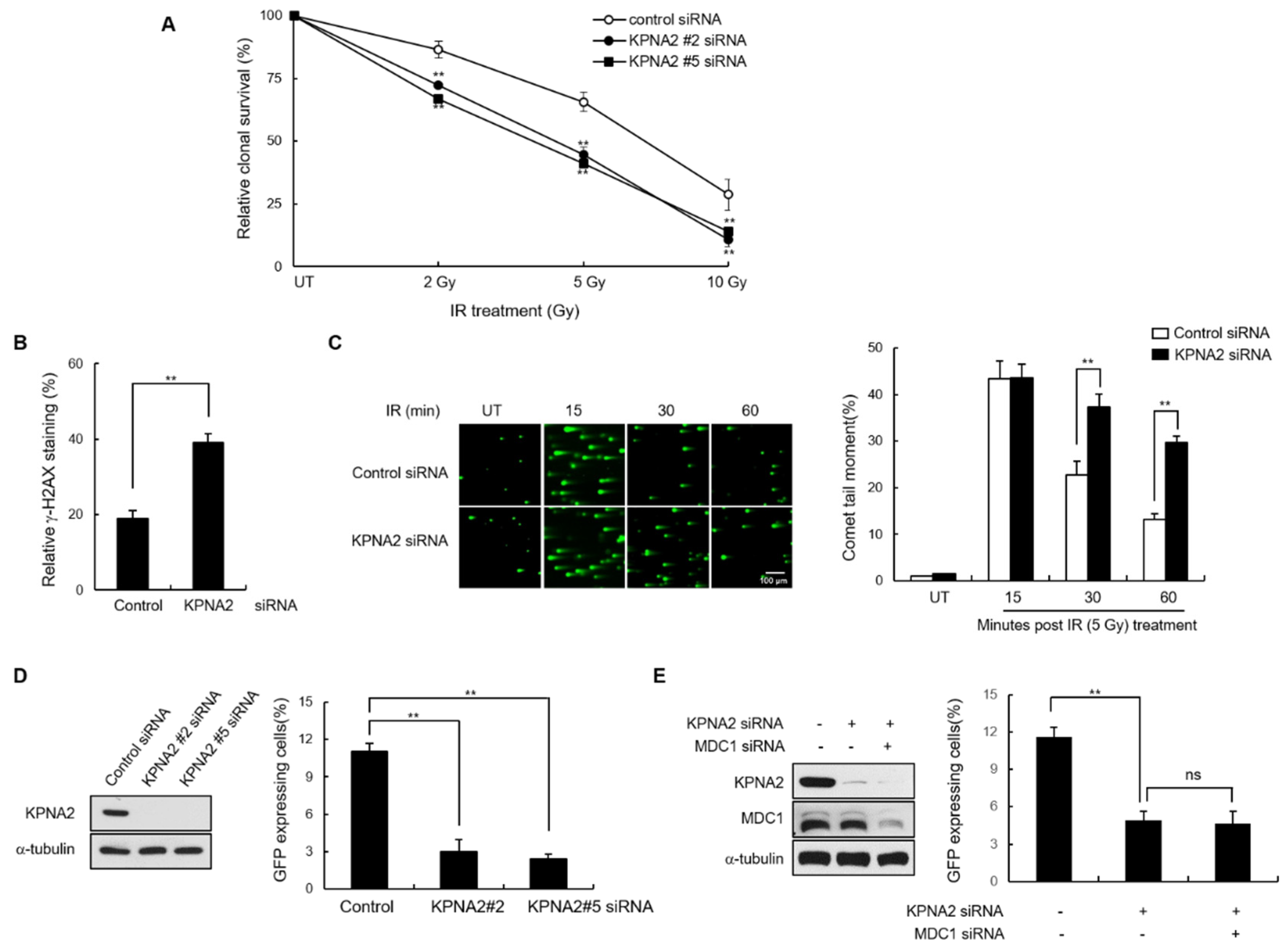
2.2. KPNA2 Knockdown Results in Impaired Nuclear Translocation of MDC1
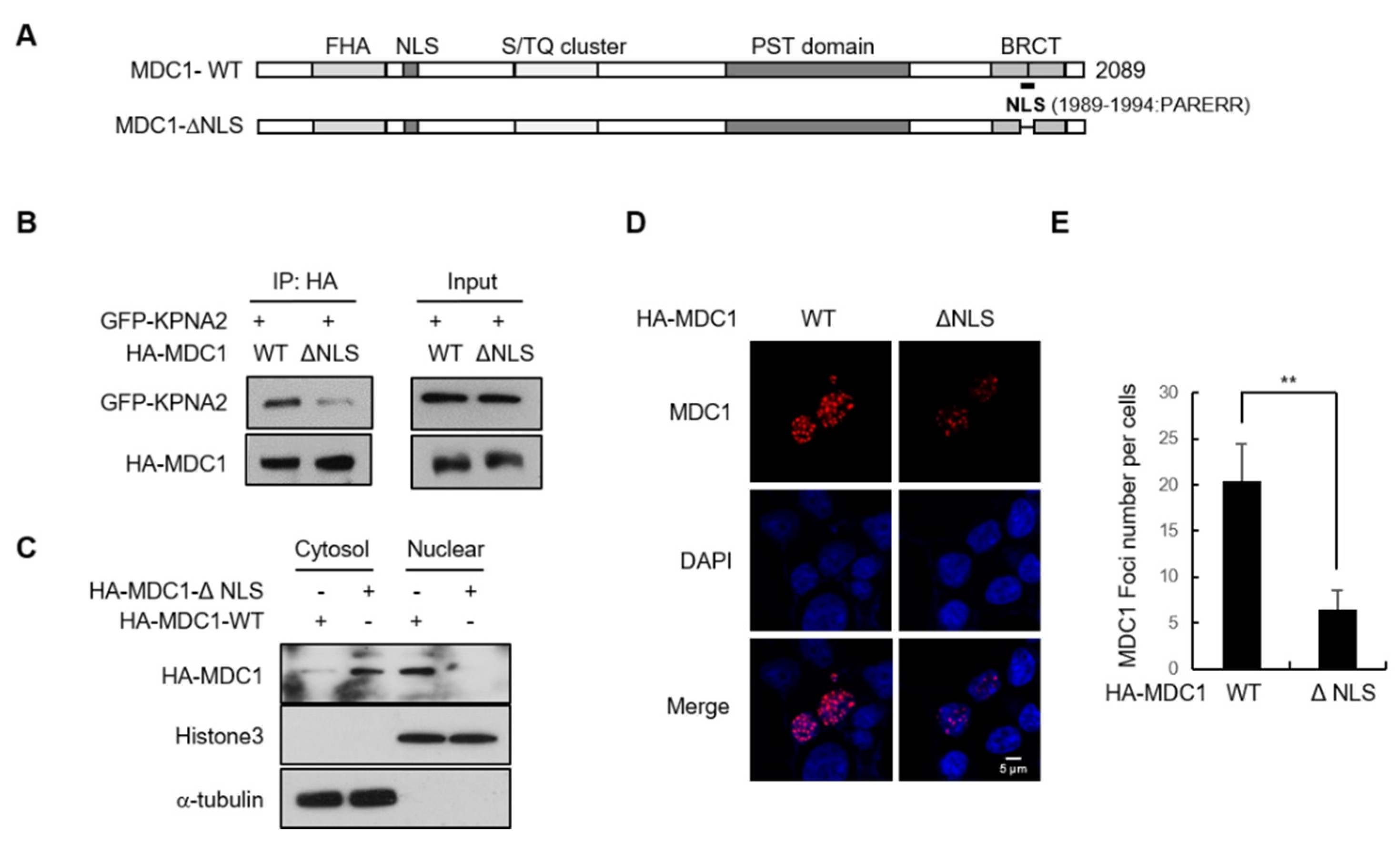
2.3. Putative NLS of MDC1 is Critical for Its Binding to KPNA2 and Contributes to Its Nuclear Import
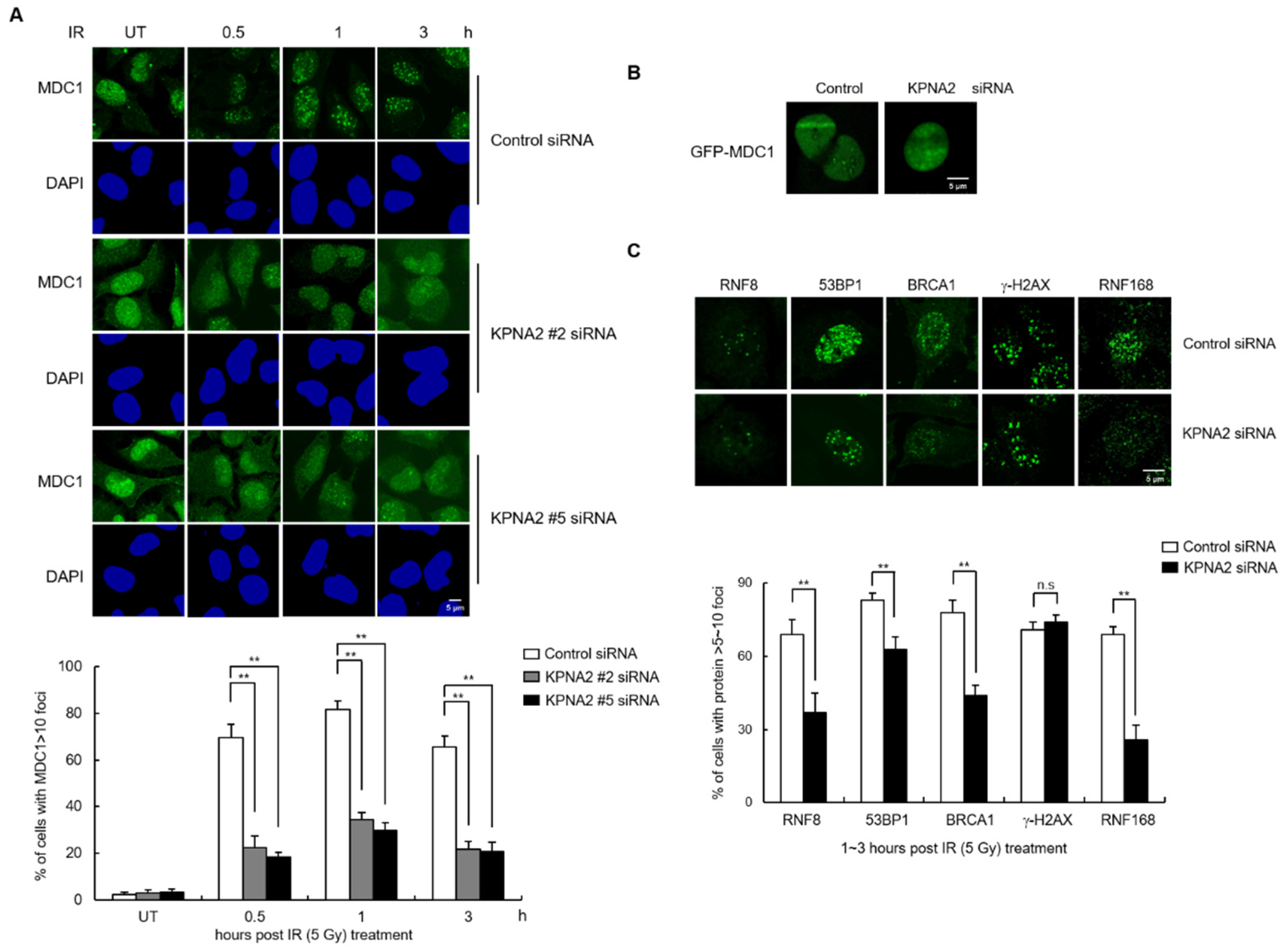
2.4. KPNA2 Knockdown Results in Reduced IR-induced MDC1 Foci Formation and Impaired Homologous Recombination (HR) Repair
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transfection of Small-Interference RNA (siRNA)
4.3. Preparation of Plasmid Construction
4.4. Western Blot Analysis
4.5. Immunoprecipitation
4.6. Immunofluorescence Microscopy
4.7. Laser Microirradiation
4.8. Antibodies
4.9. Neutral Comet Assay
4.10. Clonal Survival Assay
4.11. DR-GFP Assay
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DDR | DNA damage response |
| DSBs | Double stranded breaks |
| FHA | Forkhead-associated |
| HR | Homologous recombination |
| KPNA2 | Karyopherin α-2 |
| MDC1 | Mediator of DNA damage checkpoint 1 |
| NLS | Nuclear localization sequence |
| tBRCT | two BRCA1 carboxyl-terminal |
References
- Lee, J.H.; Park, S.J.; Hariharasudhan, G.; Kim, M.J.; Jung, S.M.; Jeong, S.Y.; Chang, I.Y.; Kim, C.; Kim, E.; Yu, J.; et al. ID3 regulates the MDC1-mediated DNA damage response in order to maintain genome stability. Nat Commun. 2017, 8, 903. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Minter-Dykhouse, K.; Wu, X.; Chen, J. MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature 2003, 421, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Stucki, M.; Clapperton, J.A.; Mohammad, D.; Yaffe, M.B.; Smerdon, S.; Jackson, S.P. MDC1 Directly Binds Phosphorylated Histone H2AX to Regulate Cellular Responses to DNA Double-Strand Breaks. Cell 2005, 123, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Jungmichel, S.; Stucki, M. MDC1: The art of keeping things in focus. Chromosom. 