Paraneoplastic Dermatoses: A Brief General Review and an Extensive Analysis of Paraneoplastic Pemphigus and Paraneoplastic Dermatomyositis
Abstract
1. Introduction
2. Obligate Paraneoplastic Dermatoses (PD)
2.1. Acanthosis Nigricans (AN)
2.2. Tripe Palms (TP)
2.3. Necrolytic Migratory Erythema (NME)
2.4. Paraneoplastic Pemphigus (PNP)
3. Facultative Paraneoplastic Dermatoses (PD)
3.1. Leser-Trélat (LT)
3.2. Pyoderma Gangrenosum (PG)
3.3. Sweet Syndrome (SS)
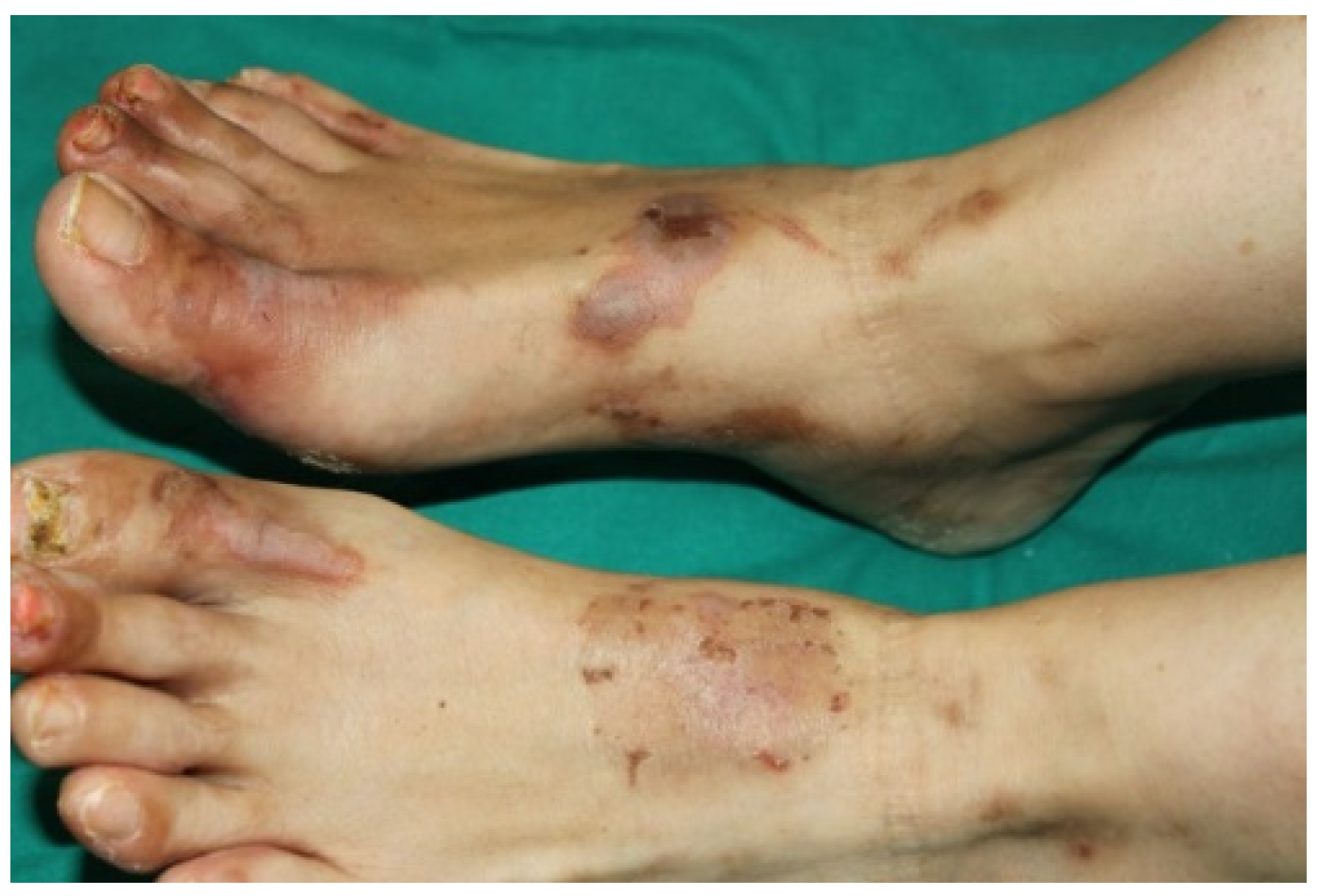
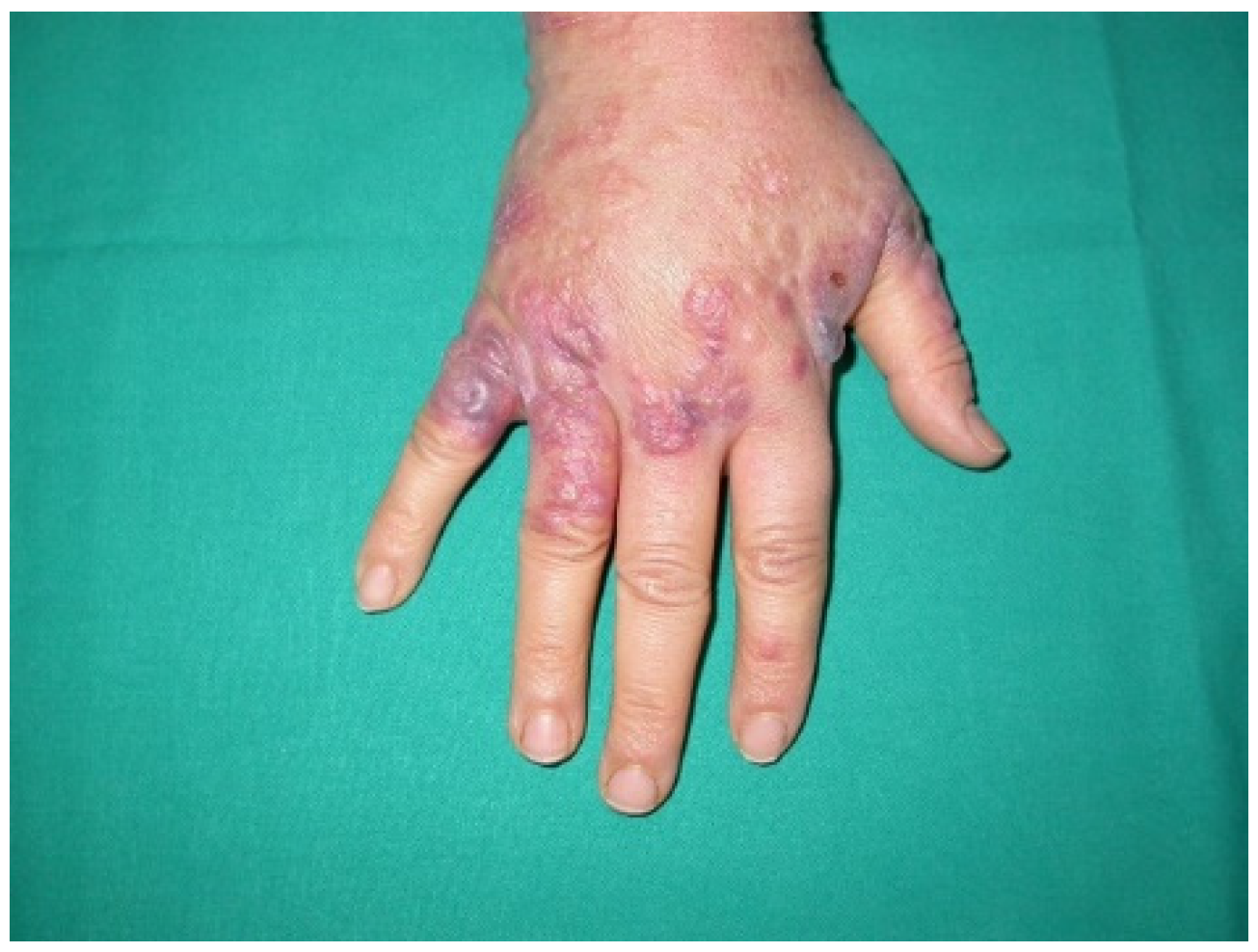
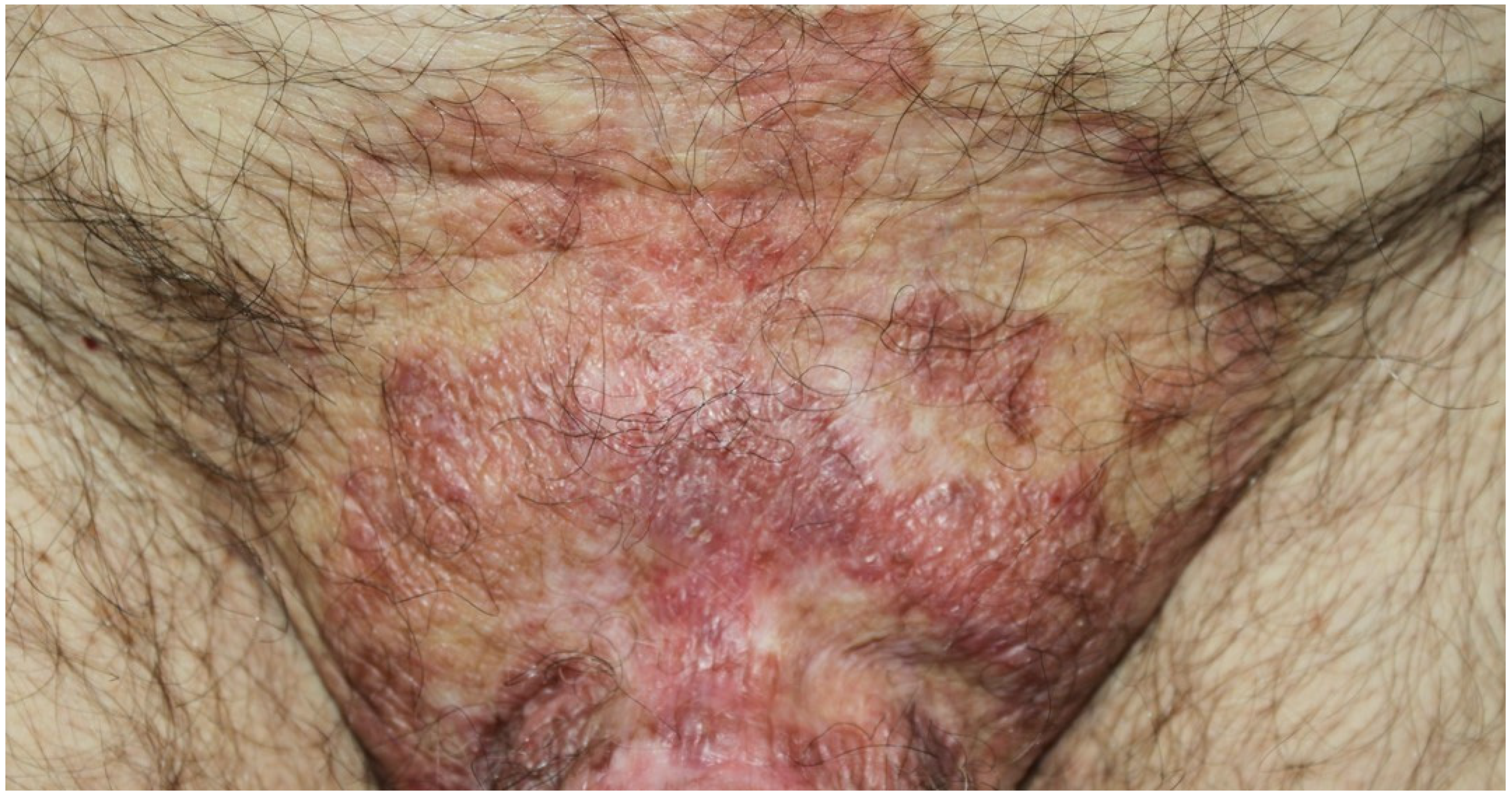
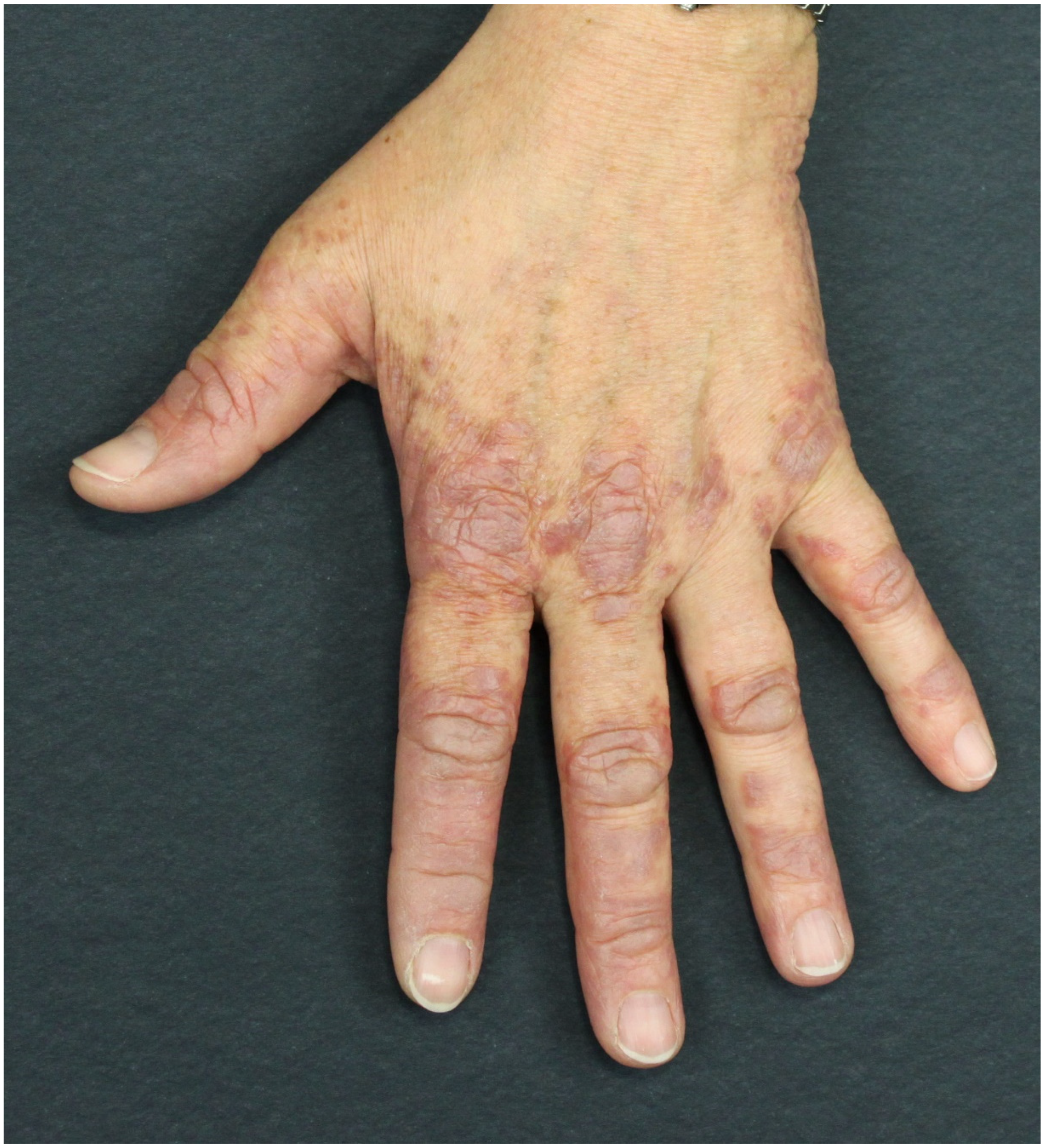
3.4. Paraneoplastic Dermatomyositis (PNDM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miyashiro, D.; Sanches, J.A. Paraneoplastic skin disorders: A review. G. Ital. Dermatol. Venereol. 2016, 151, 55–76. [Google Scholar] [PubMed]
- Gualtieri, B.; Hertl, M. Recognize rare diseases on the skin. Internist 2019, 60, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Khoschbin, T.; Loser, C.; Dippel, E. Paraneoplastic skin diseases. Internist 2019, 60, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R.; Kurzrock, R. Sweet’s syndrome revisited: A review of disease concepts. Int. J. Dermatol. 2003, 42, 761–778. [Google Scholar] [CrossRef]
- Silva, J.A.; Mesquita Kde, C.; Igreja, A.C.; Lucas, I.C.; Freitas, A.F.; Oliveira, S.M.; Costa, I.M.; Campbell, I.T. Paraneoplastic cutaneous manifestations: Concepts and updates. An. Bras. Dermatol. 2013, 88, 9–22. [Google Scholar] [CrossRef]
- Anhalt, G.J.; Kim, S.C.; Stanley, J.R.; Korman, N.J.; Jabs, D.A.; Kory, M.; Izumi, H.; Ratrie, H., 3rd; Mutasim, D.; Ariss-Abdo, L.; et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N. Engl. J. Med. 1990, 323, 1729–1735. [Google Scholar] [CrossRef]
- Paolino, G.; Didona, D.; Magliulo, G.; Iannella, G.; Didona, B.; Mercuri, S.R.; Moliterni, E.; Donati, M.; Ciofalo, A.; Granata, G.; et al. Paraneoplastic Pemphigus: Insight into the Autoimmune Pathogenesis, Clinical Features and Therapy. Int. J. Mol. Sci. 2017, 18, 2532. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Ndoye, A.; Bassler, K.D.; Shultz, L.D.; Shields, M.C.; Ruben, B.S.; Webber, R.J.; Pittelkow, M.R.; Lynch, P.J.; Grando, S.A. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: A reappraisal of paraneoplastic pemphigus. Arch. Dermatol. 2001, 137, 193–206. [Google Scholar]
