Expression of Sucrose Transporters from Vitis vinifera Confer High Yield and Enhances Drought Resistance in Arabidopsis
Abstract
1. Introduction
2. Results
2.1. Amino Acids Sequences Analysis of VvSUC Proteins
2.2. SUT Overexpression Alters Morphological Phenotypes and the Germination Rate of Arabidopsis Seeds
2.3. VvSUC-OE Lines Have Developmental Phenotypes When Grown on Sucrose
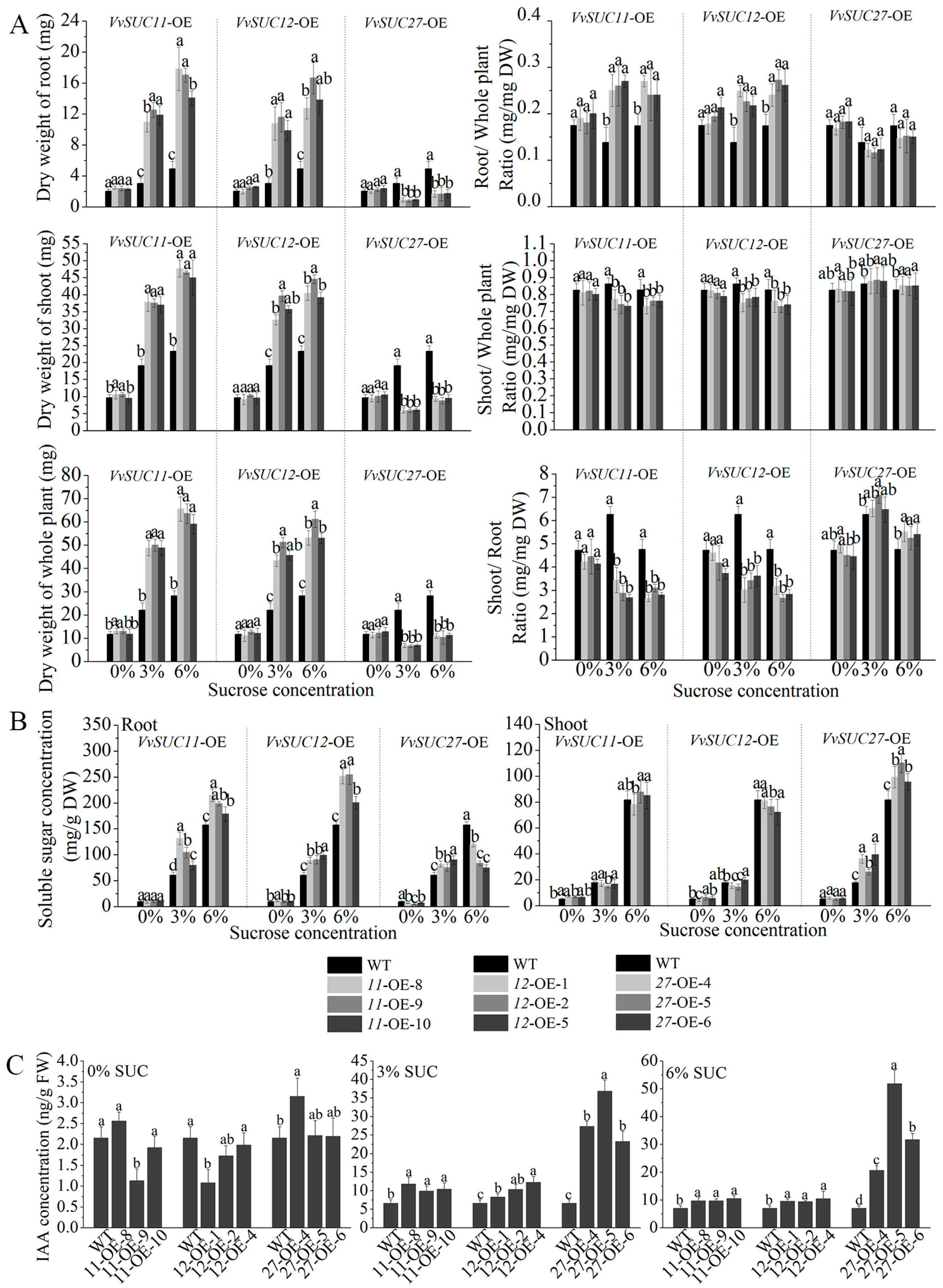
2.4. Dry Weight, Soluble Sugar Concentrations, and Endogenous Plant Growth Hormone Levels Were Altered in the VvSUC-OE Lines
2.5. VvSUC-OE Lines Have Developmental Phenotypes When Grown in Soil
2.6. VvSUC-OE Altered Leaf and Root Structures, Soluble Sugar Concentration, and Endogenous Auxin Levels in Plants Grown in Soil
2.7. VvSUC-OE Lines Altered Arabidopsis Drought Resistance When Grown in Soil
3. Discussion
3.1. VvSUC11 and VvSUC12 Regulate SUT-Dependent Sucrose Transport in Similar Way
3.2. VvSUC27 Shows the Strong Sucrose Transport Capacity Along the Phloem Path
3.3. Potential Role for SUTs in Crop Field and Drought Resistance Improvement
4. Materials and Methods
4.1. Phylogeny and Sequence Similarities Analysis
4.2. Plant Material and Growth Conditions
4.3. Generation of Transgene Constructs and Plant Transformation
4.4. qRT-PCR
4.5. Scanning Electron Microscopy
4.6. Anatomical Section Analysis
4.7. Dry Weight Determination
4.8. Soluble Sugar Measurements
4.9. Determination of Endogenous Phytohormone Concentration
4.10. Drought Stress Treatment
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SUT or SUC | sucrose transporter |
| SE | sieve element |
| LAHC | low-affinity/high capacity |
| OE | overexpressing |
| WT | wild type |
| MS | Murashige and Skoog |
| DW | dry weight |
| FW | fresh weight |
| x | xylem |
| c&p | cambium and phloem |
| p | periderm |
| IAA | indole-3-acetic acid |
| qRT-PCR | quantitative real-time polymerase chain reaction |
References
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Riesmeier, J.W.; Willmitzer, L.; Frommer, W.B. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992, 11, 4705–4713. [Google Scholar] [CrossRef] [PubMed]
- Weise, A.; Lalonde, S.; Kuhn, C.; Frommer, W.B.; Ward, J.M. Introns control expression of sucrose transporter LeSUT1 in trichomes, companion cells and in guard cells. Plant Mol. Biol. 2008, 68, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Qi, X.X.; Huang, X.S.; Xu, L.L.; Jin, C.; Wu, J.; Zhang, S.L. Overexpression of sucrose transporter gene PbSUT2 from Pyrus bretschneideri, enhances sucrose content in Solanum lycopersicum fruit. Plant Physiol. Biochem. 2016, 105, 150–161. [Google Scholar] [CrossRef]
- Sun, Y.; Ward, J.M. Arg188 in rice sucrose transporter OsSUT1 is crucial for substrate transport. BMC Biochem. 2012, 13, 26. [Google Scholar] [CrossRef]
- Scofield, G.N.; Hirose, T.; Aoki, N.; Furbank, R.T. Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J. Exp. Bot. 2007, 58, 3155–3169. [Google Scholar] [CrossRef]
