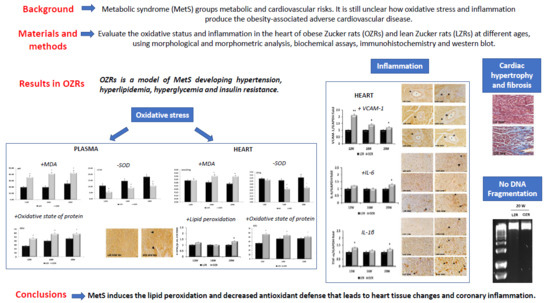
Cardiovascular Changes Related to Metabolic Syndrome: Evidence in Obese Zucker Rats
Abstract
1. Introduction
2. Results
2.1. Physiological and Blood Parameters
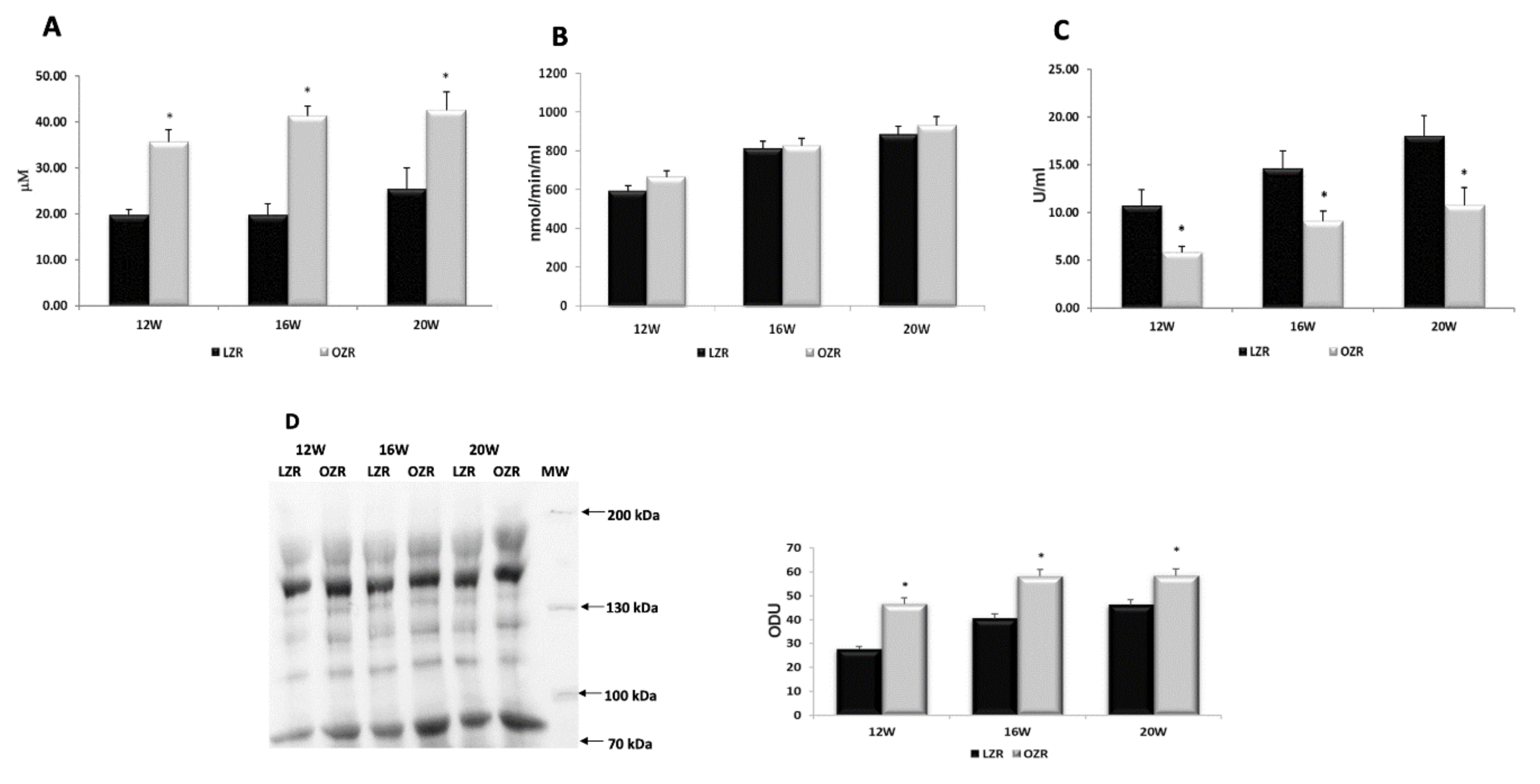
2.2. Oxidative Stress
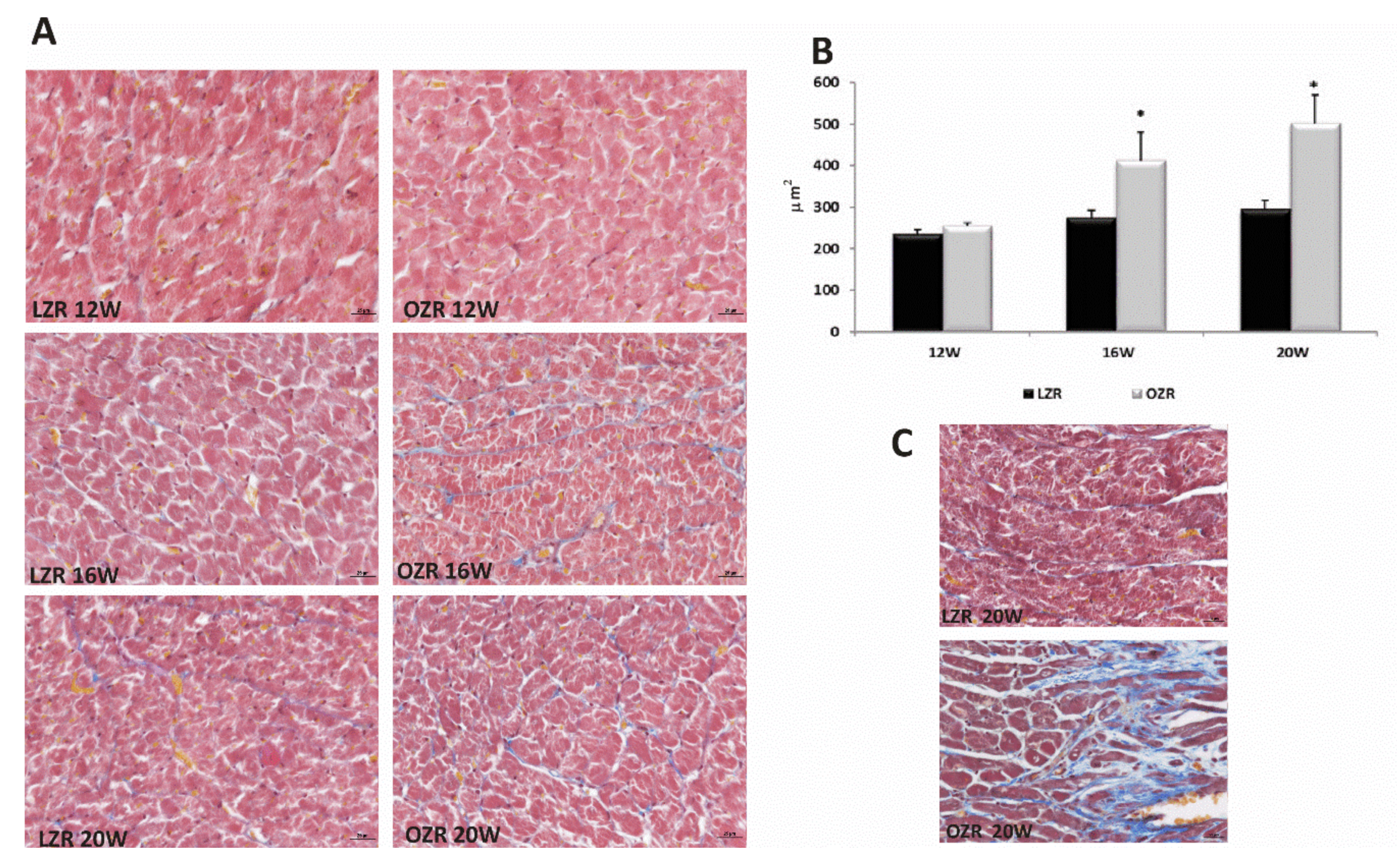
2.3. Heart Morphology
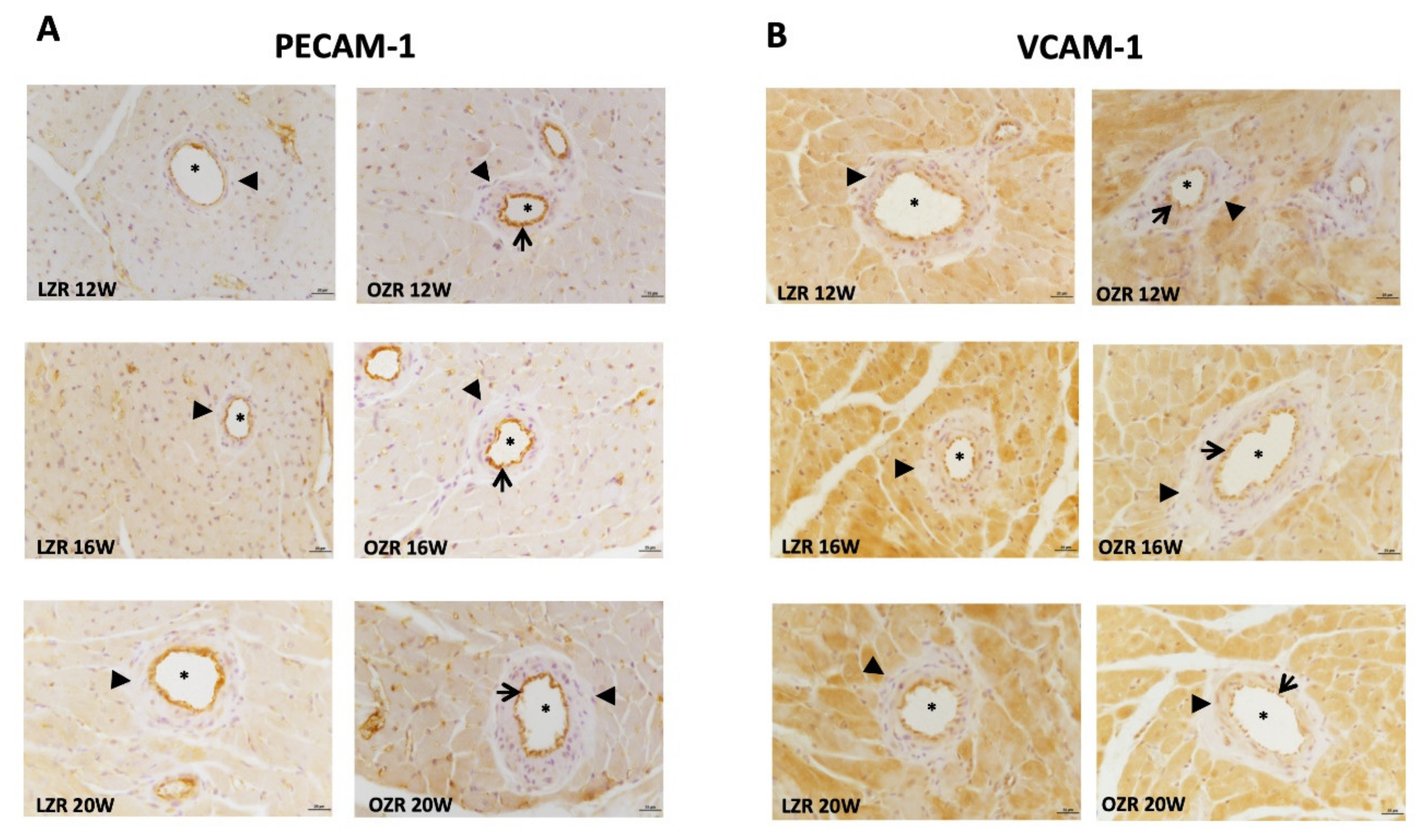
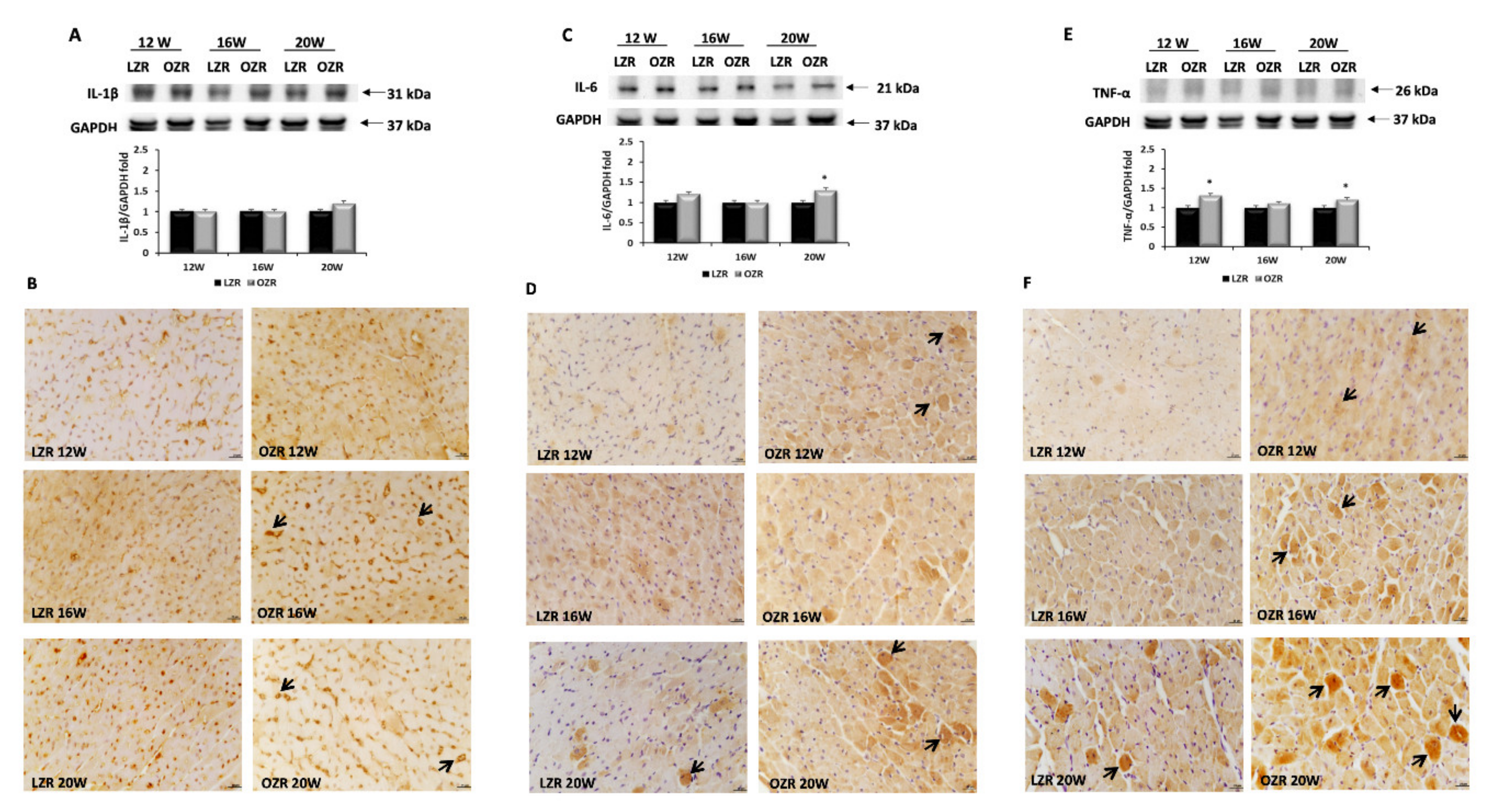
2.4. Inflammation
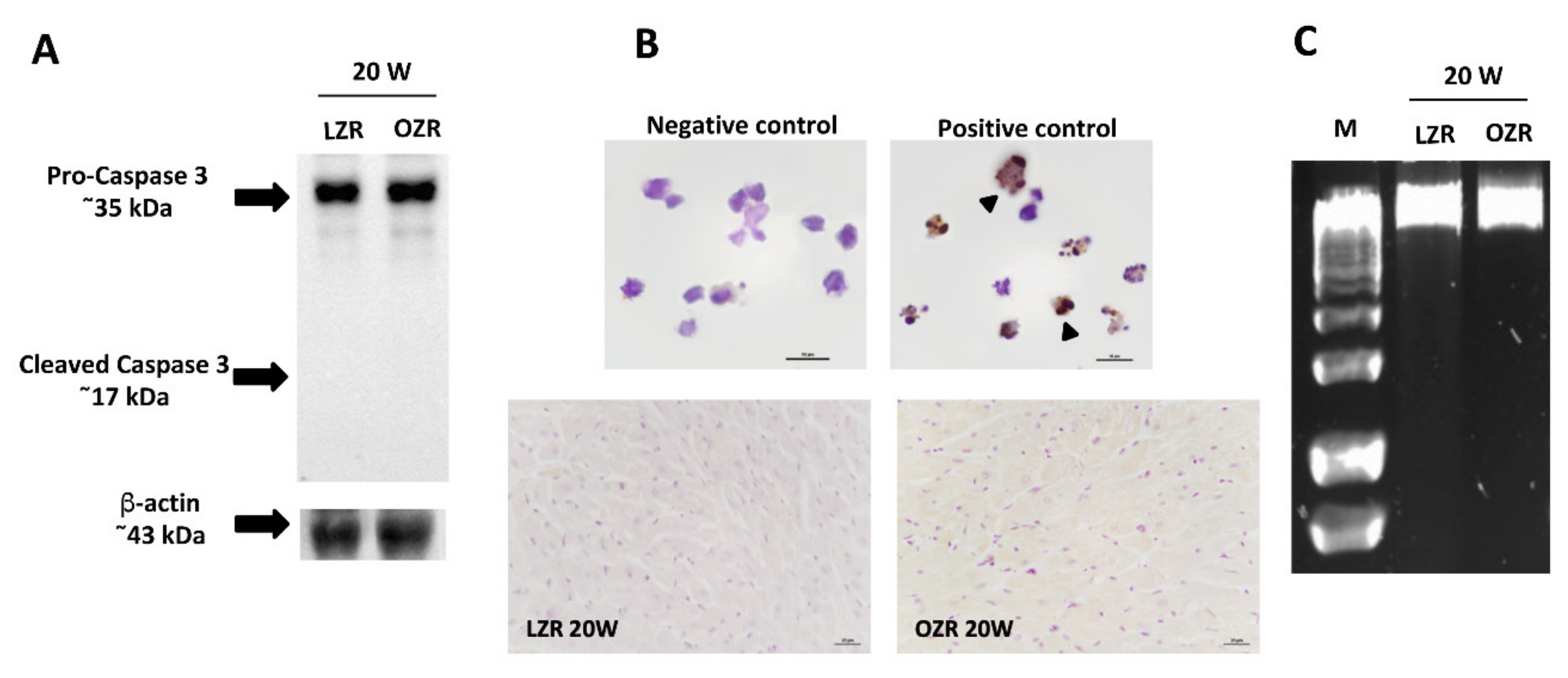
2.5. Cardiomyocytes Death Evaluation
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Morphological Aspects
4.3. Western Blot
4.4. Immunohistochemistry
4.5. TUNELAssay
4.6. DNA Ladder Evaluation
4.7. Biomarkers of Oxidative Stress
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| 8-oxo-dG | 8-oxo-2′-deoxyguanosine |
| CVD | cardiovascular diseases |
| GPx | glutathione peroxidase |
| ICAM-1 | intercellular cell adhesion molecule-1 |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| IR | insulin resistance |
| LZRs | lean Zucker rats |
| MDA | malondialdehyde |
| MetS | metabolic syndrome |
| OZRs | obese Zucker rats |
| PECAM-1 | platelet cell adhesion molecule-1 |
| SOD | superoxide dismutase |
| TBARSTdT | thiobarbituric acid reactive substancesterminal deoxynucleotidyl transferase |
| TNF-α | tumor necrosis factor-α |
| VCAM-1 | vascular cell adhesion molecule-1 |
References
- Sikaris, K.A. The clinical biochemistry of obesity. Clin. Biochem. Rev. 2004, 25, 165–181. [Google Scholar] [PubMed]
- Malnick, S.D.; Knobler, H. The medical complications of obesity. QJM 2006, 99, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Tomassoni, D.; Moruzzi, M.; Traini, E.; Amenta, F.; Tayebati, S.K. Obesity and Metabolic Syndrome Affect the Cholinergic Transmission and Cognitive Functions. CNS Neurol. Disord. Drug Targets 2017, 16, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, N.S.; Osborne, M.T.; Gupta, A.; Tavakkoli, A.; Bravo, P.E.; Vita, T.; Bibbo, C.F.; Hainer, J.; Dorbala, S.; Blankstein, R.; et al. Coronary Microvascular Dysfunction and Cardiovascular Risk in Obese Patients. J. Am. Coll. Cardiol. 2018, 72, 707–717. [Google Scholar] [CrossRef]
