Injection Molded Capsules for Colon Delivery Combining Time-Controlled and Enzyme-Triggered Approaches
Abstract
1. Introduction
2. Results and Discussion
2.1. Hot-Processability of the Starting Materials
2.2. Performance of Molded Disks
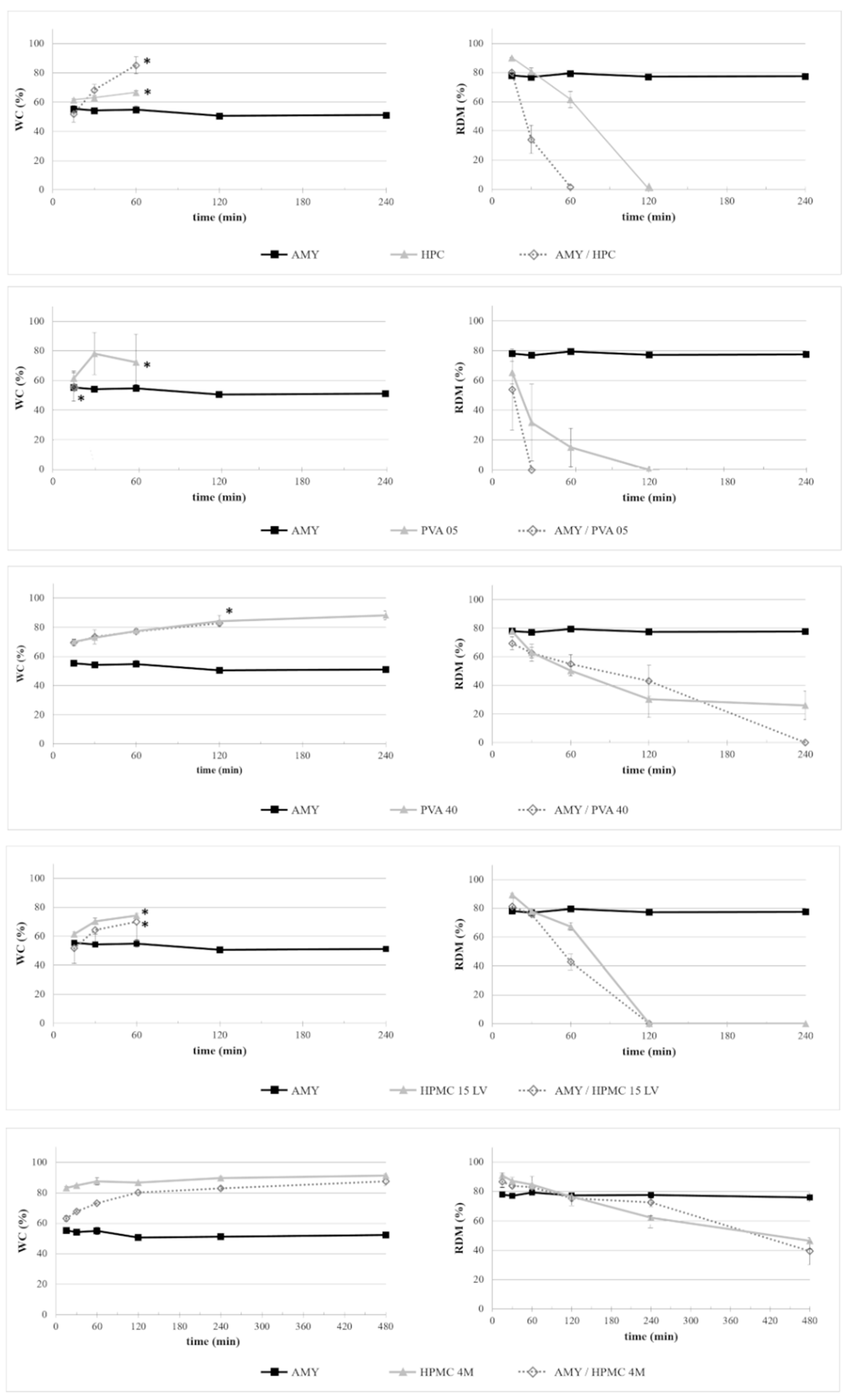
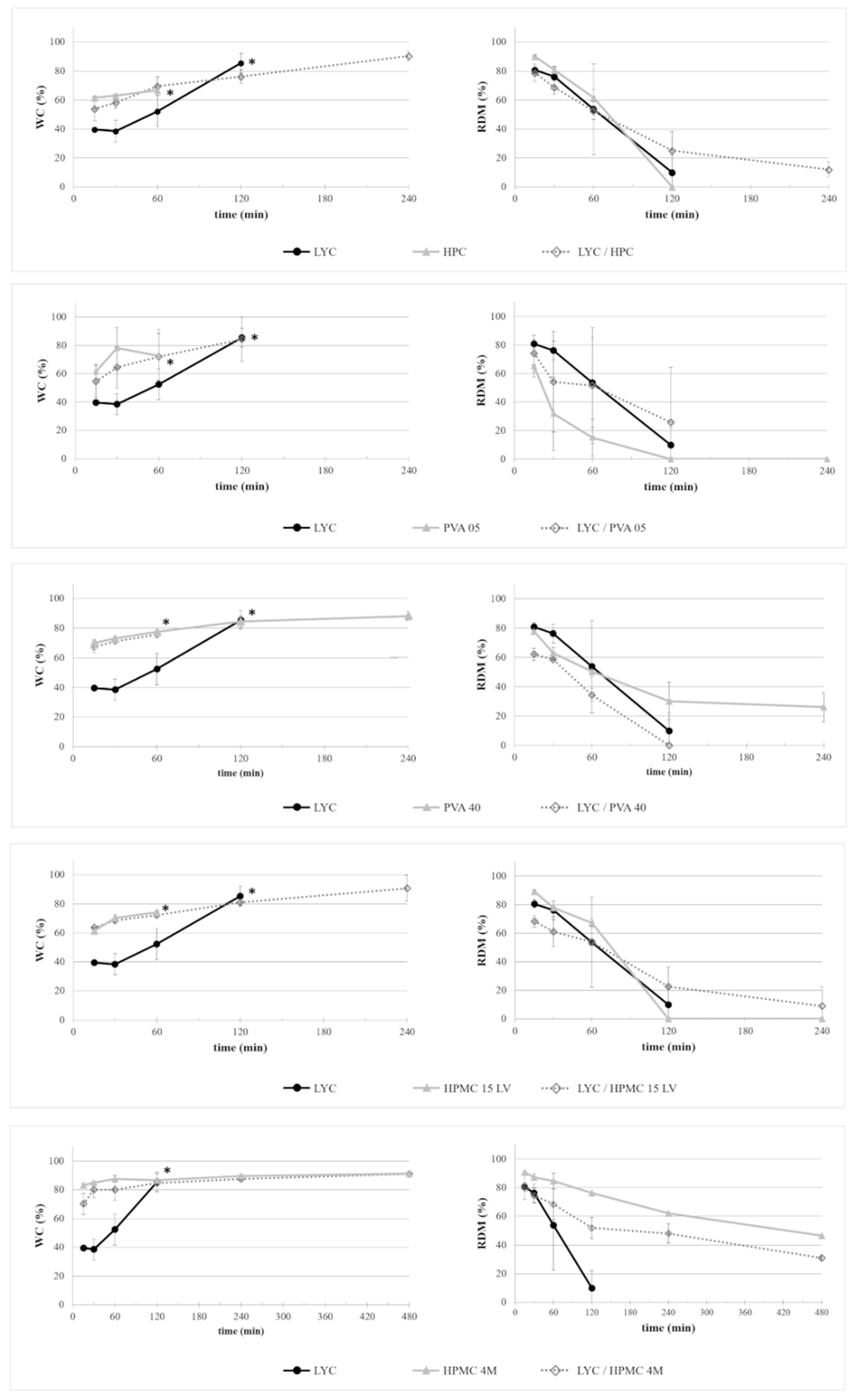
2.2.1. Interaction with Aqueous Fluids
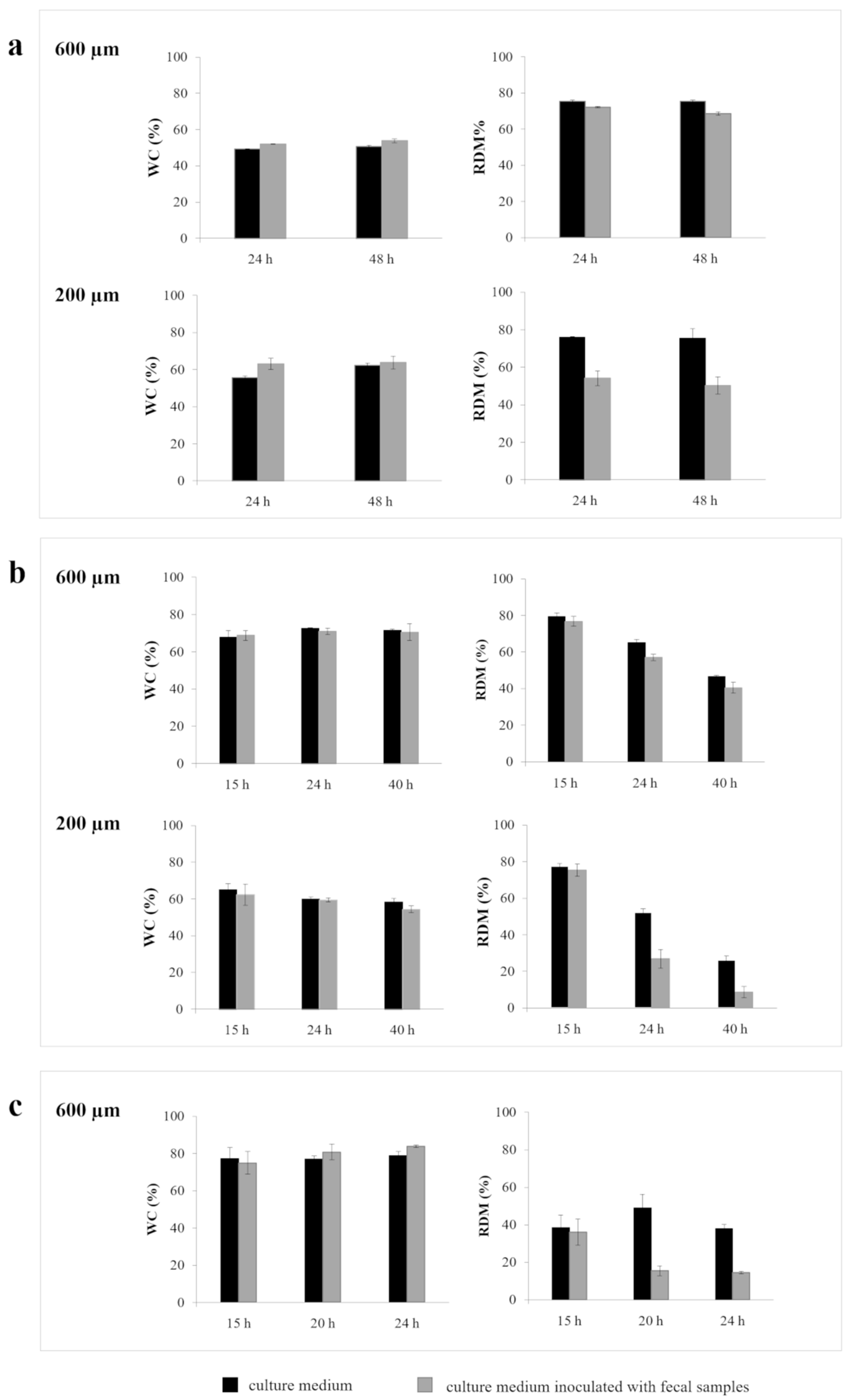
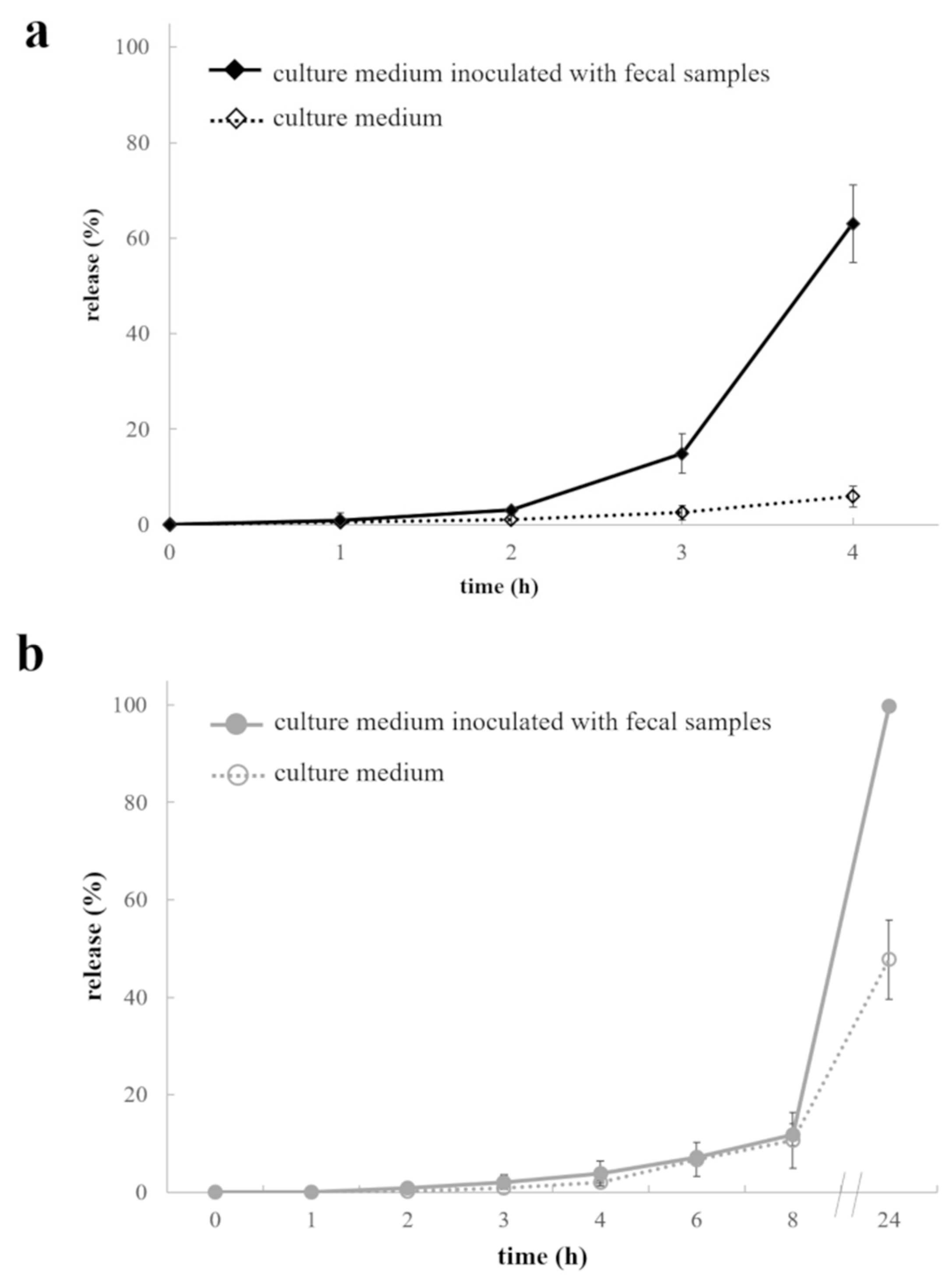
2.2.2. Interactions with Culture Medium +/- Fecal Samples
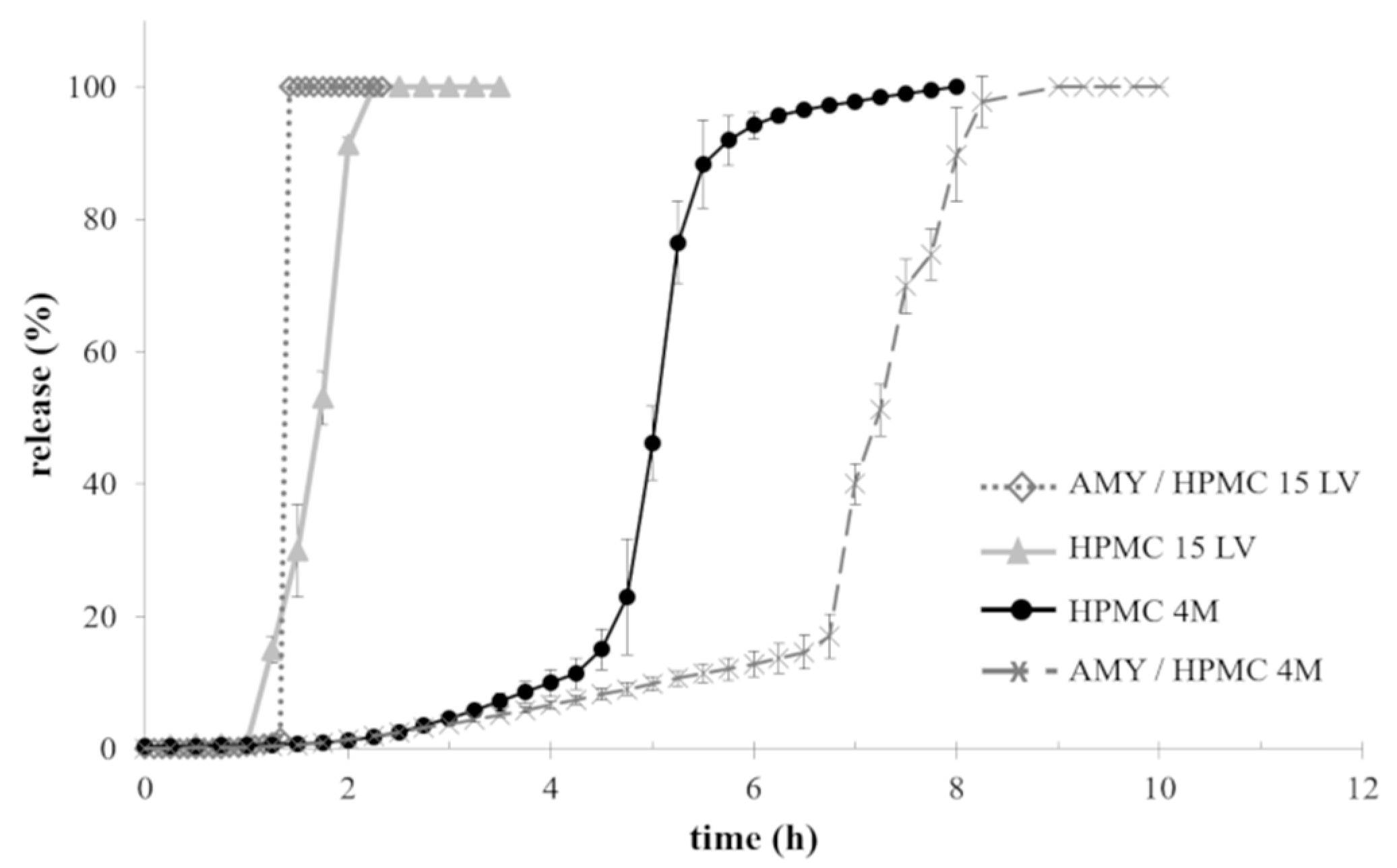
2.3. Manufacturing of and Drug Release from Capsules
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Polymeric Formulations
3.2.2. HME
3.2.3. IM
3.2.4. WC and RDM
3.2.5. Barrier Performance of Molded Disks
3.2.6. In Vitro Release from Capsules
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMY | Amylo® N-460 |
| CFU | Colony-forming unit |
| 3D | Three-dimensional |
| DDSs | Drug delivery systems |
| GLY | Glycerol |
| HME | Hot melt extrusion |
| HPC | Hydroxypropyl cellulose |
| HPMC | Hydroxypropyl methylcellulose |
| HPLC | High pressure liquid chromatography |
| IM | Injection molding |
| LYC | Lycoat® RS780 |
| PEG | Polyethylene glycol |
| PVA | Poly(vinyl alcohol) |
| RDM | Residual dry mass |
| USP | United States Pharmacopeia |
| WC | Water content |
References
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-targeted oral drug delivery systems: Design trends and approaches. AAPSPharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, A.; Giordano, F.; Sangalli, M.E.; Zema, L. Oral colon-specific drug delivery: Design strategies. STP Pharma Prat. 1994, 4, 336–343. [Google Scholar]
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Maroni, A.; Del Curto, M.D.; Salmaso, S.; Zema, L.; Melocchi, A.; Caliceti, P.; Gazzaniga, A. In vitro and in vivo evaluation of an oral multiple-unit formulation for colonic delivery of insulin. Eur. J. Pharm. Biopharm. 2016, 108, 76–82. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Zema, L.; Foppoli, A.; Gazzaniga, A. Retentive drug delivery systems based on shape memory materials. J. Appl. Polym. Sci. 2020. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Inverardi, N.; Briatico-Vangosa, F.; Baldi, F.; Pandini, S.; Scalet, G.; Auricchio, F.; Cerea, M.; Foppoli, A.; et al. Expandable drug delivery system for gastric retention based on shape memory polymers: Development via 4D printing and extrusion. Int. J. Pharm. 2019, 571, 118700. [Google Scholar] [CrossRef]
