Lessons Learned from Somatic Cell Nuclear Transfer
Abstract
1. Introduction
2. Abnormalities in Cloned Animals
3. Early SCNT Protocol Deficiencies and Optimizations
4. Donor Cell Type and Cell Cycle Synchrony
5. Epigenetic Reprogramming in SCNT
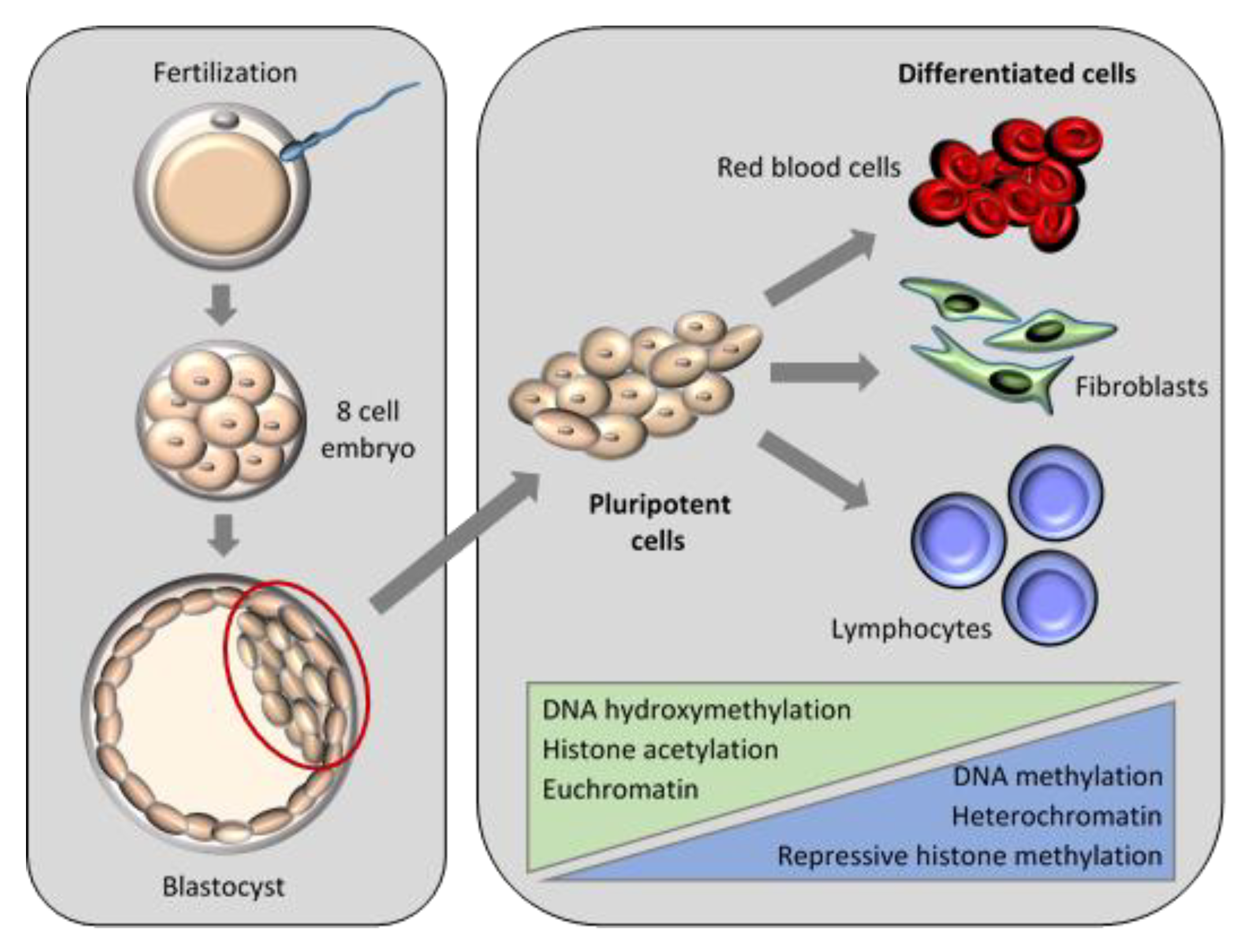
5.1. Nuclear Remodelling and Reprogramming in Embryogenesis
5.1.1. DNA Methylation
5.1.2. Histone Modifications
5.1.3. Associations of Epigenetic Events
5.2. Nuclear Remodelling and Reprogramming in SCNT Embryos
5.3. Improving SCNT with Chromatin Remodelling Agents
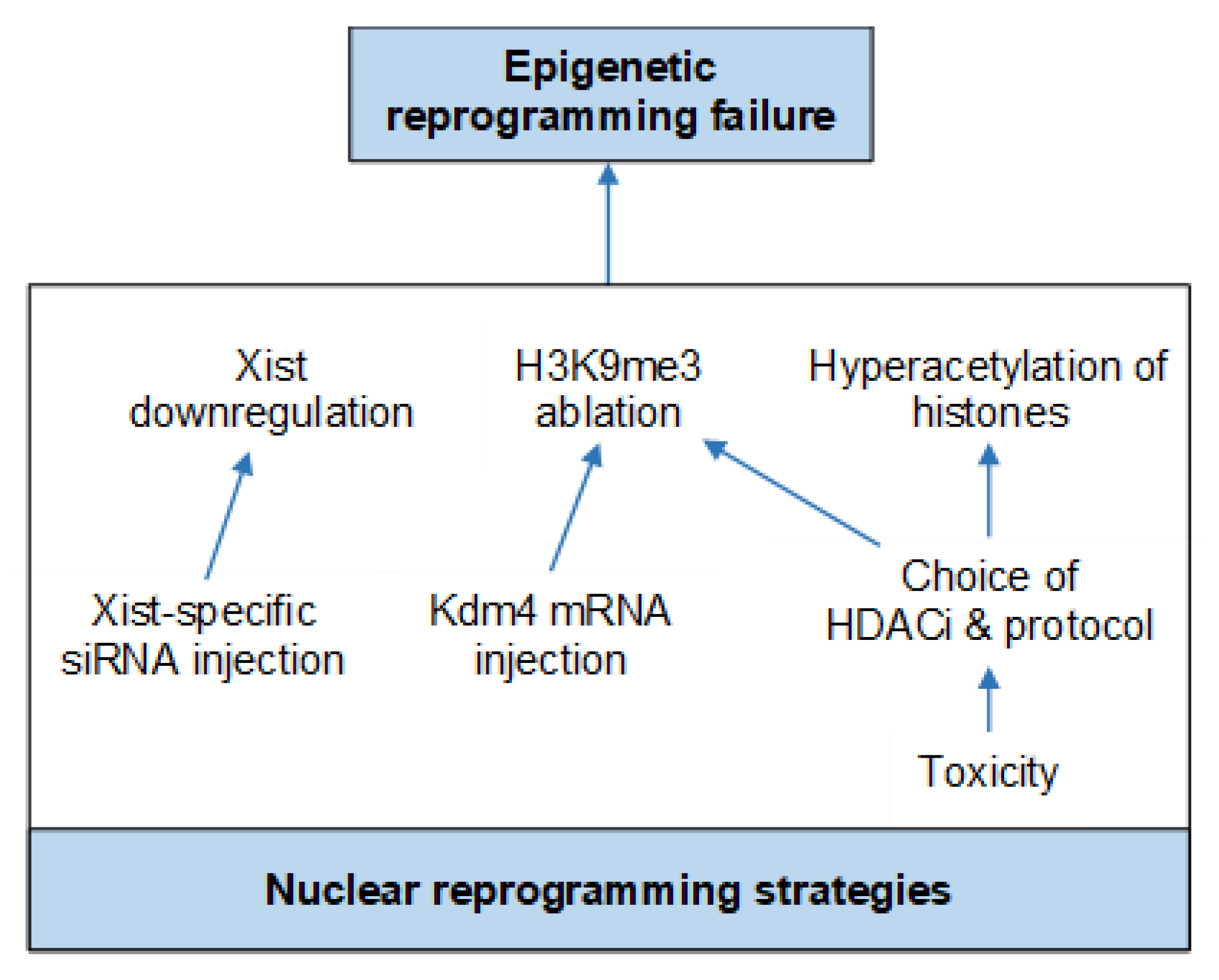
6. Current Protocol Optimizations
Nuclear Reprogramming Strategies
7. SCNT Applications
8. Ethical and Legal Implications of SCNT in Humans
9. Conclusions
9.1. Recommendations
9.1.1. Equipment and Oocyte Handling
- The optimal donor oocytes to use for high developmental competence, minimal susceptibility to in vitro handling and micromanipulation and visibility of the meiotic spindle complex, are 8–12-week-old B6D2F1 mice [61].
- Undesirable fluctuations in the culture environment of oocytes through exposure outside of the incubator for longer than 20–30 min can be prevented by adjusting the number of oocytes undergoing experimentation to the level of experience of the operator [70].