- Vazzana, N.; Santilli, F.; Sestili, S.; Cuccurullo, C.; Davi, G. Determinants of increased cardiovascular disease in obesity and metabolic syndrome. Curr. Med. Chem. 2011, 18, 5267–5280. [Google Scholar] [CrossRef]
- Wang, Z.; Nakayama, T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010, 2010, 535918. [Google Scholar] [CrossRef] [PubMed]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Barbaro, G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm. Metab. Res. 2008, 40, 442–445. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Alicka, M.; Marycz, K. The Effect of Chronic Inflammation and Oxidative and Endoplasmic Reticulum Stress in the Course of Metabolic Syndrome and Its Therapy. Stem Cells Int. 2018, 2018, 4274361. [Google Scholar] [CrossRef] [PubMed]
- Adrielle Lima Vieira, R.; Nascimento de Freitas, R.; Volp, A.C. Adhesion molecules and chemokines; relation to anthropometric, body composition, biochemical and dietary variables. Nutr. Hosp. 2014, 30, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative stress and inflammation interactions in human obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Hensley, K.; Robinson, K.A.; Gabbita, S.P.; Salsman, S.; Floyd, R.A. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Chandel, N.S.; Schumacker, P.T.; Arch, R.H. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J. Biol. Chem. 2001, 276, 42728–42736. [Google Scholar] [CrossRef]
- Hajjar, D.P.; Gotto, A.M., Jr. Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. Am. J. Pathol. 2013, 182, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, E.; Baldassari, F.; Bononi, A.; Wieckowski, M.R.; Pinton, P. Oxidative stress in cardiovascular diseases and obesity: Role of p66Shc and protein kinase C. Oxid. Med. Cell. Longev. 2013, 2013, 564961. [Google Scholar] [CrossRef]
