Recent Strategic Advances in CFTR Drug Discovery: An Overview
Abstract
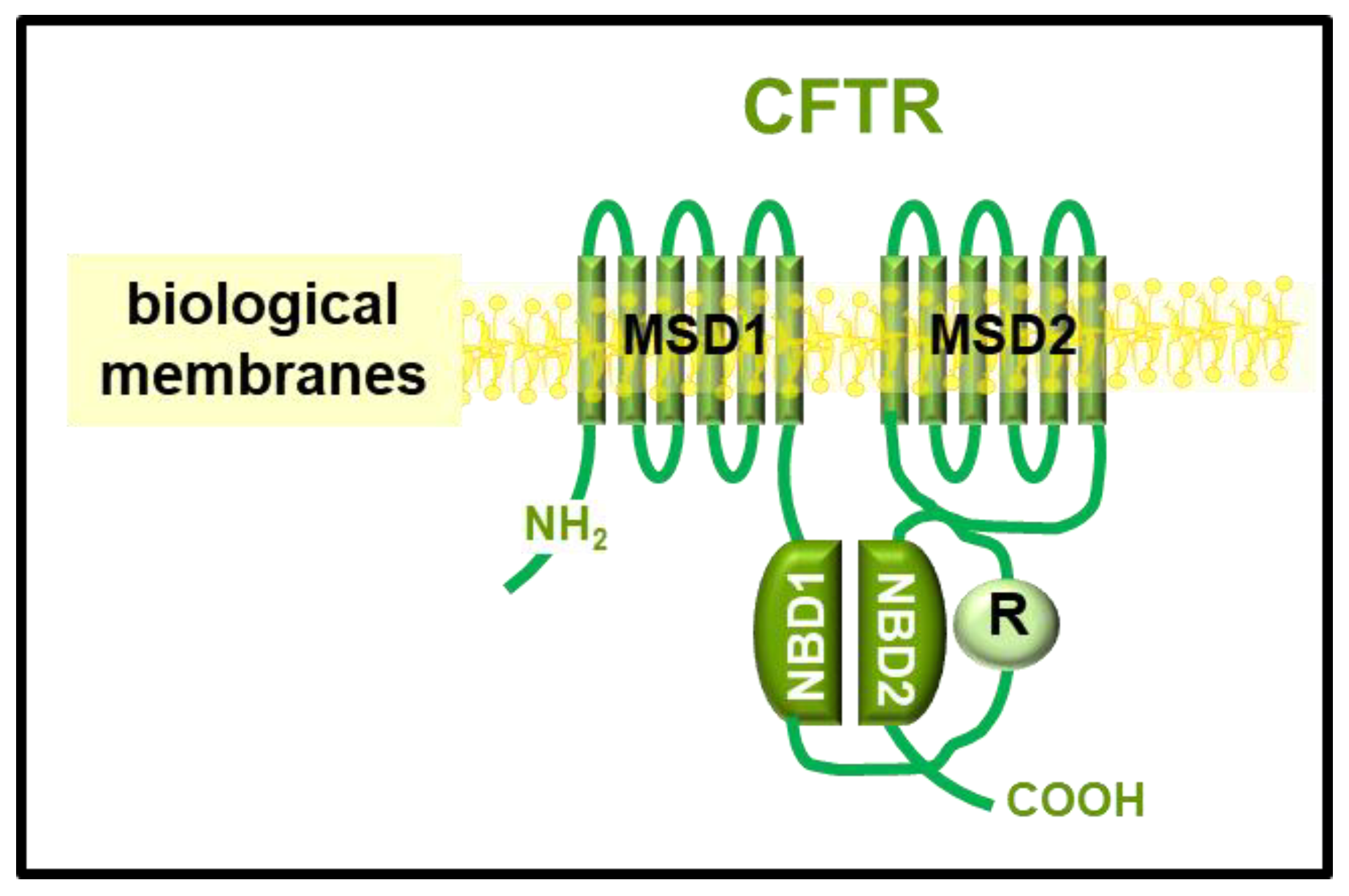
1. Cystic Fibrosis: Pathogenesis, Clinical and Therapeutic Implications
2. CFTR Experimental Structures
- (i)
- Some of the NBD1 structures available (Table 1) contain stabilizing or solubilizing point mutations introduced to get the crystals.
- (ii)
- All the reported structures of intact CFTR (Table 2) refer to the WT protein and not to disease variants, thus information concerning the different flexibility in the 3D structure of the mutated pathological proteins (e.g., F508del-NBD1 or -CFTR) are scarcely available for drug discovery. Additionally, the chicken structures show several mutations, deriving from the thermostabilization process of the protein, necessary for performing the structural studies [35];
- (iii)
- The dephosphorylated structures, ATP-free or ATP-bound refer to an inactive state of the protein, being phosphorylation a fundamental step in CFTR activation;
- (iv)
- All structures miss the secondary structure assignation of large parts of the protein (Phe409-Gly437, Gln637-Trp845 and Gly1173-Asp1202) and in some cases the R-domain, which contains multiple phosphorylation sites, essentials for regulating the channel activation after phosphorylation;
- (v)
- Different outward-occluded or inward-facing protein conformations can be observed under very similar experimental conditions [38]. As a consequence, the flexibility of the whole protein, as well as that of its subdomains, deserve further studies, being it an aspect of great importance in understanding the CFTR–ligand molecular mechanism of interaction. Figure 2 shows the X-Ray structures of CFTR colored by B-factor values, which indicate the static or dynamic mobility of an atom, with higher values corresponding to large fluctuations.
3. CFTR Computational Models
4. Biochemical and Biological Assays to Screen for CFTR Modulators
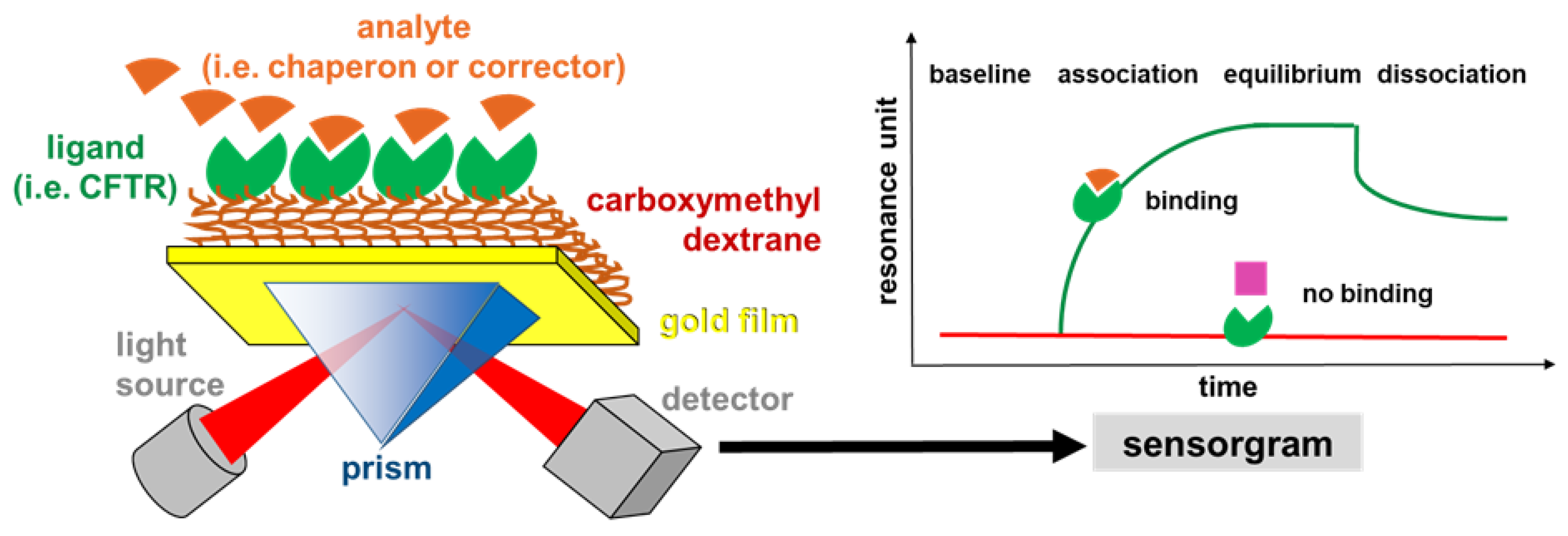
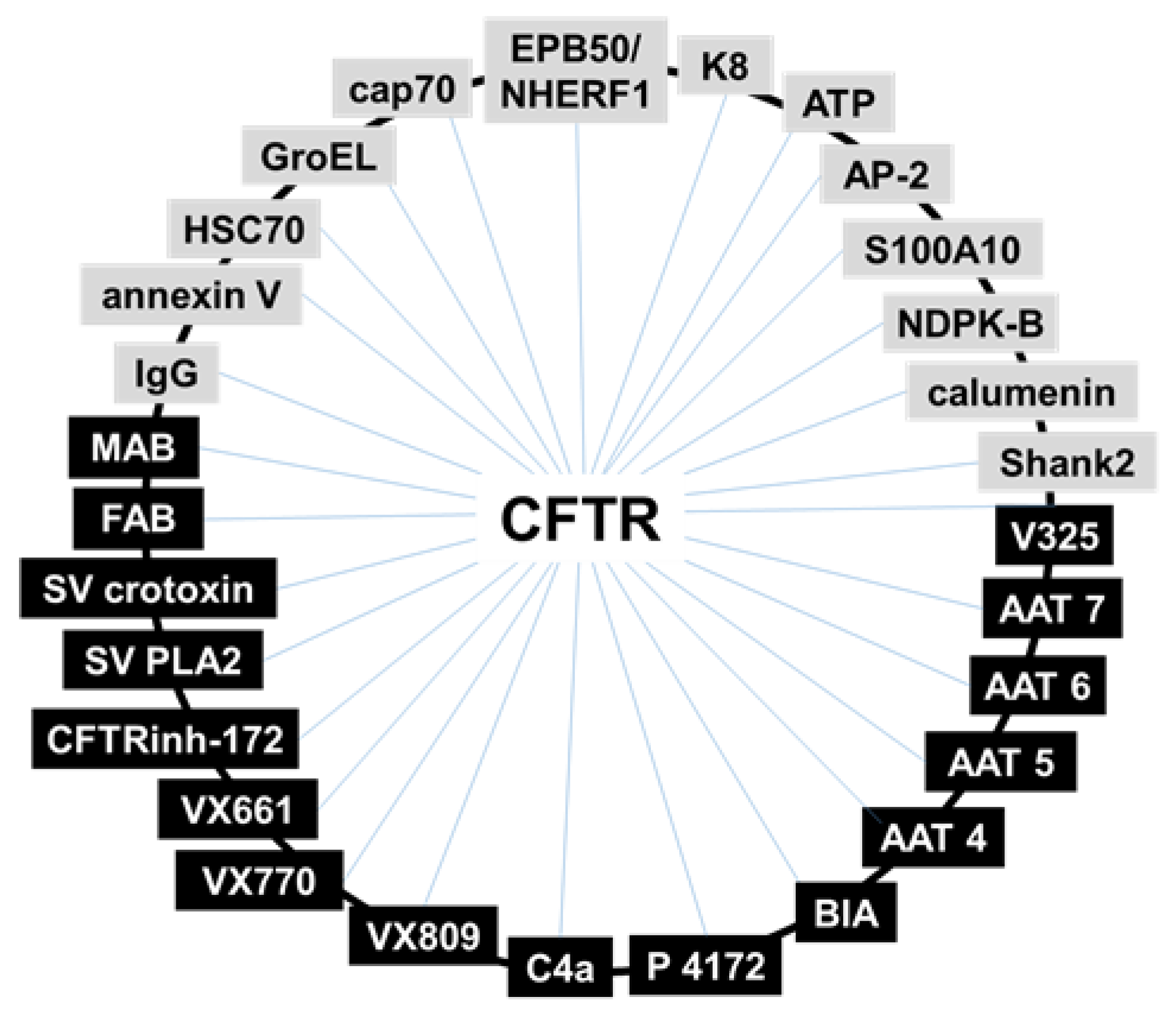
4.1. Binding Assays: Surface Plasmon Resonance (SPR) Spectroscopy
- (i)
- In search for new CFTR-correctors/activators, SPR was used to evaluate the binding of CFTR to crotoxin from Crotalus durissus terrificus and the Viperidae snake venom PLA2, known to possess a large spectrum of pharmacological functions [72].
- (ii)
- SPR has been used to the study of the direct interaction with mutated CFTR of correctors VRT-325 and Corr-4a [14] and of either F508del-NBD1 [52] or intact F508del-CFTR [48] with a panel of AAT derivatives. Additionally, the pyrazole compound 4172 was demonstrated to bind F508del-CFTR with an affinity that is 25 times higher than that of the first identified type III corrector BIA [73].
- (iii)
- Some monoclonal antibodies have been analyzed by SPR for their interaction with a different domain of CFTR [14,70]. In particular, Gakhal et al. exploited SPR to characterize synthetic antigen-binding fragments (FABs) isolated from phage-displayed library specifically directed against different domains of CFTR [74].
- (iv)
- As already mentioned above, the binding of mutated CFTR to chaperones responsible for its retention is considered a therapeutic target to rescue CFTR activity. Relevantly, when tested in SPR competition assays, Corr-4a, VRT-325 and CFTRinh-172 effectively inhibit the binding of HSC70 to sensorchip immobilized F508del-CFTR [14,68].
4.2. Biochemical Functional Assays: Thermostability Assay
4.3. Biological Assays to Screen for CFTR Modulators
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAT | Aminoarylthiazole |
| ABC | ATP-binding cassette superfamily |
| CF | cystic fibrosis |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| Corr-4a | corrector-4a |
| CPM | N-[4-(7-diethylamino-4-methyl-3-coumarinyl)phenyl]maleimide |
| cryo-EM | cryoelectron microscopy |
| C-ter | carboxy-terminal |
| dTTP | thymidine-5′-triphosphate |
| dUTP | deoxyuridine-5’-triphosphate |
| FAB | antigen-binding fragment |
| F508del | deletion of phenylalanine at position 508 |
| HPC | high performance computing |
| HS-YFP | halide -sensitive Yellow Fluorescent Protein |
| HSC70 | heat shock cognate70 |
| Kd | dissociation constant |
| K8 | cytokeratin 8 |
| MDs | molecular dynamic studies |
| MSDs | transmembrane domains |
| NBD1 | nucleotide-binding domain 1 |
| PDB | protein data bank |
| R-domain | regulatory domain |
| SPR | surface plasmon resonance |
| WT | wild type |
References
- Dechecchi, M.C.; Tamanini, A.; Cabrini, G. Molecular basis of cystic fibrosis: From bench to bedside. Ann. Transl. Med. 2018, 6, 334. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K.; Amaral, M.D. Progress in therapies for cystic fibrosis. Lancet. Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef]
- Wang, W.Y.; El Hiani, Y.; Rubaiy, H.N.; Linsdell, P. Relative contribution of different transmembrane segments to the CFTR chloride channel pore. Pflug Arch. Eur J. Phy 2014, 466, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Mornon, J.P.; Lehn, P.; Callebaut, I. Molecular models of the open and closed states of the whole human CFTR protein. Cell. Mol. Life Sci. 2009, 66, 3469–3486. [Google Scholar] [CrossRef]
- Serohijos, A.W.R.; Hegedus, T.; Aleksandrov, A.A.; He, L.; Cui, L.; Dokholyan, N.V.; Riordan, J.R. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc. Natl. Acad. Sci. USA 2008, 105, 3256–3261. [Google Scholar] [CrossRef]