- If the meiotic spindle complex cannot be visualized at first, it can be identified using Hoechst and UV illumination, which would be for practice purposes only [70].
9.1.2. Medium Supplementation
- Supplementing the culture medium with an antioxidant such as vitamin C (that protects cells against reactive oxygen species) for at least 16 h after activation should be considered, which in combination with Lat-A during micromanipulation and activation may increase the rates of blastocyst formation [123].
9.1.3. Quality Control and Training
- It is important to create laboratory-based standard operating procedures and training manuals for establishing and optimizing methods of SCNT.
- Maintaining a stable environment for embryo development including temperature, pH and medium optimization should never be underestimated and is of utmost importance.
- Non-enucleated oocytes should be activated and cultured in parallel with reconstructed oocytes as parthenogenetic controls for the activation protocol and culture conditions [70].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASRM | American Society for Reproductive Medicine |
| DNA | Deoxyribonucleic acid |
| ESCs | Embryonic stem cells |
| HDACi | Histone deacetylase inhibitor |
| H3K9me3 | Histone 3 lysine 9 trimethylation |
| HVJ | Hemagglutinating virus of Japan |
| ICM | Inner cell mass |
| iPSC | Induced pluripotent stem cell |
| IVF | In vitro fertilization |
| Kdm4 | Lysine demethylase 4 |
| MAPK | Mitogen-activated protein kinase |
| MPF | Maturation promoting factor |
| mtDNA | Mitochondrial DNA |
| MII | Metaphase II |
| NEBD | Nuclear envelope breakdown |
| PCC | Premature chromosome condensation |
| RNA | Ribonucleic acid |
| SASREG | Southern African Society for Reproductive Medicine and Gynaecological Endoscopy |
| SCNT | Somatic cell nuclear transfer |
| si | Short interfering |
| TE | Trophectoderm |
| TSA | Trichostatin A |
| UK | United Kingdom |
| US | United States |
| UV | Ultraviolet |
References
- Simerly, C.; Dominko, T.; Navara, C.; Payne, C.; Capuano, S.; Gosman, G.; Chong, K.Y.; Takahashi, D.; Chace, C.; Compton, D. Molecular correlates of primate nuclear transfer failures. Science 2003, 300, 297. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Estrada, J.; Piedrahita, J.A. A comparative study on the efficiency of two enucleation methods in pig somatic cell nuclear transfer: Effects of the squeezing and the aspiration methods. Anim. Biotechnol. 2008, 19, 71–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fulka, J., Jr.; Loi, P.; Fulka, H.; Ptak, G.; Nagai, T. Nucleus transfer in mammals: Noninvasive approaches for the preparation of cytoplasts. Trends Biotechnol. 2004, 22, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.P.; Cibelli, J.B.; West, M.D. Prospects for the use of nuclear transfer in human transplantation. Nat. Biotechnol. 1999, 17, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Spemann, H.; Mangold, H. Induction of embryonic primordia by implantation of organizers from a different species. Int. J. Dev. Biol. 2001, 45, 13–38. [Google Scholar] [PubMed]
- Briggs, R.; King, T.J. Transplantation of Living Nuclei From Blastula Cells into Enucleated Frogs‘ Eggs. Proc. Natl. Acad. Sci. USA 1952, 38, 455–463. [Google Scholar] [CrossRef]
- Gurdon, J.B. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Development 1962, 10, 622–640. [Google Scholar]
- Wilmut, I.; Schnieke, A.E.; McWhir, J.; Kind, A.J.; Campbell, K.H.S. Viable offspring derived from fetal and adult mammalian cells. Nature 1997, 385, 810–813. [Google Scholar] [CrossRef]
- Wakayama, T.; Perry, A.C.; Zuccotti, M.; Johnson, K.R.; Yanagimachi, R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998, 394, 369–374. [Google Scholar] [CrossRef]
- Iager, A.E.; Ragina, N.P.; Ross, P.J.; Beyhan, Z.; Cunniff, K.; Rodriguez, R.M.; Cibelli, J.B. Trichostatin A improves histone acetylation in bovine somatic cell nuclear transfer early embryos. Cloning Stem Cells 2008, 10, 371–380. [Google Scholar] [CrossRef]
- Ding, X.; Wang, Y.; Zhang, D.; Guo, Z.; Zhang, Y. Increased pre-implantation development of cloned bovine embryos treated with 5-aza-2′-deoxycytidine and trichostatin A. Theriogenology 2008, 70, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Akagi, S.; Matsukawa, K.; Mizutani, E.; Fukunari, K.; Kaneda, M.; Watanabe, S.; Takahashi, S. Treatment with a histone deacetylase inhibitor after nuclear transfer improves the preimplantation development of cloned bovine embryos. J. Reprod. Dev. 2011, 57, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, X.; An, Z.; Wang, L.; Liu, J.; Quan, F.; Hua, S.; Zhang, Y. Production of cloned calves by combination treatment of both donor cells and early cloned embryos with 5-aza-2/-deoxycytidine and trichostatin A. Theriogenology 2011, 75, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Sawai, K.; Fujii, T.; HirAyAmA, H.; HASHizumE, T.; Minamihashi, A. Epigenetic status and full-term development of bovine cloned embryos treated with trichostatin A. J. Reprod. Dev. 2012, 58, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Kishigami, S.; Mizutani, E.; Ohta, H.; Hikichi, T.; Van Thuan, N.; Wakayama, S.; Bui, H.-T.; Wakayama, T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem. Biophys. Res. Commun. 2006, 340, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Van Thuan, N.; Bui, H.T.; Kim, J.H.; Hikichi, T.; Wakayama, S.; Kishigami, S.; Mizutani, E.; Wakayama, T. The histone deacetylase inhibitor scriptaid enhances nascent mRNA production and rescues full-term development in cloned inbred mice. Soc. Reprod. Fertil. 2009, 138, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Kishigami, S.; Bui, H.-T.; Wakayama, S.; Tokunaga, K.; Van Thuan, N.; Hikichi, T.; Mizutani, E.; Ohta, H.; Suetsugu, R.; Sata, T. Successful mouse cloning of an outbred strain by trichostatin A treatment after somatic nuclear transfer. J. Reprod. Dev. 2007, 53, 165–170. [Google Scholar] [CrossRef]