- Lavrovsky, Y.; Chatterjee, B.; Clark, R.A.; Roy, A.K. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp. Gerontol. 2000, 35, 521–532. [Google Scholar] [CrossRef]
- Otani, H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid. Redox Signal. 2011, 15, 1911–1926. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; Guagnano, M.T.; Vazzana, N.; La Barba, S.; Davi, G. Oxidative stress drivers and modulators in obesity and cardiovascular disease: From biomarkers to therapeutic approach. Curr. Med. Chem. 2015, 22, 582–595. [Google Scholar] [CrossRef]
- Murase, T.; Hattori, T.; Ohtake, M.; Abe, M.; Amakusa, Y.; Takatsu, M.; Murohara, T.; Nagata, K. Cardiac remodeling and diastolic dysfunction in DahlS.Z-Lepr(fa)/Lepr(fa) rats: A new animal model of metabolic syndrome. Hypertens Res. 2012, 35, 186–193. [Google Scholar] [CrossRef]
- Riojas-Hernández, A.; Bernal-Ramírez, J.; Rodríguez-Mier, D.; Morales-Marroquín, F.E.; Domínguez-Barragán, E.M.; Borja-Villa, C.; Rivera-Álvarez, I.; García-Rivas, G.; Altamirano, J.; García, N. Enhanced oxidative stress sensitizes the mitochondrial permeability transition pore to opening in heart from Zucker Fa/fa rats with type 2 diabetes. Life Sci. 2015, 141, 32–43. [Google Scholar] [CrossRef]
- Ferenczyova, K.; Kalocayova, B.; Kindernay, L.; Jelemensky, M.; Balis, P.; Berenyiova, A.; Zemancikova, A.; Farkasova, V.; Sykora, M.; Tothova, L.; et al. Quercetin Exerts Age-Dependent Beneficial Effects on Blood Pressure and Vascular Function, But Is Inefficient in Preventing Myocardial Ischemia-Reperfusion Injury in Zucker Diabetic Fatty Rats. Molecules 2020, 25, E187. [Google Scholar] [CrossRef]
- Aleixandre de Artiñano, A.; Miguel Castro, M. Experimental rat models to study the metabolic syndrome. Br. J. Nutr. 2009, 102, 1246–1253. [Google Scholar] [CrossRef]