- Chincinska, I.A.; Liesche, J.; Krugel, U.; Michalska, J.; Geigenberger, P.; Grimm, B.; Kuhn, C. Sucrose transporter StSUT4 from potato affects flowering, tuberization, and shade avoidance response. Plant Physiol. 2008, 146, 515–528. [Google Scholar] [CrossRef]
- Kuhn, C.; Grof, C.P. Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 2010, 13, 288–298. [Google Scholar] [CrossRef]
- Kuhn, C.; Franceschi, V.R.; Schulz, A.; Lemoine, R.; Frommer, W.B. Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 1997, 275, 1298–1300. [Google Scholar] [CrossRef]
- Hackel, A.; Schauer, N.; Carrari, F.; Fernie, A.R.; Grimm, B.; Kuhn, C. Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J. 2006, 45, 180–192. [Google Scholar] [CrossRef]
- Barker, L.; Kuhn, C.; Weise, A.; Schulz, A.; Gebhardt, C.; Hirner, B.; Hellmann, H.; Schulze, W.; Ward, J.M.; Frommer, W.B. SUT2, a putative sucrose sensor in sieve elements. Plant Cell 2000, 12, 1153–1164. [Google Scholar] [CrossRef]
- Knop, C.; Stadler, R.; Sauer, N.; Lohaus, G. AmSUT1, a sucrose transporter in collection and transport phloem of the putative symplastic phloem loader Alonsoa meridionalis. Plant Physiol. 2004, 134, 204–214. [Google Scholar] [CrossRef]
- Schulze, W.; Weise, A.; Frommer, W.B.; Ward, J.M. Function of the cytosolic N-terminus of sucrose transporter AtSUT2 in substrate affinity. FEBS Lett. 2000, 485, 189–194. [Google Scholar] [CrossRef]
- Barth, I.; Meyer, S.; Sauer, N. PmSUC3: Characterization of a SUT2/SUC3-type sucrose transporter from Plantago major. Plant Cell 2003, 15, 1375–1385. [Google Scholar] [CrossRef]
- Meyer, S.; Lauterbach, C.; Niedermeier, M.; Barth, I.; Sjolund, R.D.; Sauer, N. Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiol. 2004, 134, 684–693. [Google Scholar] [CrossRef]
- Leach, K.A.; Tran, T.M.; Slewinski, T.L.; Meeley, R.B.; Braun, D.M. Sucrose transporter2 contributes to maize growth, development, and crop yield. J. Integr. Plant Biol. 2017, 59, 390–408. [Google Scholar] [CrossRef]
- Weise, A.; Barker, L.; Kuhn, C.; Lalonde, S.; Buschmann, H.; Frommer, W.B.; Ward, J.M. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 2000, 12, 1345–1355. [Google Scholar] [CrossRef]
- Chincinska, I.; Gier, K.; Krugel, U.; Liesche, J.; He, H.; Grimm, B.; Harren, F.J.; Cristescu, S.M.; Kuhn, C. Photoperiodic regulation of the sucrose transporter StSUT4 affects the expression of circadian-regulated genes and ethylene production. Front. Plant Sci. 2013, 4, 26. [Google Scholar] [CrossRef]
- Endler, A.; Meyer, S.; Schelbert, S.; Schneider, T.; Weschke, W.; Peters, S.W.; Keller, F.; Baginsky, S.; Martinoia, E.; Schmidt, U.G. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol. 2006, 141, 196–207. [Google Scholar] [CrossRef]
- Chen, Q.; Diao, L.; Song, H.; Zhu, X. Vitis amurensis Rupr: A review of chemistry and pharmacology. Phytomed. Int. J. Phytother. Phytopharm. 2018, 49, 111–122. [Google Scholar] [CrossRef]
- Chen, J.; Wang, N.; Fang, L.C.; Liang, Z.C.; Li, S.H.; Wu, B.H. Construction of a high-density genetic map and QTLs mapping for sugars and acids in grape berries. BMC Plant Biol. 2015, 15, 28. [Google Scholar] [CrossRef]