- Tsuji, Y.; Kato, Y.; Tsunoda, Y. The developmental potential of mouse somatic cell nuclear-transferred oocytes treated with trichostatin A and 5-aza-2′-deoxycytidine. Zygote 2009, 17, 109–115. [Google Scholar] [CrossRef]
- Ono, T.; Li, C.; Mizutani, E.; Terashita, Y.; Yamagata, K.; Wakayama, T. Inhibition of class IIb histone deacetylase significantly improves cloning efficiency in mice. Biol. Reprod. 2010, 83, 929–937. [Google Scholar] [CrossRef]
- Dai, X.; Hao, J.; Hou, X.-j.; Hai, T.; Fan, Y.; Yu, Y.; Jouneau, A.; Wang, L.; Zhou, Q. Somatic nucleus reprogramming is significantly improved by m-carboxycinnamic acid bishydroxamide, a histone deacetylase inhibitor. J. Biol. Chem. 2010, 285, 31002–31010. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Villemoes, K.; Pedersen, A.M.; Purup, S.; Vajta, G. An epigenetic modifier results in improved in vitro blastocyst production after somatic cell nuclear transfer. Cloning Stem Cells 2007, 9, 357–363. [Google Scholar] [CrossRef]
- Li, J.; Svarcova, O.; Villemoes, K.; Kragh, P.M.; Schmidt, M.; Bøgh, I.B.; Zhang, Y.; Du, Y.; Lin, L.; Purup, S. High in vitro development after somatic cell nuclear transfer and trichostatin A treatment of reconstructed porcine embryos. Theriogenology 2008, 70, 800–808. [Google Scholar] [CrossRef]
- Zhao, J.; Ross, J.W.; Hao, Y.; Spate, L.D.; Walters, E.M.; Samuel, M.S.; Rieke, A.; Murphy, C.N.; Prather, R.S. Significant improvement in cloning efficiency of an inbred miniature pig by histone deacetylase inhibitor treatment after somatic cell nuclear transfer. Biol. Reprod. 2009, 81, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hao, Y.; Ross, J.W.; Spate, L.D.; Walters, E.M.; Samuel, M.S.; Rieke, A.; Murphy, C.N.; Prather, R.S. Histone deacetylase inhibitors improve in vitro and in vivo developmental competence of somatic cell nuclear transfer porcine embryos. Cell. Reprogram. 2010, 12, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Kang, E.J.; Maeng, G.H.; Kim, M.J.; Park, J.K.; Kim, T.S.; Hyun, S.H.; Lee, E.S.; Rho, G.J. Developmental ability of miniature pig embryos cloned with mesenchymal stem cells. J. Reprod. Dev. 2010, 56, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ahn, K.S.; Kim, M.; Shim, H. Comparison of potency between histone deacetylase inhibitors trichostatin A and valproic acid on enhancing in vitro development of porcine somatic cell nuclear transfer embryos. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Vassiliev, I.; Vassilieva, S.; Truong, K.P.; Beebe, L.F.; McIlfatrick, S.M.; Harrison, S.J.; Nottle, M.B. Isolation and in vitro characterization of putative porcine embryonic stem cells from cloned embryos treated with trichostatin A. Cell. Reprogram. 2011, 13, 205–213. [Google Scholar] [CrossRef]
- Shi, L.H.; Miao, Y.L.; Ouyang, Y.C.; Huang, J.C.; Lei, Z.L.; Yang, J.W.; Han, Z.M.; Song, X.F.; Sun, Q.Y.; Chen, D.Y. Trichostatin A (TSA) improves the development of rabbit-rabbit intraspecies cloned embryos, but not rabbit-human interspecies cloned embryos. Dev. Dyn. 2008, 237, 640–648. [Google Scholar] [CrossRef]
- Meng, Q.; Polgar, Z.; Liu, J.; Dinnyes, A. Live birth of somatic cell-cloned rabbits following trichostatin A treatment and cotransfer of parthenogenetic embryos. Cloning Stem Cells 2009, 11, 203–208. [Google Scholar] [CrossRef]
- Sparman, M.; Dighe, V.; Sritanaudomchai, H.; Ma, H.; Ramsey, C.; Pedersen, D.; Clepper, L.; Nighot, P.; Wolf, D.; Hennebold, J. Epigenetic reprogramming by somatic cell nuclear transfer in primates. Stem Cells 2009, 27, 1255–1264. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Cai, Y.; Wang, Y.; Nie, Y.; Zhang, C.; Xu, Y.; Zhang, X.; Lu, Y.; Wang, Z.; Poo, M. Cloning of macaque monkeys by somatic cell nuclear transfer. Cell 2018, 172, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.P.; Mitalipov, S. Primate and human somatic cell nuclear transfer. In Biology and Pathology of the Oocyte: Role in Fertility, Medicine, and Nuclear Reprogramming, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012; pp. 274–284. [Google Scholar]
- Long, C.R.; Westhusin, M.E.; Golding, M.C. Reshaping the transcriptional frontier: Epigenetics and somatic cell nuclear transfer. Mol. Reprod. Dev. 2014, 81, 183–193. [Google Scholar] [CrossRef] [PubMed]
- French, A.J.; Adams, C.A.; Anderson, L.S.; Kitchen, J.R.; Hughes, M.R.; Wood, S.H. Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem Cells 2008, 26, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Amato, P.; Sparman, M.; Gutierrez, N.M.; Tippner-Hedges, R.; Ma, H.; Kang, E.; Fulati, A.; Lee, H.S.; Sritanaudomchai, H. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 2013, 153, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Egli, D.; Chia, G. A Protocol for Embryonic Stem Cell Derivation by Somatic Cell Nuclear Transfer into Human Oocytes. Protocol Exchange. Available online: https://protocolexchange.researchsquare.com/article/nprot-3117/v1 (accessed on 25 August 2014).