- Solimani, F.; Maglie, R.; Pollmann, R.; Schmidt, T.; Schmidt, A.; Ishii, N.; Tackenberg, B.; Kirschbaum, A.; Didona, D.; Pickert, J.; et al. Thymoma-Associated Paraneoplastic Autoimmune Multiorgan Syndrome-From Pemphigus to Lichenoid Dermatitis. Front. Immunol. 2019, 10, 1413. [Google Scholar] [CrossRef]
- Vassileva, S.; Drenovska, K.; Manuelyan, K. Autoimmune blistering dermatoses as systemic diseases. Clin. Dermatol. 2014, 32, 364–375. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.C. Paraneoplastic Pemphigus: Paraneoplastic Autoimmune Disease of the Skin and Mucosa. Front. Immunol. 2019, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, D.; Anhalt, G.J.; Lazarova, Z.; Aho, S.; Kazerounian, S.; Kouba, D.J.; Mascaro, J.M., Jr.; Nousari, H.C. Paraneoplastic pemphigus in children and adolescents. Br. J. Dermatol. 2002, 147, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, C.; Ruhrberg, C.; Nie, Z.; Karashima, T.; Mori, O.; Nishikawa, T.; Green, K.J.; Anhalt, G.J.; DiColandrea, T.; Watt, F.M.; et al. Envoplakin and periplakin are components of the paraneoplastic pemphigus antigen complex. J. Investig. Dermatol. 1998, 111, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Oursler, J.R.; Labib, R.S.; Ariss-Abdo, L.; Burke, T.; O’Keefe, E.J.; Anhalt, G.J. Human autoantibodies against desmoplakins in paraneoplastic pemphigus. J. Clin. Investig. 1992, 89, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Borradori, L.; Trueb, R.M.; Jaunin, F.; Limat, A.; Favre, B.; Saurat, J.H. Autoantibodies from a patient with paraneoplastic pemphigus bind periplakin, a novel member of the plakin family. J. Investig. Dermatol. 1998, 111, 338–340. [Google Scholar] [CrossRef]
- Lambert, J.; Bracke, S.; van Roy, F.; Pas, H.H.; Bonne, S.; De Schepper, S. Serum plakophilin-3 autoreactivity in paraneoplastic pemphigus. Br. J. Dermatol. 2010, 163, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Brandt, O.; Rafei, D.; Podstawa, E.; Niedermeier, A.; Jonkman, M.F.; Terra, J.B.; Hein, R.; Hertl, M.; Pas, H.H.; Muller, R. Differential IgG recognition of desmoglein 3 by paraneoplastic pemphigus and pemphigus vulgaris sera. J. Investig. Dermatol. 2012, 132, 1738–1741. [Google Scholar] [CrossRef]
- Amagai, M.; Nishikawa, T.; Nousari, H.C.; Anhalt, G.J.; Hashimoto, T. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J. Clin. Investig. 1998, 102, 775–782. [Google Scholar] [CrossRef]
- Zimmermann, J.; Bahmer, F.; Rose, C.; Zillikens, D.; Schmidt, E. Clinical and immunopathological spectrum of paraneoplastic pemphigus. J. Dtsch. Dermatol. Ges. 2010, 8, 598–606. [Google Scholar] [CrossRef]