- Tomassoni, D.; Nwankwo, I.E.; Gabrielli, M.G.; Bhatt, S.; Muhammad, A.B.; Lokhandwala, M.F.; Tayebati, S.K.; Amenta, F. Astrogliosis in the brain of obese Zucker rat: A model of metabolic syndrome. Neurosci. Lett. 2013, 543, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I. The accumulation of extracellular matrix proteins and collagen deposition were not found in subendocardial region at the level of the apex in the younger OZRs. School of Medicinal and Health Products Sciences, University of Camerino: Camerino, Italy, 2003; Unpublished work. [Google Scholar]
- Mittwede, P.N.; Clemmer, J.S.; Bergin, P.F.; Xiang, L. Obesity and critical illness: Insights from animal models. Shock 2016, 45, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ebenezer, P.J.; Mariappan, N.; Elks, C.M.; Haque, M.; Francis, J. Diet-induced renal changes in Zucker rats are ameliorated by the superoxide dismutase mimetic TEMPOL. Obesity 2009, 17, 1994–2002. [Google Scholar] [CrossRef]
- Hansel, B.; Giral, P.; Nobecourt, E.; Chantepie, S.; Bruckert, E.; Chapman, M.J.; Kontush, A. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab. 2004, 89, 4963–4971. [Google Scholar] [CrossRef] [PubMed]
- Fortuño, A.; San José, G.; Moreno, M.U.; Beloqui, O.; Díez, J.; Zalba, G. Phagocytic NADPH oxidase overactivity underlies oxidative stress in metabolic syndrome. Diabetes 2006, 55, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Wilson, P.W.; Grundy, S.M. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation 2004, 109, 2818–2825. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Banday, A.A.; Marwaha, A.; Tallam, L.S.; Lokhandwala, M.F. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes 2005, 54, 2219–2226. [Google Scholar] [CrossRef]
- Russo, I.; Del Mese, P.; Doronzo, G.; Mattiello, L.; Viretto, M.; Bosia, A.; Anfossi, G.; Trovati, M. Resistance to the nitric oxide/cyclic guanosine 5′-monophosphate/protein kinase G pathway in vascular smooth muscle cells from the obese Zucker rat, a classical animal model of insulin resistance: Role of oxidative stress. Endocrinology 2008, 149, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Dhalla, A.K.; Singal, P.K. Antioxidant changes in hypertrophied and failing guinea pig hearts. Am. J. Physiol. 1994, 266, H1280–H1285. [Google Scholar] [CrossRef]
