Modulation of the In Vivo Inflammatory Response by Pro- Versus Anti-Inflammatory Intervertebral Disc Treatments
Abstract
:1. Introduction
2. Results
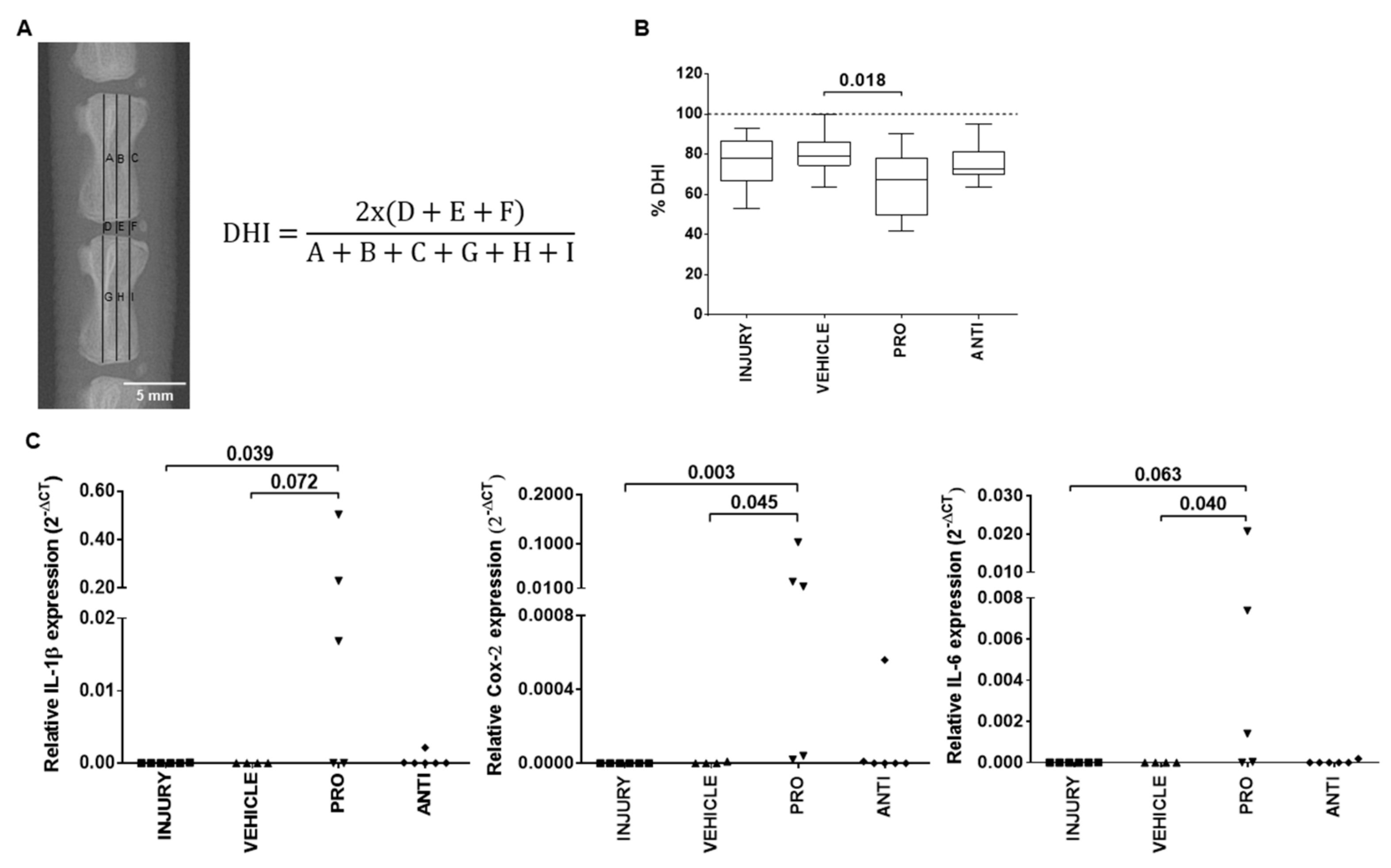
2.1. Comparison of Intradiscal Pro-/Anti-Inflammatory Treatments in