Genetic Transformation of Tribonema minus, a Eukaryotic Filamentous Oleaginous Yellow-Green Alga
Abstract
1. Introduction
2. Results
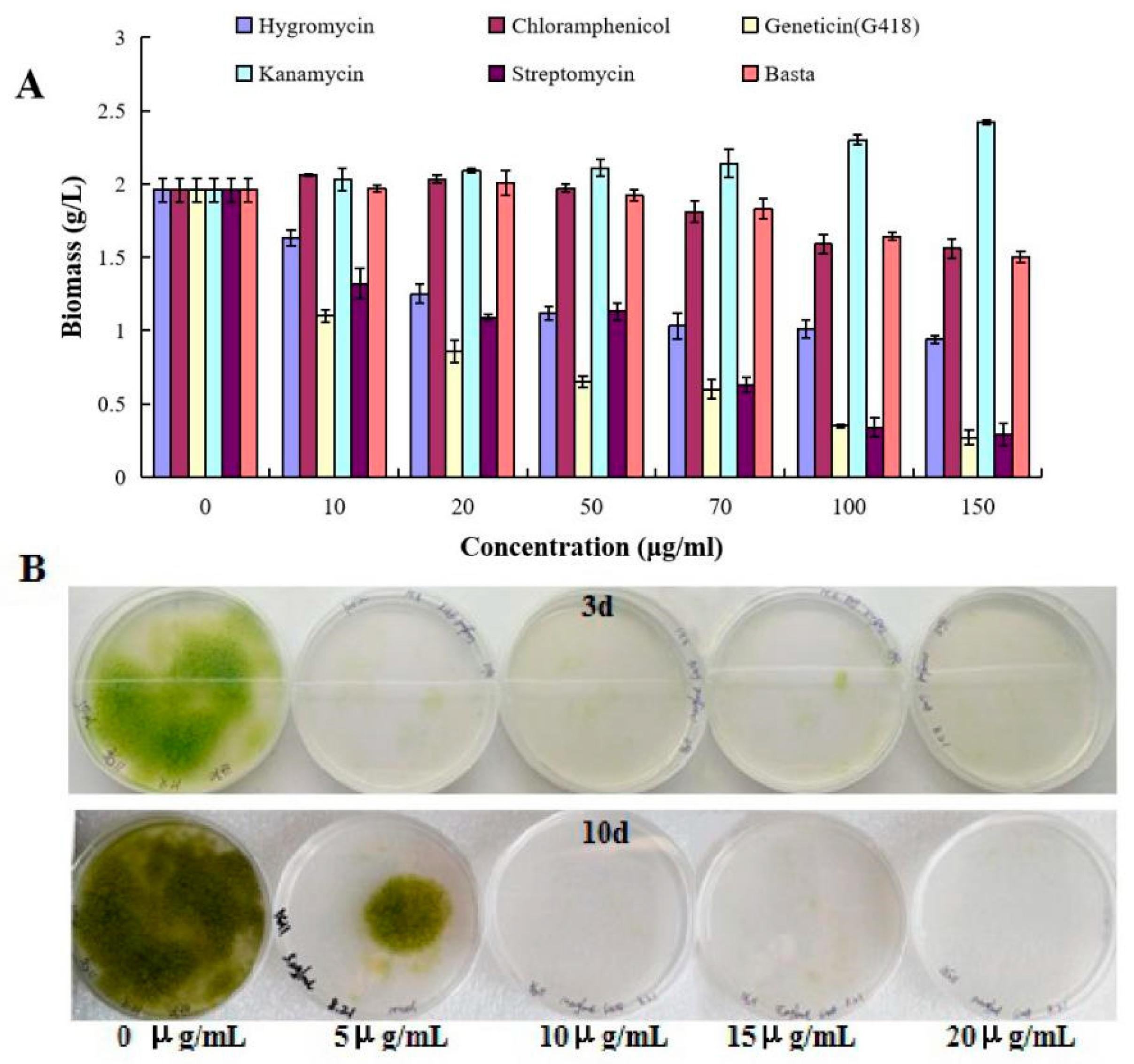
2.1. Growth Characterization of T. minus in Response to Herbicides and Antibiotics
2.2. Construction of Plasmids pSimple-tub-eGFP and pEASY-tub- nptⅡ
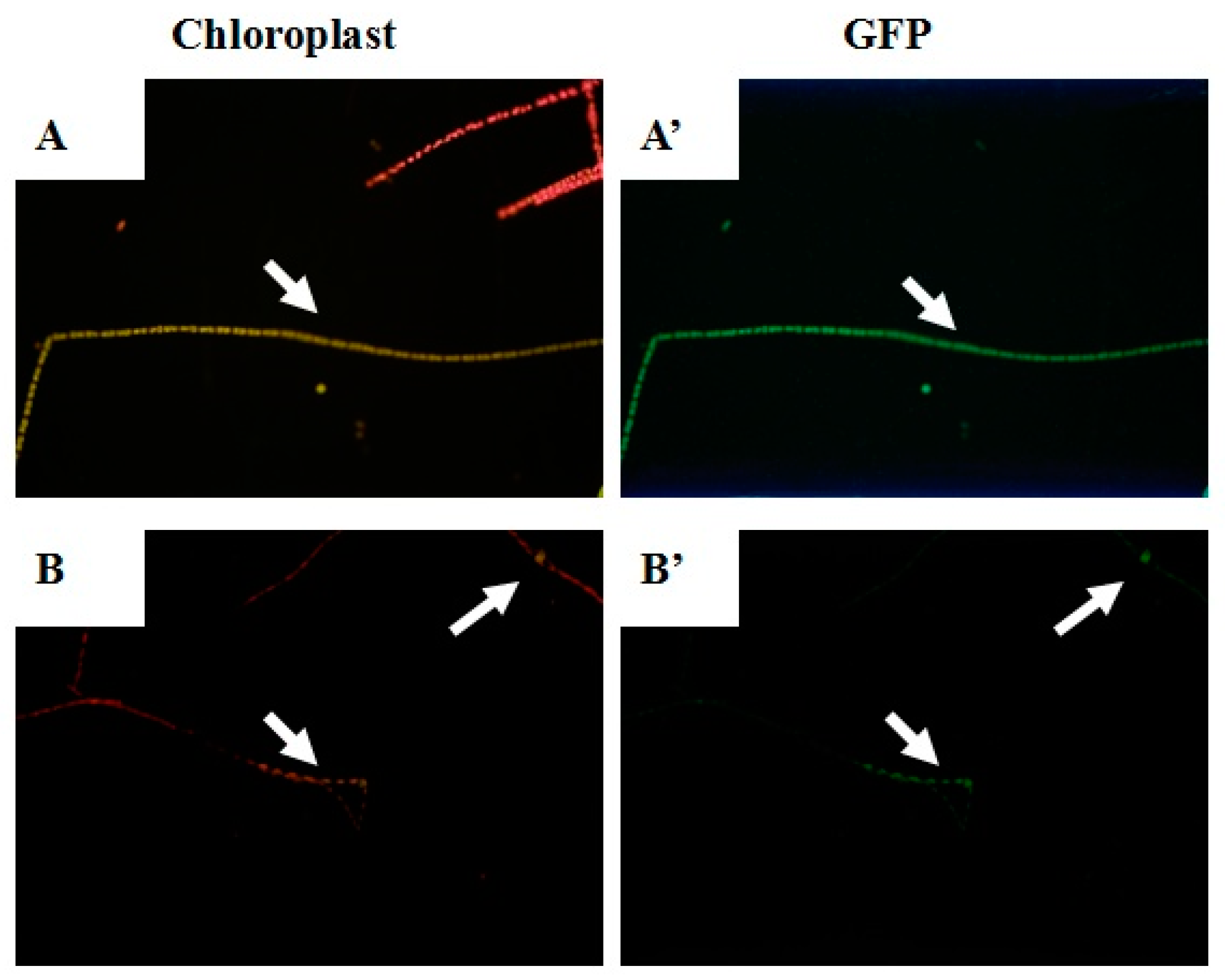
2.3. pEASY-Tub-nptⅡ Transformant Selection
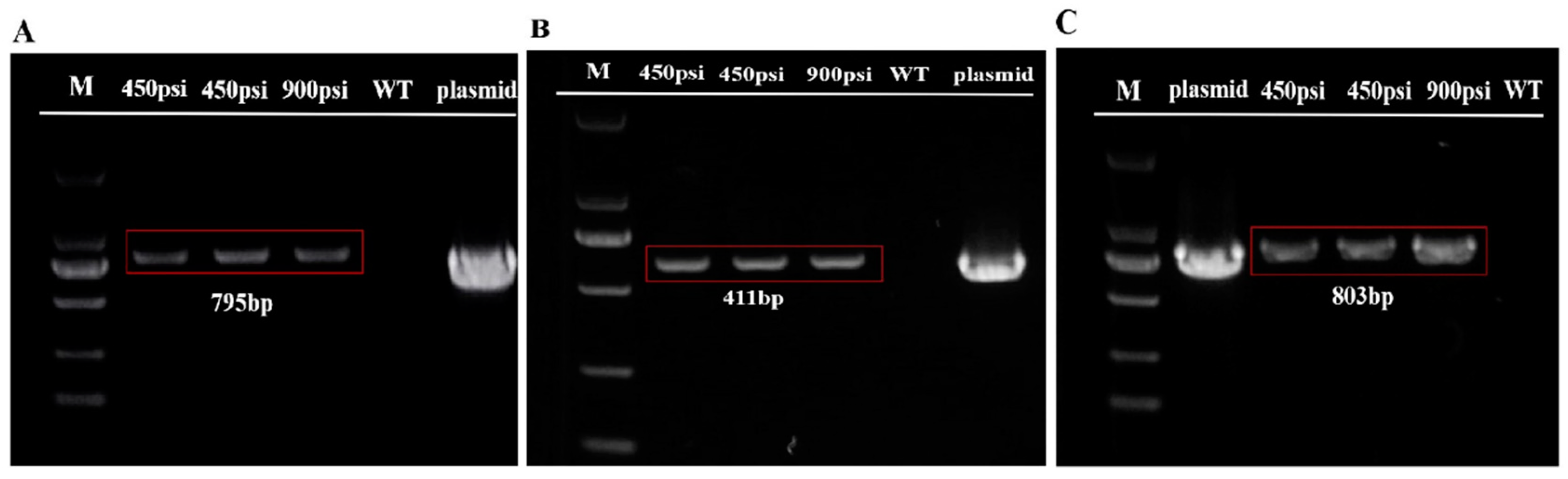
2.4. Confirmation of Transformation into the T. minus Genome
2.5. Quantitative Analysis of the nptⅡ Gene in the Transformants
3. Discussion
4. Materials and Methods
4.1. Strain and Growth Conditions
4.2. Sensitivity to Herbicide and Antibiotics
4.3. Endogenous Promoter and Terminator Clone
4.4. Construction of Plasmids for Transformation
4.5. Particle Bombardment
4.6. Fluorescence Detection of eGFP Gene Expression
4.7. Preparation of Genomic DNA and PCR Analysis
4.8. Continuous Cultivation of Transformants
4.9. Quantitative Real-Time PCR Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglcerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Irina, A.G.; John, L.H. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar]
- Liang, J.B.; Wen, F.; Liu, J.H. Transcriptomic and lipidomic analysis of an EPA-containing Nannochloropsis sp. PJ 12 in response to nitrogen deprivation. Sci. Rep. 2019, 9, 4540. [Google Scholar] [CrossRef]
- William, P.L.I.B.; Laurens, L.M.L. Microalgae as biodiesel and biomass feedstocks: Review and analysis of the biochemistry, energetics and economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar] [CrossRef]