- Numata, S.; Teye, K.; Tsuruta, D.; Sogame, R.; Ishii, N.; Koga, H.; Natsuaki, Y.; Tsuchisaka, A.; Hamada, T.; Karashima, T.; et al. Anti-alpha-2-macroglobulin-like-1 autoantibodies are detected frequently and may be pathogenic in paraneoplastic pemphigus. J. Investig. Dermatol. 2013, 133, 1785–1793. [Google Scholar] [CrossRef]
- Schepens, I.; Jaunin, F.; Begre, N.; Laderach, U.; Marcus, K.; Hashimoto, T.; Favre, B.; Borradori, L. The protease inhibitor alpha-2-macroglobulin-like-1 is the p170 antigen recognized by paraneoplastic pemphigus autoantibodies in human. PLoS ONE 2010, 5, e12250. [Google Scholar] [CrossRef] [PubMed]
- Billet, S.E.; Grando, S.A.; Pittelkow, M.R. Paraneoplastic autoimmune multiorgan syndrome: Review of the literature and support for a cytotoxic role in pathogenesis. Autoimmunity 2006, 39, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, V.N.; Srivastava, G. Paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome. Int. J. Dermatol. 2009, 48, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Brinck, U.; Letschert, M.; Blaschke, V.; Dames, K.; Braess, J.; Wormann, B.; Runger, T.M.; Neumann, C. Graft-versus-host disease-like immunophenotype and apoptotic keratinocyte death in paraneoplastic pemphigus. Br. J. Dermatol. 1999, 141, 739–746. [Google Scholar] [CrossRef]
- Cummins, D.L.; Mimouni, D.; Tzu, J.; Owens, N.; Anhalt, G.J.; Meyerle, J.H. Lichenoid paraneoplastic pemphigus in the absence of detectable antibodies. J. Am. Acad. Dermatol. 2007, 56, 153–159. [Google Scholar] [CrossRef]
- Wade, M.S.; Black, M.M. Paraneoplastic pemphigus: A brief update. Australas. J. Dermatol. 2005, 46, 1–8. [Google Scholar] [CrossRef]
- Camisa, C.; Helm, T.N. Paraneoplastic pemphigus is a distinct neoplasia-induced autoimmune disease. Arch. Dermatol. 1993, 129, 883–886. [Google Scholar] [CrossRef]
- Joly, P.; Richard, C.; Gilbert, D.; Courville, P.; Chosidow, O.; Roujeau, J.C.; Beylot-Barry, M.; D’Incan, M.; Martel, P.; Lauret, P.; et al. Sensitivity and specificity of clinical, histologic, and immunologic features in the diagnosis of paraneoplastic pemphigus. J. Am. Acad. Dermatol. 2000, 43, 619–626. [Google Scholar] [CrossRef]
- Helou, J.; Allbritton, J.; Anhalt, G.J. Accuracy of indirect immunofluorescence testing in the diagnosis of paraneoplastic pemphigus. J. Am. Acad. Dermatol. 1995, 32, 441–447. [Google Scholar] [CrossRef]