- Davies, C.; Wolf, T.; Robinson, S.P. Three putative sucrose transporters are differentially expressed in grapevine tissues. Plant Sci. 1999, 147, 93–100. [Google Scholar] [CrossRef]
- Manning, K.; Davies, C.; Bowen, H.C.; White, P.J. Functional characterization of two ripening-related sucrose transporters from grape berries. Ann. Bot. 2001, 87, 125–129. [Google Scholar] [CrossRef]
- Ageorges, A.; Issaly, R.; Picaud, S.; Delrot, S.; Romieu, C. Identification and functional expression in yeast of a grape berry sucrose carrier. Plant Physiol. Biochem. 2000, 38, 177–185. [Google Scholar] [CrossRef]
- Afoufa-Bastien, D.; Medici, A.; Jeauffre, J.; Coutos-Thevenot, P.; Lemoine, R.; Atanassova, R.; Laloi, M. The Vitis vinifera sugar transporter gene family: Phylogenetic overview and macroarray expression profiling. BMC Plant Biol. 2010, 10, 1741–1763. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Meng, Q.Y.; Zhu, H.L.; Guo, Y.; Gao, H.Y.; Luo, Y.B.; Lu, J.A. Functional characterization of a LAHC sucrose transporter isolated from grape berries in yeast. Plant Growth Regul. 2008, 54, 71–79. [Google Scholar] [CrossRef]
- Cai, Y.; Tu, W.; Zu, Y.; Jing, Y.; Xu, Z.; Lu, J.; Zhang, Y. Overexpression of a Grapevine Sucrose Transporter (VvSUC27) in Tobacco Improves Plant Growth Rate in the Presence of Sucrose In vitro. Front. Plant Sci. 2017, 8, 1069. [Google Scholar] [CrossRef]
- Moore, C.R.; Gronwall, D.S.; Miller, N.D.; Spalding, E.P. Mapping quantitative trait loci affecting Arabidopsis thaliana seed morphology features extracted computationally from images. G3 2013, 3, 109–118. [Google Scholar] [CrossRef]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef]
- Shi, H.; Liu, G.; Wei, Y.; Chan, Z. The zinc-finger transcription factor ZAT6 is essential for hydrogen peroxide induction of anthocyanin synthesis in Arabidopsis. Plant Mol. Biol. 2018, 97, 165–176. [Google Scholar] [CrossRef]
- Zhang, X.; Abrahan, C.; Colquhoun, T.A.; Liu, C.J. A Proteolytic Regulator Controlling Chalcone Synthase Stability and Flavonoid Biosynthesis in Arabidopsis. Plant Cell 2017, 29, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, W.J.; Vu, T.T.; Jeong, C.Y.; Hong, S.W.; Lee, H. High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Rep. 2017, 36, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Z.; Maximova, S.; Payne, M.J.; Guiltinan, M.J. Proanthocyanidin synthesis in Theobroma cacao: Genes encoding anthocyanidin synthase, anthocyanidin reductase, and leucoanthocyanidin reductase. BMC Plant Biol. 2013, 13, 202. [Google Scholar] [CrossRef]
- Jameson, P.E.; Dhandapani, P.; Novak, O.; Song, J. Cytokinins and Expression of SWEET, SUT, CWINV and AAP Genes Increase as Pea Seeds Germinate. Int. J. Mol. Sci. 2016, 17, 2013. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Srivastava, A.C.; Ganesan, S.; Ismail, I.O.; Ayre, B.G. Functional characterization of the Arabidopsis AtSUC2 Sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol. 2008, 148, 200–211. [Google Scholar] [CrossRef]
