LY75 Ablation Mediates Mesenchymal-Epithelial Transition (MET) in Epithelial Ovarian Cancer (EOC) Cells Associated with DNA Methylation Alterations and Suppression of the Wnt/β-Catenin Pathway
Abstract
1. Introduction
2. Results
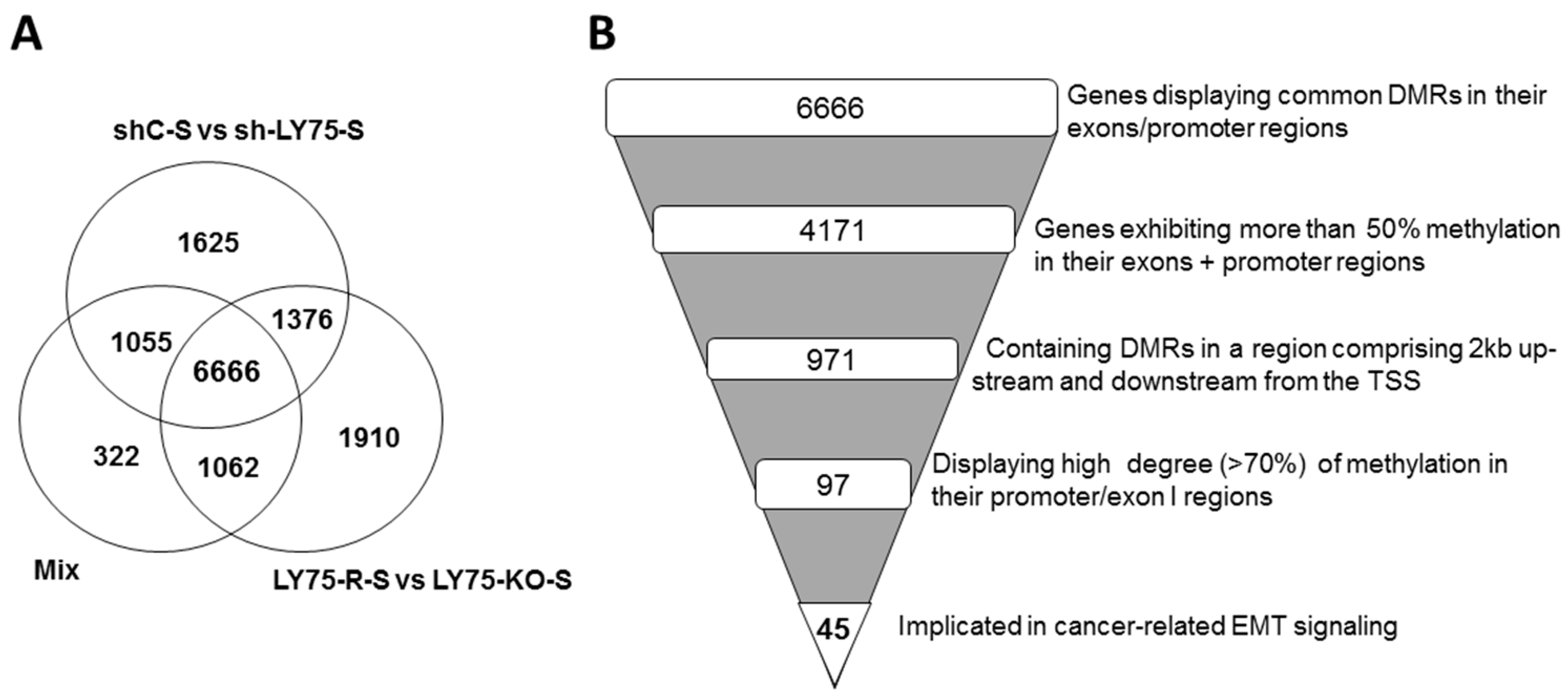
2.1. Reduced Representation Bisulfite Sequencing (RRBS) Analysis of Altered DNA Methylation Patterns during LY75-Mediated EMT in SKOV3 Cells; Identification of Novel Genes Displaying EMT-Associated DNA Methylation Variations
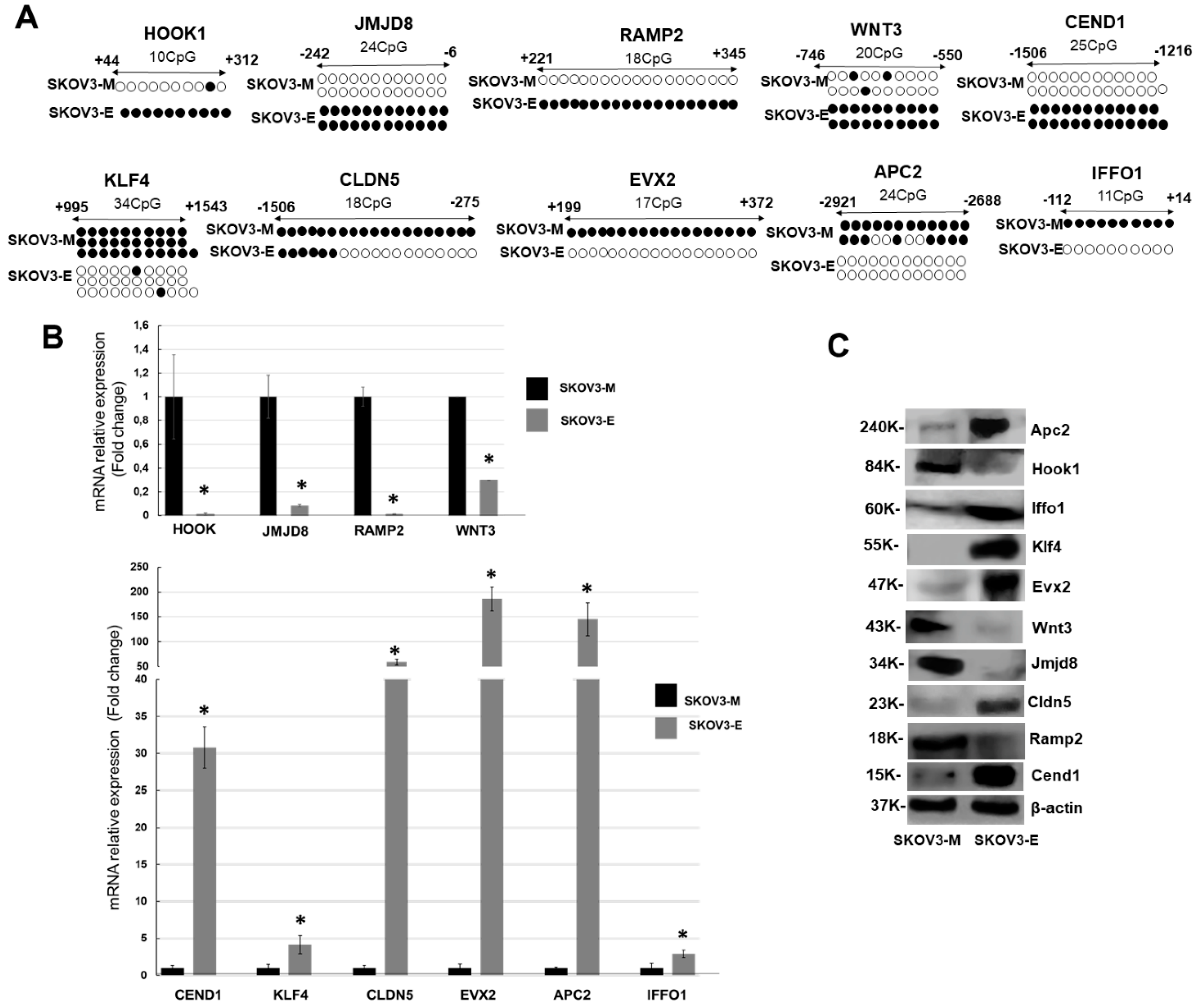
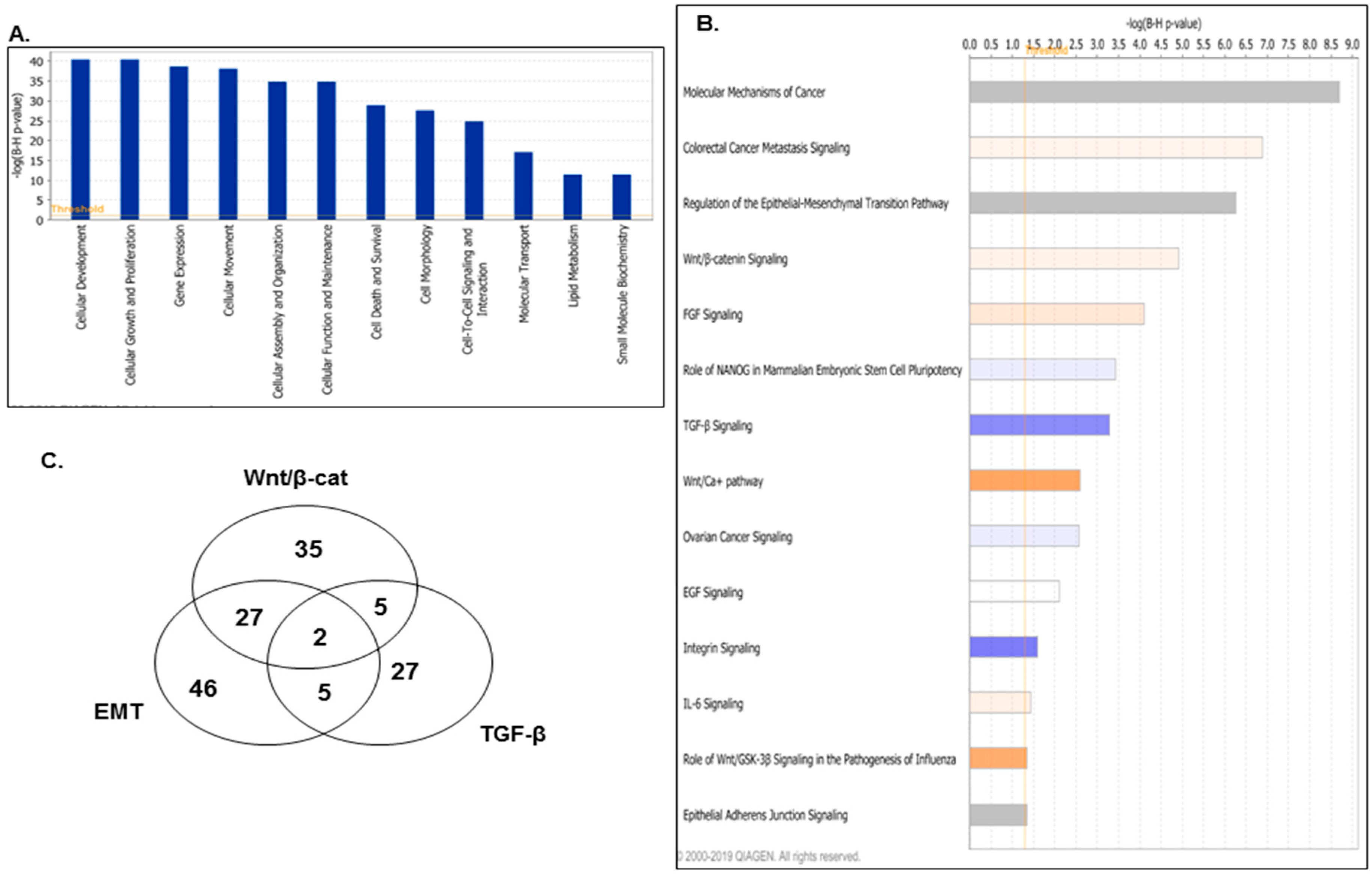
2.2. LY75-Mediated EMT Alterations in EOC Cells are Associated with Predominant Epigenetic Regulation of Members of the Wnt/β-Catenin Pathway
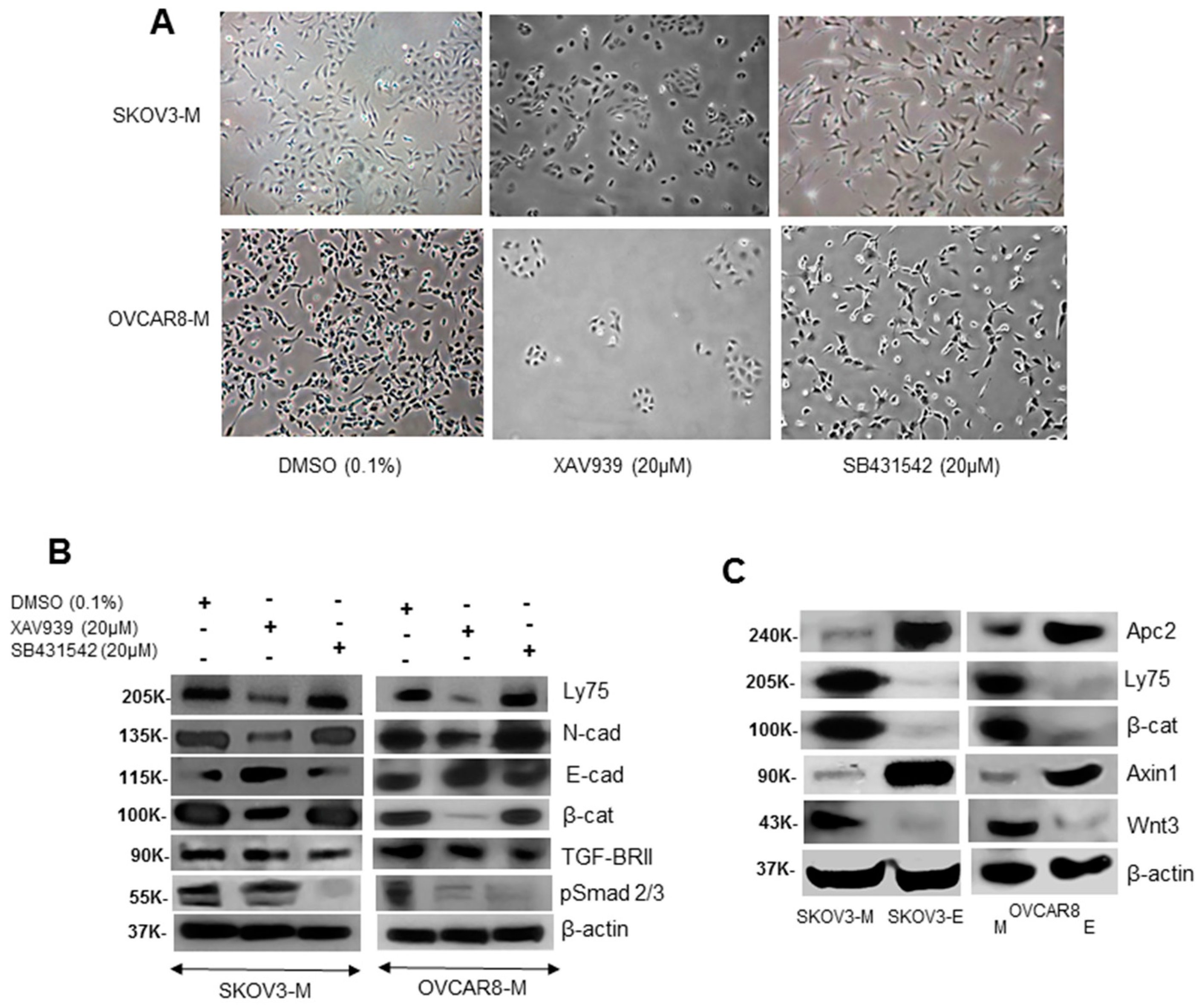
2.3. LY75 Expression Modulates the Wnt/β-Catenin Pathway Activity in EOC Cells
2.4. The Identification of Specific LY75-Interaction Proteins Supports the LY75 Role in Modulation of Wnt/β-Catenin Pathway Activity
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Reduced Representation Bisulfite Sequencing (RRBS) Analysis
4.3. Bisulfite Sequencing PCR (BSP) Analysis
4.4. Short Hairpin RNA (shRNA)- ediated LY75 Knockdown in EOC Cells
4.5. Western Blot Analysis
4.6. Quantitative PCR (qPCR)
4.7. Immunoprecipitation and Consecutive Mass Spectrometry (MS) Analysis
4.8. Immunofluorescence
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EOC | Epithelia ovarian cancer |
| EMT | Epithelial–mesenchymal transition |
| MET | Mesenchymal–epithelial transition |
| ncRNA | Noncoding RNA |
| RRBS | Reduced Representation Bisulfite Sequencing |
| IPA | Ingenuity Pathway Analysis |
| DMRs | differentially methylated regions |
| IGV | Integrative Genomic Viewer |
| BSP | Bisulfite-sequencing PCR |
| shRNA | Short hairpin RNA |
| q-PCR | Quantitative PCR |
| LC-MS/MS | liquid chromatography–mass spectrometry |
Appendix A
| EMT Genes | Wnt/β-Cat Genes | TGF-β Genes | Common EMT/Wnt/β-Cat Genes | Common EMT/TGF-β Genes | ||||
|---|---|---|---|---|---|---|---|---|
| WNT3 | PDGFD | CDKN2A | EP300 | INHA | WNT3 | SMAD3 | ||
| AXIN1 | NOTCH1 | WNT3 | AKT1 | BMP4 | AXIN1 | HRAS | ||
| PIK3R1 | WNT1 | AXIN1 | JUN | SMAD3 | WNT6 | MAP2K2 | ||
| SMAD3 | FGF5 | PPP2R5B | CDH3 | SKI | WNT7B | SOS1 | ||
| HRAS | RELA | TLE1 | UBA52 | HRAS | WNT4 | GRB2 | ||
| PARD6G | FZD10 | WNT6 | DKK3 | BMPR1B | WNT5B | |||
| WNT6 | FGF8 | SOX13 | TGFB2 | MAPK13 | AKT2 | |||
| PIK3R4 | TWIST1 | MYC | SOX14 | MAPK11 | WNT9A | |||
| FGFR3 | KLB | TGFB1 | CSNK2B | TLX2 | FZD9 | |||
| FOXC2 | HNF1A | PPM1J | SOX7 | TGIF1 | TCF3 | |||
| MAP2K2 | WNT2 | WNT7B | LRP5 | EP300 | CDH1 | |||
| TGFB1 | mir34 | RARA | WNT9B | BCL2 | CDH12 | |||
| WNT7B | FGF13 | WNT4 | CSNK1G3 | JUN | FZD6 | |||
| FGF12 | FGF4 | MAP4K1 | WNT2B | MAP2K2 | LEF1 | |||
| WNT4 | AKT1 | WNT5B | DVL1 | TGFB1 | WNT1 | |||
| IRS2 | KL | AXIN2 | DKKL1 | SOS1 | FZD10 | |||
| WNT5B | SOS1 | AKT2 | TCF7L1 | TGFB2 | HNF1A | |||
| FGF19 | TGFB2 | CSNK1G2 | SOX11 | MAP4K1 | WNT2 | |||
| PIK3C2B | FGF23 | WNT9A | FZD8 | VDR | AKT1 | |||
| AKT2 | PIK3R2 | CREBBP | WNT3A | HNF4A | WNT9B | |||
| TWIST2 | FGF3 | CSNK1D | WNT10A | RUNX3 | WNT2B | |||
| JAG2 | EGFR | FZD9 | SOX6 | GRB2 | DVL1 | |||
| WNT9A | MAP2K7 | TCF3 | APC2 | FOXH1 | TCF7L1 | |||
| TYK2 | ESRP2 | ACVR1B | NR5A2 | CREBBP | FZD8 | |||
| FGFR2 | WNT9B | CDH1 | PPP2R5E | SMAD6 | WNT3A | |||
| FZD9 | GRB2 | CDH12 | SOX15 | SMAD7 | WNT10A | |||
| MMP2 | EGR1 | TLE3 | WNT11 | MAPK9 | WNT11 | |||
| NFKB2 | WNT2B | FZD6 | LRP1 | PITX2 | ||||
| ZEB1 | DVL1 | LEF1 | SOX3 | MAPK12 | ||||
| TCF3 | FGF14 | SOX8 | ACVR1B | |||||
| TLR9 | TCF7L1 | SFRP1 | INHBB | |||||
| MET | FGF21 | WNT1 | TRAF6 | |||||
| CDH1 | FZD8 | FZD10 | NKX2-5 | |||||
| CDH12 | WNT3A | SFRP2 | FOS | |||||
| IRS1 | WNT10A | FRZB | ZNF423 | |||||
| mir-192 | ZEB2 | SOX1 | IRF7 | |||||
| FZD6 | FGF20 | FRAT1 | SMURF2 | |||||
| LEF1 | FGF11 | MARK2 | BMP7 | |||||
| FGFRL1 | RBPJ | HNF1A | PMEPA1 | |||||
| WNT11 | JAK3 | WNT2 | ||||||
| Gene | q-PCR Primers | Sequence (5′->3′) |
|---|---|---|
| HOOK1 (NM_015888) | Forward | AGTGAGTTGACACCCTGTGG |
| Reverse | TGGTATATGTACTCAAGCCTCCC | |
| Product length (pb) | 103 | |