- Poot, A.M.; Diercks, G.F.; Kramer, D.; Schepens, I.; Klunder, G.; Hashimoto, T.; Borradori, L.; Jonkman, M.F.; Pas, H.H. Laboratory diagnosis of paraneoplastic pemphigus. Br. J. Dermatol. 2013, 169, 1016–1024. [Google Scholar] [CrossRef]
- Probst, C.; Schlumberger, W.; Stocker, W.; Recke, A.; Schmidt, E.; Hashimoto, T.; Zhu, X.J.; Zillikens, D.; Komorowski, L. Development of ELISA for the specific determination of autoantibodies against envoplakin and periplakin in paraneoplastic pemphigus. Clin. Chim. Acta 2009, 410, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Nishimoto, S.; Nagao, K.; Takahashi, H.; Yoshida, K.; Ohyama, M.; Yamada, T.; Asano, K.; Amagai, M. Ectopic expression of epidermal antigens renders the lung a target organ in paraneoplastic pemphigus. J. Immunol. 2013, 191, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.S.M.; Carvalho, J.C.; Carneiro, S.C. Cutaneous paraneoplasia. Clin. Dermatol. 2011, 29, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Negus, D.; Huang, W. Pyoderma gangrenosum: A review of pathogenesis and treatment. Expert Rev. Clin. Immunol. 2018, 14, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ashchyan, H.J.; Nelson, C.A.; Stephen, S.; James, W.D.; Micheletti, R.G.; Rosenbach, M. Neutrophilic dermatoses: Pyoderma gangrenosum and other bowel- and arthritis-associated neutrophilic dermatoses. J. Am. Acad. Dermatol. 2018, 79, 1009–1022. [Google Scholar] [CrossRef]
- Papi, M.; Didona, B.; Chinni, L.M.; Gobello, T.; Mazzanti, C.; De Pita, O.; Cavalieri, R. Koebner phenomenon in an ANCA-positive patient with pyoderma gangrenosum. J. Dermatol. 1997, 24, 583–586. [Google Scholar] [CrossRef]
- Alavi, A.; French, L.E.; Davis, M.D.; Brassard, A.; Kirsner, R.S. Pyoderma Gangrenosum: An Update on Pathophysiology, Diagnosis and Treatment. Am. J. Clin. Dermatol. 2017, 18, 355–372. [Google Scholar] [CrossRef]
- Marzano, A.V.; Fanoni, D.; Antiga, E.; Quaglino, P.; Caproni, M.; Crosti, C.; Meroni, P.L.; Cugno, M. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet’s syndrome. Clin. Exp. Immunol. 2014, 178, 48–56. [Google Scholar] [CrossRef]
- Antiga, E.; Maglie, R.; Volpi, W.; Bianchi, B.; Berti, E.; Marzano, A.V.; Caproni, M. T helper type 1-related molecules as well as interleukin-15 are hyperexpressed in the skin lesions of patients with pyoderma gangrenosum. Clin. Exp. Immunol. 2017, 189, 383–391. [Google Scholar] [CrossRef]
- Caproni, M.; Antiga, E.; Volpi, W.; Verdelli, A.; Venegoni, L.; Quaglino, P.; Fabbri, P.; Marzano, A.V. The Treg/Th17 cell ratio is reduced in the skin lesions of patients with pyoderma gangrenosum. Br. J. Dermatol. 2015, 173, 275–278. [Google Scholar] [CrossRef]