- Yamada, M.; Johannesson, B.; Sagi, I.; Burnett, L.C.; Kort, D.H.; Prosser, R.W.; Paull, D.; Nestor, M.W.; Freeby, M.; Greenberg, E. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature 2014, 510, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Chia, G.; Agudo, J.; Treff, N.; Sauer, M.V.; Billing, D.; Brown, B.D.; Baer, R.; Egli, D. Genomic instability during reprogramming by nuclear transfer is DNA replication dependent. Nat. Cell. Biol. 2017, 19, 282–291. [Google Scholar] [CrossRef]
- Tian, X.C.; Kubota, C.; Enright, B.; Yang, X. Cloning animals by somatic cell nuclear transfer–biological factors. Reprod. Biol. Endocrinol. 2003, 1, 98. [Google Scholar] [CrossRef]
- Tanaka, S.; Oda, M.; Toyoshima, Y.; Wakayama, T.; Tanaka, M.; Yoshida, N.; Hattori, N.; Ohgane, J.; Yanagimachi, R.; Shiota, K. Placentomegaly in Cloned Mouse Concepti Caused by Expansion of the Spongiotrophoblast Layer1. Biol. Reprod. 2001, 65, 1813–1821. [Google Scholar] [CrossRef]
- Whitworth, K.M.; Prather, R.S. Somatic cell nuclear transfer efficiency: How can it be improved through nuclear remodeling and reprogramming? Mol. Reprod. Dev. 2010, 77, 1001–1015. [Google Scholar] [CrossRef]
- Young, L.E.; Sinclair, K.D.; Wilmut, I. Large offspring syndrome in cattle and sheep. Rev. Reprod. 1998, 3, 155–163. [Google Scholar] [CrossRef]
- Sinclair, K.; Young, L.; Wilmut, I.; McEvoy, T. In-utero overgrowth in ruminants following embryo culture: Lessons from mice and a warning to men. Hum. Reprod. 2000, 15, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Wrenzycki, C.; Lucas-Hahn, A.; Brambrink, T.; Kues, W.; Carnwath, J. Gene expression patterns in bovine in vitro-produced and nuclear transfer-derived embryos and their implications for early development. Cloning Stem Cells 2002, 4, 29–38. [Google Scholar] [CrossRef]
- Constant, F.; Guillomot, M.; Heyman, Y.; Vignon, X.; Laigre, P.; Servely, J.; Renard, J.; Chavatte-Palmer, P. Large offspring or large placenta syndrome? Morphometric analysis of late gestation bovine placentomes from somatic nuclear transfer pregnancies complicated by hydrallantois. Biol. Reprod. 2006, 75, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.A.; Carter, D.B.; Korte, S.W.; Prather, R.S. Evaluation of the acute phase response in cloned pigs following a lipopolysaccharide challenge. Domest. Anim. Endocrinol. 2005, 29, 564–572. [Google Scholar] [CrossRef]
- Tamashiro, K.L.K.; Wakayama, T.; Akutsu, H.; Yamazaki, Y.; Lachey, J.L.; Wortman, M.D.; Seeley, R.J.; D’Alessio, D.A.; Woods, S.C.; Yanagimachi, R.; et al. Cloned mice have an obese phenotype not transmitted to their offspring. Nat. Med. 2002, 8, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.; Sommer, J.; Collins, B.; Mir, B.; Martin, A.; York, A.; Petters, R.M.; Piedrahita, J.A. Swine generated by somatic cell nuclear transfer have increased incidence of intrauterine growth restriction (IUGR). Cloning Stem Cells 2007, 9, 229–236. [Google Scholar] [CrossRef]
- Wakisaka-Saito, N.; Kohda, T.; Inoue, K.; Ogonuki, N.; Miki, H.; Hikichi, T.; Mizutani, E.; Wakayama, T.; Kaneko-Ishino, T.; Ogura, A. Chorioallantoic placenta defects in cloned mice. Biochem. Biophys. Res. Commun. 2006, 349, 106–114. [Google Scholar] [CrossRef]
- Jouneau, A.; Zhou, Q.; Camus, A.; Brochard, V.; Maulny, L.; Collignon, J.; Renard, J.P. Developmental abnormalities of NT mouse embryos appear early after implantation. Development 2006, 133, 1597–1607. [Google Scholar] [CrossRef]
- Wakayama, T.; Yanagimachi, R. Cloning of male mice from adult tail-tip cells. Nat. Genet. 1999, 22, 127–128. [Google Scholar] [CrossRef]
- Wakisaka, N.; Inoue, K.; Ogonuki, N.; Miki, H.; Sekita, Y.; Hanaki, K.; Akatsuka, A.; Kaneko-Ishino, T.; Ishino, F.; Ogura, A. Ultrastructure of placental hyperplasia in mice: Comparison of placental phenotypes with three different etiologies. Placenta 2008, 29, 753–759. [Google Scholar] [CrossRef]
- Dean, W.; Santos, F.; Stojkovic, M.; Zakhartchenko, V.; Walter, J.; Wolf, E.; Reik, W. Conservation of methylation reprogramming in mammalian development: Aberrant reprogramming in cloned embryos. Proc. Natl. Acad. Sci. USA 2001, 98, 13734–13738. [Google Scholar] [CrossRef]
- Kang, Y.K.; Koo, D.B.; Park, J.S.; Choi, Y.H.; Chung, A.S.; Lee, K.K.; Han, Y.M. Aberrant methylation of donor genome in cloned bovine embryos. Nat. Genet. 2001, 28, 173–177. [Google Scholar] [CrossRef]
- Ohgane, J.; Wakayama, T.; Kogo, Y.; Senda, S.; Hattori, N.; Tanaka, S.; Yanagimachi, R.; Shiota, K. DNA methylation variation in cloned mice. Genesis 2001, 30, 45–50. [Google Scholar] [CrossRef]
- Renard, J.-P.; Chastant, S.; Chesné, P.; Richard, C.; Marchal, J.; Cordonnier, N.; Chavatte, P.; Vignon, X. Lymphoid hypoplasia and somatic cloning. Lancet 1999, 353, 1489–1491. [Google Scholar] [CrossRef]
- Wakayama, T.; Rodriguez, I.; Perry, A.C.F.; Yanagimachi, R.; Mombaerts, P. Mice cloned from embryonic stem cells. Proc. Natl. Acad. Sci. USA 1999, 96, 14984–14989. [Google Scholar] [CrossRef]
- Ogonuki, N.; Inoue, K.; Yamamoto, Y.; Noguchi, Y.; Tanemura, K.; Suzuki, O.; Nakayama, H.; Doi, K.; Ohtomo, Y.; Satoh, M.; et al. Early death of mice cloned from somatic cells. Nat. Genet. 2002, 30, 253–254. [Google Scholar] [CrossRef]