| JMJD8 (NM_001005920) | Forward | TCATCACCCTCGTGGTTTCG |
| Reverse | ATCTGGCCAGGTCCATCTCT | |
| Product length (pb) | 83 | |
| RAMP2 (NM_005854) | Forward | GCACGAGCTTCTCAACAACC |
| Reverse | CAACCCTGGCTTCCATTCCC | |
| Product length (pb) | 134 | |
| KRT7 (NM_005556) | Forward | ATTCCACTGGTGGCAGTAGC |
| Reverse | TGGAGAAGCTCAGGGCATTG | |
| Product length (pb) | 83 | |
| APC2 (NM_001351273) | Forward | GCTCCGACAGCATTACCTCA |
| Reverse | AGACCCGGTACAGAAACGTG | |
| Product length (pb) | 115 | |
| CEND1 (NM_016564) | Forward | TACATACGCCCCCAACACAC |
| Reverse | TCTTTCTGCCCTGGAGGTTG | |
| Product length (pb) | 92 | |
| CLDN5 (NM_001130861) | Forward | GGATTTCGCTTCCCCTCCAA |
| Reverse | GTACACATCTTCCGGTGGGG | |
| Product length (pb) | 119 | |
| EVX2 (NM_001080458) | Forward | GGGAGAACTATGTGTCGCGG |
| Reverse | CGGTTCTGGAACCACACCTT | |
| Product length (pb) | 91 | |
| KLF4 (NM_004235.6) | Forward | AGAACAGATGGGGTCTGTGAC |
| Reverse | TCCACAACTTCCAGTCACCC | |
| Product length (pb) | 106 | |
| WNT3 (NM_030753) | Forward | ATCTACGACGTGCACACCTG |
| Reverse | TGCTTCCCATGAGACTTCGC | |
| Product length (pb) | 95 | |
| 18S (NR_003278) | Forward | AACCCGTTGAACCCCATT |
| Reverse | CCATCCAATCGGTAGTAGCG | |
| Product length (pb) | 119 |
| Genes | Primers | Outer PCR-Sequence (5′->3′) | Inner PCR-Sequence (5′->3′) |
|---|---|---|---|
| HOOK1 (NM_015888) | Forward | GGTTTAGGTGTTTGGTAGYG 3 | GGTTTAGGTGTTTGGTAGYG |
| Reverse | ACCCAAATCATAAAACTATCRC | ACCRACTCTCCTCCAAAAA | |
| Product length (pb) | 318 | 204 | |
| JMJD8 (NM_001005920) | Forward | ATGAAGTGGTTGGAAGGTAGTT | GGAGGTTGGAATTTGAGATTT |
| Reverse | CRAAAATAACCTCCTTTAACCC | CRAAAATAACCTCCTTTAACCC | |
| Product length (pb) | 367 | 231 | |
| RAMP2 (NM_005854) | Forward | TTTTTTTTTTGTTGGGYG | TTTTTTTTTTGTTGGGYG |
| Reverse | CTTATCACTCACACCCAAACC | CCCCTCATCTCTAACCAACTT | |
| Product length (pb) | 318 | 291 | |
| KRT7 (NM_005556) | Forward | TTTGGATTGAAAGTTTGG | TGGTAGTAGAGAAAGGTGGTT |
| Reverse | AACCCCCATAAACAAAAC | AACCCCCATAAACAAAAC | |
| Product length (pb) | 396 | 325 | |
| APC2 (NM_001351273) | Forward | TTGGTTGTTGTTATGGTATTAGT | TTGGTTGTTGTTATGGTATTAGT |
| Reverse | AACTCAATTTCCCCTCCAA | CCTCCAACTCCCACTCTAA | |
| Product length (pb) | 447 | 435 | |
| CEND1 (NM_016564) | Forward | AGTAGTGTATTGTGGGAAATTTT | AGTAGTGTATTGTGGGAAATTTT |
| Reverse | ACTACTACCACCTCCCAAA | CCCAATAACCTTCAAAACC | |
| Product length (pb) | 391 | 191 | |
| CLDN5 (NM_001130861) | Forward | GTAAATTTTGGTTAGGGAAGTG | GTAAATTTTGGTTAGGGAAGTG |
| Reverse | CACCTCCTAAATCTACCAACTC | ACCAATCACAAAACCTCTAACAA | |
| Product length (pb) | 436 | 310 | |
| EVX2 (NM_001080458) | Forward | GGGTTATTGTGATATTTTTAAGAA | TGGAGAGAGGGTTGTATAGTT |
| Reverse | ATTACCTTTACCATTATTTTCCTT | ATTACCTTTACCATTATTTTCCTT | |
| Product length (pb) | 463 | 398 | |
| KLF4 (NM_004235.6) | Forward | ATTTTTTGGATTTGGATTTTATT | ATTTTTTGGATTTGGATTTTATT |
| Reverse | AAATATACACCRAATCCAATTC | AAACRAACTCCCTACCATA | |
| Product length (pb) | 339 | 266 | |
| WNT3 (NM_030753) | Forward | AGGAAATGTAAAGGTAGTAGGAG | AGGAAATGTAAAGGTAGTAGGAG |
| Reverse | AAAAACACAAAAATATTTCCAA | ACAAAAATATTTCCAAAAACCC | |
| Product length (pb) | 286 | 280 |
References
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA A Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Marchetti, C.; Pisano, C.; Facchini, G.; Bruni, G.S.; Magazzino, F.P.; Losito, S.; Pignata, S. First-line treatment of advanced ovarian cancer: Current research and perspectives. Expert Rev. Anticancer Ther. 2010, 10, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Sheta, R.; Woo, C.M.; Roux-Dalvai, F.; Fournier, F.; Bourassa, S.; Droit, A.; Bertozzi, C.R.; Bachvarov, D. A metabolic labeling approach for glycoproteomic analysis reveals altered glycoprotein expression upon GALNT3 knockdown in ovarian cancer cells. J. Proteom. 2016, 145, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Fruscio, R.; Corso, S.; Ceppi, L.; Garavaglia, D.; Garbi, A.; Floriani, I.; Franchi, D.; Cantu, M.G.; Bonazzi, C.M.; Milani, R.; et al. Conservative management of early-stage epithelial ovarian cancer: Results of a large retrospective series. Ann. Oncol. 2013, 24, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Alouini, S. Management of ovarian cancer has changed. Gynecol. Oncol. 2012, 126, 313. [Google Scholar] [CrossRef]