- Quaglino, P.; Fava, P.; Caproni, M.; Antiga, E.; De Simone, C.; Papini, M.; Parodi, A.; Novelli, M.; Osella-Abate, S.; Ribero, S.; et al. Phenotypical characterization of circulating cell subsets in pyoderma gangrenosum patients: The experience of the Italian immuno-pathology group. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Bendewald, M.J.; Wetter, D.A.; Li, X.; Davis, M.D. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: A population-based study in Olmsted County, Minnesota. Arch. Dermatol. 2010, 146, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Callen, J.P. Dermatomyositis. Lancet 2000, 355, 53–57. [Google Scholar] [CrossRef]
- Callen, J.P.; Wortmann, R.L. Dermatomyositis. Clin. Dermatol 2006, 24, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Yamada, H.; Ohkubo, M.; Yamasaki, Y.; Yamasaki, M.; Mizushima, M.; Ozaki, S. Incidence and predictive factors for malignancies in 136 Japanese patients with dermatomyositis, polymyositis and clinically amyopathic dermatomyositis. Mod. Rheumatol. 2011, 21, 178–183. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 1975, 292, 403–407. [Google Scholar] [CrossRef]
- Bohan, A.; Peter, J.B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 1975, 292, 344–347. [Google Scholar] [CrossRef]
- Auriemma, M.; Capo, A.; Meogrossi, G.; Amerio, P. Cutaneous signs of classical dermatomyositis. G. Ital. Dermatol. Venereol. 2014, 149, 505–517. [Google Scholar]
- Iaccarino, L.; Ghirardello, A.; Bettio, S.; Zen, M.; Gatto, M.; Punzi, L.; Doria, A. The clinical features, diagnosis and classification of dermatomyositis. J. Autoimmun. 2014, 48–49, 122–127. [Google Scholar] [CrossRef]
- Muro, Y.; Sugiura, K.; Akiyama, M. Cutaneous Manifestations in Dermatomyositis: Key Clinical and Serological Features-a Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 293–302. [Google Scholar] [CrossRef]
- Santmyire-Rosenberger, B.; Dugan, E.M. Skin involvement in dermatomyositis. Curr. Opin. Rheumatol. 2003, 15, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Mainetti, C.; Terziroli Beretta-Piccoli, B.; Selmi, C. Cutaneous Manifestations of Dermatomyositis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 53, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Balin, S.J.; Wetter, D.A.; Andersen, L.K.; Davis, M.D. Calcinosis cutis occurring in association with autoimmune connective tissue disease: The Mayo Clinic experience with 78 patients, 1996–2009. Arch. Dermatol. 2012, 148, 455–462. [Google Scholar] [PubMed]