- Adrme-Vega, T.C.; Lim, D.K.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A proming approach towards sustainable omega-3 fatty acid production. Microb. Cell Facotries 2012, 11, 96. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L.L.; Chen, L.; Guo, F.J.; Liu, T.Z. Integration process of biodiesel production from filamentous oleaginous microalgae Tribonema minus. Bioresour. Technol. 2013, 142, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, W.J.; Shao, H.M.; Liu, T.Z. A comparative analysis of biomass and lipid content in five Tribonema sp. strains at autotrophic, heterotrophic and mixotrophic cultivation. Algal Res. 2017, 24, 284–289. [Google Scholar] [CrossRef]
- Zhou, W.J.; Wang, H.; Chen, L.; Cheng, W.T.; Liu, T.Z. Heterotrophy of filamentous oleaginous microalgae Tribonema minus for potential production of lipid and palmitoleic acid. Bioresour. Technol. 2017, 239, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Zhou, W.J.; Noppol, L.; Liu, T.Z. Mechanism and enhancement of lipid accumulation in filamentous oleaginous microalgae Tribonema minus under heterotrophic condition. Biotechnol. Biofuels 2018, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Couso, A.; Vila, M.; Rodriguez, H.; Vargas, M.A.; Leon, R. Overexpression of an exogenous phytoene synthase gene in the unicellular alga Chlamydomonas reinhardtii leads to an increase in the content of carotenoids. Biotechnol. Prog. 2011, 27, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Mussgnug, J.H. Genetic tools and techniques for Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2015, 99, 5407–5418. [Google Scholar] [CrossRef] [PubMed]
- Ajjwai, I.; Verruto, J.; Aqui, M.; Soriage, L.B.; Coppersmith, J.; Kwok, K. Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat. Biotechnol. 2017, 35, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Vieler, A.; Wu, G.X.; Tsai, C.H. Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genet. 2012, 8, e1003064. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Jeon, J.; Choi, J.; Kim, S.R. Rapid and efficient genetic transformation of the green microalga Chlorella vulgaris. J. Appl. Phycol. 2018, 30, 1735–1745. [Google Scholar] [CrossRef]