- Faddaoui, A.; Bachvarova, M.; Plante, M.; Gregoire, J.; Renaud, M.C.; Sebastianelli, A.; Gobeil, S.; Morin, C.; Macdonald, E.; Vanderhyden, B.; et al. The mannose receptor LY75 (DEC205/CD205) modulates cellular phenotype and metastatic potential of ovarian cancer cells. Oncotarget 2016, 7, 14125–14142. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Takai, M.; Terai, Y.; Kawaguchi, H.; Ashihara, K.; Fujiwara, S.; Tanaka, T.; Tsunetoh, S.; Tanaka, Y.; Sasaki, H.; Kanemura, M.; et al. The EMT (epithelial-mesenchymal-transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J. Ovarian Res. 2014, 7, 1757–2215. [Google Scholar] [CrossRef]
- Davidson, B.; Trope, C.G.; Reich, R. Epithelial-mesenchymal transition in ovarian carcinoma. Front Oncol 2012, 2. [Google Scholar] [CrossRef]
- Klymenko, Y.; Kim, O.; Stack, M.S. Complex Determinants of Epithelial: Mesenchymal Phenotypic Plasticity in Ovarian Cancer. Cancers 2017, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.L.; Weinberg, R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013, 19, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Bedi, U.; Mishra, V.K.; Wasilewski, D.; Scheel, C.; Johnsen, S.A. Epigenetic plasticity: A central regulator of epithelial-to-mesenchymal transition in cancer. Oncotarget 2014, 5, 2016–2029. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fang, J. Epigenetic regulation of epithelial-mesenchymal transition. Cell. Mol. Life Sci. 2016, 73, 4493–4515. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Smith, Z.D.; Bock, C.; Boyle, P.; Gnirke, A.; Meissner, A. Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat. Protoc. 2011, 6, 468–481. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Petersen, I.; Yang, L.; Zhang, Q.; Knösel, T.; Cui, T.; Chen, Y.; Albring, K.F.; Huber, O. Desmoplakin acts as a tumor suppressor by inhibition of the Wnt/β-catenin signaling pathway in human lung cancer. Carcinogenesis 2012, 33, 1863–1870. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Dai, D.; Zhou, Q.; Guo, X.; Tian, Z.; Huang, X.; Yang, L.; Tang, H.; Xie, X. AHNAK suppresses tumour proliferation and invasion by targeting multiple pathways in triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2017, 36, 65. [Google Scholar] [CrossRef]
- Zhi, X.; Lin, L.; Yang, S.; Bhuvaneshwar, K.; Wang, H.; Gusev, Y.; Lee, M.-H.; Kallakury, B.; Shivapurkar, N.; Cahn, K.; et al. βII-Spectrin (SPTBN1) suppresses progression of hepatocellular carcinoma and Wnt signaling by regulation of Wnt inhibitor kallistatin. Hepatology 2015, 61, 598–612. [Google Scholar] [CrossRef]
- Keita, M.; Wang, Z.Q.; Pelletier, J.F.; Bachvarova, M.; Plante, M.; Gregoire, J.; Renaud, M.C.; Mes-Masson, A.M.; Paquet, E.R.; Bachvarov, D. Global methylation profiling in serous ovarian cancer is indicative for distinct aberrant DNA methylation signatures associated with tumor aggressiveness and disease progression. Gynecol. Oncol. 2013, 128, 356–363. [Google Scholar] [CrossRef]
- Faddaoui, A.; Sheta, R.; Bachvarova, M.; Plante, M.; Gregoire, J.; Renaud, M.C.; Sebastianelli, A.; Gobeil, S.; Morin, C.; Ghani, K.; et al. Suppression of the grainyhead transcription factor 2 gene (GRHL2) inhibits the proliferation, migration, invasion and mediates cell cycle arrest of ovarian cancer cells. Cell Cycle 2017, 16, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Sheta, R.; Wang, Z.Q.; Bachvarova, M.; Plante, M.; Gregoire, J.; Renaud, M.C.; Sebastianelli, A.; Gobeil, S.; Morin, C.; Macdonald, E.; et al. Hic-5 regulates epithelial to mesenchymal transition in ovarian cancer cells in a TGFbeta1-independent manner. Oncotarget 2017, 8, 82506–82530. [Google Scholar] [CrossRef] [PubMed]
- Keita, M.; Bachvarova, M.; Morin, C.; Plante, M.; Gregoire, J.; Renaud, M.C.; Sebastianelli, A.; Trinh, X.B.; Bachvarov, D. The RUNX1 transcription factor is expressed in serous epithelial ovarian carcinoma and contributes to cell proliferation, migration and invasion. Cell Cycle 2013, 12, 972–986. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lai, Q.; He, C.; Fang, Y.; Yan, Q.; Zhang, Y.; Wang, X.; Gu, C.; Wang, Y.; Ye, L.; et al. RUNX1 promotes tumour metastasis by activating the Wnt/beta-catenin signalling pathway and EMT in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 334. [Google Scholar] [CrossRef]