- Rouge, N.; Buri, P.; Doelker, E. Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int. J. Pharm. 1996, 136, 117–139. [Google Scholar] [CrossRef]
- Maroni, A.; Zema, L.; Del Curto, M.D.; Loreti, G.; Gazzaniga, A. Oral pulsatile delivery: Rationale and chronopharmaceutical formulations. Int. J. Pharm. 2010, 398, 1–8. [Google Scholar] [CrossRef]
- Patel, M.M. Getting into the colon: Approaches to target colorectal cancer. Expert Opin. Drug Deliv. 2014, 11, 1343–1350. [Google Scholar] [CrossRef]
- Van den Mooter, G. Colon drug delivery. Expert Opin. Drug Deliv. 2006, 3, 111–125. [Google Scholar] [CrossRef]
- Pawara, V.K.; Mehera, J.G.; Singh, Y.; Chaurasia, M.; Reddy, B.S.; Chourasia, M.K. Targeting of gastrointestinal tract for amended delivery of protein/peptide therapeutics: Strategies and industrial perspectives. J. Control. Release 2014, 196, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M. Colon targeting: An emerging frontier for oral insulin delivery. Expert Opin. Drug Deliv. 2013, 10, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Varamini, P. Recent advances in oral delivery of peptide hormones. Expert Opin. Drug Deliv. 2016, 13, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, R.; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar] [CrossRef]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- McConnell, E.L.; Short, M.D.; Basit, A.W. An in vivo comparison of intestinal pH and bacteria as physiological trigger mechanisms for colonic targeting in man. J. Control. Release 2008, 130, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Amin, A. Recent trends in microbially and/or enzymatically driven colon-specific drug delivery systems. Crit. Rev. Ther. Drug Carr. Syst. 2011, 28, 489–552. [Google Scholar] [CrossRef]
- Maroni, A.; Moutaharrik, S.; Zema, L.; Gazzaniga, A. Enteric coatings for colonic drug delivery: State of the art. Exp. Opin. Drug Deliv. 2017, 14, 1027–1029. [Google Scholar] [CrossRef]