- Qiang, J.K.; Kim, W.B.; Baibergenova, A.; Alhusayen, R. Risk of Malignancy in Dermatomyositis and Polymyositis. J. Cutan. Med. Surg. 2017, 21, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; Zhang, Y.; Sigurgeirsson, B.; Pukkala, E.; Mellemkjaer, L.; Airio, A.; Evans, S.R.; Felson, D.T. Frequency of specific cancer types in dermatomyositis and polymyositis: A population-based study. Lancet 2001, 357, 96–100. [Google Scholar] [CrossRef]
- Liu, W.C.; Ho, M.; Koh, W.P.; Tan, A.W.; Ng, P.P.; Chua, S.H.; Tan, S.H.; Tang, M.B. An 11-year review of dermatomyositis in Asian patients. Ann. Acad. Med. Singapore 2010, 39, 843–847. [Google Scholar] [PubMed]
- Stockton, D.; Doherty, V.R.; Brewster, D.H. Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: A Scottish population-based cohort study. Br. J. Cancer 2001, 85, 41–45. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, F.; Qin, B.; Liang, Y.; Zhong, R. Polymyositis/dermatomyositis and malignancy risk: A metaanalysis study. J. Rheumatol. 2015, 42, 282–291. [Google Scholar] [CrossRef]
- Buchbinder, R.; Forbes, A.; Hall, S.; Dennett, X.; Giles, G. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann. Intern. Med. 2001, 134, 1087–1095. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wu, C.Y.; Huang, Y.L.; Wang, C.B.; Shen, J.L.; Chang, Y.T. Cancer risks of dermatomyositis and polymyositis: A nationwide cohort study in Taiwan. Arthritis Res. Ther. 2010, 12, R70. [Google Scholar] [CrossRef]
- Olazagasti, J.M.; Baez, P.J.; Wetter, D.A.; Ernste, F.C. Cancer risk in dermatomyositis: A meta-analysis of cohort studies. Am. J. Clin. Dermatol. 2015, 16, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Mannion, M.L.; Beukelman, T. What is the background incidence of malignancy in children with rheumatic disease? Curr. Rheumatol. Rep. 2013, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.; Dare, J. Juvenile dermatomyositis as a paraneoplastic phenomenon: An update. J. Pediatr. Hematol. Oncol. 2010, 32, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Volc-Platzer, B. Dermatomyositis-update. Hautarzt 2015, 66, 604–610. [Google Scholar] [CrossRef]
- Requena, C.; Traves, V.; Llombart, B.; Guillen, C. Incipient merkel cell carcinoma: A report of 2 cases. Actas Dermosifiliogr. 2013, 104, 71–74. [Google Scholar] [CrossRef]