- Rabeh, W.M.; Bossard, F.; Xu, H.J.; Okiyoneda, T.; Bagdany, M.; Mulvihill, C.M.; Du, K.; di Bernardo, S.; Liu, Y.H.; Konermann, L.; et al. Correction of Both NBD1 Energetics and Domain Interface Is Required to Restore Delta F508 CFTR Folding and Function. Cell 2012, 148, 150–163. [Google Scholar] [CrossRef]
- Thibodeau, P.H.; Brautigam, C.A.; Machius, M.; Thomas, P.J. Side chain and backbone contributions of Phe508 to CFTR folding. Nat. Struct Mol. Biol. 2005, 12, 10–16. [Google Scholar] [CrossRef]
- Serohijos, A.W.R.; Hegedus, T.; Riordan, J.R.; Dokholyan, N.V. Diminished Self-Chaperoning Activity of the Delta F508 Mutant of CFTR Results in Protein Misfolding. PLoS Comput. Biol. 2008, 4, 2. [Google Scholar] [CrossRef]
- Cheng, S.H.; Gregory, R.J.; Marshall, J.; Paul, S.; Souza, D.W.; White, G.A.; Oriordan, C.R.; Smith, A.E. Defective Intracellular-Transport and Processing of Cftr Is the Molecular-Basis of Most Cystic-Fibrosis. Cell 1990, 63, 827–834. [Google Scholar] [CrossRef]
- Denning, G.M.; Anderson, M.P.; Amara, J.F.; Marshall, J.; Smith, A.E.; Welsh, M.J. Processing of Mutant Cystic-Fibrosis Transmembrane Conductance Regulator Is Temperature-Sensitive. Nature 1992, 358, 761–764. [Google Scholar] [CrossRef]
- Lukacs, G.L.; Mohamed, A.; Kartner, N.; Chang, X.B.; Riordan, J.R.; Grinstein, S. Conformational Maturation of Cftr but Not Its Mutant Counterpart (Delta-F508) Occurs in the Endoplasmic-Reticulum and Requires Atp. Embo J. 1994, 13, 6076–6086. [Google Scholar] [CrossRef]
- Dalemans, W.; Barbry, P.; Champigny, G.; Jallat, S.; Dott, K.; Dreyer, D.; Crystal, R.G.; Pavirani, A.; Lecocq, J.P.; Lazdunski, M. Altered Chloride-Ion Channel Kinetics Associated with the Delta-F508 Cystic-Fibrosis Mutation. Nature 1991, 354, 526–528. [Google Scholar] [CrossRef]
- Rivas Caldas, R.; Boisrame, S. Upper aero-digestive contamination by Pseudomonas aeruginosa and implications in Cystic Fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2015, 14, 6–15. [Google Scholar] [CrossRef]
- Scott-Ward, T.S.; Amaral, M.D. Deletion of Phe508 in the first nucleotide-binding domain of the cystic fibrosis transmembrane conductance regulator increases its affinity for the heat shock cognate 70 chaperone. Febs J. 2009, 276, 7097–7109. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.D.; Farinha, C.M. Rescuing mutant CFTR: A multi-task approach to a better outcome in treating cystic fibrosis. Curr Pharm Des. 2013, 19, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Ponzano, S.; Nigrelli, G.; Fregonese, L.; Eichler, I.; Bertozzi, F.; Bandiera, T.; Galietta, L.J.V.; Papaluca, M. A European regulatory perspective on cystic fibrosis: Current treatments, trends in drug development and translational challenges for CFTR modulators. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2018, 27, 170124. [Google Scholar] [CrossRef] [PubMed]
- Dukovski, D.; Villella, A.; Bastos, C.; King, R.; Finley, D.; Kelly, J.W.; Morimoto, R.I.; Hartl, F.U.; Munoz, B.; Lee, P.S.; et al. Amplifiers co-translationally enhance CFTR biosynthesis via PCBP1-mediated regulation of CFTR mRNA. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2020, in press. [Google Scholar] [CrossRef]
- Habib, A.R.; Kajbafzadeh, M.; Desai, S.; Yang, C.L.; Skolnik, K.; Quon, B.S. A Systematic Review of the Clinical Efficacy and Safety of CFTR Modulators in Cystic Fibrosis. Sci. Rep. 2019, 9, 7234. [Google Scholar] [CrossRef]
- Thafar, M.; Raies, A.B.; Albaradei, S.; Essack, M.; Bajic, V.B. Comparison Study of Computational Prediction Tools for Drug-Target Binding Affinities. Front. Chem. 2019, 7, 782. [Google Scholar] [CrossRef]
- Dhiman, N.; Kingshott, P.; Sumer, H.; Sharma, C.S.; Rath, S.N. On-chip anticancer drug screening - Recent progress in microfluidic platforms to address challenges in chemotherapy. Biosens. Bioelectron. 2019, 137, 236–254. [Google Scholar] [CrossRef]
- Callebaut, I.; Hoffmann, B.; Mornon, J.P. The implications of CFTR structural studies for cystic fibrosis drug development. Curr. Opin. Pharmacol. 2017, 34, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Sigoillot, M.; Overtus, M.; Grodecka, M.; Scholl, D.; Garcia-Pino, A.; Laeremans, T.; He, L.; Pardon, E.; Hildebrandt, E.; Urbatsch, I.; et al. Domain-interface dynamics of CFTR revealed by stabilizing nanobodies. Nat. Commun. 2019, 10, 2636. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.D.; Wang, H.; Byrnes, L.J.; Shanker, S.; Wang, K.; Efremov, I.V.; Chong, P.A.; Forman-Kay, J.D.; Aulabaugh, A.E. Binding screen for cystic fibrosis transmembrane conductance regulator correctors finds new chemical matter and yields insights into cystic fibrosis therapeutic strategy. Protein Sci. A Publ. Protein Soc. 2016, 25, 360–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atwell, S.; Brouillette, C.G.; Conners, K.; Emtage, S.; Gheyi, T.; Guggino, W.B.; Hendle, J.; Hunt, J.F.; Lewis, H.A.; Lu, F.; et al. Structures of a minimal human CFTR first nucleotide-binding domain as a monomer, head-to-tail homodimer, and pathogenic mutant. Protein Eng. Des. Sel. Peds 2010, 23, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H.A.; Wang, C.; Zhao, X.; Hamuro, Y.; Conners, K.; Kearins, M.C.; Lu, F.; Sauder, J.M.; Molnar, K.S.; Coales, S.J.; et al. Structure and dynamics of NBD1 from CFTR characterized using crystallography and hydrogen/deuterium exchange mass spectrometry. J. Mol. Biol. 2010, 396, 406–430. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H.A.; Zhao, X.; Wang, C.; Sauder, J.M.; Rooney, I.; Noland, B.W.; Lorimer, D.; Kearins, M.C.; Conners, K.; Condon, B.; et al. Impact of the deltaF508 mutation in first nucleotide-binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J. Biol. Chem. 2005, 280, 1346–1353. [Google Scholar] [CrossRef]