- Hill, M.F.; Singal, P.K. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am. J. Pathol. 1996, 148, 291–300. [Google Scholar]
- Li, R.K.; Sole, M.J.; Mickle, D.A.G.; Schimmer, J.; Goldstein, D. Vitamin E and oxidative stress in the heart of the cardiomyopathic syrian hamster. Free Radic. Biol. Med. 1998, 24, 252–258. [Google Scholar] [CrossRef]
- Freeman, L.M.; Brown, D.J.; Rush, J.E. Assessment of degree of oxidative stress and antioxidant concentrations in dogs with idiopathic dilated cardiomyopathy. J. Am. Vet. Med. Assoc. 1999, 215, 644–646. [Google Scholar] [PubMed]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J.; et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N. Engl. J. Med. 2003, 349, 1605–1613. [Google Scholar] [CrossRef]
- Woodiwiss, A.J.; Norton, G.R. Obesity and left ventricular hypertrophy: The hypertension connection. Curr. Hypertens Rep. 2015, 17, 539. [Google Scholar] [CrossRef]
- Maulik, S.K.; Kumar, S. Oxidative stress and cardiac hypertrophy: A review. Toxicol. Mech. Methods. 2012, 22, 359–366. [Google Scholar] [CrossRef]
- Bender, S.B.; DeMarco, V.G.; Padilla, J.; Jenkins, N.T.; Habibi, J.; Garro, M.; Pulakat, L.; Aroor, A.R.; Jaffe, I.Z.; Sowers, J.R. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension 2015, 65, 1082–1088. [Google Scholar] [CrossRef]
- Deshpande, M.; Mali, V.R.; Pan, G.; Xu, J.; Yang, X.P.; Thandavarayan, R.A.; Palaniyandi, S.S. Increased 4-hydroxy-2-nonenal-induced proteasome dysfunction is correlated with cardiac damage in streptozotocin-injected rats with isoproterenol infusion. Cell Biochem. Funct. 