2010, 119, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Stucki, M.; Falck, J.; D’Amours, D.; Rahman, D.; Pappin, D.J.; Blow, J.J.; Jackson, S.P. MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature. 2003, 421, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.S.; Wang, B.; Bignell, C.R.; Taylor, A.M.; Elledge, S.J. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature 2003, 421, 961–966. [Google Scholar] [CrossRef]
- Lukas, C.; Melander, F.; Stucki, M.; Falck, J.; Bekker-Jensen, S.; Goldberg, M.; Lerenthal, Y.; Jackson, S.P.; Bartek, J.; Lukas, J. Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention. EMBO J. 2004, 23, 2674–2683. [Google Scholar] [CrossRef]
- Stucki, M.; Jackson, S.P. MDC1/NFBD1: a key regulator of the DNA damage response in higher eukaryotes. DNA Repair 2004, 3, 953–957. [Google Scholar] [CrossRef]
- Kim, J.-E.; Chen, J.; Minter-Dykhouse, K. Signaling networks controlled by the MRN complex and MDC1 during early DNA damage responses. Mol. Carcinog. 2006, 45, 403–408. [Google Scholar] [CrossRef]
- Holthausen, J.T.; Wyman, C.; Kanaar, R. Regulation of DNA strand exchange in homologous recombination. DNA Repair 2010, 9, 1264–1272. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z.; Treszezamsky, A.; Powell, S.N. MDC1 interacts with Rad51 and facilitates homologous recombination. Nat. Struct. Mol. Boil. 2005, 12, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Görlich, D. Isolation of a protein that is essential for the first step of nuclear protein import. Cell 1994, 79, 767–778. [Google Scholar] [CrossRef]
- Görlich, D.; Henklein, P.; Laskey, R.A.; Hartmann, E. A 41 amino acid motif in importin-alpha confers binding to importin-beta and hence transit into the nucleus. EMBO J. 1996, 15, 1810–1817. [Google Scholar] [CrossRef]