- Liu, L.; Oldenbourg, R.; Trimarchi, J.R.; Keefe, D.L. A reliable, noninvasive technique for spindle imaging and enucleation of mammalian oocytes. Nat. Biotechnol. 2000, 18, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.A.; Pedersen, D.A.; Clepper, L.L.; Nelson, M.; Sanger, W.G.; Gokhale, S.; Wolf, D.P.; Mitalipov, S.M. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 2007, 450, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Kishigami, S.; Wakayama, S.; Van Thuan, N.; Ohta, H.; Mizutani, E.; Hikichi, T.; Bui, H.T.; Balbach, S.; Ogura, A.; Boiani, M. Production of cloned mice by somatic cellnuclear transfer. Nat. Protoc. 2006, 1, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Sparman, M.; Mitalipov, S. Chromosome transfer in mature oocytes. Nat. Protoc. 2010, 5, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Greggains, G.D.; Lister, L.M.; Tuppen, H.A.L.; Zhang, Q.; Needham, L.H.; Prathalingam, N.; Hyslop, L.A.; Craven, L.; Polanski, Z.; Murdoch, A.P.; et al. Therapeutic potential of somatic cell nuclear transfer for degenerative disease caused by mitochondrial DNA mutations. Sci. Rep. 2014, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Sparman, M.; Sritanaudomchai, H.; Ma, H.; Clepper, L.; Woodward, J.; Li, Y.; Ramsey, C.; Kolotushkina, O.; Mitalipov, S. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 2009, 461, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Mitalipov, S.; Zhou, Q.; Byrne, J.; Ji, W.; Norgren, R.; Wolf, D. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum. Reprod. 2007, 22, 2232–2242. [Google Scholar] [CrossRef]
- Fulka, J.; Notarianni, E.; Passoni, L.; Moor, R.M. Early changes in embryonic nuclei fused to chemically enucleated mouse oocytes. Int. J. Dev. Biol. 1993, 37, 433. [Google Scholar] [PubMed]
- Campbell, K.H.; McWhir, J.; Ritchie, W.A.; Wilmut, I. Sheep cloned by nuclear transfer from a cultured cell line. Nature 1996, 380, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Egli, D.; Chen, A.E.; Saphier, G.; Ichida, J.; Fitzgerald, C.; Go, K.J.; Acevedo, N.; Patel, J.; Baetscher, M.; Kearns, W.G. Reprogramming within hours following nuclear transfer into mouse but not human zygotes. Nat. Commun. 2011, 2, 488. [Google Scholar] [CrossRef]
- Susko-Parrish, J.; Leibfried-Rutledge, M.; Northey, D.; Schutzkus, V.; First, N. Inhibition of protein kinases after an induced calcium transient causes transition of bovine oocytes to embryonic cycles without meiotic completion. Dev. Biol. 1994, 166, 729–739. [Google Scholar] [CrossRef]
- Egli, D.; Eggan, K. Nuclear transfer into mouse oocytes. J. Vis. Exp. 2006, 1, e116. [Google Scholar] [CrossRef]
- Ono, Y.; Shimozawa, N.; Ito, M.; Kono, T. Cloned mice from fetal fibroblast cells arrested at metaphase by a serial nuclear transfer. Biol. Reprod. 2001, 64, 44–50. [Google Scholar] [CrossRef][Green Version]
- Ogura, A.; Inoue, K.; Ogonuki, N.; Noguchi, A.; Takano, K.; Nagano, R.; Suzuki, O.; Lee, J.; Ishino, F.; Matsuda, J. Production of male cloned mice from fresh, cultured, and cryopreserved immature Sertoli cells. Biol. Reprod. 2000, 62, 1579–1584. [Google Scholar] [CrossRef]
- Rideout Iii, W.M.; Wakayama, T.; Wutz, A.; Eggan, K.; Jackson-Grusby, L.; Dausman, J.; Yanagimachi, R.; Jaenisch, R. Generation of mice from wild-type and targeted ES cells by nuclear cloning. Nat. Genet. 2000, 24, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, E.; Ohta, H.; Kishigami, S.; Van Thuan, N.; Hikichi, T.; Wakayama, S.; Kosaka, M.; Sato, E.; Wakayama, T. Developmental ability of cloned embryos from neural stem cells. Reproduction 2006, 132, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Ogonuki, N.; Miki, H.; Hirose, M.; Noda, S.; Kim, J.M.; Aoki, F.; Miyoshi, H.; Ogura, A. Inefficient reprogramming of the hematopoietic stem cell genome following nuclear transfer. J. Cell. Sci. 2006, 119, 1985–1991. [Google Scholar] [CrossRef][Green Version]
- Inoue, K.; Noda, S.; Ogonuki, N.; Miki, H.; Inoue, S.; Katayama, K.; Mekada, K.; Miyoshi, H.; Ogura, A. Differential developmental ability of embryos cloned from tissue-specific stem cells. Stem Cells 2007, 25, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Greco, V.; Guasch, G.; Fuchs, E.; Mombaerts, P. Mice cloned from skin cells. Proc. Natl. Acad. Sci. USA 2007, 104, 2738–2743. [Google Scholar] [CrossRef]
- Sung, L.Y.; Gao, S.; Shen, H.; Yu, H.; Song, Y.; Smith, S.L.; Chang, C.C.; Inoue, K.; Kuo, L.; Lian, J. Differentiated cells are more efficient than adult stem cells for cloning by somatic cell nuclear transfer. Nat. Genet. 2006, 38, 1323–1328. [Google Scholar] [CrossRef]
- Kato, Y.; Tani, T.; Tsunoda, Y. Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J. Reprod. Fertil. 2000, 120, 231–237. [Google Scholar] [CrossRef][Green Version]
- Forsberg, E.J.; Strelchenko, N.S.; Augenstein, M.L.; Betthauser, J.M.; Childs, L.A.; Eilertsen, K.J.; Enos, J.M.; Forsythe, T.M.; Golueke, P.J.; Koppang, R.W. Production of cloned cattle from in vitro systems. Biol. Reprod. 2002, 67, 327–333. [Google Scholar] [CrossRef][Green Version]
- Tsunoda, Y.; Kato, Y. Nuclear Transfer Technologies. In Transgenic Animal Technology: A Laboratory Handbook, 2nd ed.; Pinkert, C.A., Ed.; Academic Press: Amsterdam, The Netherlands, 2002; pp. 195–229. [Google Scholar]
- Wakayama, T.; Yanagimachi, R. Mouse cloning with nucleus donor cells of different age and type. Mol. Reprod. Dev. 2001, 58, 376–383. [Google Scholar] [CrossRef]
- Inoue, K.; Ogonuki, N.; Mochida, K.; Yamamoto, Y.; Takano, K.; Kohda, T.; Ishino, F.; Ogura, A. Effects of donor cell type and genotype on the efficiency of mouse somatic cell cloning. Biol. Reprod. 2003, 69, 1394–1400. [Google Scholar] [CrossRef]