- Wang, C.; Aleksandrov, A.A.; Yang, Z.; Forouhar, F.; Proctor, E.A.; Kota, P.; An, J.; Kaplan, A.; Khazanov, N.; Boel, G.; et al. Ligand binding to a remote site thermodynamically corrects the F508del mutation in the human cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2018, 293, 17685–17704. [Google Scholar] [CrossRef]
- Mendoza, J.L.; Schmidt, A.; Li, Q.; Nuvaga, E.; Barrett, T.; Bridges, R.J.; Feranchak, A.P.; Brautigam, C.A.; Thomas, P.J. Requirements for efficient correction of DeltaF508 CFTR revealed by analyses of evolved sequences. Cell 2012, 148, 164–174. [Google Scholar] [CrossRef]
- Lewis, H.A.; Buchanan, S.G.; Burley, S.K.; Conners, K.; Dickey, M.; Dorwart, M.; Fowler, R.; Gao, X.; Guggino, W.B.; Hendrickson, W.A.; et al. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. Embo J. 2004, 23, 282–293. [Google Scholar] [CrossRef]
- Stevers, L.M.; Lam, C.V.; Leysen, S.F.; Meijer, F.A.; van Scheppingen, D.S.; de Vries, R.M.; Carlile, G.W.; Milroy, L.G.; Thomas, D.Y.; Brunsveld, L.; et al. Characterization and small-molecule stabilization of the multisite tandem binding between 14-3-3 and the R domain of CFTR. Proc. Natl. Acad. Sci. USA 2016, 113, E1152–E1161. [Google Scholar] [CrossRef]
- Jun, S.; Ro, H.J.; Bharda, A.; Kim, S.I.; Jeoung, D.; Jung, H.S. Advances in Cryo-Correlative Light and Electron Microscopy: Applications for Studying Molecular and Cellular Events. Protein J. 2019, 38, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Z.; Csanady, L.; Gadsby, D.C.; Chen, J. Molecular Structure of the Human CFTR Ion Channel. Cell 2017, 169, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, F.; Chen, J. Molecular structure of the ATP-bound, phosphorylated human CFTR. Proc. Natl. Acad. Sci. USA 2018, 115, 12757–12762. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Z.; Levit, A.; Levring, J.; Touhara, K.K.; Shoichet, B.K.; Chen, J. Structural identification of a hotspot on CFTR for potentiation. Science 2019, 364, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Fay, J.F.; Aleksandrov, L.A.; Jensen, T.J.; Cui, L.L.; Kousouros, J.N.; He, L.; Aleksandrov, A.A.; Gingerich, D.S.; Riordan, J.R.; Chen, J.Z. Cryo-EM Visualization of an Active High Open Probability CFTR Anion Channel. Biochemistry 2018, 57, 6234–6246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, J. Atomic Structure of the Cystic Fibrosis Transmembrane Conductance Regulator. Cell 2016, 167, 1586–1597. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, F.; Chen, J. Conformational Changes of CFTR upon Phosphorylation and ATP Binding. Cell 2017, 170, 483–491. [Google Scholar] [CrossRef]
- Meng, X.; Clews, J.; Ciuta, A.D.; Martin, E.R.; Ford, R.C. CFTR structure, stability, function and regulation. Biol. Chem. 2019, 400, 1359–1370. [Google Scholar] [CrossRef]
- Dawson, R.J.; Locher, K.P. Structure of a bacterial multidrug ABC transporter. Nature 2006, 443, 180–185. [Google Scholar] [CrossRef]
- Mornon, J.P.; Lehn, P.; Callebaut, I. Atomic model of human cystic fibrosis transmembrane conductance regulator: Membrane-spanning domains and coupling interfaces. Cell. Mol. Life Sci. 2008, 65, 2594–2612. [Google Scholar] [CrossRef]
- Dalton, J.; Kalid, O.; Schushan, M.; Ben-Tal, N.; Villa-Freixa, J. New model of cystic fibrosis transmembrane conductance regulator proposes active channel-like conformation. J. Chem. Inf. Modeling 2012, 52, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.S.; Cui, G.; Harvey, S.C.; McCarty, N.A. Modeling the conformational changes underlying channel opening in CFTR. PLoS ONE 2013, 8, e74574. [Google Scholar] [CrossRef] [PubMed]
- Furukawa-Hagiya, T.; Furuta, T.; Chiba, S.; Sohma, Y.; Sakurai, M. The power stroke driven by ATP binding in CFTR as studied by molecular dynamics simulations. J. Phys. Chem. B 2013, 117, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Mornon, J.P.; Hoffmann, B.; Jonic, S.; Lehn, P.; Callebaut, I. Full-open and closed CFTR channels, with lateral tunnels from the cytoplasm and an alternative position of the F508 region, as revealed by molecular dynamics. Cell. Mol. Life Sci. 2015, 72, 1377–1403. [Google Scholar] [CrossRef]
- Belmonte, L.; Moran, O. On the interactions between nucleotide binding domains and membrane spanning domains in cystic fibrosis transmembrane regulator: A molecular dynamic study. Biochimie 2015, 111, 19–29. [Google Scholar] [CrossRef]
- Corradi, V.; Vergani, P.; Tieleman, D.P. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR): Closed and open state channel models. J. Biol. Chem. 2015, 290, 22891–22906. [Google Scholar] [CrossRef]
- Simhaev, L.; McCarty, N.A.; Ford, R.C.; Senderowitz, H. Molecular Dynamics Flexible Fitting Simulations Identify New Models of the Closed State of the Cystic Fibrosis Transmembrane Conductance Regulator Protein. J. Chem. Inf. Modeling 2017, 57, 1932–1946. [Google Scholar] [CrossRef]