- Cingolani, G.; Petosa, C.; Weis, K.; Müller, C.W. Structure of importin-β bound to the IBB domain of importin-α. Nature 1999, 399, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Rexach, M.; Blobel, G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell 1995, 83, 683–692. [Google Scholar] [CrossRef]
- Goldfarb, D.S.; Corbett, A.H.; Mason, D.A.; Harreman, M.T.; Adam, S.A. Importin α: a multipurpose nuclear-transport receptor. Trends Cell Boil. 2004, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Ba, A.N.N.; Pogoutse, A.; Provart, N.J.; Moses, A.M. NLStradamus: a simple Hidden Markov Model for nuclear localization signal prediction. BMC Bioinform. 2009, 10, 202. [Google Scholar]
- Lou, Z.; Minter-Dykhouse, K.; Franco, S.; Gostissa, M.; Rivera, M.A.; Celeste, A.; Manis, J.P.; Van Deursen, J.; Nussenzweig, A.; Paull, T.T.; et al. MDC1 Maintains Genomic Stability by Participating in the Amplification of ATM-Dependent DNA Damage Signals. Mol. Cell 2006, 21, 187–200. [Google Scholar] [CrossRef]
- Coster, G.; Goldberg, M. The cellular response to DNA damage: a focus on MDC1 and its interacting proteins. Nucleus 2009, 1, 166–178. [Google Scholar] [CrossRef]
- Tseng, S.-F.; Chang, C.-Y.; Wu, K.-J.; Teng, S.-C. Importin KPNA2 Is Required for Proper Nuclear Localization and Multiple Functions of NBS1. J. Boil. Chem. 2005, 280, 39594–39600. [Google Scholar] [CrossRef]
- Zannini, L.; Lecis, D.; Lisanti, S.; Benetti, R.; Buscemi, G.; Schneider, C.; Delia, D. Karyopherin-α2 Protein Interacts with Chk2 and Contributes to Its Nuclear Import. J. Boil. Chem. 2003, 278, 42346–42351. [Google Scholar] [CrossRef] [PubMed]
- Schuchner, S.; Tembe, V.; Rodriguez, J.A.; Henderson, B.R. Nuclear Targeting and Cell Cycle Regulatory Function of Human BARD1. J. Boil. Chem. 2005, 280, 8855–8861. [Google Scholar] [CrossRef] [PubMed]