- Maldonado-Baez, L.; Cole, N.B.; Kramer, H.; Donaldson, J.G. Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1. J. Cell Biol. 2013, 201, 233–247. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.; Chen, W.; Hu, Q.; Lou, Y.; Fu, Q.-H.; Zhang, J.-Y.; Chen, Y.-W.; Ye, L.-Y.; Wang, Y.; et al. Hook1 inhibits malignancy and epithelial–mesenchymal transition in hepatocellular carcinoma. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Cao, J.; Huang, Y.-Q.; Jiao, S.; Lan, X.-B.; Ge, M.-H. Clinicopathological and prognostic significance of SHP2 and Hook1 expression in patients with thyroid carcinoma. Hum. Pathol. 2018, 81, 105–112. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Zhao, Q.; Liu, Y.; He, L.; Xu, Q.; Sun, X.; Teng, L.; Cheng, H.; Ke, Y. SHP2 positively regulates TGFbeta1-induced epithelial-mesenchymal transition modulated by its novel interacting protein Hook1. J. Biol. Chem. 2014, 289, 34152–34160. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef]
- Nouguerède, E.; Berenguer, C.; Garcia, S.; Bennani, B.; Delfino, C.; Nanni, I.; Dahan, L.; Gasmi, M.; Seitz, J.-F.; Martin, P.-M.; et al. Expression of adrenomedullin in human colorectal tumors and its role in cell growth and invasion in vitro and in xenograft growth in vivo. Cancer Med. 2013, 2, 196–207. [Google Scholar] [CrossRef]
- Yue, W.; Dacic, S.; Sun, Q.; Landreneau, R.; Guo, M.; Zhou, W.; Siegfried, J.M.; Yu, J.; Zhang, L. Frequent Inactivation of RAMP2, EFEMP1 and Dutt1 in Lung Cancer by Promoter Hypermethylation. Clin. Cancer Res. 2007, 13, 4336–4344. [Google Scholar] [CrossRef] [PubMed]
- Accari, S.L.; Fisher, P.R. Emerging Roles of JmjC Domain-Containing Proteins. Int. Rev. Cell Mol. Biol. 2015, 319, 165–220. [Google Scholar] [CrossRef] [PubMed]
- Boeckel, J.N.; Derlet, A.; Glaser, S.F.; Luczak, A.; Lucas, T.; Heumuller, A.W.; Kruger, M.; Zehendner, C.M.; Kaluza, D.; Doddaballapur, A.; et al. JMJD8 Regulates Angiogenic Sprouting and Cellular Metabolism by Interacting With Pyruvate Kinase M2 in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Yeo, K.S.; Tan, M.C.; Wong, W.Y.; Loh, S.W.; Lam, Y.L.; Tan, C.L.; Lim, Y.-Y.; Ea, C.-K. JMJD8 is a positive regulator of TNF-induced NF-κB signaling. Sci. Rep. 2016, 6, 34125. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, F.; Ma, F.; Zhang, B. MiR-873-5p suppresses cell proliferation and epithelial-mesenchymal transition via directly targeting Jumonji domain-containing protein 8 through the NF-kappaB pathway in colorectal cancer. J. Cell Commun. Signal. 2019, 13, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, J. JmjC domain-containing protein 8 (JMJD8) represses Ku70/Ku80 expression via attenuating AKT/NF-kappaB/COX-2 signaling. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118541. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Xia, F.; Liu, Y.; Zhou, Y.; Ye, W.; Hean, P.; Meng, J.; Liu, H.; Liu, L.; Wen, J.; et al. Downregulation of Wnt3 Suppresses Colorectal Cancer Development Through Inhibiting Cell Proliferation and Migration. Front. Pharmacol. 2019, 10, 1110. [Google Scholar] [CrossRef]
- Wang, H.S.; Nie, X.; Wu, R.B.; Yuan, H.W.; Ma, Y.H.; Liu, X.L.; Zhang, J.Y.; Deng, X.L.; Na, Q.; Jin, H.Y.; et al. Downregulation of human Wnt3 in gastric cancer suppresses cell proliferation and induces apoptosis. Oncotargets Ther. 2016, 9, 3849–3860. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.; Ginther, C.; Kim, J.; Mosher, N.; Chung, S.; Slamon, D.; Vadgama, J.V. Expression of Wnt3 activates Wnt/beta-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol. Cancer Res. 2012, 10, 1597–1606. [Google Scholar] [CrossRef]