- D’Ursi, P.; Uggeri, M.; Urbinati, C.; Millo, E.; Paiardi, G.; Milanesi, L.; Ford, R.C.; Clews, J.; Meng, X.; Bergese, P.; et al. Exploitation of a novel biosensor based on the full-length human F508de1-CFTR with computational studies, biochemical and biological assays for the characterization of a new Lumacaftor/Tezacaftor analogue. Sens. Actuat B-Chem 2019, 301, 127131. [Google Scholar]
- Froux, L.; Elbahnsi, A.; Boucherle, B.; Billet, A.; Baatallah, N.; Hoffmann, B.; Alliot, J.; Zelli, R.; Zeinyeh, W.; Haudecoeur, R.; et al. Targeting different binding sites in the CFTR structures allows to synergistically potentiate channel activity. Eur. J. Med. Chem. 2020, 190, 112116. [Google Scholar] [CrossRef]
- Moran, O.; Galietta, L.J.; Zegarra-Moran, O. Binding site of activators of the cystic fibrosis transmembrane conductance regulator in the nucleotide binding domains. Cell. Mol. Life Sci. 2005, 62, 446–460. [Google Scholar] [CrossRef]
- Kalid, O.; Mense, M.; Fischman, S.; Shitrit, A.; Bihler, H.; Ben-Zeev, E.; Schutz, N.; Pedemonte, N.; Thomas, P.J.; Bridges, R.J.; et al. Small molecule correctors of F508del-CFTR discovered by structure-based virtual screening. J. Comput. -Aided Mol. Des. 2010, 24, 971–991. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rusnati, M.; Sala, D.; Orro, A.; Bugatti, A.; Trombetti, G.; Cichero, E.; Urbinati, C.; Di Somma, M.; Millo, E.; Galietta, L.J.V.; et al. Speeding Up the Identification of Cystic Fibrosis Transmembrane Conductance Regulator-Targeted Drugs: An Approach Based on Bioinformatics Strategies and Surface Plasmon Resonance. Molecules 2018, 23, 120. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.I.; Qiu, L.; Sohma, Y.; Conrath, K.; Zou, X.; Hwang, T.C. Identifying the molecular target sites for CFTR potentiators GLPG1837 and VX-770. J. Gen. Physiol. 2019, 151, 912–928. [Google Scholar] [CrossRef] [PubMed]
- Piliarik, M.; Vaisocherova, H.; Homola, J. Surface plasmon resonance biosensing. Methods Mol. Biol 2009, 503, 65–88. [Google Scholar]
- Rusnati, M.; Presta, M. Angiogenic growth factors interactome and drug discovery: The contribution of surface plasmon resonance. Cytokine Growth Factor Rev. 2015, 26, 293–310. [Google Scholar] [CrossRef]
- Rusnati, M.; Chiodelli, P.; Bugatti, A.; Urbinati, C. Bridging the past and the future of virology: Surface plasmon resonance as a powerful tool to investigate virus/host interactions. Crit. Rev. Microbiol. 2015, 41, 238–260. [Google Scholar] [CrossRef]
- Renaud, J.P.; Chung, C.W.; Danielson, U.H.; Egner, U.; Hennig, M.; Hubbard, R.E.; Nar, H. Biophysics in drug discovery: Impact, challenges and opportunities. Nat. Rev. Drug Discov. 2016, 15, 679–698. [Google Scholar] [CrossRef]
- Meneghello, A.; Antognoli, A.; Sonato, A.; Zacco, G.; Ruffato, G.; Cretaio, E.; Romanato, F. Label-free efficient and accurate detection of cystic fibrosis causing mutations using an azimuthally rotated GC-SPR platform. Anal. Chem. 2014, 86, 11773–11781. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ju, J.H.; Orlova, N.; Khajeh, J.A.; Cowburn, D.; Bu, Z. Ligand-induced dynamic changes in extended PDZ domains from NHERF1. J. Mol. Biol. 2013, 425, 2509–2528. [Google Scholar] [CrossRef]
- Lee, J.H.; Richter, W.; Namkung, W.; Kim, K.H.; Kim, E.; Conti, M.; Lee, M.G. Dynamic regulation of cystic fibrosis transmembrane conductance regulator by competitive interactions of molecular adaptors. J. Biol. Chem. 2007, 282, 10414–10422. [Google Scholar] [CrossRef]
- Wang, S.; Raab, R.W.; Schatz, P.J.; Guggino, W.B.; Li, M. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR). Febs Lett. 1998, 427, 103–108. [Google Scholar] [CrossRef]
- Weixel, K.M.; Bradbury, N.A. Mu 2 binding directs the cystic fibrosis transmembrane conductance regulator to the clathrin-mediated endocytic pathway. J. Biol. Chem. 2001, 276, 46251–46259. [Google Scholar] [CrossRef] [PubMed]
- Borot, F.; Vieu, D.L.; Faure, G.; Fritsch, J.; Colas, J.; Moriceau, S.; Baudouin-Legros, M.; Brouillard, F.; Ayala-Sanmartin, J.; Touqui, L.; et al. Eicosanoid release is increased by membrane destabilization and CFTR inhibition in Calu-3 cells. PLoS ONE 2009, 4, e7116. [Google Scholar] [CrossRef]
- Borthwick, L.A.; Kerbiriou, M.; Taylor, C.J.; Cozza, G.; Lascu, I.; Postel, E.H.; Cassidy, D.; Trouve, P.; Mehta, A.; Robson, L.; et al. Role of Interaction and Nucleoside Diphosphate Kinase B in Regulation of the Cystic Fibrosis Transmembrane Conductance Regulator Function by cAMP-Dependent Protein Kinase A. PLoS ONE 2016, 11, e0149097. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Kerbiriou, M.; Taiya, M.; Le Hir, S.; Mignen, O.; Benz, N.; Trouve, P.; Ferec, C. Proteomic identification of calumenin as a G551D-CFTR associated protein. PLoS ONE 2012, 7, e40173. [Google Scholar] [CrossRef]
- Colas, J.; Faure, G.; Saussereau, E.; Trudel, S.; Rabeh, W.M.; Bitam, S.; Guerrera, I.C.; Fritsch, J.; Sermet-Gaudelus, I.; Davezac, N.; et al. Disruption of cytokeratin-8 interaction with F508del-CFTR corrects its functional defect. Hum. Mol. Genet. 2012, 21, 623–634. [Google Scholar] [CrossRef]
- Urbinati, C. (Facoltà di Medicina e Chirurgia, Università degli Studi di Brescia, Brescia, Italy). Personal communication, 2018.