- Gong, X.; Liu, M.; Zhang, L.; Ruan, Y.; Ding, R.; Ji, Y.; Zhang, N.; Zhang, S.; Farmer, J.; Wang, C. Arabidopsis AtSUC2 and AtSUC4, encoding sucrose transporters, are required for abiotic stress tolerance in an ABA-dependent pathway. Physiol. Plant 2015, 153, 119–136. [Google Scholar] [CrossRef]
- Gottwald, J.R.; Krysan, P.J.; Young, J.C.; Evert, R.F.; Sussman, M.R. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13979–13984. [Google Scholar] [CrossRef]
- Gibson, S.I. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef]
- Reinders, A.; Schulze, W.; Kuhn, C.; Barker, L.; Schulz, A.; Ward, J.M.; Frommer, W.B. Protein-protein interactions between sucrose transporters of different affinities colocalized in the same enucleate sieve element. Plant Cell 2002, 14, 1567–1577. [Google Scholar] [CrossRef]
- Sivitz, A.B.; Reinders, A.; Ward, J.M. Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol. 2008, 147, 92–100. [Google Scholar] [CrossRef]
- Schneider, S.; Hulpke, S.; Schulz, A.; Yaron, I.; Holl, J.; Imlau, A.; Schmitt, B.; Batz, S.; Wolf, S.; Hedrich, R.; et al. Vacuoles release sucrose via tonoplast-localised SUC4-type transporters. Plant Biol. 2012, 14, 325–336. [Google Scholar] [CrossRef]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef]
- Schulze, S.; Westhoff, P.; Gowik, U. Glycine decarboxylase in C3, C4 and C3-C4 intermediate species. Curr. Opin. Plant Biol. 2016, 31, 29–35. [Google Scholar] [CrossRef]
- Brautigam, A.; Gowik, U. Photorespiration connects C3 and C4 photosynthesis. J. Exp. Bot. 2016, 67, 2953–2962. [Google Scholar] [CrossRef]
- Hibberd, J.M.; Covshoff, S. The regulation of gene expression required for C4 photosynthesis. Annu. Rev. Plant Biol. 2010, 61, 181–207. [Google Scholar] [CrossRef]
- Wang, L.; Czedik-Eysenberg, A.; Mertz, R.A.; Si, Y.; Tohge, T.; Nunes-Nesi, A.; Arrivault, S.; Dedow, L.K.; Bryant, D.W.; Zhou, W.; et al. Comparative analyses of C(4) and C(3) photosynthesis in developing leaves of maize and rice. Nat. Biotechnol. 2014, 32, 1158–1165. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Chen, S.Y.; Ren, Y.J.; Chen, S.L.; Liesche, J. Regulation of Sucrose Transporters and Phloem Loading in Response to Environmental Cues. Plant Physiol. 2018, 176, 930–945. [Google Scholar] [CrossRef]
- Reinders, A.; Sivitz, A.B.; Ward, J.M. Evolution of plant sucrose uptake transporters. Front. Plant Sci. 2012, 3, 22. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, J.; Li, Q.; Deng, Z.; Liu, S.; Lu, J.; Zhang, Y. Sucrose transporters of resistant grapevine are involved in stress resistance. Plant Mol. Biol. 2019, 100, 111–132. [Google Scholar] [CrossRef]
- Ma, Q.J.; Sun, M.H.; Lu, J.; Kang, H.; You, C.X.; Hao, Y.J. An apple sucrose transporter MdSUT2.2 is a phosphorylation target for protein kinase MdCIPK22 in response to drought. Plant Biotechnol. J. 2019, 17, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Bent, A. Arabidopsis thaliana floral dip transformation method. Methods Mol. Biol. 2006, 343, 87–103. [Google Scholar] [PubMed]