- Terashita, Y.; Wakayama, S.; Yamagata, K.; Li, C.; Sato, E.; Wakayama, T. Latrunculin a can improve the birth rate of cloned mice and simplify the nuclear transfer protocol by gently inhibiting actin polymerization. Biol. Reprod. 2012, 86, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Inoue, K.; Takano, K.; Wakayama, T.; Yanagimachi, R. Birth of mice after nuclear transfer by electrofusion using tail tip cells. Mol. Reprod. Dev. 2000, 57, 55–59. [Google Scholar] [CrossRef]
- Amano, T.; Tani, T.; Kato, Y.; Tsunoda, Y. Mouse cloned from embryonic stem (ES) cells synchronized in metaphase with nocodazole. J. Exp. Zool. 2001, 289, 139–145. [Google Scholar] [CrossRef]
- Kasinathan, P.; Knott, J.G.; Wang, Z.; Jerry, D.J.; Robl, J.M. Production of calves from G1 fibroblasts. Nat. Biotechnol. 2001, 19, 1176. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Kato, Y.; Tsunoda, Y. Direct exposure of chromosomes to nonactivated ovum cytoplasm is effective for bovine somatic cell nucleus reprogramming. Biol. Reprod. 2001, 64, 324–330. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, I.; Campbell, K.H.S. Treatment of ovine oocytes with caffeine increases the accessibility of DNase I to the donor chromatin and reduces apoptosis in somatic cell nuclear transfer embryos. Reprod. Fertil. Dev. 2010, 22, 1000–1014. [Google Scholar] [CrossRef]
- Tani, T.; Kato, Y. Mitogen-Activated Protein Kinase Activity Is Not Essential for the First Step of Nuclear Reprogramming in Bovine Somatic Cell Nuclear Transfer. Cell. Reprogram. 2017, 19, 95–106. [Google Scholar] [CrossRef]
- Krishnakumar, R.; Blelloch, R.H. Epigenetics of cellular reprogramming. Curr. Opin. Genet. Dev. 2013, 23, 548–555. [Google Scholar] [CrossRef]
- Niemann, H. Epigenetic reprogramming in mammalian species after SCNT-based cloning. Theriogenology 2016, 86, 80–90. [Google Scholar] [CrossRef]
- Latham, K.E. Mechanisms and control of embryonic genome activation in mammalian embryos. Int. Rev. Cytol. 1999, 193, 71–124. [Google Scholar]
- Braude, P.; Bolton, V.; Moore, S. Human gene expression first occurs between the four-and eight-cell stages of preimplantation development. Nature 1988, 332, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Prather, R.S.; Schatten, G. Construction of the nuclear matrix at the transition from maternal to zygotic control of development in the mouse: An immunocytochemical study. Mol. Reprod. Dev. 1992, 32, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Reik, W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature 2007, 447, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.D.; Santos, F.; Green, K.; Dean, W.; Reik, W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 2005, 14, 47–58. [Google Scholar] [CrossRef]
- Santos, F.; Hendrich, B.; Reik, W.; Dean, W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev. Biol. 2002, 241, 172–182. [Google Scholar] [CrossRef]
- Mayer, W.; Niveleau, A.; Walter, J.; Fundele, R.; Haaf, T. Embryogenesis: Demethylation of the zygotic paternal genome. Nature 2000, 403, 501–502. [Google Scholar] [CrossRef]
- Cloos, P.A.; Christensen, J.; Agger, K.; Helin, K. Erasing the methyl mark: Histone demethylases at the center of cellular differentiation and disease. Genes. Dev. 2008, 22, 1115–1140. [Google Scholar] [CrossRef]
- Adenot, P.G.; Mercier, Y.; Renard, J.P.; Thompson, E.M. Differential H4 acetylation of paternal and maternal chromatin precedes DNA replication and differential transcriptional activity in pronuclei of 1-cell mouse embryos. Development 1997, 124, 4615–4625. [Google Scholar]
- Prather, R.S.; Ross, J.W.; Isom, S.C.; Green, J.A. Transcriptional, post-transcriptional and epigenetic control of porcine oocyte maturation and embryogenesis. Soc. Reprod. Fertil. Suppl. 2009, 66, 165–176. [Google Scholar]
- Geiman, T.M.; Robertson, K.D. Chromatin remodeling, histone modifications, and DNA methylation—How does it all fit together? J. Cell. Biochem. 2002, 87, 117–125. [Google Scholar] [CrossRef]
- Armstrong, L.; Lako, M.; Dean, W.; Stojkovic, M. Epigenetic modification is central to genome reprogramming in somatic cell nuclear transfer. Stem Cells 2006, 24, 805–814. [Google Scholar] [CrossRef]
- Bui, H.T.; Van Thuan, N.; Wakayama, T.; Miyano, T. Chromatin remodeling in somatic cells injected into mature pig oocytes. Reproduction 2006, 131, 1037–1049. [Google Scholar] [CrossRef]
- Gao, S.; Chung, Y.G.; Parseghian, M.H.; King, G.J.; Adashi, E.Y.; Latham, K.E. Rapid H1 linker histone transitions following fertilization or somatic cell nuclear transfer: Evidence for a uniform developmental program in mice. Dev. Biol. 2004, 266, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Gao, S.; Sung, L.Y.; Corry, G.N.; Ma, Y.; Nagy, Z.P.; Tian, X.C.; Rasmussen, T.P. Rapid elimination of the histone variant MacroH2A from somatic cell heterochromatin after nuclear transfer. Cell. Reprogram. 2010, 12, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, M.; Garagna, S.; Redi, C.A. Nuclear transfer, genome reprogramming and novel opportunities in cell therapy. J. Endocrinol. Investig. 2000, 23, 623–629. [Google Scholar] [CrossRef]
- Moreira, P.N.; Robl, J.M.; Collas, P. Architectural defects in pronuclei of mouse nuclear transplant embryos. J. Cell. Sci. 2003, 116, 3713–3720. [Google Scholar] [CrossRef] [PubMed]
- Beaujean, N.; Taylor, J.; Gardner, J.; Wilmut, I.; Meehan, R.; Young, L. Effect of limited DNA methylation reprogramming in the normal sheep embryo on somatic cell nuclear transfer. Biol. Reprod. 2004, 71, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Zakhartchenko, V.; Stojkovic, M.; Peters, A.; Jenuwein, T.; Wolf, E.; Reik, W.; Dean, W. Epigenetic marking correlates with developmental potential in cloned bovine preimplantation embryos. Curr. Biol. 2003, 13, 1116–1121. [Google Scholar] [CrossRef]