2016, 34, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Taddei, S.; Ghiadoni, L.; Virdis, A.; Versari, D.; Salvetti, A. Mechanisms of endothelial dysfunction: Clinical significance and preventive non-pharmacological therapeutic strategies. Curr. Pharm. Des. 2003, 9, 2385–2402. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Giugliano, D. The metabolic syndrome and inflammation: Association or causation? Nutr. Metab. Cardiovasc. Dis. 2004, 14, 228–232. [Google Scholar] [CrossRef]
- Uosaki, H.; Fukushima, H.; Takeuchi, A.; Matsuoka, S.; Nakatsuji, N.; Yamanaka, S.; Yamashita, J.K. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS ONE 2011, 6, e23657. [Google Scholar] [CrossRef]
- Tayebati, S.K.; Tomassoni, D.; Di Cesare Mannelli, L.; Amenta, F. Effect of treatment with the antioxidant alpha-lipoic (thioctic) acid on heart and kidney microvasculature in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2016, 38, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rifai, N.; Pfeffer, M.; Sacks, F.; Lepage, S.; Braunwald, E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 2000, 101, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Picchi, A.; Gao, X.; Belmadani, S.; Potter, B.J.; Focardi, M.; Chilian, W.M.; Zhang, C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ. Res. 2006, 99, 69–77. [Google Scholar] [CrossRef]
- Maedler, K.; Dharmadhikari, G.; Schumann, D.M.; Størling, J. Interleukin-targeted therapy for metabolic syndrome and type 2 diabetes. Handb. Exp. Pharmacol. 2011, 203, 257–278. [Google Scholar] [CrossRef]
- Zanni, M.V.; Stanley, T.L.; Makimura, H.; Chen, C.Y.; Grinspoon, S.K. Effects of TNF-alpha antagonism on E-selectin in obese subjects with metabolic dysregulation. Clin. Endocrinol. 2010, 73, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Porres, J.M.; Constantino, J.; Kapravelou, G.; Lopez-Chaves, C.; Galisteo, M.; Aranda, P.; López-Jurado, M.; Martínez, R. The combined treatment with lentil protein hydrolysate and a mixed training protocol is an efficient lifestyle intervention to manage cardiovascular and renal alterations in obese Zucker rats. Eur. J. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Francisqueti, F.V.; Chiaverini, L.C.; Santos, K.C.; Minatel, I.O.; Ronchi, C.B.; Ferron, A.J.; Ferreira, A.L.; Corrêa, C.R. The role of oxidative stress on the pathophysiology of metabolic syndrome. Rev. Assoc. Med. Bras. 2017, 63, 85–91. [Google Scholar] [CrossRef]
- Palmieri, V.O.; Grattagliano, I.; Portincasa, P.; Palasciano, G. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J. Nutr. 2006, 136, 3022–3026. [Google Scholar] [CrossRef]
- Armutcu, F.; Ataymen, M.; Atmaca, H.; Gurel, A. Oxidative stress markers, C-reactive protein and heat shock protein 70 levels in subjects with metabolic syndrome. Clin. Chem. Lab. Med. 2008, 46, 785–790. [Google Scholar] [CrossRef]
- Després, J.P.; Lemieux, I.; Bergeron, J.; Pibarot, P.; Mathieu, P.; Larose, E.; Rodés-Cabau, J.; Bertrand, O.F.; Poirier, P. Abdominal obesity and the metabolic syndrome: Contribution to global cardiometabolic risk. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1039–1049. [Google Scholar] [CrossRef]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity; Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef]
- Amenta, F.; Peleg, E.; Tomassoni, D.; Sabbatini, M.; Rosenthal, T. Effect of treatment with lercanidipine on heart of Cohen-Rosenthal diabetic hypertensive rats. Hypertension 2003, 41, 1330–1335. [Google Scholar] [CrossRef] [PubMed]


| Parameter | 12 Weeks | 16 Weeks | 20 Weeks | |||
|---|---|---|---|---|---|---|
| LZR | OZR | LZR | OZR | LZR | OZR | |
| Body weight (g) | 284.7 ± 6.1 | 411.3 ± 5.4 * | 356.1 ± 6.2 | 519.8 ± 7.8 * | 377.1 ± 8.7 | 566.2 ± 19.6 * |
| 24 h Food intake (g) | 26.8 ± 0.9 | 36.7 ± 1.5 * | 24.4 ± 0.7 | 31.1 ± 0.7 * | 25.6 ± 1.8 | 33.2 ± 3.1 * |
| Systolic blood pressure (mmHg) | 103.6 ± 9.1 | 120.1 ± 13.7 | 104.3 ± 6.4 | 140.8 ± 5.6 * | 99.8 ± 2.2 | 137.3 ± 4.2 * |