- Chu, Y.; Fan, W.; Guo, W.; Zhang, Y.; Wang, L.; Guo, L.; Duan, X.; Wei, J.; Xu, G. miR-1247-5p functions as a tumor suppressor in human hepatocellular carcinoma by targeting Wnt3. Oncol. Rep. 2017, 38, 343–351. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, H.Y.; Su, W.Y.; Liu, Y.F.; Wang, X.X.; Zhan, P.; Lv, T.F.; Song, Y. Wnt3 knockdown sensitizes human non-small cell type lung cancer (NSCLC) cells to cisplatin via regulating the cell proliferation and apoptosis. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Poppova, L.; Janovska, P.; Plevova, K.; Radova, L.; Plesingerova, H.; Borsky, M.; Kotaskova, J.; Kantorova, B.; Hlozkova, M.; Figulova, J.; et al. Decreased WNT3 expression in chronic lymphocytic leukaemia is a hallmark of disease progression and identifies patients with worse prognosis in the subgroup with mutated IGHV. Br. J. Haematol. 2016, 175, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; King, M.L.; Ran, S.; Okuda, H.; MacLean, J.A., 2nd; McAsey, M.E.; Sugino, N.; Brard, L.; Watabe, K.; Hayashi, K. WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/beta-catenin pathway. Mol. Cancer Res. 2012, 10, 469–482,. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Morales, J.L.; Schulte, C.J.; Pezoa, S.A.; Vallejo, G.K.; Hilinski, W.C.; England, S.J.; de Jager, S.; Lewis, K.E. Evx1 and Evx2 specify excitatory neurotransmitter fates and suppress inhibitory fates through a Pax2-independent mechanism. Neural Dev. 2016, 11, 5. [Google Scholar] [CrossRef]
- Rauch, T.A.; Wang, Z.; Wu, X.; Kernstine, K.H.; Riggs, A.D.; Pfeifer, G.P. DNA methylation biomarkers for lung cancer. Tumor Biol. 2012, 33, 287–296. [Google Scholar] [CrossRef]
- Liu, F.; Koval, M.; Ranganathan, S.; Fanayan, S.; Hancock, W.S.; Lundberg, E.K.; Beavis, R.C.; Lane, L.; Duek, P.; McQuade, L.; et al. Systems Proteomics View of the Endogenous Human Claudin Protein Family. J. Proteome Res. 2016, 15, 339–359. [Google Scholar] [CrossRef]
- Cherradi, S.; Martineau, P.; Gongora, C.; Del Rio, M. Claudin gene expression profiles and clinical value in colorectal tumors classified according to their molecular subtype. Cancer Manag. Res. 2019, 11, 1337–1348. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Suzuki, S.; Higashi, H.; Inaba, K.; Nakamura, S.; Baba, S.; Kato, T.; Konno, H. Expression of tight junction protein claudin-5 in tumor vessels and sinusoidal endothelium in patients with hepatocellular carcinoma. J. Surg. Res. 2008, 147, 123–131. [Google Scholar] [CrossRef]
- Kudinov, A.E.; Deneka, A.; Nikonova, A.S.; Beck, T.N.; Ahn, Y.H.; Liu, X.; Martinez, C.F.; Schultz, F.A.; Reynolds, S.; Yang, D.H.; et al. Musashi-2 (MSI2) supports TGF-beta signaling and inhibits claudins to promote non-small cell lung cancer (NSCLC) metastasis. Proc. Natl. Acad. Sci. USA 2016, 113, 6955–6960. [Google Scholar] [CrossRef]
- Karnati, H.K.; Panigrahi, M.; Shaik, N.A.; Greig, N.H.; Bagadi, S.A.; Kamal, M.A.; Kapalavayi, N. Down regulated expression of Claudin-1 and Claudin-5 and up regulation of beta-catenin: Association with human glioma progression. Cns Neurol. Disord. Drug Targets 2014, 13, 1413–1426. [Google Scholar] [CrossRef]
- Escudero-Esparza, A.; Jiang, W.G.; Martin, T.A. Claudin-5 is involved in breast cancer cell motility through the N-WASP and ROCK signalling pathways. J. Exp. Clin. Cancer Res. 2012, 31, 43. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Eskelinen, M.; Juvonen, P.; Karja, V.; Haapasaari, K.M.; Saarela, A.; Karihtala, P. Strong claudin 5 expression is a poor prognostic sign in pancreatic adenocarcinoma. Tumour Biol. 2014, 35, 3803–3808. [Google Scholar] [CrossRef] [PubMed]
- Takala, H.; Saarnio, J.; Wiik, H.; Soini, Y. Claudins 1, 3, 4, 5 and 7 in esophageal cancer: Loss of claudin 3 and 4 expression is associated with metastatic behavior. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2007, 115, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Talvensaari-Mattila, A. Expression of claudins 1, 4, 5, and 7 in ovarian tumors of diverse types. Int. J. Gynecol. Pathol. 2006, 25, 330–335. [Google Scholar] [CrossRef]
- Turunen, M.; Talvensaari-Mattila, A.; Soini, Y.; Santala, M. Claudin-5 overexpression correlates with aggressive behavior in serous ovarian adenocarcinoma. Anticancer Res. 2009, 29, 5185–5189. [Google Scholar]
- Nissi, R.; Talvensaari-Mattila, A.; Kuvaja, P.; Paakko, P.; Soini, Y.; Santala, M. Claudin-5 is associated with elevated TATI and CA125 levels in mucinous ovarian borderline tumors. Anticancer Res. 2015, 35, 973–976. [Google Scholar]