- Radakovits, R.; Eduafo, P.M.; Posewitz, M.C. Genetic engineering of fatty acid chain length in Phaeodactylum tricornutum. Metab. Eng. 2011, 13, 89–95. [Google Scholar] [CrossRef]
- Perin, G.; Bellan, A.; Segalla, A.; Meneghesso, A.; Alboresi, A.; Morosinotto, T. Generation of random mutants to improve light-use efficiency of Nannochloropsis gaditata cultures for biofuels production. Biotechnol. Biofuels 2015, 8, 161. [Google Scholar] [CrossRef]
- Wei, L.; Xin, Y.; Wang, Q.T.; Yang, J.H.; Hu, H.; Xu, J. RNAi-based targeted gene knockdown in the model oleaginous microalgae Nannochloropsis oceanica. Plant J. 2017, 89, 1236–1250. [Google Scholar] [CrossRef]
- Berrios, H.; Zapata, M.; Rivas, M. A method for genetic transformation of Botryococcus braunii using a cellulase pretreatment. J. Appl. Phycol. 2016, 28, 201–208. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhang, Y.; Chen, X.; Zhang, P.; Ma, S. Development of a new method for genetic transformation of the green alga Chlorella ellipsoidea. Mol. Biotechnol. 2013, 54, 211–219. [Google Scholar] [CrossRef]
- Hamilton, M.L.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Metabolic engineering of Phaeodactylum ricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. MET Eng. 2014, 22, 3–9. [Google Scholar] [CrossRef]
- Zaslavskaia, L.A.; Lippmeier, J.C.; Kroth, P.G.; Grossman, A.R.; Apt, K.E. Transformation of the diatom Phaeodactylum tricornutum (Bacillaripphyceae) with a variety of selectable marker and reporter genes. J. Phycol. 2000, 36, 379–386. [Google Scholar] [CrossRef]
- Anami, S.; Njuguna, E.; Coussens, G.; Aesaert, S.; van Lijsebettens, M. Higher plant transformation: Principles and molecular tools. Int. J. Dev. Biol. 2013, 57, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Radakovits, R.; Jinkerson, R.; Darzins, A.; Posewitz, M. Genetic engineering of algae for enhanced biofule prodution. Eukayot. Cell 2010, 9, 486. [Google Scholar] [CrossRef] [PubMed]