- Wu, X.J.; Sun, S.; Xing, G.M.; Wang, G.L.; Wang, F.; Xu, Z.S.; Tian, Y.S.; Hou, X.L.; Xiong, A.S. Elevated Carbon Dioxide Altered Morphological and Anatomical Characteristics, Ascorbic Acid Accumulation, and Related Gene Expression during Taproot Development in Carrots. Front. Plant Sci. 2016, 7, 2026. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, S.W.; Gan, Y.T.; Xu, B.L. Application of Plant-Growth-Promoting Fungi Trichoderma longibrachiatum T6 Enhances Tolerance of Wheat to Salt Stress through Improvement of Antioxidative Defense System and Gene Expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef] [PubMed]
- Somani, B.L.; Khanade, J.; Sinha, R. A modified anthrone-sulfuric acid method for the determination of fructose in the presence of certain proteins. Anal. Biochem. 1987, 167, 327–330. [Google Scholar] [CrossRef]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]





| Line | Seeds Number Per Silique | Silique Number | Yield Per Plant (mg) |
|---|---|---|---|
| VvSUC11-OE-8 | 44.71 ± 0.43 d | 402.29 ± 31.76 a | 339.97 ± 15.83 a,b |
| VvSUC11-OE-9 | 44.29 ± 0.21 d,e | 350.89 ± 15.49 a,b,c | 292.75 ± 28.72 a,b,c |
| VvSUC11-OE-10 | 46.83 ± 0.13 a,b | 383.72 ± 28.84 a,b,c | 342.39 ± 20.31 a |
| VvSUC12-OE-1 | 46.31 ± 0.24 a,b,c | 381.39 ± 31.07 a,b,c | 330.12 ± 23.50 a,b |
| VvSUC12-OE-2 | 45.89 ± 0.13 c | 392.81 ± 28.53 a,b | 341.58 ± 35.02 a,b |
| VvSUC12-OE-4 | 43.92 ± 0.26 e | 414.3 ± 37.51 a | 345.47 ± 29.17 a |
| VvSUC27-OE-4 | 47.02 ± 0.25 a | 318.39 ± 27.07 b,c,d | 282.12 ± 19.72 a,b,c |
| VvSUC27-OE-5 | 44.74 ± 0.33 d | 322.81 ± 22.53 b,c,d | 276.58 ± 26.15 a,b,c |
| VvSUC27-OE-6 | 46.13 ± 0.24 b,c | 308.19 ± 19.51 c,d | 270.54 ± 21.43 b,c |
| WT | 46.72 ± 0.24 a,b | 268.57 ± 16.79 d | 244.84 ± 22.91 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Yan, J.; Tu, W.; Deng, Z.; Dong, W.; Gao, H.; Xu, J.; Zhang, N.; Yin, L.; Meng, Q.; et al. Expression of Sucrose Transporters from Vitis vinifera Confer High Yield and Enhances Drought Resistance in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 2624. https://doi.org/10.3390/ijms21072624
Cai Y, Yan J, Tu W, Deng Z, Dong W, Gao H, Xu J, Zhang N, Yin L, Meng Q, et al. Expression of Sucrose Transporters from Vitis vinifera Confer High Yield and Enhances Drought Resistance in Arabidopsis. International Journal of Molecular Sciences. 2020; 21(7):2624. https://doi.org/10.3390/ijms21072624
Chicago/Turabian StyleCai, Yumeng, Jing Yan, Wenrui Tu, Zhefang Deng, Wenjie Dong, Han Gao, Jinxu Xu, Nan Zhang, Ling Yin, Qingyong Meng, and et al. 2020. "Expression of Sucrose Transporters from Vitis vinifera Confer High Yield and Enhances Drought Resistance in Arabidopsis" International Journal of Molecular Sciences 21, no. 7: 2624. https://doi.org/10.3390/ijms21072624
APA StyleCai, Y., Yan, J., Tu, W., Deng, Z., Dong, W., Gao, H., Xu, J., Zhang, N., Yin, L., Meng, Q., & Zhang, Y. (2020). Expression of Sucrose Transporters from Vitis vinifera Confer High Yield and Enhances Drought Resistance in Arabidopsis. International Journal of Molecular Sciences, 21(7), 2624. https://doi.org/10.3390/ijms21072624