- Hong, S.M.; Kelly, D.; Griffith, M.; Omura, N.; Li, A.; Li, C.P.; Hruban, R.H.; Goggins, M. Multiple genes are hypermethylated in intraductal papillary mucinous neoplasms of the pancreas. Mod. Pathol. 2008, 21, 1499–1507. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Yang, V.W. Kruppel-like factor 4 (KLF4): What we currently know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef]
- Tetreault, M.P.; Yang, Y.; Katz, J.P. Kruppel-like factors in cancer. Nat. Rev. Cancer 2013, 13, 701–713. [Google Scholar] [CrossRef]
- Yu, M.; Hao, B.; Zhan, Y.; Luo, G. Kruppel-like factor 4 expression in solid tumor prognosis: A meta-analysis. Clin. Chim. Acta 2018, 485, 50–59. [Google Scholar] [CrossRef]
- Park, C.S.; Lewis, A.; Chen, T.; Lacorazza, D. Concise Review: Regulation of Self-Renewal in Normal and Malignant Hematopoietic Stem Cells by Kruppel-Like Factor 4. Stem Cells Transl. Med. 2019, 8, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Niu, Y.; Huang, C. Role of FoxM1 in the Progression and Epithelial to Mesenchymal Transition of Gastrointestinal Cancer. Recent Pat. Anti-Cancer Drug Discov. 2017, 12, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shi, M.; Quan, M.; Xie, K. Regulation of EMT by KLF4 in gastrointestinal cancer. Curr. Cancer Drug Targets 2013, 13, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Zhang, Y.; Luo, C.; Zhu, T.; Zhang, R.; Yao, R. LINC01210 accelerates proliferation, invasion and migration in ovarian cancer through epigenetically downregulating KLF4. Biomed. Pharmacother. = Biomed. Pharmacother. 2019, 119, 109431. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, A.; Ouyang, X.; Zhao, G.; Du, Z.; Huo, W.; Zhang, T.; Wang, Y.; Yang, C.; Dong, P.; et al. KLF4 expression enhances the efficacy of chemotherapy drugs in ovarian cancer cells. Biochem. Biophys. Res. Commun. 2017, 484, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.; Liu, W.; Zhao, G.; Lee, S.; Balogh, A.; Zou, Y.; Guo, Y.; Zhang, Z.; Gu, W.; et al. Doxycycline inducible Kruppel-like factor 4 lentiviral vector mediates mesenchymal to epithelial transition in ovarian cancer cells. PLoS ONE 2014, 9, e105331. [Google Scholar] [CrossRef]
- Roberts, D.M.; Pronobis, M.I.; Poulton, J.S.; Kane, E.G.; Peifer, M. Regulation of Wnt signaling by the tumor suppressor adenomatous polyposis coli does not require the ability to enter the nucleus or a particular cytoplasmic localization. Mol. Biol. Cell 2012, 23, 2041–2056. [Google Scholar] [CrossRef]
- Xing, R. miR-3648 Promotes Prostate Cancer Cell Proliferation by Inhibiting Adenomatous Polyposis Coli 2. J. Nanosci. Nanotechnol. 2019, 19, 7526–7531. [Google Scholar] [CrossRef]
- Geng, Y.; Zheng, X.; Hu, W.; Wang, Q.; Xu, Y.; He, W.; Wu, C.; Zhu, D.; Jiang, J. Hsa_circ_0009361 acts as the sponge of miR-582 to suppress colorectal cancer progression by regulating APC2 expression. Clin. Sci. 2019, 133, 1197–1213. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Z.; Zhang, L.; Chen, Y.; Li, N.; Lv, Y.; Li, Y.; Xu, X. miR-4326 promotes lung cancer cell proliferation through targeting tumor suppressor APC2. Mol. Cell. Biochem. 2018, 443, 151–157. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, W.; Jiang, C. Overexpressing circular RNA hsa_circ_0002052 impairs osteosarcoma progression via inhibiting Wnt/beta-catenin pathway by regulating miR-1205/APC2 axis. Biochem. Biophys. Res. Commun. 2018, 502, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Beta, M.; Chitipothu, S.; Khetan, V.; Biswas, J.; Krishnakumar, S. Hypermethylation of adenomatosis polyposis coli-2 and its tumor suppressor role in retinoblastoma. Curr. Eye Res. 2015, 40, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Li, G.; Xu, C.; Hui, Y. MicroRNA miR-1249 downregulates adenomatous polyposis coli 2 expression and promotes glioma cells proliferation. Am. J. Transl. Res. 2018, 10, 1324–1336. [Google Scholar] [PubMed]