- Premchandar, A.; Kupniewska, A.; Bonna, A.; Faure, G.; Fraczyk, T.; Roldan, A.; Hoffmann, B.; Faria da Cunha, M.; Herrmann, H.; Lukacs, G.L.; et al. New insights into interactions between the nucleotide-binding domain of CFTR and keratin 8. Protein Sci. A Publ. Protein Soc. 2017, 26, 343–354. [Google Scholar] [CrossRef]
- Naik, S.; Zhang, N.; Gao, P.; Fisher, M.T. On the design of broad based screening assays to identify potential pharmacological chaperones of protein misfolding diseases. Curr. Top. Med. Chem. 2012, 12, 2504–2522. [Google Scholar] [CrossRef]
- Trouve, P.; Le Drevo, M.A.; Kerbiriou, M.; Friocourt, G.; Fichou, Y.; Gillet, D.; Ferec, C. Annexin V is directly involved in cystic fibrosis transmembrane conductance regulator’s chloride channel function. Biochim. Et Biophys. Acta 2007, 1772, 1121–1133. [Google Scholar] [CrossRef][Green Version]
- Treharne, K.J.; Cassidy, D.; Goddard, C.; Colledge, W.H.; Cassidy, A.; Mehta, A. Epithelial IgG and its relationship to the loss of F508 in the common mutant form of the cystic fibrosis transmembrane conductance regulator. Febs Lett. 2009, 583, 2493–2499. [Google Scholar] [CrossRef][Green Version]
- Faure, G.; Bakouh, N.; Lourdel, S.; Odolczyk, N.; Premchandar, A.; Servel, N.; Hatton, A.; Ostrowski, M.K.; Xu, H.; Saul, F.A.; et al. Rattlesnake Phospholipase A2 Increases CFTR-Chloride Channel Current and Corrects F508CFTR Dysfunction: Impact in Cystic Fibrosis. J. Mol. Biol. 2016, 428, 2898–2915. [Google Scholar] [CrossRef]
- Veit, G.; Xu, H.; Dreano, E.; Avramescu, R.G.; Bagdany, M.; Beitel, L.K.; Roldan, A.; Hancock, M.A.; Lay, C.; Li, W.; et al. Structure-guided combination therapy to potently improve the function of mutant CFTRs. Nat. Med. 2018, 24, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Gakhal, A.K.; Jensen, T.J.; Bozoky, Z.; Roldan, A.; Lukacs, G.L.; Forman-Kay, J.; Riordan, J.R.; Sidhu, S.S. Development and characterization of synthetic antibodies binding to the cystic fibrosis conductance regulator. mAbs 2016, 8, 1167–1176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pollock, N.; Cant, N.; Rimington, T.; Ford, R.C. Purification of the cystic fibrosis transmembrane conductance regulator protein expressed in Saccharomyces cerevisiae. J. Vis. Exp. Jove 2014, e51447. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, Y.; Wang, X.; Wrennall, J.A.; Rimington, T.L.; Li, H.; Cai, Z.; Ford, R.C.; Sheppard, D.N. Two Small Molecules Restore Stability to a Subpopulation of the Cystic Fibrosis Transmembrane Conductance Regulator with the Predominant Disease-causing Mutation. J. Biol. Chem. 2017, 292, 3706–3719. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Clews, J.; Kargas, V.; Wang, X.; Ford, R.C. The cystic fibrosis transmembrane conductance regulator (CFTR) and its stability. Cell. Mol. Life Sci. 2017, 74, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.P.; Fleming, K.G. Using tryptophan fluorescence to measure the stability of membrane proteins folded in liposomes. Methods Enzym. 2011, 492, 189–211. [Google Scholar]
- Kohlstaedt, M.; von der Hocht, I.; Hilbers, F.; Thielmann, Y.; Michel, H. Development of a Thermofluor assay for stability determination of membrane proteins using the Na(+)/H(+) antiporter NhaA and cytochrome c oxidase. Acta Cryst. D Biol Cryst. 2015, 71, 1112–1122. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Collnot, E.M.; Baldes, C.; Becker, U.; Laue, M.; Kim, K.J.; Lehr, C.M. Towards an in vitro model of cystic fibrosis small airway epithelium: Characterisation of the human bronchial epithelial cell line CFBE41o. Cell Tissue Res. 2006, 323, 405–415. [Google Scholar] [CrossRef]
- Tomati, V.; Pesce, E.; Caci, E.; Sondo, E.; Scudieri, P.; Marini, M.; Amato, F.; Castaldo, G.; Ravazzolo, R.; Galietta, L.J.V.; et al. High-throughput screening identifies FAU protein as a regulator of mutant cystic fibrosis transmembrane conductance regulator channel. J. Biol. Chem. 2018, 293, 1203–1217. [Google Scholar] [CrossRef]
- Norez, C.; Heda, G.D.; Jensen, T.; Kogan, I.; Hughes, L.K.; Auzanneau, C.; Derand, R.; Bulteau-Pignoux, L.; Li, C.; Ramjeesingh, M.; et al. Determination of CFTR chloride channel activity and pharmacology using radiotracer flux methods. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2004, 3 (Suppl. 2), 119–121. [Google Scholar] [CrossRef]