Disc Height Index and Local Inflammatory Response
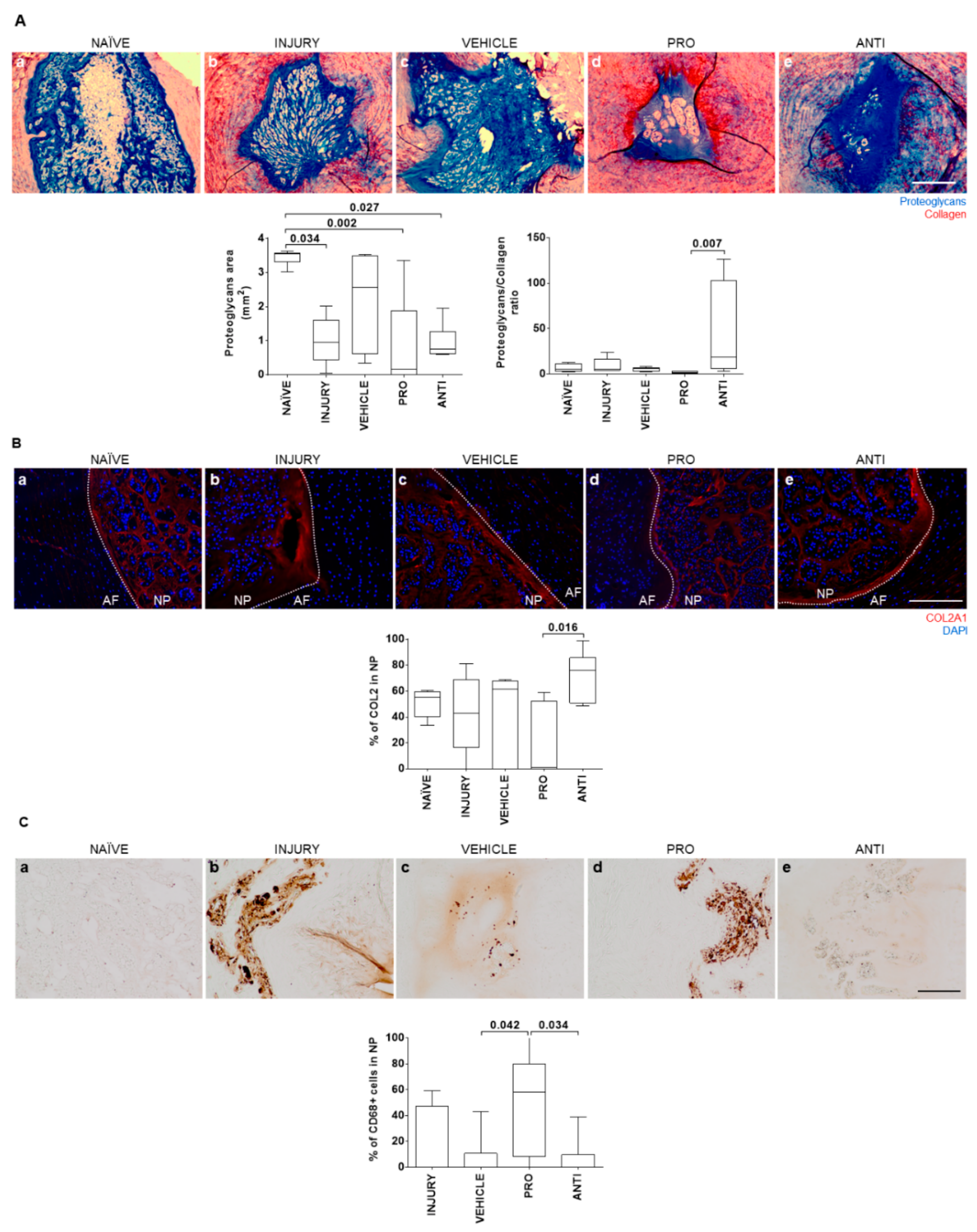
2.2. Comparison of Intradiscal Pro-/Anti-Inflammatory Treatments in NP ECM
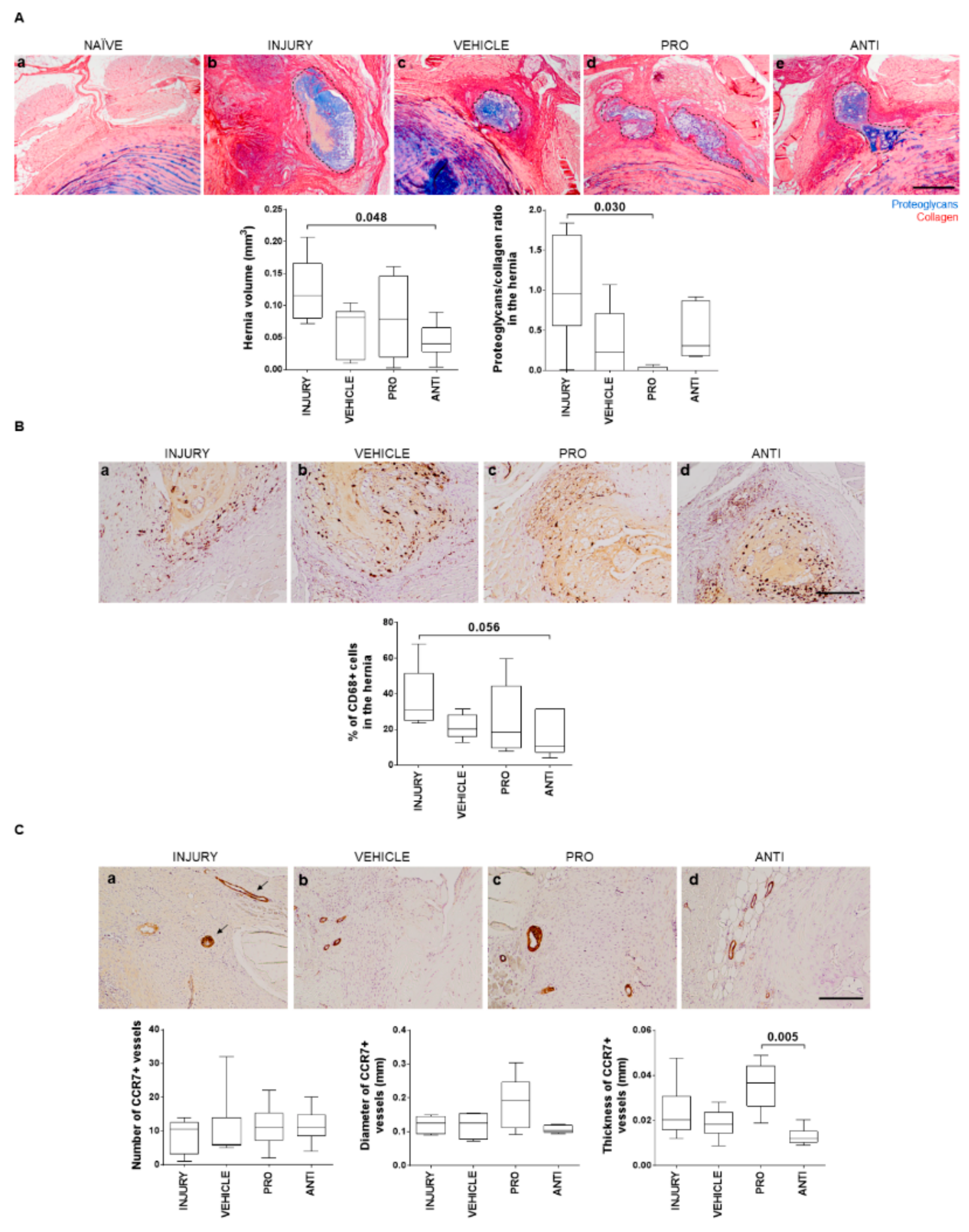
2.3. Comparison of Intradiscal Pro-/Anti-Inflammatory Treatments in Disc Herniation