- Schellekens, R.C.A.; Baltink, J.H.; Woesthuis, E.M.; Stellaard, F.; Kosterink, J.G.W.; Woerdenbag, H.J.; Frijlink, H.W. Film coated tablets (ColoPulse technology) for targeted delivery in the lower intestinal tract: Influence of the core composition on release characteristics. Pharm. Dev. Technol. 2012, 17, 40–47. [Google Scholar] [CrossRef]
- Cerchiara, T.; Abruzzo, A.; Parolin, C.; Vitali, B.; Bigucci, F.; Gallucci, M.C.; Nicoletta, F.P.; Luppi, B. Microparticles based on chitosan/carboxymethylcellulose polyelectrolyte complexes for colon delivery of vancomycin. Carbohydr. Polym. 2016, 143, 124–130. [Google Scholar] [CrossRef]
- Seeli, D.S.; Prabaharan, M. Guar gum succinate as a carrier for colon-specific drug delivery. Int. J. Biol. Macromol. 2016, 84, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.T.; Pham, T.M.H.; Nguyen, T.H.; Pham, T.T.; Nguyen, T.Q. Pectin/HPMC dry powder coating formulations for colon specific targeting tablets of metronidazole. J. Drug Deliv. Sci. Technol. 2016, 33, 19–27. [Google Scholar] [CrossRef]
- Cummings, J.H.; Milojevic, S.; Harding, M.; Coward, W.A.; Gibson, G.R.; Botham, L.R.; Ring, S.G.; Wraight, E.P.; Stockham, M.A.; Allwood, M.C.; et al. In vivo studies of amylose- and ethylcellulose-coated [13C]glucose microspheres as a model for drug delivery to the colon. J. Control. Release 1996, 40, 123–131. [Google Scholar] [CrossRef]
- Freire, C.; Podczeck, F.; Veiga, F.; Sousa, J. Starch-based coatings for colon-specific delivery. Part II: Physicochemical properties and in vitro drug release from high amylose maize starch films. Eur. J. Pharm. Biopharm. 2009, 72, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Karrout, Y.; Dubuquoy, L.; Piveteau, C.; Siepmann, F.; Moussa, E.; Wils, D.; Beghyn, T.; Neut, C.; Flament, M.-P.; Guerin-Deremaux, L.; et al. In vivo efficacy of microbiota-sensitive coatings for colon targeting: A promising tool for IBD therapy. J. Control. Release 2015, 197, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, A.; Palugan, L.; Foppoli, A.; Sangalli, M.E. Oral pulsatile delivery systems based on swellable hydrophilic polymers. Eur. J. Pharm. Biopharm. 2008, 68, 11–18. [Google Scholar] [CrossRef]