- Judith, N.; Alic, M.; Lenka, B.; Jason, D.; Atle, M.B.; Roman, S. Tools for biotechnological studies of the freshwater alga Nannochloropsis limnetica: Antibiotic resistance and protoplast production. J. Appl. Phycol. 2017, 29, 853–863. [Google Scholar]
- Yuan, G.H.; Xu, X.Y.; Zhang, W.; Zhang, W.L.; Cui, Y.L.; Qin, S.; Liu, T.Z. Biolistic transformation of Haemotococcus pluvialis with constructs based on the flanking sequences of its endogenous alpha tubulin gene. Front. Microbiol. 2019, 10, 1749. [Google Scholar] [CrossRef]
- Ivan, K.K.; Katya, N.V.; Krasimira, D.D. Species composition and distribution of genus Tribonema (Xanthophyceae) in Bulgaria. Phytol. Balc. 2011, 17, 273–277. [Google Scholar]
- Zhou, W.J.; Wang, H.; Zheng, L.; Cheng, W.T.; Gao, L.L.; Liu, T.Z. Comparison of lipid and palmitoleic acid induction of Tribonema minus under heterotrophic and phototrophic regimes by using high-density fermented seeds. Int. J. Mol. Sci. 2019, 20, 4356. [Google Scholar] [CrossRef]
- Teng, C.Y.; Qin, S.; Liu, J.; Yu, D.Z.; Liang, C.W.; Tseng, C.K. Transient expression of lacZ in bombarded unicellular green alga Haematococcis pluvialis. J. Appl. Phycol. 2002, 14, 497–500. [Google Scholar] [CrossRef]
- Kathiresan, S.; Sarada, R. Towards genetic improvement of commercially imporant microalga Haematococcus pluvialis for biotech applications. J. Appl. Phycol. 2009, 21, 553–558. [Google Scholar] [CrossRef]
- Sharon-Gojman, R.; Maimon, E.; Leu, S.; Zarka, A.; Boussiba, S. Advanced methods for genetic engineering of Haeatococcus pluvialis (Chlorophyceae, Volvocales). Algal Res. 2015, 10, 8–15. [Google Scholar] [CrossRef]