- Moudry, P.; Lukas, C.; Macurek, L.; Neumann, B.; Hériché, J.-K.; Pepperkok, R.; Ellenberg, J.; Hodny, Z.; Lukas, J.; Blow, J.J. Nucleoporin NUP153 guards genome integrity by promoting nuclear import of 53BP1. Cell Death Differ. 2011, 19, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Michael, W.M.; Yan, S. Importin β-dependent nuclear import of TopBP1 in ATR-Chk1 checkpoint in Xenopus egg extracts. Cell. Signal. 2014, 26, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luo, K.; Lou, Z.; Chen, J. MDC1 regulates intra-S-phase checkpoint by targeting NBS1 to DNA double-strand breaks. Proc. Natl. Acad. Sci USA 2008, 105, 11200–11205. [Google Scholar] [CrossRef]
- Friedrich, B.; Quensel, C.; Sommer, T.; Hartmann, E.; Kohler, M. Nuclear Localization Signal and Protein Context both Mediate Importin α Specificity of Nuclear Import Substrates. Mol. Cell. Boil. 2006, 26, 8697–8709. [Google Scholar] [CrossRef] [PubMed]
- Chook, Y. Karyopherins and nuclear import. Curr. Opin. Struct. Boil. 2001, 11, 703–715. [Google Scholar] [CrossRef]
- Guirouilh-Barbat, J.; Lambert, S.; Bertrand, P.; Lopez, B.S.; Guirouilh-Barbat, J. Is homologous recombination really an error-free process? Front. Genet. 2014, 5, 175. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Cingolani, G. Diversification of importin-α isoforms in cellular trafficking and disease states. Biochem. J. 2015, 466, 13–28. [Google Scholar] [CrossRef]
- Kelley, J.B.; Talley, A.M.; Spencer, A.; Gioeli, D.; Paschal, B.M. Karyopherin α7 (KPNA7), a divergent member of the importin α family of nuclear import receptors. BMC Cell Boil. 2010, 11, 63. [Google Scholar]
- Hu, J.; Wang, F.; Yuan, Y.; Zhu, X.; Wang, Y.; Zhang, Y.; Kou, Z.; Wang, S.; Gao, S. Novel Importin-α Family Member Kpna7 Is Required for Normal Fertility and Fecundity in the Mouse*. J. Boil. Chem. 2010, 285, 33113–33122. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.; Chang, C.-W.; Rona, G.; Smith, K.; Stewart, A.G.; Takeda, A.A.; Fontes, M.R.D.M.; Stewart, M.; Vértessy, B.G.; Forwood, J.K.; et al. Structural Biology and Regulation of Protein Import into the Nucleus. J. Mol. Boil. 2016, 428, 2060–2090. [Google Scholar] [CrossRef] [PubMed]