- Foppoli, A.; Maroni, A.; Moutaharrik, S.; Melocchi, A.; Zema, L.; Palugan, L.; Cerea, M.; Gazzaniga, A. In vitro and human pharmacoscintigraphic evaluation of an oral 5-ASA delivery system for colonic release. Int. J. Pharm. 2019, 572, 118723. [Google Scholar] [CrossRef]
- Davis, S.S.; Hardy, J.G.; Fara, J.W. Transit of pharmaceutical dosage forms through the small intestine. Gut 1986, 27, 886–892. [Google Scholar] [CrossRef]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef]
- Maroni, A.; Zema, L.; Cerea, M.; Foppoli, A.; Palugan, L.; Gazzaniga, A. Erodible drug delivery systems for time-controlled release into the gastrointestinal tract. J. Drug Deliv. Sci. Technol. 2016, 32, 229–235. [Google Scholar] [CrossRef]
- Foppoli, A.; Maroni, A.; Cerea, M.; Zema, L.; Gazzaniga, A. Dry coating of solid dosage forms: An overview of processes and applications. Drug Dev. Ind. Pharm. 2017, 43, 1919–1931. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Parietti, F.; Cerea, M.; Foppoli, A.; Palugan, L.; Gazzaniga, A.; Maroni, A.; Zema, L. Lego-inspired capsular devices for the development of personalized dietary supplements: Proof of concept with multimodal release of caffeine. J. Pharm. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zema, L.; Loreti, G.; Melocchi, A.; Maroni, A.; Palugan, L.; Gazzaniga, A. Gastroresistant capsular device prepared by injection molding. Int. J. Pharm. 2013, 440, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Uboldi, M.; Maroni, A.; Foppoli, A.; Palugan, L.; Zema, L.; Gazzaniga, A. 3D printing by fused deposition modeling of single- and multi-compartment hollow systems for oral delivery—A review. Int. J. Pharm. 2020, 579, 119155. [Google Scholar] [CrossRef]
- Gazzaniga, A.; Cerea, M.; Cozzi, A.; Foppoli, A.; Maroni, A.; Zema, L. A novel injection-molded capsular device for oral pulsatile delivery based on swellable/erodible polymers. AAPSPharmSciTech 2011, 12, 295–303. [Google Scholar]
- Zema, L.; Loreti, G.; Macchi, E.; Foppoli, A.; Maroni, A.; Gazzaniga, A. Injection-molded capsular device for oral pulsatile release: Development of a novel mold. J. Pharm. Sci. 2013, 102, 489–499. [Google Scholar] [CrossRef]