- Lu, X.; Yang, H.; Shu, X.; Chen, F.; Zhang, Y.; Zhang, S.; Peng, Q.; Tian, X.; Wang, G. Factors predicting malignancy in patients with polymyositis and dermatomyostis: A systematic review and meta-analysis. PLoS ONE 2014, 9, e94128. [Google Scholar] [CrossRef]


| Criterion |
|---|
| 1. The onset of dermatosis must be near to the beginning of the neoplasia |
| 2. Both must follow parallel courses |
| 3. The dermatosis must not be part of any genetic syndrome |
| 4. A specific dermatosis accompanies a specific tumor |
| 5. The cutaneous disease is rare in general population |
| 6. There is a high grade of association with the neoplasia |
| Paraneoplastic Dermatosis | Involved Molecular Factors and Detected Antibodies |
|---|---|
| Acanthosis nigricans [1,2,3] | FGF, IGF-1, MSHα, TGF-α |
| Tripe palms [1,2,3] | EGF-α and TGF-α |
| Necrolytic migratory erythema [1,2,3] | Increased level of arachidonic acid and deficit of niacin |
| Paraneoplastic pemphigus [1,2,3] | Circulating autoantibodies against α-2-macroglobulin-like-1, bullous pemphigoid antigen, desmocollins 1 and 3, desmogleins 1 and 3, desmoplakins 1 and 2, envoplakin, and periplakin, and plakophilin 3 |
| Leser-Trélat [1,2,3] | EGF-α, IGF-1, and TGF-α |
| Pyoderma gangrenosum [1,2,3] | Fas, FasL, IL1b, IL-8, IL-17, IL-23 |
| Sweet syndrome [1,3,4,5] | G-CSF, GM-CSF, IL-1, IL-3, IL-6, IL-8 |
| Paraneoplastic dermatomyositis [1,2,3] | Circulating autoantibodies against NXP-2 and TIF1-γ |
| Neoplasia | Obligate PD |
| Gastrointestinal tract | Acanthosis nigricans maligna Necrolytic migratory erythema Leser–Trelat’s syndrome Trousseau’s syndrome |
| Myeloproliferative and lymphoproliferative disorders | Paraneoplastic pemphigus |
| Miscellaneous | Acquired hypertricosis lanuginose Acrokeratosis paraneoplastica of Bazex Erythema gyratum repens |
| Neoplasia | Facultative PD |
| Low respiratory tract and gastrointestinal tract | Paraneoplastic dermatomyositis |
| Esophageal tract | Acquired palmo-plantar keratoderma |
| Myeloproliferative and lymphoproliferative disorders | Sweet syndrome Pyoderma ganrenosum |
| Miscellaneous | Pytiriasis rotunda Multicentric reticulohistiocytosis Anti-laminina 332 bullous pemphigoid |
| Criterion | Details |
|---|---|
| Clinical features | Painful mucosal erosions with or without a multiform skin eruption characterized by blisters and erosions, occurring in association with an occult or evident neoplasm |
| Pathology | Suprabasal intraepithelial acantholysis, interface dermatitis, and necrosis of keratinocytes |