- Bandziulis, R.J.; Rosenbaum, J.L. Novel control elements in the alpha-1 tubulin gene promoter from Chlamydomonas reinhardii. Mol. Gen. Genet. 1988, 214, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Elghavi, Z.; Ruf, S.; Bock, R. Biolistic co-transformation of the nuclear and plastid genomes. Plant J. 2011, 67, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Iammura, S.; Hagiwara, D.; Suzuki, F.; Kurano, N.; Harayama, S. Genetic transformation of Pseudochoricystis ellipsoidea, an aliphatic hydrocarbon-producing green alga. J. Gen. Appl. Microbiol. 2012, 58, 1–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, S.L.; Zhao, X.Q.; Tang, Y.; Wan, C.; Alam, M.A.; Ho, S.H.; Bai, F.W.; Chang, J.S. Establishment of an efficient genetic transformation system in Scenedesmus obliquus. J. Biotechnol. 2013, 163, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zorin, B.; Grundman, O.; Khozin-Goldberg, I.; Leu, S.; Shapira, M.; Kaye, Y.; Tourasse, N.; Vallon, O.; Boussiba, S. Development of a nuclear transformation system for oleaginous green alga Lobosphaera (Prietochloris) incisa and genetic complementation of a mutant strains, deficient in arachldonic acid biosynthesis. PLoS ONE 2014, 9, e105223. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematocottus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Li, H.X.; Ding, D.Q.; Cao, Y.S.; Yu, B.; Guo, L.; Liu, X.H. Partially overlapping primer-based PCR for genome walking. PLoS ONE 2015, 10, e0120139. [Google Scholar] [CrossRef]
- Siemering, K.R.; Golbik, R.; Sever, R.; Haseloff, J. Mutations that suppress the thermosensitivity of green fluorescent protein. Curr. Biol. 1996, 6, 1653–1663. [Google Scholar] [CrossRef]



| Primers | Sequence (5’-3’) |
|---|---|
| tub-F | ATGCGTGAATGCATCAGCATCC |
| tub-R | CGGCACACGTCGTACAGGGCC |
| Ptub-sp1 | AGTAGAGCTCCCAGCAGGCATT |
| Ptub-sp2 | TTTCAGGGCATCGCTGCTTCAGTA |
| Ptub-sp3 | AGCGGAACTCATGCCGATCAGGTA |
| Ttub-sp1 | TAACCCCACTCCTCCTCGTCGCTTT |