- Macchi, E.; Zema, L.; Maroni, A.; Gazzaniga, A.; Felton, L.A. Enteric-coating of pulsatile-release HPC capsules prepared by injection molding. Eur. J. Pharm. Sci. 2015, 70, 1–11. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Loreti, G.; Maroni, A.; Gazzaniga, A.; Zema, L. 3D printing by fused deposition modeling (FDM) of a swellable/erodible capsular device for oral pulsatile release of drugs. J. Drug Deliv. Sci. Technol. 2015, 30 Pt B, 360–367. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maccagnan, S.; Ortenzi, M.A.; Antenucci, S.; Briatico-Vangosa, F.; Maroni, A.; Gazzaniga, A.; Zema, L. Industrial development of a 3D-printed nutraceutical delivery platform in the form of a multicompartment HPC capsule. AAPSPharmSciTech 2018, 19, 3343–3354. [Google Scholar] [CrossRef]
- Ibekwe, V.C.; Khela, M.K.; Evans, D.F.; Basit, A.W. A new concept in colonic drug targeting: A combined pH-responsive and bacterially-triggered drug delivery technology. Aliment. Pharmacol. Ther. 2008, 28, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Loreti, G.; Maroni, A.; Del Curto, M.D.; Melocchi, A.; Gazzaniga, A.; Zema, L. Evaluation of hot-melt extrusion technique in the preparation of HPC matrices for prolonged release. Eur. J. Pharm. Sci. 2014, 52, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharma-grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef]
- Melocchi, A.; Inverardi, N.; Uboldi, M.; Baldi, F.; Maroni, A.; Pandini, S.; Briatico-Vangosa, F.; Zema, L.; Gazzaniga, A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly (vinyl alcohol): Design concept and 4D printing feasibility. Int. J. Pharm. 2019, 559, 299–311. [Google Scholar] [CrossRef]
- Sangalli, M.E.; Maroni, A.; Foppoli, A.; Zema, L.; Giordano, F.; Gazzaniga, A. Different HPMC viscosity grades as coating agents for an oral time and/or site-controlled delivery system: A study on process parameters and in vitro performances. J. Pharm. Sci. 2004, 22, 469–476. [Google Scholar] [CrossRef]
- Available online: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_0954/0901b803809543f4.pdf?filepath=dowwolff/pdfs/noreg/198–02327.pdf&fromPage=GetDoc (accessed on 25 February 2020).