- Mokarram, P.; Kumar, K.; Brim, H.; Naghibalhossaini, F.; Saberi-firoozi, M.; Nouraie, M.; Green, R.; Lee, E.; Smoot, D.T.; Ashktorab, H. Distinct high-profile methylated genes in colorectal cancer. PLoS ONE 2009, 4, e7012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, J.; Yang, L.; Yuan, Y.C.; Tong, T.R.; Wu, J.; Yun, X.; Bonner, M.; Pangeni, R.; Liu, Z.; et al. Targeting histone methyltransferase G9a inhibits growth and Wnt signaling pathway by epigenetically regulating HP1alpha and APC2 gene expression in non-small cell lung cancer. Mol. Cancer 2018, 17, 153. [Google Scholar] [CrossRef]
- Mohamed, N.E.; Hay, T.; Reed, K.R.; Smalley, M.J.; Clarke, A.R. APC2 is critical for ovarian WNT signalling control, fertility and tumour suppression. BMC Cancer 2019, 19, 677. [Google Scholar] [CrossRef]
- Ying, X.; Li-ya, Q.; Feng, Z.; Yin, W.; Ji-hong, L. MiR-939 promotes the proliferation of human ovarian cancer cells by repressing APC2 expression. Biomed. Pharmacother. = Biomed. Pharmacother. 2015, 71, 64–69. [Google Scholar] [CrossRef]
- Seagle, B.L.; Eng, K.H.; Yeh, J.Y.; Dandapani, M.; Schiller, E.; Samuelson, R.; Odunsi, K.; Shahabi, S. Discovery of candidate tumor biomarkers for treatment with intraperitoneal chemotherapy for ovarian cancer. Sci. Rep. 2016, 6, 21591. [Google Scholar] [CrossRef]
- Gaitanou, M.; Segklia, K.; Matsas, R. Cend1, a Story with Many Tales: From Regulation of Cell Cycle Progression/Exit of Neural Stem Cells to Brain Structure and Function. Stem Cells Int. 2019, 2019, 2054783. [Google Scholar] [CrossRef]
- Tsioras, K.; Papastefanaki, F.; Politis, P.K.; Matsas, R.; Gaitanou, M. Functional Interactions between BM88/Cend1, Ran-binding protein M and Dyrk1B kinase affect cyclin D1 levels and cell cycle progression/exit in mouse neuroblastoma cells. PLoS ONE 2013, 8, e82172. [Google Scholar] [CrossRef]
- Fleischer, T.; Frigessi, A.; Johnson, K.C.; Edvardsen, H.; Touleimat, N.; Klajic, J.; Riis, M.L.; Haakensen, V.D.; Wärnberg, F.; Naume, B.; et al. Genome-wide DNA methylation profiles in progression to in situ and invasive carcinoma of the breast with impact on gene transcription and prognosis. Genome Biol. 2014, 15, 435. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, R.; Hutchison, C.; Lane, B. Intermediate filament proteins. Protein Profile 1994, 1, 779–911. [Google Scholar] [PubMed]
- Li, W.; Bai, X.; Li, J.; Zhao, Y.; Liu, J.; Zhao, H.; Liu, L.; Ding, M.; Wang, Q.; Shi, F.Y.; et al. The nucleoskeleton protein IFFO1 immobilizes broken DNA and suppresses chromosome translocation during tumorigenesis. Nat. Cell Biol. 2019, 21, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Campan, M.; Moffitt, M.; Houshdaran, S.; Shen, H.; Widschwendter, M.; Daxenbichler, G.; Long, T.; Marth, C.; Laird-Offringa, I.A.; Press, M.F.; et al. Genome-scale screen for DNA methylation-based detection markers for ovarian cancer. PLoS ONE 2011, 6, e28141. [Google Scholar] [CrossRef]
- Christie, M.; Jorissen, R.N.; Mouradov, D.; Sakthianandeswaren, A.; Li, S.; Day, F.; Tsui, C.; Lipton, L.; Desai, J.; Jones, I.T.; et al. Different APC genotypes in proximal and distal sporadic colorectal cancers suggest distinct WNT/beta-catenin signalling thresholds for tumourigenesis. Oncogene 2013, 32, 4675–4682. [Google Scholar] [CrossRef]
- Kypta, R.M.; Waxman, J. Wnt/beta-catenin signalling in prostate cancer. Nat. Rev. Urol. 2012, 9, 418–428. [Google Scholar] [CrossRef]
- Lin, S.Y.; Xia, W.; Wang, J.C.; Kwong, K.Y.; Spohn, B.; Wen, Y.; Pestell, R.G.; Hung, M.C. Beta-catenin, a novel prognostic marker for breast cancer: Its roles in cyclin D1 expression and cancer progression. Proc. Natl. Acad. Sci. USA 2000, 97, 4262–4266. [Google Scholar] [CrossRef]
- Kovacs, D.; Migliano, E.; Muscardin, L.; Silipo, V.; Catricala, C.; Picardo, M.; Bellei, B. The role of Wnt/beta-catenin signaling pathway in melanoma epithelial-to-mesenchymal-like switching: Evidences from patients-derived cell lines. Oncotarget 2016, 7, 43295–43314. [Google Scholar] [CrossRef]
- Teeuwssen, M.; Fodde, R. Wnt Signaling in Ovarian Cancer Stemness, EMT, and Therapy Resistance. J. Clin. Med. 2019, 8, 1658. [Google Scholar] [CrossRef]
- Nguyen, V.H.L.; Hough, R.; Bernaudo, S.; Peng, C. Wnt/beta-catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. J. Ovarian Res. 2019, 12, 122. [Google Scholar] [CrossRef]