| Direct immunofluorescence | Combined presence of IgG and complement granular-linear deposition within the epidermal intercellular spaces and along the basement-membrane zone |
| Indirect immunofluorescence | Presence of circulating antibodies directed against the intercellular zone of stratified squamous or transitional epithelia |
| Immunoprecipitation | Complex of proteins, including desmoplakin 1 (250 kDa), bullous pemphigoid antigen (230 kDa), envoplakin (210 kDa), desmoplakin 2 (210 kDa), periplakin (190 kDa) and α-2-macroglobulin-like-1 (170 kDa) |
| Criterion | Details |
|---|---|
| 1. Symmetric proximal muscle weakness | Dysphagia and/or diaphragmatic weakness can be present |
| 2. Increase of skeletal muscle enzymes | High level of skeletal muscle enzymes, such as creatine kinase, aspartate transaminase, alanine transaminase, andlactate dehydrogenase |
| 3. Alteration at EMG | Several abnormalities can be detected, including positive sharp waves, and repetitive high-frequency discharges |
| 4. Alterations showed in muscle biopsy | Several pattern can be showed, including loss of capillaries, deposits of C5b–C9 on the capillaries, and endothelial microtubular inclusions |
| 5. Typical skin rash | Heliotrope rash or Gottron’s sign |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didona, D.; Fania, L.; Didona, B.; Eming, R.; Hertl, M.; Di Zenzo, G. Paraneoplastic Dermatoses: A Brief General Review and an Extensive Analysis of Paraneoplastic Pemphigus and Paraneoplastic Dermatomyositis. Int. J. Mol. Sci. 2020, 21, 2178. https://doi.org/10.3390/ijms21062178
Didona D, Fania L, Didona B, Eming R, Hertl M, Di Zenzo G. Paraneoplastic Dermatoses: A Brief General Review and an Extensive Analysis of Paraneoplastic Pemphigus and Paraneoplastic Dermatomyositis. International Journal of Molecular Sciences. 2020; 21(6):2178. https://doi.org/10.3390/ijms21062178
Chicago/Turabian StyleDidona, Dario, Luca Fania, Biagio Didona, Rüdiger Eming, Michael Hertl, and Giovanni Di Zenzo. 2020. "Paraneoplastic Dermatoses: A Brief General Review and an Extensive Analysis of Paraneoplastic Pemphigus and Paraneoplastic Dermatomyositis" International Journal of Molecular Sciences 21, no. 6: 2178. https://doi.org/10.3390/ijms21062178
APA StyleDidona, D., Fania, L., Didona, B., Eming, R., Hertl, M., & Di Zenzo, G. (2020). Paraneoplastic Dermatoses: A Brief General Review and an Extensive Analysis of Paraneoplastic Pemphigus and Paraneoplastic Dermatomyositis. International Journal of Molecular Sciences, 21(6), 2178. https://doi.org/10.3390/ijms21062178