- Huang, S.; O’Donnell, K.P.; Keen, J.M.; Rickard, M.A.; McGinity, J.W.; Williams, R.O. A new extrudable form of hypromellose: Affinisol™ HPMC HME. AAPSPharmSciTech 2016, 17, 106–119. [Google Scholar] [CrossRef]
- Karrout, Y.; Neut, C.; Wils, D.; Siepmann, F.; Deremaux, L.; Dubreuil, L.; Desreumaux, P.; Siepmann, J. Colon targeting with bacteria-sensitive films adapted to the disease state. Eur. J. Pharm. Biopharm. 2009, 73, 74–81. [Google Scholar] [CrossRef]
- Karrout, Y.; Neut, C.; Wils, D.; Siepmann, F.; Deremaux, L.; Flament, M.-P.; Dubreuil, L.; Desreumaux, P.; Siepmann, J. Novel polymeric film coatings for colon targeting: Drug release from coated pellets. Eur. J. Pharm. Sci. 2009, 37, 427–433. [Google Scholar] [CrossRef]
- LaFountaine, J.S.; McGinity, J.W.; Williams, R.O. III Challenges and strategies in thermal processing of amorphous solid dispersions: A review. AAPSPharmSciTech 2016, 17, 43–55. [Google Scholar]
- Launa, B.; Lisch, J.M. Twin-screw extrusion cooking of starches: Flow behaviour of starch pastes, expansion and mechanical properties of extrudates. J. Food Eng. 1983, 2, 259–280. [Google Scholar] [CrossRef]
- Forssell, P.M.; Mikkilä, J.M.; Moates, G.K.; Parker, R. Phase and glass transition behaviour of concentrated barley starch-glycerol-water mixtures, a model for thermoplastic starch. Carbohydr. Polym. 1997, 34, 275–282. [Google Scholar] [CrossRef]
- Da Róz, A.L.; Carvalho, A.J.F.; Gandini, A.; Curvelo, A.A.S. The effect of plasticizers on thermoplastic starch compositions obtained by melt processing. Carbohydr. Polym. 2006, 63, 417–424. [Google Scholar] [CrossRef]
- Shogren, R.L.; Swanson, C.L.; Thomson, A.R. Extrudates of cornstarch with urea and glycols: Structure/mechanical property relations. Starch-Starke 1992, 44, 335–338. [Google Scholar] [CrossRef]
- Yang, L.; Chu, J.S.; Fix, J.A. Colon-specific drug delivery: New approaches and in vitro/in vivo evaluation. Int. J. Pharm. 2002, 235, 1–15. [Google Scholar] [CrossRef]
- Eith, L.; Stepto, R.F.T.; Tomka, I.; Wittwer, F. The injection-moulded capsule. Drug Dev. Ind. Pharm. 1986, 12, 2113–2126. [Google Scholar] [CrossRef]