- Enright, B.P.; Sung, L.Y.; Chang, C.C.; Yang, X.; Tian, X.C. Methylation and acetylation characteristics of cloned bovine embryos from donor cells treated with 5-aza-2′-deoxycytidine. Biol. Reprod. 2005, 72, 944–948. [Google Scholar] [CrossRef][Green Version]
- Kishigami, S.; Wakayama, S.; Wakayama, T. Enhancing SCNT with Chromatin Remodeling Agents. In Principles of Cloning, 2nd ed.; Cibelli, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 137–148. [Google Scholar]
- Yoshida, M.; Kijima, M.; Akita, M.; Beppu, T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem. 1990, 265, 17174–17179. [Google Scholar]
- Hattori, N.; Nishino, K.; Ko, Y.G.; Hattori, N.; Ohgane, J.; Tanaka, S.; Shiota, K. Epigenetic control of mouse Oct-4 gene expression in embryonic stem cells and trophoblast stem cells. J. Biol. Chem. 2004, 279, 17063–17069. [Google Scholar] [CrossRef] [PubMed]
- Rybouchkin, A.; Kato, Y.; Tsunoda, Y. Role of histone acetylation in reprogramming of somatic nuclei following nuclear transfer. Biol. Reprod. 2006, 74, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Van Thuan, N.; Kishigami, S.; Wakayama, T. How to improve the success rate of mouse cloning technology. J. Reprod. Dev. 2010, 56, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Sparman, M.L.; Tachibana, M.; Mitalipov, S.M. Cloning of non-human primates: The road “less traveled by”. Int. J. Dev. Biol. 2010, 54, 1671–1678. [Google Scholar] [CrossRef]
- Svensson, K.; Mattsson, R.; James, T.C.; Wentzel, P.; Pilartz, M.; MacLaughlin, J.; Miller, S.J.; Olsson, T.; Eriksson, U.J.; Ohlsson, R. The paternal allele of the H19 gene is progressively silenced during early mouse development: The acetylation status of histones may be involved in the generation of variegated expression patterns. Development 1998, 125, 61–69. [Google Scholar]
- Ogura, A.; Inoue, K.; Wakayama, T. Recent advancements in cloning by somatic cell nuclear transfer. Philos. Trans. R. Soc. Lond. B 2013, 368, 20110329. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Dufort, I.; Nieminen, J.; Moulavi, F.; Ghanaei, H.R.; Hajian, M.; Jafarpour, F.; Forouzanfar, M.; Gourbai, H.; Shahverdi, A.H. Epigenetic modification with trichostatin A does not correct specific errors of somatic cell nuclear transfer at the transcriptomic level; highlighting the non-random nature of oocyte-mediated reprogramming errors. BMC Genom. 2016, 17, 1–21. [Google Scholar] [CrossRef]
- Noggle, S.; Fung, H.-L.; Gore, A.; Martinez, H.; Satriani, K.C.; Prosser, R.; Oum, K.; Paull, D.; Druckenmiller, S.; Freeby, M. Human oocytes reprogram somatic cells to a pluripotent state. Nature 2011, 478, 70–75. [Google Scholar] [CrossRef]
- Mallol, A.; Santaló, J.; Ibáñez, E. Improved development of somatic cell cloned mouse embryos by vitamin C and latrunculin A. PLoS ONE 2015, 10, e0120033. [Google Scholar] [CrossRef]
- Loi, P.; Iuso, D.; Czernik, M.; Ogura, A. A new, dynamic era for somatic cell nuclear transfer? Trends Biotechnol. 2016, 34, 791–797. [Google Scholar] [CrossRef]
- Inoue, K.; Kohda, T.; Sugimoto, M.; Sado, T.; Ogonuki, N.; Matoba, S.; Shiura, H.; Ikeda, R.; Mochida, K.; Fujii, T.; et al. Impeding Xist expression from the active X chromosome improves mouse somatic cell nuclear transfer. Science 2010, 330, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Inoue, K.; Kohda, T.; Sugimoto, M.; Mizutani, E.; Ogonuki, N.; Nakamura, T.; Abe, K.; Nakano, T.; Ishino, F.; et al. RNAi-mediated knockdown of Xist can rescue the impaired postimplantation development of cloned mouse embryos. Proc. Natl. Acad. Sci. USA 2011, 108, 20621–20626. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Liu, Y.; Lu, F.; Iwabuchi, K.A.; Shen, L.; Inoue, A.; Zhang, Y. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell 2014, 159, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.; Oback, F.; Chamley, L.W.; Oback, B.; Laible, G. Transient JMJD2B-mediated reduction of H3K9me3 levels improves reprogramming of embryonic stem cells into cloned embryos. Mol. Cell. Biol. 2013, 33, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.G.; Matoba, S.; Liu, Y.; Eum, J.H.; Lu, F.; Jiang, W.; Lee, J.E.; Sepilian, V.; Cha, K.Y.; Lee, D.R. Histone demethylase expression enhances human somatic cell nuclear transfer efficiency and promotes derivation of pluripotent stem cells. Cell Stem Cell 2015, 17, 758–766. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Wang, C.; Gao, Y.; Gao, R.; Kou, X.; Zhao, Y.; Li, J.; Wu, Y.; Xiu, W. Identification of key factors conquering developmental arrest of somatic cell cloned embryos by combining embryo biopsy and single-cell sequencing. Cell. Discov. 2016, 2, 16010. [Google Scholar] [CrossRef]
- Wolf, D.P.; Morey, R.; Kang, E.; Ma, H.; Hayama, T.; Laurent, L.C.; Mitalipov, S. Concise review: Embryonic stem cells derived by somatic cell nuclear transfer: A horse in the race? Stem Cells 2017, 35, 26–34. [Google Scholar] [CrossRef]
- Yang, X.; Smith, S.L.; Tian, X.C.; Lewin, H.A.; Renard, J.P.; Wakayama, T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat. Genet. 2007, 39, 295–302. [Google Scholar] [CrossRef]