| Weight of the heart (g) | 1.02 ± 0.04 | 1.18 ± 0.02 * | 1.14 ± 0.05 | 1.32 ± 0.02 * | 1.15 ± 0.03 | 1.31 ± 0.04 * |
| Glucose (mmol/l) | 5.28 ± 0.26 | 6.56 ± 0.21 * | 4.47 ± 0.25 | 6.22 ± 0.40 * | 5.22 ± 0.15 | 6.39 ± 0.30 * |
| Insulin (pmol/l) | 5.2 ± 1.7 | 444.8 ± 65.6 * | 13.8 ± 3.4 | 751.7 ± 63.8 * | 12.1 ± 1.7 | 598.3 ± 44.8 * |
| Triglycerides (mmol/l) | 0.53 ± 0.02 | 3.69 ± 0.33 * | 0.54 ± 0.03 | 3.91 ± 0.33 * | 0.65 ± 0.07 | 4.55 ± 0.36 * |
| Total cholesterol (mmol/l) | 2.33 ± 0.05 | 3.76 ± 0.13 * | 2.2 ± 0.10 | 2.58 ± 0.26 * | 2.58 ± 0.08 | 5.09 ± 0.22 * |
| LDL cholesterol (nmol/dl) | 0.28 ± 0.02 | 0.22 ± 0.01 | 0.19 ± 0.01 | 0.24 ± 0.02 * | 0.27 ± 0.02 | 0.40 ± 0.03 * |
| HDL cholesterol (nmol/dl) | 0.82 ± 0.03 | 1.16 ± 0.04 * | 0.65 ± 0.02 | 0.98 ± 0.02 * | 0.78 ± 0.02 | 1.09 ± 0.02 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelli, I.; Tomassoni, D.; Moruzzi, M.; Roy, P.; Cifani, C.; Amenta, F.; Tayebati, S.K. Cardiovascular Changes Related to Metabolic Syndrome: Evidence in Obese Zucker Rats. Int. J. Mol. Sci. 2020, 21, 2035. https://doi.org/10.3390/ijms21062035
Martinelli I, Tomassoni D, Moruzzi M, Roy P, Cifani C, Amenta F, Tayebati SK. Cardiovascular Changes Related to Metabolic Syndrome: Evidence in Obese Zucker Rats. International Journal of Molecular Sciences. 2020; 21(6):2035. https://doi.org/10.3390/ijms21062035
Chicago/Turabian StyleMartinelli, Ilenia, Daniele Tomassoni, Michele Moruzzi, Proshanta Roy, Carlo Cifani, Francesco Amenta, and Seyed Khosrow Tayebati. 2020. "Cardiovascular Changes Related to Metabolic Syndrome: Evidence in Obese Zucker Rats" International Journal of Molecular Sciences 21, no. 6: 2035. https://doi.org/10.3390/ijms21062035
APA StyleMartinelli, I., Tomassoni, D., Moruzzi, M., Roy, P., Cifani, C., Amenta, F., & Tayebati, S. K. (2020). Cardiovascular Changes Related to Metabolic Syndrome: Evidence in Obese Zucker Rats. International Journal of Molecular Sciences, 21(6), 2035. https://doi.org/10.3390/ijms21062035