- Idrissi, S.; Dumesnil, R.; Michel, L.; Traisnel, M. Capill: Substitution of gelatin by starch. Pharm. Acta Helv. 1991, 66, 246–252. [Google Scholar]
- Vilivalam, V.D.; Illum, L.; Iqbal, K. Starch capsules: An alternative system for oral drug delivery. Pharm. Sci. Technol. Today 2000, 3, 64–69. [Google Scholar] [CrossRef]

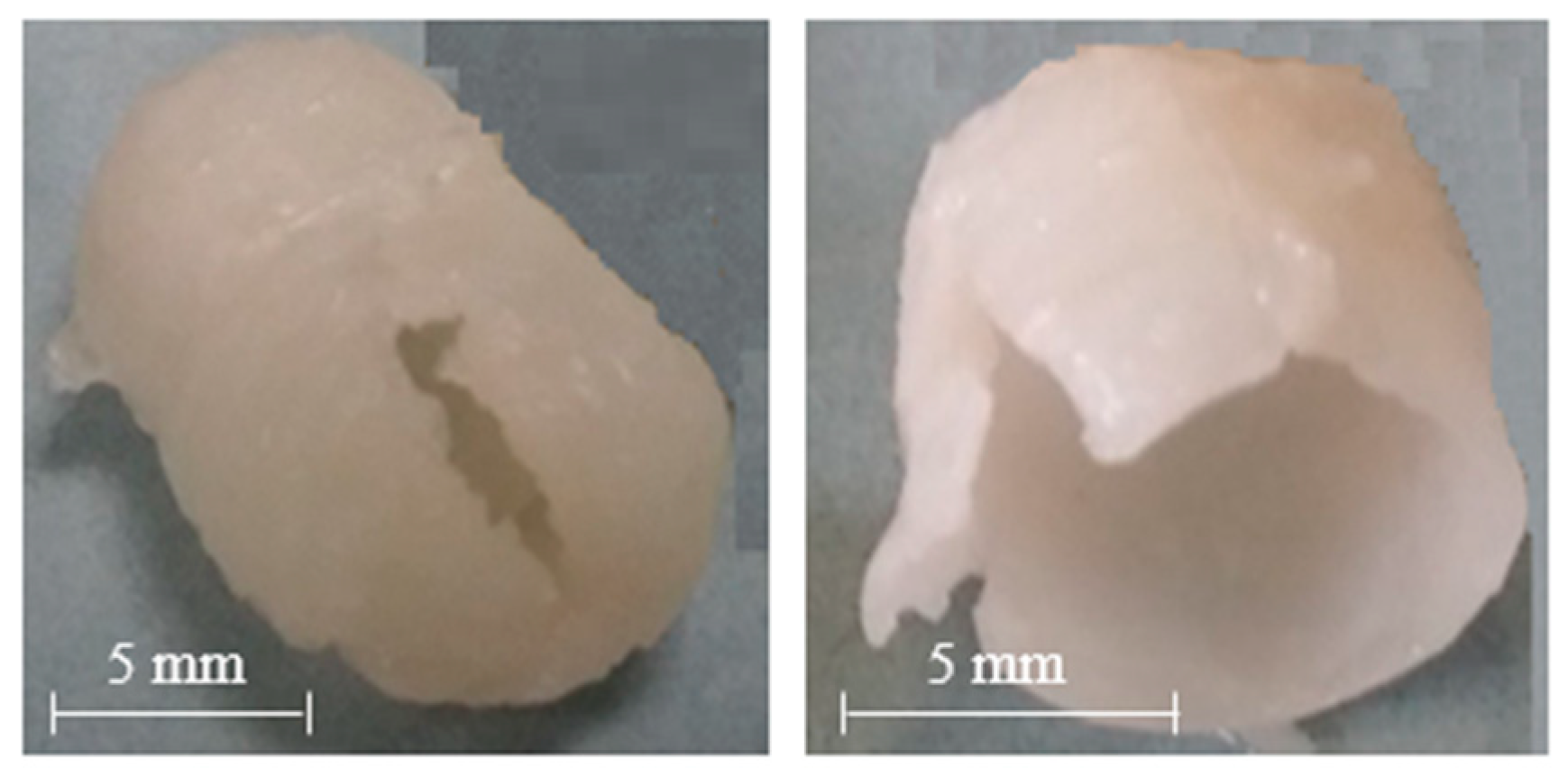
| HPC | HPMC 15LV | HPMC 4M | PVA 05 | PVA 40 | AMY | LYC | |
|---|---|---|---|---|---|---|---|
| Plasticizer (% w/w) | PEG 1500 (10) | PEG 1500 (10) | PEG 1500 (10) | GLY (15) | GLY (15) | GLY (20) + water (15) | GLY (10) + water (5) |
| Temperature (°C) | 150 | 155 | 160 | 170 | 190 | 105 | 100 |
| Screw Speed (rpm) | 50 | 80 | 80 | 50 | 30 | 75 | 50 |
| Torque (N·cm) | 25 | 60 | 80 | 40 | 95 | 60 | 80 |
 |  |  |  |  |  |  |
| Plasticized Formulation Based on | Process Parameters | Processability * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Injection Pressures, P1–P2 (bar) | Injection Times, t1–t2 (s) | Injection Rates, v1–v2 (%) | ||||||
| Compression Zone | Metering Zone | Nozzle | |||||||
| AMY | Powder | 110 | 115 | 130 | 70-60 | 0.8-0.3 | 40-20 | -/+ |  |
| Extruded pellets | 110 | 120 | 140 | 70-60 | 0.8-0.3 | 40-20 | -/+ | ||
| LYC | 100 | 125 | 135 | 70-60 | 0.8-0.3 | 50-30 | - |  | |
| HPC | 145 | 150 | 165 | 50-40 | 0.8-0.3 | 50-40 | ++ |  | |
| HPMC 15LV | 155 | 165 | 175 | 50-30 | 0.8-0.3 | 30-10 | ++ |  | |
| HPMC 4M | 175 | 180 | 185 | 60-50 | 1.5-1.0 | 60-50 | ++ |  | |
| PVA 05 | 155 | 165 | 170 | 50-30 | 0.8-0.3 | 50-30 | + |  | |
| PVA 40 | 170 | 175 | 180 | 70-50 | 0.8-0.3 | 70-50 | +/- |  | |
| AMY/HPC | 125 | 125 | 135 | 60-50 | 0.8-0.3 | 30-20 | + |  | |
| AMY/HPMC 15LV | 125 | 125 | 135 | 30-20 | 2.0-1.5 | 30-10 | + |  | |
| AMY/HPMC 4M | 135 | 135 | 155 | 60-50 | 0.8-0.3 | 60-40 | + |  | |
| AMY/PVA 05 | 160 | 170 | 175 | 75-60 | 1.5-1.0 | 40-30 | +/- |  | |
| AMY/PVA 40 | 150 | 155 | 160 | 60-50 | 1.0-0.8 | 40-30 | - |  | |
| LYC/HPC | 125 | 130 | 135 | 60-50 | 0.8-0.3 | 30-20 | +/- |  | |
| LYC/HPMC 15LV | 125 | 135 | 145 | 80-60 | 0.8-0.3 | 60-50 | +/- |  | |
| LYC/HPMC 4M | 135 | 140 | 150 | 50-30 | 0.8-0.3 | 50-30 | +/- |  | |