- Chidgey, A.P.; Layton, D.; Trounson, A.; Boyd, R.L. Tolerance strategies for stem-cell-based therapies. Nature 2008, 453, 330–337. [Google Scholar] [CrossRef]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Parham, L.; Alvarez-Buylla, A.; Cedars, M.; Conklin, B.; Fisher, S.; Gates, E.; Giudice, L.; Halme, D.G.; Hershon, W. Cloning mice and men: Prohibiting the use of iPS cells for human reproductive cloning. Cell Stem Cell 2010, 6, 16–20. [Google Scholar] [CrossRef] [PubMed]
- United Nations General Assembly. United Nations Declaration on Human Cloning. Available online: https://digitallibrary.un.org/record/541409/files/A_C.6_59_L.27_Add.1-EN.pdf (accessed on 31 January 2018).
- The Council of Europe. Additional Protocol to the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine, on the Prohibition of Cloning Human Beings. Available online: https://rm.coe.int/168007f2ca (accessed on 31 January 2018).
- International Society for Stem Cell Research. Guidelines for the Conduct of Human Embryonic Stem Cell Research. Available online: https://www.forth.gr/_gfx/pdf/ISSCRhESCguidelines2006.pdf (accessed on 31 January 2018).
- Daar, J.; Amato, P.; Benward, J.; Collins, L.R.; Davis, J.; Francis, L.; Gates, E.; La Barbera, A.; McCullough, L.; Klipstein, S. Human somatic cell nuclear transfer and reproductive cloning: An Ethics Committee opinion. Fertil. Steril. 2016, 105, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Human Fertilisation and Embryology Act. Embryo Project Encyclopedia. Available online: https://hpsrepository.asu.edu/handle/10776/8270 (accessed on 31 January 2018).
- Pepper, M.S.; Gouveia, C.; Slabbert, M.N. Legislation governing pluripotent stem cells in South Africa. S. Afr. J. Bioeth. Law. 2015, 8, 23–31. [Google Scholar] [CrossRef]
- Hyun, I. Moving human SCNT research forward ethically. Cell Stem Cell 2011, 9, 295. [Google Scholar] [CrossRef]
- Zacher, A. Oocyte Donor Compensation for Embryonic Stem Cell Research: An Analysis of New York‘s Payment for Eggs Program. Alb. Law. J. Sci. Technol. 2011, 21, 323–352. [Google Scholar]
- Safier, L.Z.; Gumer, A.; Kline, M.; Egli, D.; Sauer, M. Compensating human subjects providing oocytes for stem cell research: 9-year experience and outcomes. J. Assist. Reprod. Genet. 2018, 35, 1219–1225. [Google Scholar] [CrossRef]
- Republic of South Africa. National Health Act 61. Government Gazette. Available online: http://www.acts.co.za/national-health-act-2003/index.html (accessed on 31 January 2018).
- Southern African Society of Reproductive Medicine and Gynaecological Endoscopy South African Society of Reproductive Science and Surgery. Guidelines for Gamete Donation. Available online: http://www.fertilitysa.org.za/Guidelines/ReproductiveMedicine/2008%20GUIDELINES%20FOR%20GAMETE%20DONATION.pdf (accessed on 31 January 2018).
- Southern African Society of Reproductive Medicine and Gynaecological Endoscopy South Africa Society of Reproductive Science and Surgery. Amendment to 2008 Guidelines for Gamete Donation. Available online: http://www.fertilitysa.org.za/Guidelines/ReproductiveMedicine/Egg%20donor%20compensation%20-%20November%202014.pdf (accessed on 31 January 2018).
- Heindryckx, B.; De Sutter, P.; Gerris, J.; Dhont, M.; Van der Elst, J. Embryo development after successful somatic cell nuclear transfer to in vitro matured human germinal vesicle oocytes. Hum. Reprod. 2007, 22, 1982–1990. [Google Scholar] [CrossRef]
- Egli, D.; Chen, A.E.; Saphier, G.; Powers, D.; Alper, M.; Katz, K.; Berger, B.; Goland, R.; Leibel, R.L.; Melton, D.A. Impracticality of egg donor recruitment in the absence of compensation. Cell Stem Cell 2011, 9, 293–294. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Whyte, J.; Prather, R.S. Effect of epigenetic regulation during swine embryogenesis and on cloning by nuclear transfer. Cell Tissue Res. 2010, 341, 13–21. [Google Scholar] [CrossRef]
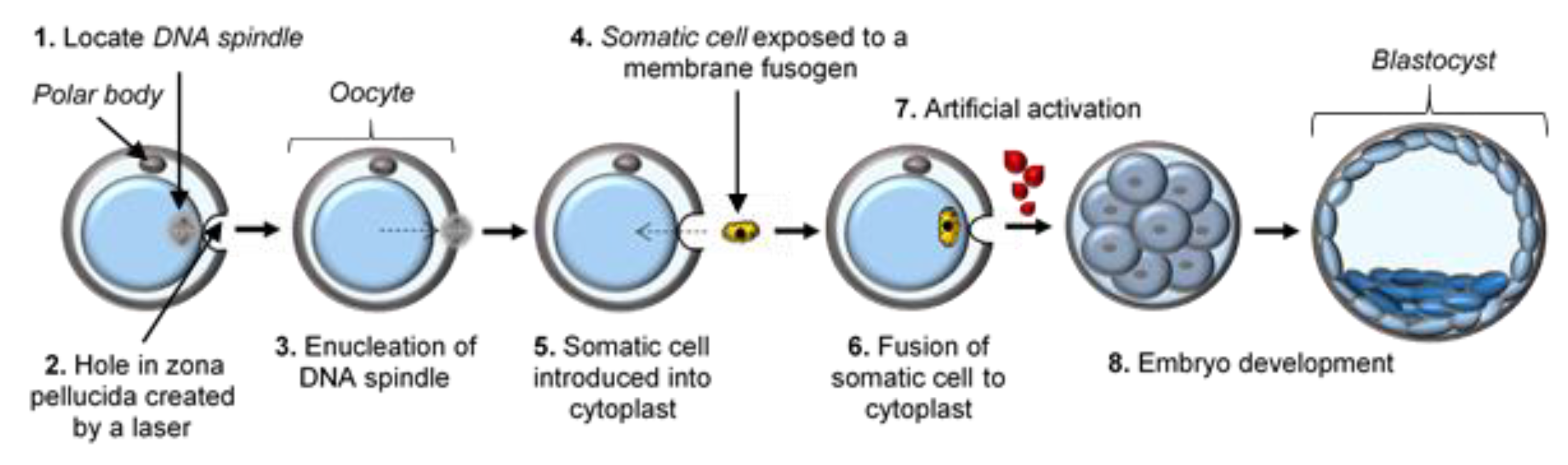
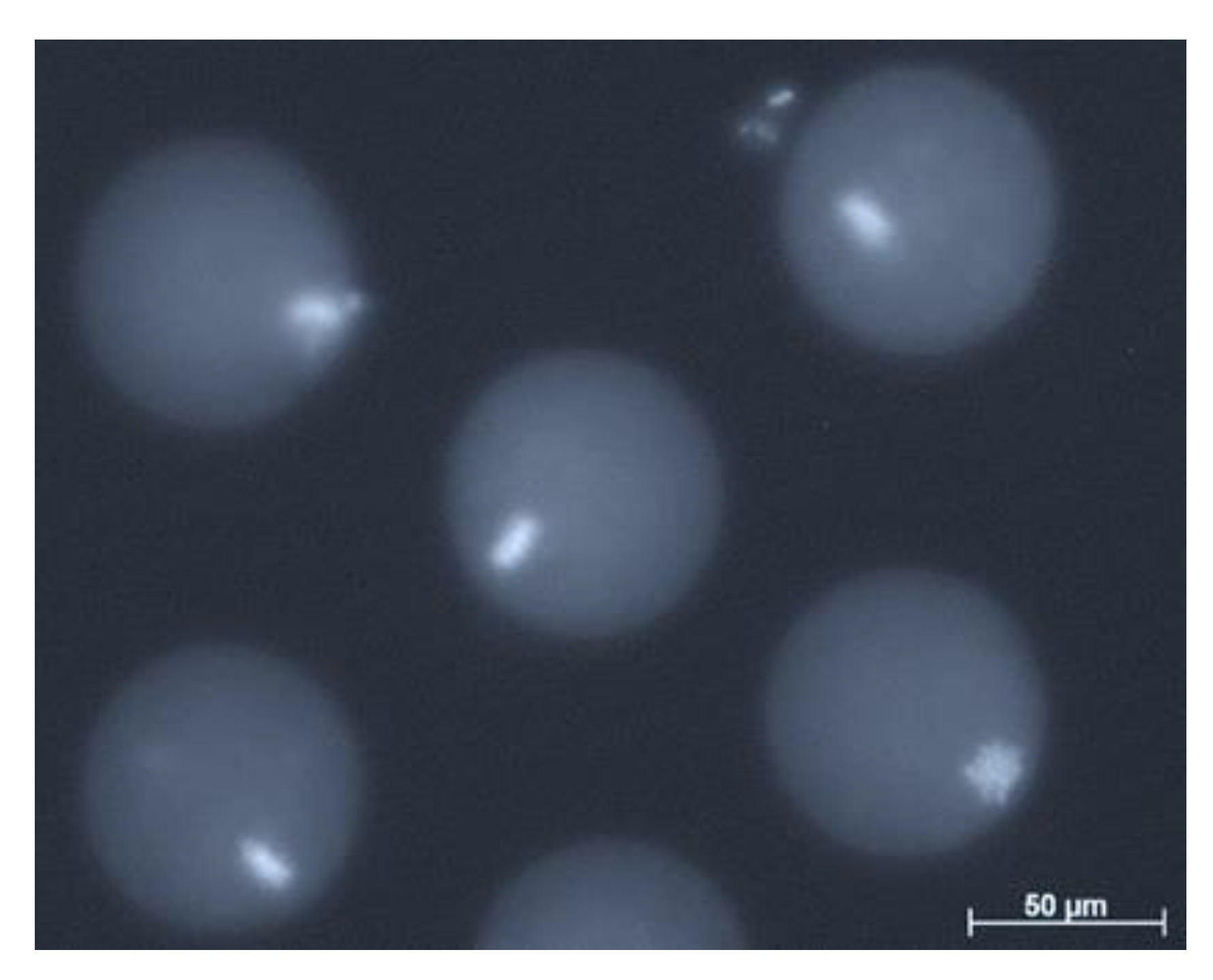
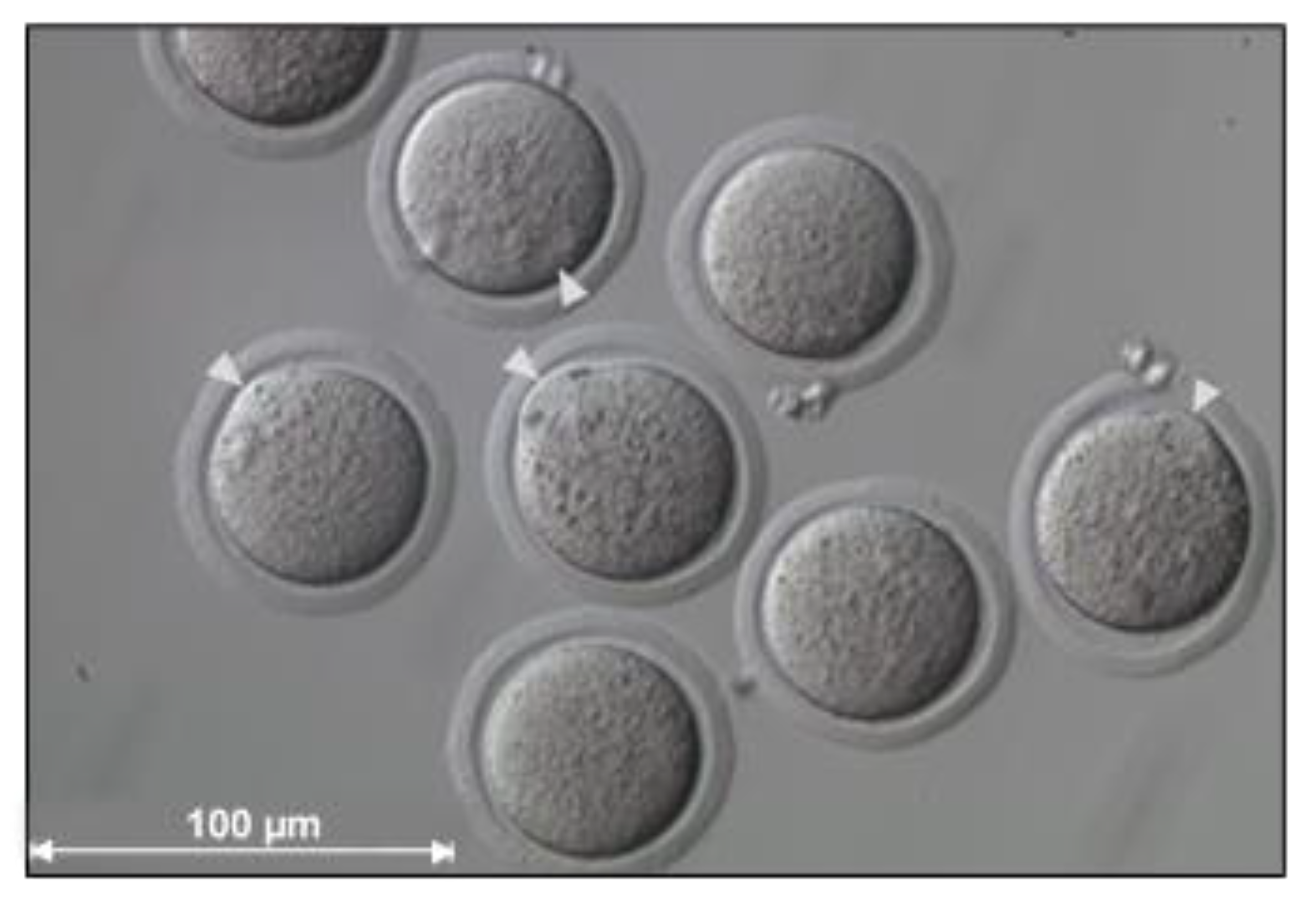
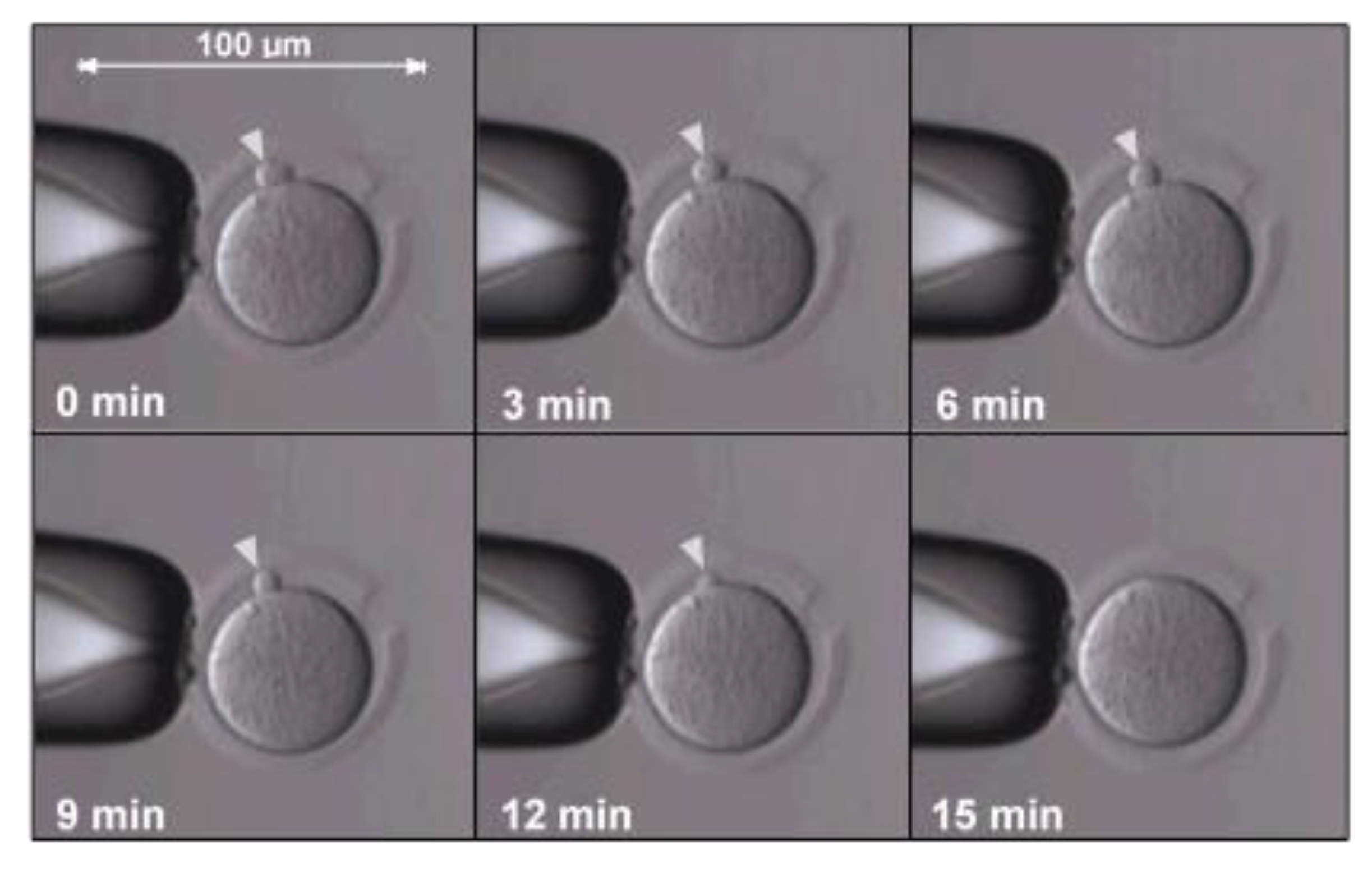
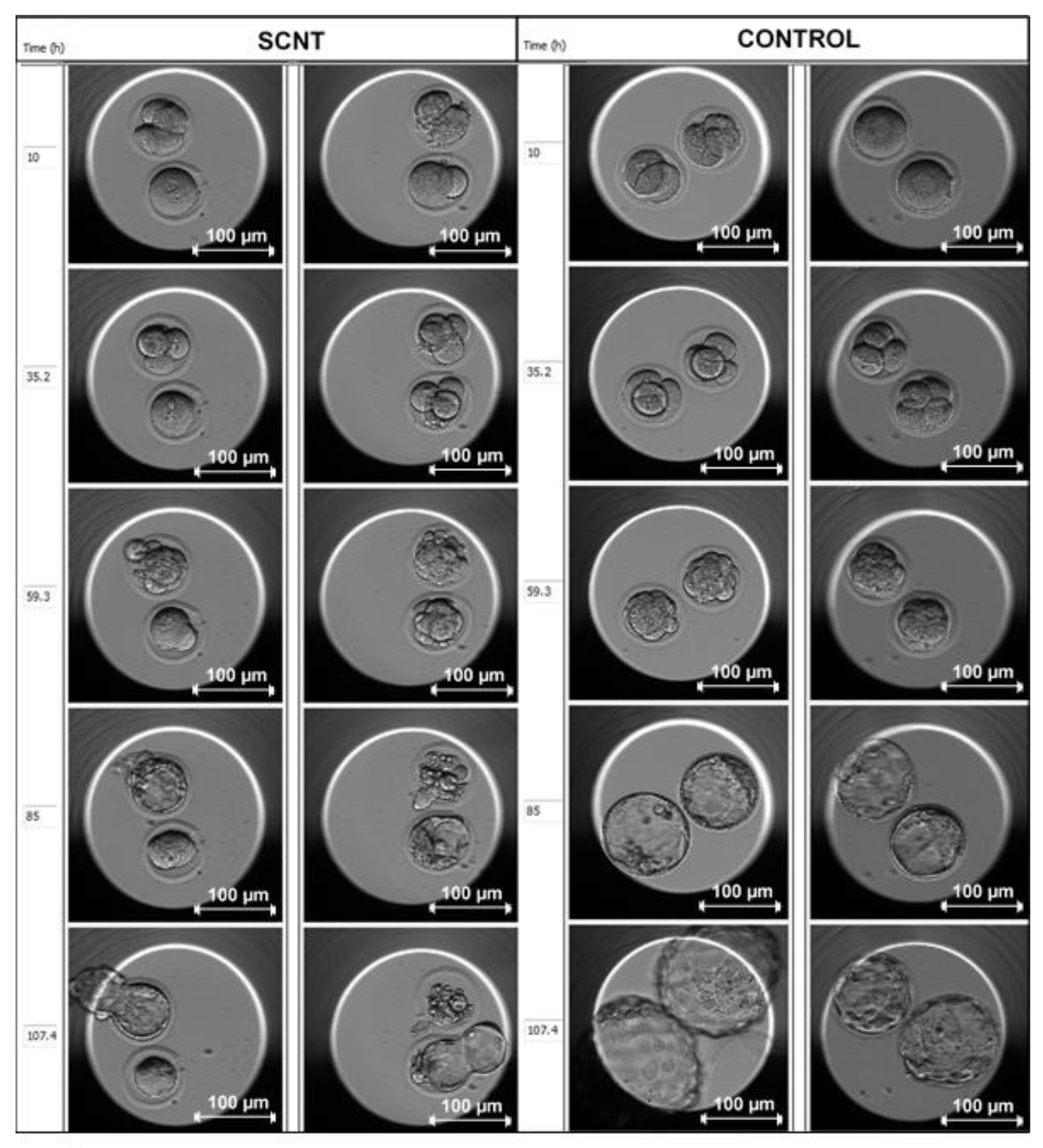
| Donor Cell Type | Donor Age | Success Rate | References |
|---|---|---|---|
| Cumulus (B6D2F1) | Adult | 2.5–4.5% | [9,82] |
| Cumulus (129B6F1) | Adult | 3.2% | [83] |
| Cumulus (BDF1x129/Sv) | Adult | 15.6% | [84] |
| Tail-tip fibroblast | Adult | 1.1–4.8% | [51,85] |
| Fetal fibroblast | Fetus | 3.0–3.7% | [71,82] |
| Sertoli (B6D2F1) | Newborn | 4.5% | [72] |
| Sertoli (B6129F1) | Newborn | 10.8% | [83] |
| Neuronal stem cell | Newborn | 0.5% | [74] |
| Neuronal stem cell | Fetus | 1.6% | [76] |
| Hematopoietic stem cell | Adult | 0.7% | [75] |
| Keratinocyte stem cell | Adult | 5.4% | [77] |
| ESC (G1 phase) | Embryonic | 12.3% | [57] |
| ESC (G2/M phase) | Embryonic | 6.4% | [57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouveia, C.; Huyser, C.; Egli, D.; Pepper, M.S. Lessons Learned from Somatic Cell Nuclear Transfer. Int. J. Mol. Sci. 2020, 21, 2314. https://doi.org/10.3390/ijms21072314
Gouveia C, Huyser C, Egli D, Pepper MS. Lessons Learned from Somatic Cell Nuclear Transfer. International Journal of Molecular Sciences. 2020; 21(7):2314. https://doi.org/10.3390/ijms21072314
Chicago/Turabian StyleGouveia, Chantel, Carin Huyser, Dieter Egli, and Michael S. Pepper. 2020. "Lessons Learned from Somatic Cell Nuclear Transfer" International Journal of Molecular Sciences 21, no. 7: 2314. https://doi.org/10.3390/ijms21072314
APA StyleGouveia, C., Huyser, C., Egli, D., & Pepper, M. S. (2020). Lessons Learned from Somatic Cell Nuclear Transfer. International Journal of Molecular Sciences, 21(7), 2314. https://doi.org/10.3390/ijms21072314