- Munkonge, F.; Alton, E.W.; Andersson, C.; Davidson, H.; Dragomir, A.; Edelman, A.; Farley, R.; Hjelte, L.; McLachlan, G.; Stern, M.; et al. Measurement of halide efflux from cultured and primary airway epithelial cells using fluorescence indicators. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2004, 3 (Suppl. 2), 171–176. [Google Scholar] [CrossRef]
- Galietta, L.V.J.; Jayaraman, S.; Verkman, A.S. Cell-based assay for high-throughput quantitative screening of CFTR chloride transport agonists. Am. J. Physiol.-Cell Physiol. 2001, 271, C1734–C1742. [Google Scholar] [CrossRef]
- Galietta, L.J.V.; Haggie, P.M.; Verkman, A.S. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. Febs Lett. 2001, 499, 220–224. [Google Scholar] [CrossRef]
- Pedemonte, N.; Lukacs, G.L.; Du, K.; Caci, E.; Zegarra-Moran, O.; Galietta, L.J.V.; Verkman, A.S. Small-molecule correctors of defective Delta F508-CFTR cellular processing identified by high-throughput screening. J. Clin. Investig. 2005, 115, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.H.; Vetrivel, L.; Yang, H.; Pedemonte, N.; Zegarra-Moran, O.; Galietta, L.J.V.; Verkman, A.S. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J. Biol. Chem. 2002, 277, 37235–37241. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.H.; Thiagarajah, J.R.; Yang, H.; Sonawane, N.D.; Folli, C.; Galietta, L.J.V.; Verkman, A.S. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Investig. 2002, 110, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shelat, A.A.; Guy, R.K.; Gopinath, V.S.; Ma, T.H.; Du, K.; Lukacs, G.L.; Taddei, A.; Folli, C.; Pedemonte, N.; et al. Nanomolar affinity small molecule correctors of defective Delta F508-CFTR chloride channel gating. J. Biol. Chem. 2003, 278, 35079–35085. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, N.; Sonawane, N.D.; Taddei, A.; Hu, J.; Zegarra-Moran, O.; Suen, Y.F.; Robins, L.I.; Dicus, C.W.; Willenbring, D.; Nantz, M.H.; et al. Phenylglycine and sulfonamide correctors of defective Delta F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol. Pharmacol. 2005, 67, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, N.; Diena, T.; Caci, E.; Nieddu, E.; Mazzei, M.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J.V. Antihypertensive 1,4-dihydropyridines as correctors of the cystic fibrosis transmembrane conductance regulator channel gating defect caused by cystic fibrosis mutations. Mol. Pharmacol. 2005, 68, 1736–1746. [Google Scholar] [CrossRef]
- Phuan, P.W.; Veit, G.; Tan, J.A.; Finkbeiner, W.E.; Lukacs, G.L.; Verkman, A.S. Potentiators of Defective Delta F508-CFTR Gating that Do Not Interfere with Corrector Action. Mol. Pharmacol. 2015, 88, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Phuan, P.W.; Yang, B.X.; Knapp, J.M.; Wood, A.B.; Lukacs, G.L.; Kurth, M.J.; Verkman, A.S. Cyanoquinolines with Independent Corrector and Potentiator Activities Restore Delta Phe508-Cystic Fibrosis Transmembrane Conductance Regulator Chloride Channel Function in Cystic Fibrosis. Mol. Pharmacol. 2011, 80, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Phuan, P.; Yang, B.; Knapp, J.; Wood, A.; Lukacs, G.; Kurth, M.; Verkman, A.S. Cyanoquinolines with Dual Corrector and Potentiator Activities Restore Delta F508-Cftr Chloride Channel Function. Pediatric Pulmonol. 2011, 46, 221. [Google Scholar]
- Yu, G.J.; Yoo, C.L.; Yang, B.; Lodewyk, M.W.; Meng, L.; El-Idreesy, T.T.; Fettinger, J.C.; Tantillo, D.J.; Verkman, A.S.; Kurth, M.J. Potent s-cis-locked bithiazole correctors of DeltaF508 cystic fibrosis transmembrane conductance regulator cellular processing for cystic fibrosis therapy. J. Med. Chem. 2008, 51, 6044–6054. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sondo, E.; Falchi, F.; Caci, E.; Ferrera, L.; Giacomini, E.; Pesce, E.; Tomati, V.; Mandrup Bertozzi, S.; Goldoni, L.; Armirotti, A.; et al. Pharmacological Inhibition of the Ubiquitin Ligase RNF5 Rescues F508del-CFTR in Cystic Fibrosis Airway Epithelia. Cell Chem. Biol. 2018, 25, 891–905. [Google Scholar] [CrossRef]
- Vijftigschild, L.A.; van der Ent, C.K.; Beekman, J.M. A novel fluorescent sensor for measurement of CFTR function by flow cytometry. Cytom. Part. A J. Int. Soc. Anal. Cytol. 2013, 83, 576–584. [Google Scholar] [CrossRef]
- Langron, E.; Simone, M.I.; Delalande, C.M.; Reymond, J.L.; Selwood, D.L.; Vergani, P. Improved fluorescence assays to measure the defects associated with F508del-CFTR allow identification of new active compounds. Br. J. Pharmacol. 2017, 174, 525–539. [Google Scholar] [CrossRef]