| LYC/PVA 05 | 160 | 170 | 175 | 60-40 | 0.8-0.3 | 40-30 | - |  | |
| LYC/PVA 40 | 150 | 155 | 165 | 50-30 | 0.8-0.3 | 50-30 | - |  | |
| Formulation | Process Parameters | Processability * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Injection Pressures, P1–P2 (bar) | Injection Times, t1–t2 (s) | Injection Rates, v1–v2 (%) | ||||||
| Compression Zone | Metering Zone | Nozzle | Hot Runner | ||||||
| AMY | 110 | 115 | 130 | 135 | 70–60 | 0.8–0.3 | 40–20 | +/− |  |
| HPMC 15LV | 165 | 170 | 180 | 190 | 30–10 | 0.5–0.3 | 30–10 | +/− |  |
| HPMC 4M | 175 | 180 | 190 | 200 | 30–10 | 0.5–0.3 | 30–10 | +/− |  |
| AMY/HPMC 15LV | 120 | 125 | 135 | 145 | 30–20 | 0.8–0.3 | 30–10 | +/− |  |
| AMY/HPMC 4M | 130 | 135 | 155 | 165 | 70–50 | 0.8–0.3 | 60–40 | +/− |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casati, F.; Melocchi, A.; Moutaharrik, S.; Uboldi, M.; Foppoli, A.; Maroni, A.; Zema, L.; Neut, C.; Siepmann, F.; Siepmann, J.; et al. Injection Molded Capsules for Colon Delivery Combining Time-Controlled and Enzyme-Triggered Approaches. Int. J. Mol. Sci. 2020, 21, 1917. https://doi.org/10.3390/ijms21061917
Casati F, Melocchi A, Moutaharrik S, Uboldi M, Foppoli A, Maroni A, Zema L, Neut C, Siepmann F, Siepmann J, et al. Injection Molded Capsules for Colon Delivery Combining Time-Controlled and Enzyme-Triggered Approaches. International Journal of Molecular Sciences. 2020; 21(6):1917. https://doi.org/10.3390/ijms21061917
Chicago/Turabian StyleCasati, Federica, Alice Melocchi, Saliha Moutaharrik, Marco Uboldi, Anastasia Foppoli, Alessandra Maroni, Lucia Zema, Christel Neut, Florence Siepmann, Juergen Siepmann, and et al. 2020. "Injection Molded Capsules for Colon Delivery Combining Time-Controlled and Enzyme-Triggered Approaches" International Journal of Molecular Sciences 21, no. 6: 1917. https://doi.org/10.3390/ijms21061917
APA StyleCasati, F., Melocchi, A., Moutaharrik, S., Uboldi, M., Foppoli, A., Maroni, A., Zema, L., Neut, C., Siepmann, F., Siepmann, J., & Gazzaniga, A. (2020). Injection Molded Capsules for Colon Delivery Combining Time-Controlled and Enzyme-Triggered Approaches. International Journal of Molecular Sciences, 21(6), 1917. https://doi.org/10.3390/ijms21061917