- Andersen, J.S.; Lyon, C.E.; Fox, A.H.; Leung, A.K.L.; Lam, Y.W.; Steen, H.; Mann, M.; Lamond, A.I. Directed Proteomic Analysis of the Human Nucleolus. Curr. Biol. 2002, 12, 1–11. [Google Scholar] [CrossRef]
- Juhling, F.; Kretzmer, H.; Bernhart, S.H.; Otto, C.; Stadler, P.F.; Hoffmann, S. metilene: Fast and sensitive calling of differentially methylated regions from bisulfite sequencing data. Genome Res. 2016, 26, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dionne, U.; Chartier, F.J.M.; Lopez de Los Santos, Y.; Lavoie, N.; Bernard, D.N.; Banerjee, S.L.; Otis, F.; Jacquet, K.; Tremblay, M.G.; Jain, M.; et al. Direct Phosphorylation of SRC Homology 3 Domains by Tyrosine Kinase Receptors Disassembles Ligand-Induced Signaling Networks. Mol. Cell 2018, 70, 995–1007.e11. [Google Scholar] [CrossRef]

| A. Description of the SKOV3 Clones Used (as Described in [14]) | Phenotype |
| shC-S: control shRNA expressed in SKOV3 cells | Mesenchymal (M) |
| sh-LY75-S: shRNA-mediated LY75-KD in SKOV3 cells | Epithelial (E) |
| LY75-KO-S: CRISPR/Cas9-mediated Ly75 KO in SKOV3 cells | Epithelial (E) |
| LY75-shR-S: sh-resistant-Ly75 cDNA expressed in sh-LY75-S cells | Mesenchymal (M) |
| B. Experimental Comparison Combinations Used for RRBS Analysis | Number of Differently Methylated Regions (Hypo + Hyper) Identified in Exons and Promoter Regions of Different Genes |
| shC-S vs. sh-LY75-S (M vs. E) | 10,722 |
| LY75-R-S vs. LY75-KO-S (M vs. E) | 11,014 |
| (mix: shC-S + LY75-R-S) vs. (mix: sh-LY75-S + LY5-KO-S) (M vs. E) | 9105 |
| Ly75 Partners Identified by Scaffold | M Weight |
|---|---|
| AHNAK: Neuroblast differentiation-associated protein | 629 kDa |
| PLEC: Isoform 4 of Plectin | 516 kDa |
| DSP: Desmoplakin | 332 kDa |
| SPTAN1: Spectrin alpha chain, nonerythrocytic | 285 kDa |
| SPTBN1: Spectrin beta chain | 275 kDa |
| CAD protein | 243 kDa |
| RRBP1: Ribosome-binding protein 1 | 152 kDa |
| ACTN4: Alpha-actinin-4 | 105 kDa |
| MVP: Major vault protein | 99 kDa |
| DNM2: Isoform 2 of Dynamin-2 | 98 kDa |
| HSD17B4: Peroxisomal multifunctional enzyme type 2 | 80 kDa |
| HNRNPM: Heterogeneous nuclear ribonucleoprotein M | 78 kDa |
| LMNA: Prelamin-A/C | 74 kDa |
| PABPC1: Polyadenylate-binding protein 1 | 71 kDa |
| SEPT9: Septin-9 | 65 kDa |
| KRT8: Keratin, type II cytoskeletal 8 | 54 kDa |
| SPATS2L: Isoform 2 of SPATS2-like protein | 54 kDa |
| KRT7: Keratin, type II cytoskeletal 7 | 51 kDa |
| KRT18: Keratin, type I cytoskeletal 18 | 48 kDa |
| KHDRBS1: KH domain-containing, RNA-binding, signal transduction-associated protein 1 | 48 kDa |
| KRT19: Keratin, type I cytoskeletal 19 | 44 kDa |
| RPL6: 60S ribosomal protein L6 | 33 kDa |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdi, S.; Bachvarova, M.; Scott-Boyer, M.-P.; Droit, A.; Bachvarov, D. LY75 Ablation Mediates Mesenchymal-Epithelial Transition (MET) in Epithelial Ovarian Cancer (EOC) Cells Associated with DNA Methylation Alterations and Suppression of the Wnt/β-Catenin Pathway. Int. J. Mol. Sci. 2020, 21, 1848. https://doi.org/10.3390/ijms21051848
Mehdi S, Bachvarova M, Scott-Boyer M-P, Droit A, Bachvarov D. LY75 Ablation Mediates Mesenchymal-Epithelial Transition (MET) in Epithelial Ovarian Cancer (EOC) Cells Associated with DNA Methylation Alterations and Suppression of the Wnt/β-Catenin Pathway. International Journal of Molecular Sciences. 2020; 21(5):1848. https://doi.org/10.3390/ijms21051848
Chicago/Turabian StyleMehdi, Sadia, Magdalena Bachvarova, Marie-Pier Scott-Boyer, Arnaud Droit, and Dimcho Bachvarov. 2020. "LY75 Ablation Mediates Mesenchymal-Epithelial Transition (MET) in Epithelial Ovarian Cancer (EOC) Cells Associated with DNA Methylation Alterations and Suppression of the Wnt/β-Catenin Pathway" International Journal of Molecular Sciences 21, no. 5: 1848. https://doi.org/10.3390/ijms21051848
APA StyleMehdi, S., Bachvarova, M., Scott-Boyer, M.-P., Droit, A., & Bachvarov, D. (2020). LY75 Ablation Mediates Mesenchymal-Epithelial Transition (MET) in Epithelial Ovarian Cancer (EOC) Cells Associated with DNA Methylation Alterations and Suppression of the Wnt/β-Catenin Pathway. International Journal of Molecular Sciences, 21(5), 1848. https://doi.org/10.3390/ijms21051848