- Pedemonte, N.; Tomati, V.; Sondo, E.; Galietta, L.J. Influence of cell background on pharmacological rescue of mutant CFTR. Am. J. Physiol. Cell Physiol. 2010, 298, C866–C874. [Google Scholar] [CrossRef]
- Farinha, C.M.; Sousa, M.; Canato, S.; Schmidt, A.; Uliyakina, I.; Amaral, M.D. Increased efficacy of VX-809 in different cellular systems results from an early stabilization effect of F508del-CFTR. Pharmacol. Res. Perspect. 2015, 3, e00152. [Google Scholar] [CrossRef]
- Dar, K.B.; Bhat, A.H.; Amin, S.; Hamid, R.; Anees, S.; Anjum, S.; Reshi, B.A.; Zargar, M.A.; Masood, A.; Ganie, S.A. Modern Computational Strategies for Designing Drugs to Curb Human Diseases: A Prospect. Curr. Top. Med. Chem. 2018, 18, 2702–2719. [Google Scholar] [CrossRef]
- Scotti, M.T.; Alves, M.F.; Herrera-Acevedo, C.A.; Scotti, L. Virtual Screening Studies for Discovery of Novel Inhibitors of Inflammatory Process Targets. Curr. Pharm. Des. 2018, 24, 1617–1638. [Google Scholar] [CrossRef]
- Jason-Moller, L.; Murphy, M.; Bruno, J. Overview of Biacore systems and their applications. Curr. Protoc. Protein Sci. 2006, 45, 19.13.1–19.13.14. [Google Scholar] [CrossRef] [PubMed]
- Sherbet, G.V. Therapeutic Potential of Thalidomide and Its Analogues in the Treatment of Cancer. Anticancer Res. 2015, 35, 5767–5772. [Google Scholar] [PubMed]
- Newman, S.P. Delivering drugs to the lungs: The history of repurposing in the treatment of respiratory diseases. Adv. Drug Deliv. Rev. 2018, 133, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Daviskas, E.; Brannan, J.D.; Chan, H.K. Repurposing excipients as active inhalation agents: The mannitol story. Adv. Drug Deliv. Rev. 2018, 133, 45–56. [Google Scholar] [CrossRef]
- Villella, V.R.; Tosco, A.; Esposito, S.; Bona, G.; Raia, V.; Maiuri, L. Mutation-specific therapies and drug repositioning in cystic fibrosis. Minerva Pediatrica 2019, 71, 287–296. [Google Scholar] [CrossRef]
- Schmidt, B.Z.; Haaf, J.B.; Leal, T.; Noel, S. Cystic fibrosis transmembrane conductance regulator modulators in cystic fibrosis: Current perspectives. Clin. Pharmacol. Adv. Appl. 2016, 8, 127–140. [Google Scholar]
- Molinski, S.V.; Ahmadi, S.; Ip, W.; Ouyang, H.; Villella, A.; Miller, J.P.; Lee, P.S.; Kulleperuma, K.; Du, K.; Di Paola, M.; et al. Orkambi(R) and amplifier co-therapy improves function from a rare CFTR mutation in gene-edited cells and patient tissue. Embo Mol. Med. 2017, 9, 1224–1243. [Google Scholar] [CrossRef]

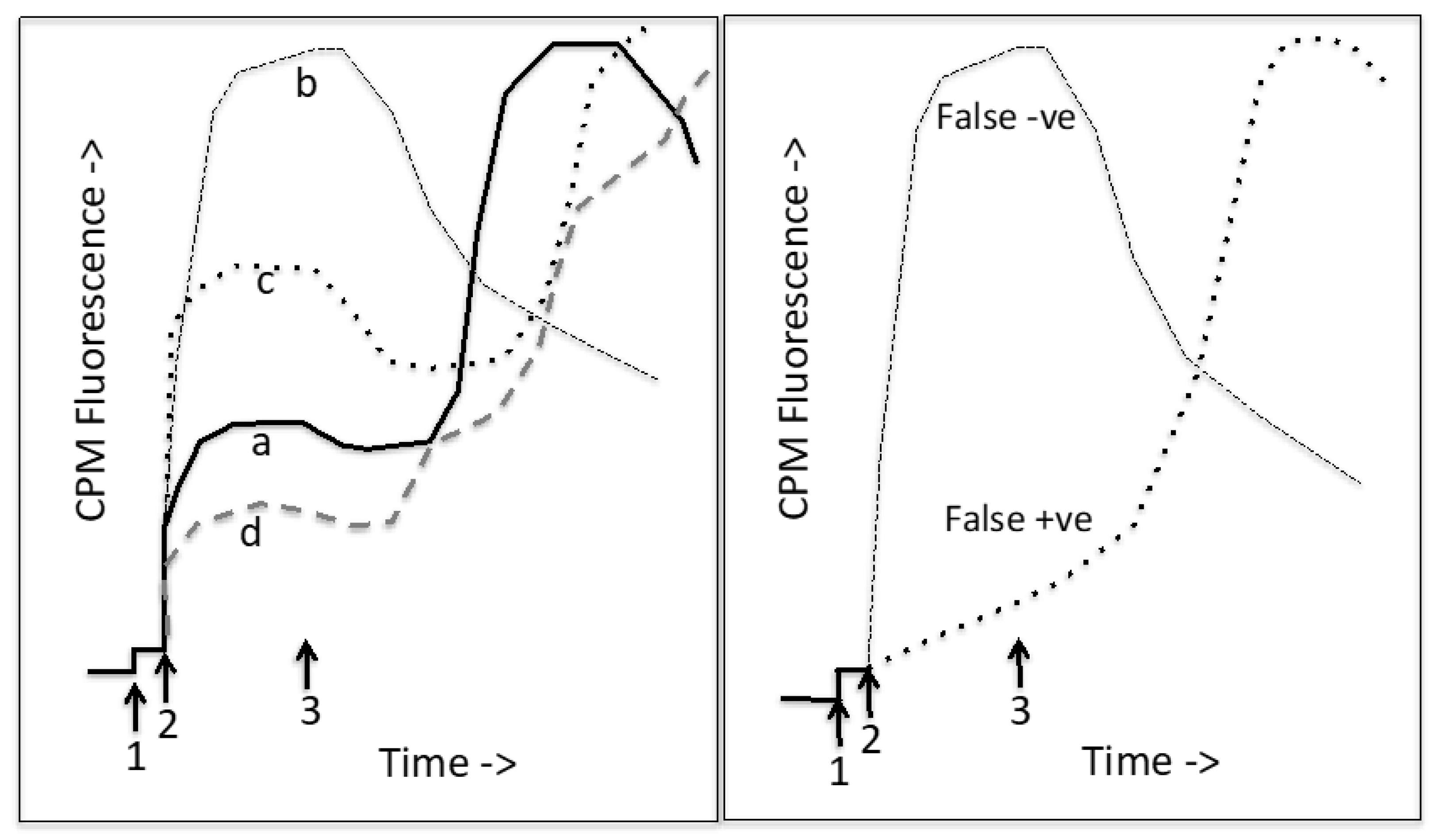
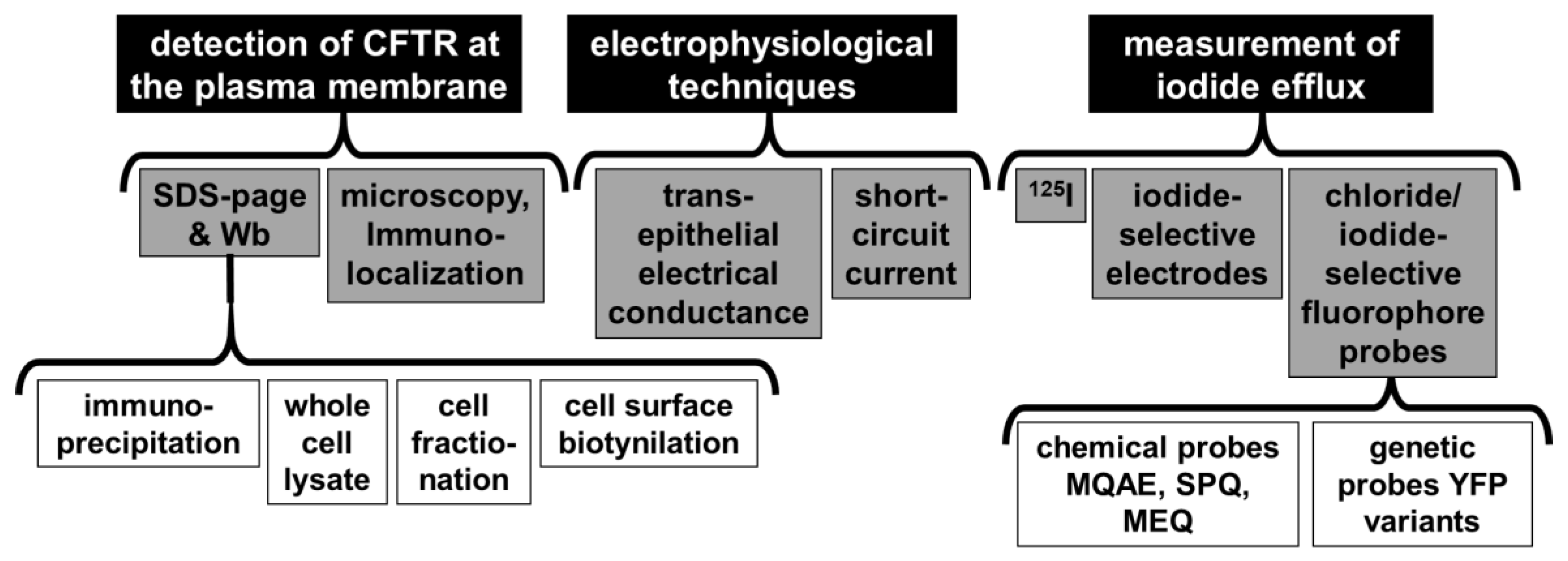
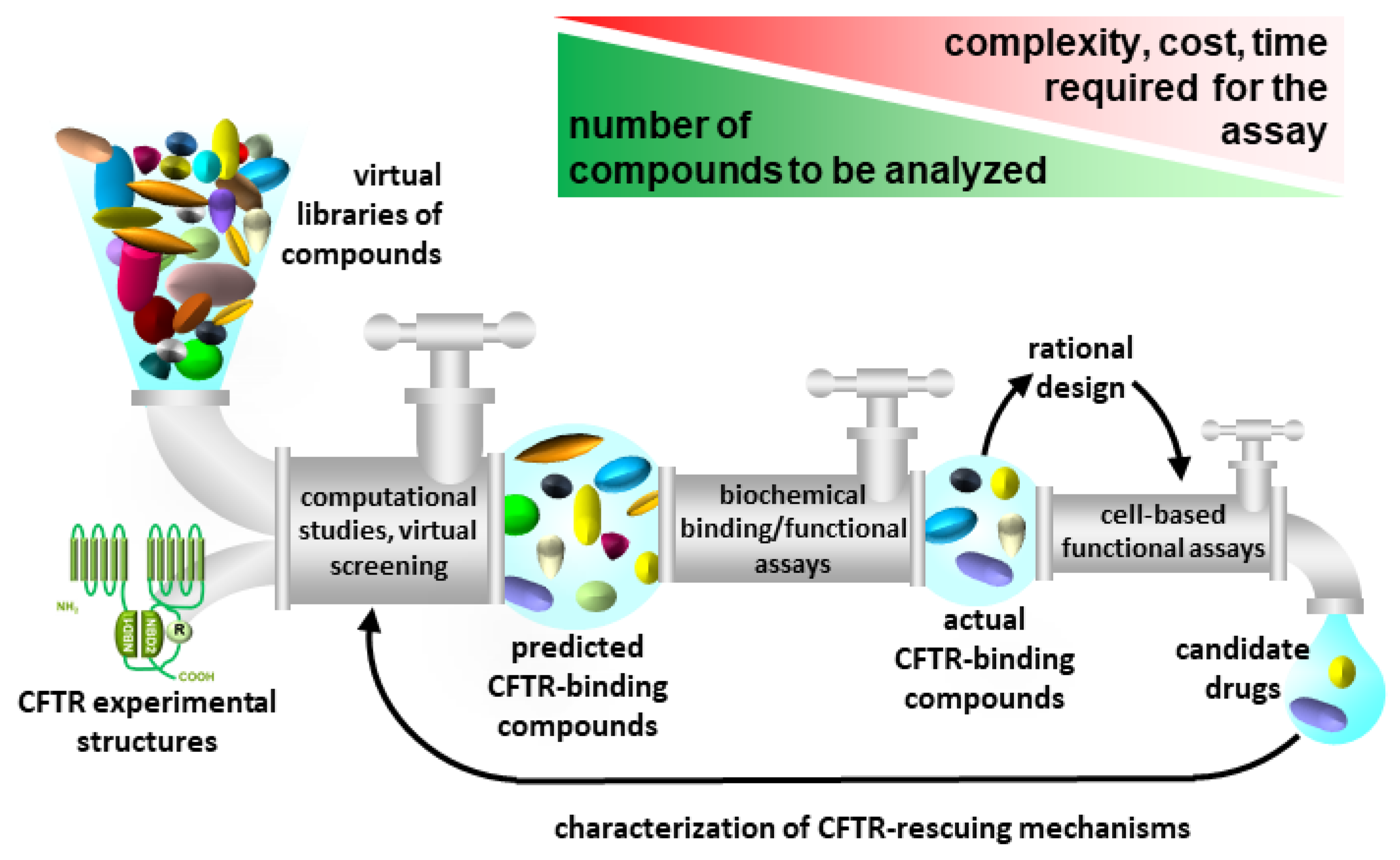
| PDB Code | Organism | Resol. (Å) | Features | Complex with | Ref. |
|---|---|---|---|---|---|
| 6GJS | H. sapiens | 1.95 | WT | ATP and 2 nanobodies | [22] |
| 6GJU | H. sapiens | 2.6 | WT | nanobody | |
| 6GJQ | H. sapiens | 2.49 | WT | nanobody | |
| 6GK4 | H. sapiens | 2.91 | WT | ATP and 2 nanobodies | |
| 6GKD | H. sapiens | 2.99 | WT | ATP and 2 nanobodies | |
| 4WZ6 | H. sapiens | 2.05 | F508del, 3 stabilizing mutations | ATP | [23] |
| 2PZE | H. sapiens | 1.7 | WT dimer | ATP | [24] |
| 2PZF | H. sapiens | 2.0 | WT/F508del dimer | ATP | |
| 2PZG | H. sapiens | 1.8 | WT | ATP | |
| 2BBO | H. sapiens | 2.55 | F508del | ATP | [25] |
| 2BBS | H. sapiens | 2.05 | WT, 3 stabilizing mutations | ATP | |
| 2BBT | H. sapiens | 2.3 | WT, 2 stabilizing mutations | ATP | |
| 1XMI | H. sapiens | 2.25 | WT | ATP | [26] |
| 1XMJ | H. sapiens | 2.3 | F508del | ATP | |
| 5TF7 | H. sapiens | 1.93 | WT | ATP | [27] |
| 5TF8 | H. sapiens | 1.86 | WT | dTTP | |
| 5TFA | H. sapiens | 1.87 | WT | dUTP | |
| 5TFB | H. sapiens | 1.87 | WT | 7-methyl-GTP | |
| 5TFC | H. sapiens | 1.92 | WT | GTP | |
| 5TFD | H. sapiens | 1.89 | WT | CTP | |
| 5TFF | H. sapiens | 1.89 | WT | UTP | |
| 5TFG | H. sapiens | 1.91 | WT | 5-methyl-UTP | |
| 5TFI | H. sapiens | 1.89 | WT | dGTP | |
| 5TFJ | H. sapiens | 1.85 | WT | dCTP | |
| 3SI7 | Mouse | 2.25 | F508del | ATP | [28] |
| 1XF9 | Mouse | 2.7 | F508S | ATP | [7] |
| 1XFA | Mouse | 3.1 | F508R | ATP | |
| 1Q3H | Mouse | 2.5 | F508R | ANP | [29] |
| 1R10 | Mouse | 3.0 | WT plus R-domain | ATP | |
| 1R0Z | Mouse | 2.35 | WT plus R-domain | ATP | |
| 1R0Y | Mouse | 2.55 | WT plus R-domain | ADP | |
| 1R0X | Mouse | 2.2 | WT plus R-domain | ATP | |
| 1R0W | Mouse | 2.2 | WT apo form | - |