| Ttub-sp2 | ATCAACTACCAGCCTCCCACCGT |
| Ttub-sp3 | ACCACAAGTTCGACCTCATGTACGCC |
| Primers | Sequence (5’–3’) |
|---|---|
| Ptub-F | CAGTGCAGTCATCTGCTGCGGGC |
| Ptub(nptⅡ)-R | GCAATCCATCTTGTTCAATCATGTTGACTGGAGAAGTGGTCCTGC |
| nptⅡ-F | GCAGGACCACTTCTCCAGTCAACATGATTGAACAAGATGGATTGC |
| nptⅡ-R | GTGCACACGTCAACAGTGCGCTCAGAAGAACTCGTCAAGAAGG |
| Ttub(nptⅡ)-F | CCTTCTTGACGAGTTCTTCTGAGCGCATGTTGACGTGTGCAC |
| Ttub-R | GGGCGATTGGGCCCTGTAGATGC |
| Primers | Sequence (5’–3’) |
|---|---|
| Ptub-F | GAATTCTCATGGCGCGACGCTGT |
| Ptub(eGFP)-R | GCTCCTCGCCCTTGCTCACCATGGTGGCCTTTAAGGGTGCTGTTTAAACTGC |
| eGFP-F | GCAGTTTAAACAGCACCCTTAAAGGCCACCATGGTGAGCAAGGGCGAGGAGC |
| eGFP-R | GTGGGCCTGAGCGTCATGTTTACTTGTACAGCTCGTCCATG |
| Ttub(eGFP)-F | CATGGACGAGCTGTACAAGTAAACATGACGCTCAGGCCCAC |
| Ttub-R | GGTTCATCTGCTGACGAGTGCT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, H.; Yang, R.; Wang, L.; Yang, G.; Liu, T. Genetic Transformation of Tribonema minus, a Eukaryotic Filamentous Oleaginous Yellow-Green Alga. Int. J. Mol. Sci. 2020, 21, 2106. https://doi.org/10.3390/ijms21062106
Zhang Y, Wang H, Yang R, Wang L, Yang G, Liu T. Genetic Transformation of Tribonema minus, a Eukaryotic Filamentous Oleaginous Yellow-Green Alga. International Journal of Molecular Sciences. 2020; 21(6):2106. https://doi.org/10.3390/ijms21062106
Chicago/Turabian StyleZhang, Yan, Hui Wang, Ruigang Yang, Lihao Wang, Guanpin Yang, and Tianzhong Liu. 2020. "Genetic Transformation of Tribonema minus, a Eukaryotic Filamentous Oleaginous Yellow-Green Alga" International Journal of Molecular Sciences 21, no. 6: 2106. https://doi.org/10.3390/ijms21062106
APA StyleZhang, Y., Wang, H., Yang, R., Wang, L., Yang, G., & Liu, T. (2020). Genetic Transformation of Tribonema minus, a Eukaryotic Filamentous Oleaginous Yellow-Green Alga. International Journal of Molecular Sciences, 21(6), 2106. https://doi.org/10.3390/ijms21062106