- Marfori, M.; Mynott, A.; Ellis, J.; Mehdi, A.M.; Saunders, N.; Curmi, P.; Forwood, J.K.; Bodén, M.; Kobe, B. Molecular basis for specificity of nuclear import and prediction of nuclear localization. Biochim. Biophys. Acta 2011, 1813, 1562–1577. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radhakrishnan, K.; Park, S.-J.; Kim, S.W.; Hariharasudhan, G.; Jeong, S.-Y.; Chang, I.Y.; Lee, J.-H. Karyopherin α-2 Mediates MDC1 Nuclear Import through a Functional Nuclear Localization Signal in the tBRCT Domain of MDC1. Int. J. Mol. Sci. 2020, 21, 2650. https://doi.org/10.3390/ijms21072650
Radhakrishnan K, Park S-J, Kim SW, Hariharasudhan G, Jeong S-Y, Chang IY, Lee J-H. Karyopherin α-2 Mediates MDC1 Nuclear Import through a Functional Nuclear Localization Signal in the tBRCT Domain of MDC1. International Journal of Molecular Sciences. 2020; 21(7):2650. https://doi.org/10.3390/ijms21072650
Chicago/Turabian StyleRadhakrishnan, Kamalakannan, Seon-Joo Park, Seok Won Kim, Gurusamy Hariharasudhan, Seo-Yeon Jeong, In Youb Chang, and Jung-Hee Lee. 2020. "Karyopherin α-2 Mediates MDC1 Nuclear Import through a Functional Nuclear Localization Signal in the tBRCT Domain of MDC1" International Journal of Molecular Sciences 21, no. 7: 2650. https://doi.org/10.3390/ijms21072650
APA StyleRadhakrishnan, K., Park, S.-J., Kim, S. W., Hariharasudhan, G., Jeong, S.-Y., Chang, I. Y., & Lee, J.-H. (2020). Karyopherin α-2 Mediates MDC1 Nuclear Import through a Functional Nuclear Localization Signal in the tBRCT Domain of MDC1. International Journal of Molecular Sciences, 21(7), 2650. https://doi.org/10.3390/ijms21072650