| PDB Code | Organ. | Resol. (Å) | (Residues Count) and Features | in Complex with | Ref. |
|---|---|---|---|---|---|
| 5UAK | H. sapiens | 3.87 | (1508), DP | - | [32] |
| 6MSM | H. sapiens | 3.2 | (1506), P, 1 stabilizing mutation | ATP | [33] |
| 6O1V | H. sapiens | 3.2 | (1489), DP, 1 solubilizing mutation | ATP and GLPG1837 | [34] |
| 6O2P | H. sapiens | 3.2 | (1489), DP, 1 solubilizing mutation | ATP and VX770 | |
| 6D3R | G. gallus. | 4.3 | (1437), DP, many stabilizing mutations | ATP | [35] |
| 6D3S | G gallus. | 6.6 | (1437), P, many stabilizing mutations | ATP | |
| 5UAR | D. reiro. | 3.73 | (1494), DP | - | [36] |
| 5W81 | D. reiro. | 3.37 | (1494), P, 1 stabilizing mutation | ATP | [37] |
| Natural Binder | CFTR Domain | Kd (μM) | Ref. |
|---|---|---|---|
| EBP50/NHERF1 PDZ1 | C-ter (a.a. 1411–1480) | 0.211–1.5 a | [59] |
| PDZ2 | 0.267–4.8 a | ||
| PDZ1 | C-ter (a.a. 1451–1480) | 0.023 | [60] |
| PDZ2 | 0.074 | ||
| PDZ1+2 | 0.022 | ||
| Shank2 PDZ | C-ter (a.a. 1451–1480) | 0.056 | [60] |
| CAP70 protein PDZ1 | C-ter (EEVQDTRL) | 0.22 | [61] |
| PDZ2 | 0.008 | ||
| PDZ3 | 0.120 | ||
| AP-2 | C-ter (KVIEENKVRQYDSIQ) | not determined | [62] |
| adaptor S100A10 | WT NBD1L | 7.8 | [63] |
| NDPK-B | WT NBD1 | not determined | [64] |
| Calumenin | WT full length | not determined | [65] |
| K8 | WT NBD1 | 0.048 | [66] |
| 0.04 | |||
| F508del-NBD1 | 0.016 | [67] | |
| 0.02 | [66] | ||
| K8 fragment (a.a. 83–105) | WT NBD1 | 31.0 | [67] |
| F508del-NBD1 | 4.6 | [68] | |
| cytokeratin 18 | WT NBD1 | no binding | [66] |
| F508del-NBD1 | no binding | ||
| HSC70 | WT NBD1 | 0.014 | [14] |
| F508del-NBD1 | 0.003 | ||
| ATP | NBD1 | 2.5 | [23] |
| GroEL chaperonin | WT NBD1 | 0.025 | [69] |
| annexin V | WT full length | not determined 0.002–0.004 b | [70] |
| WT NBD1 | |||
| Albumin | WT full length | no binding | [70] |
| human immunoglobulin G | WT NBD1 (a.a. 503–519) | 0.069 | [71] |
| F508del-NBD1 (a.a. 503–518) | 0.086 | ||
| NBD2 (a.a.1237–1253) | no binding |
| Putative CFTR-Rescuing Molecules | CFTR Domain | Kd (μM) | Ref. |
|---|---|---|---|
| intact crotoxin | WT NBD1 | 0.004–0.118 a | [72] |
| crotoxin subunit CBa2 | F508del-NBD1 | 0.028 | |
| Viperidae snake venom PLA2 | WT NBD1 | 0.035 | [72] |
| F508del-NBD1 | 0.037 | ||
| Corr-4a | F508del-NBD1 | not determined | [14] |
| VRT-325 | not determined | ||
| CFTRinh-172 | no binding | ||
| pyrazole compound 4172 | F508del-NBD1 | 38.0 | [73] |
| BIA | > 1,000 | ||
| aminothiazole compound 3152 | no binding | ||
| sulfamoyl-pyrrol compound 6258 | no binding | ||
| VX770 | F508del-NBD1 | no binding | [52] |
| Corr-4a | no binding | ||
| VX809 | 24.2 | ||
| AAT compound 4 | 99.3 | ||
| AAT compound 5 | 40.3 | ||
| AAT compound 6 | 197.9 | ||
| AAT compound 7 | no binding | ||
| VX809 | F508del-CFTR | 72.8 | [48] |
| Corr-4a | 18.6 | ||
| VX661 | 206.1 | ||
| AAT compound 4 | 146.6 | ||
| AAT compound 5 | 19.7 | ||
| AAT compound 6 | 44.1 | ||
| AAT compound 7 | 4.5 | ||
| compound EN503 | 9.2 | ||
| anti-R-domain 1660 MAB | WT CFTR | not determined | [70] |
| anti-R-domain AG6 FAB | R-domain | 0.032–0.009 b | [74] |
| anti-NBD1 L12B4 MAB | WT NBD1 | not determined | [14,70] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusnati, M.; D’Ursi, P.; Pedemonte, N.; Urbinati, C.; Ford, R.C.; Cichero, E.; Uggeri, M.; Orro, A.; Fossa, P. Recent Strategic Advances in CFTR Drug Discovery: An Overview. Int. J. Mol. Sci. 2020, 21, 2407. https://doi.org/10.3390/ijms21072407
Rusnati M, D’Ursi P, Pedemonte N, Urbinati C, Ford RC, Cichero E, Uggeri M, Orro A, Fossa P. Recent Strategic Advances in CFTR Drug Discovery: An Overview. International Journal of Molecular Sciences. 2020; 21(7):2407. https://doi.org/10.3390/ijms21072407
Chicago/Turabian StyleRusnati, Marco, Pasqualina D’Ursi, Nicoletta Pedemonte, Chiara Urbinati, Robert C. Ford, Elena Cichero, Matteo Uggeri, Alessandro Orro, and Paola Fossa. 2020. "Recent Strategic Advances in CFTR Drug Discovery: An Overview" International Journal of Molecular Sciences 21, no. 7: 2407. https://doi.org/10.3390/ijms21072407
APA StyleRusnati, M., D’Ursi, P., Pedemonte, N., Urbinati, C., Ford, R. C., Cichero, E., Uggeri, M., Orro, A., & Fossa, P. (2020). Recent Strategic Advances in CFTR Drug Discovery: An Overview. International Journal of Molecular Sciences, 21(7), 2407. https://doi.org/10.3390/ijms21072407