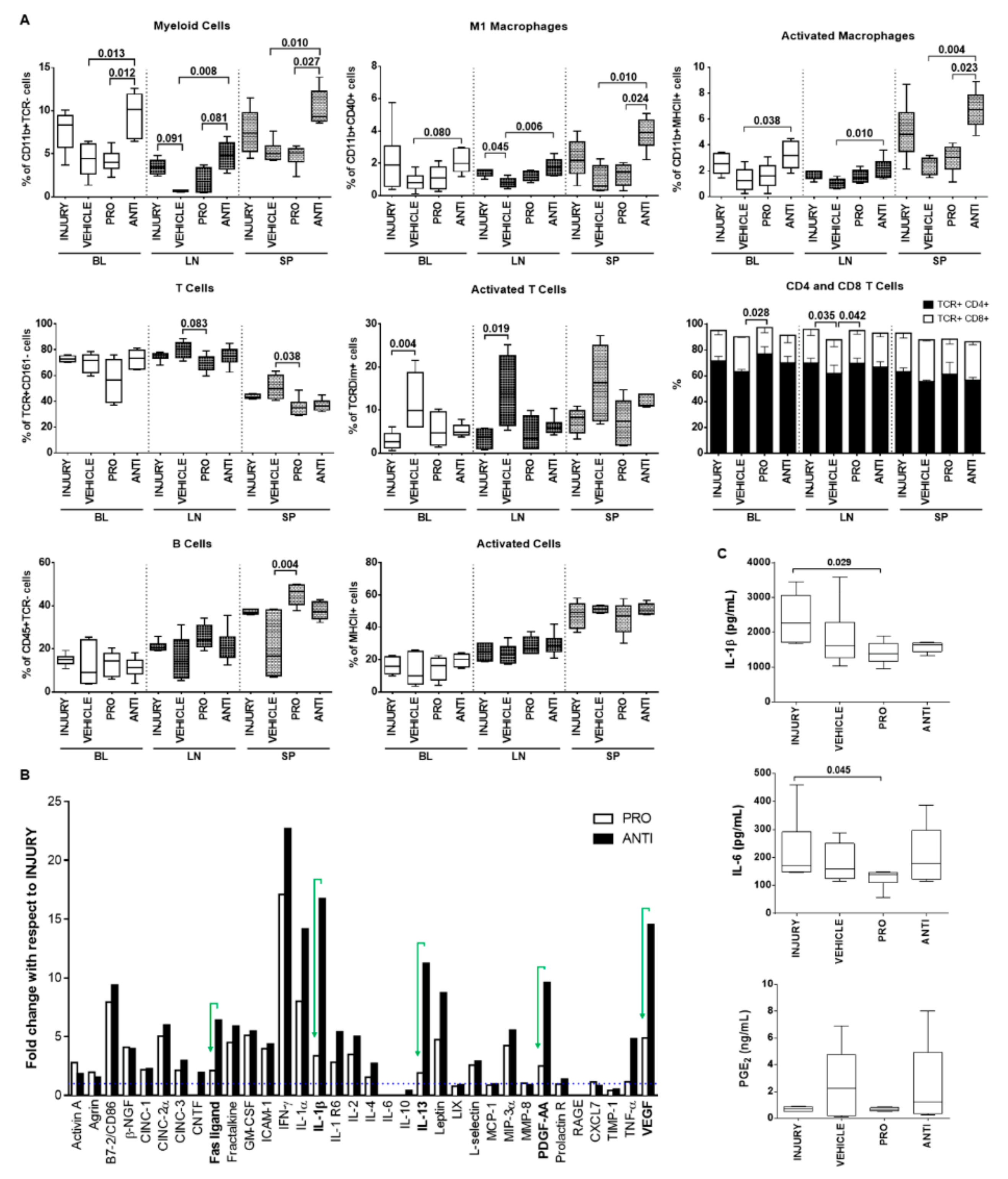
2.4. Comparison of Intradiscal Pro-/Anti-Inflammatory Treatments in Systemic Immune Response
3. Discussion
4. Materials and Methods
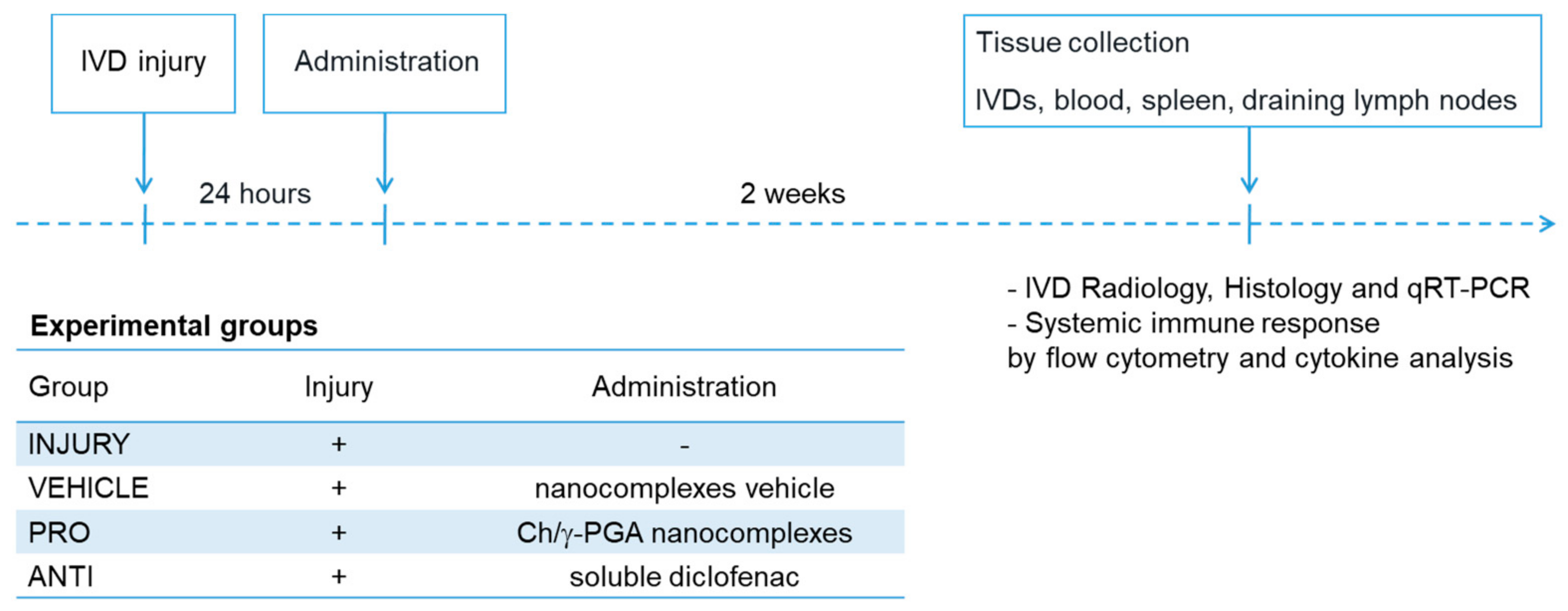
4.1. Animal Experimentation
4.2. Determination of the Disc Height Index
4.3. RNA Isolation and Quantitative Real-Time qPCR
4.4. IVD Collection and Histological Analysis
4.5. Detection of Collagen Type II in the IVD
4.6. Detection of CD68+ and CCR7+ Cells
4.7. Flow Cytometry Analysis of Systemic Immune Cell Populations
4.8. Plasma Cytokine Quantification
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IVD | Intervertebral disc |
| Df | Diclofenac |
| Ch/γ-PGA | Chitosan/poly-γ-glutamic acid |
| NCs | Nanocomplexes |
Appendix A
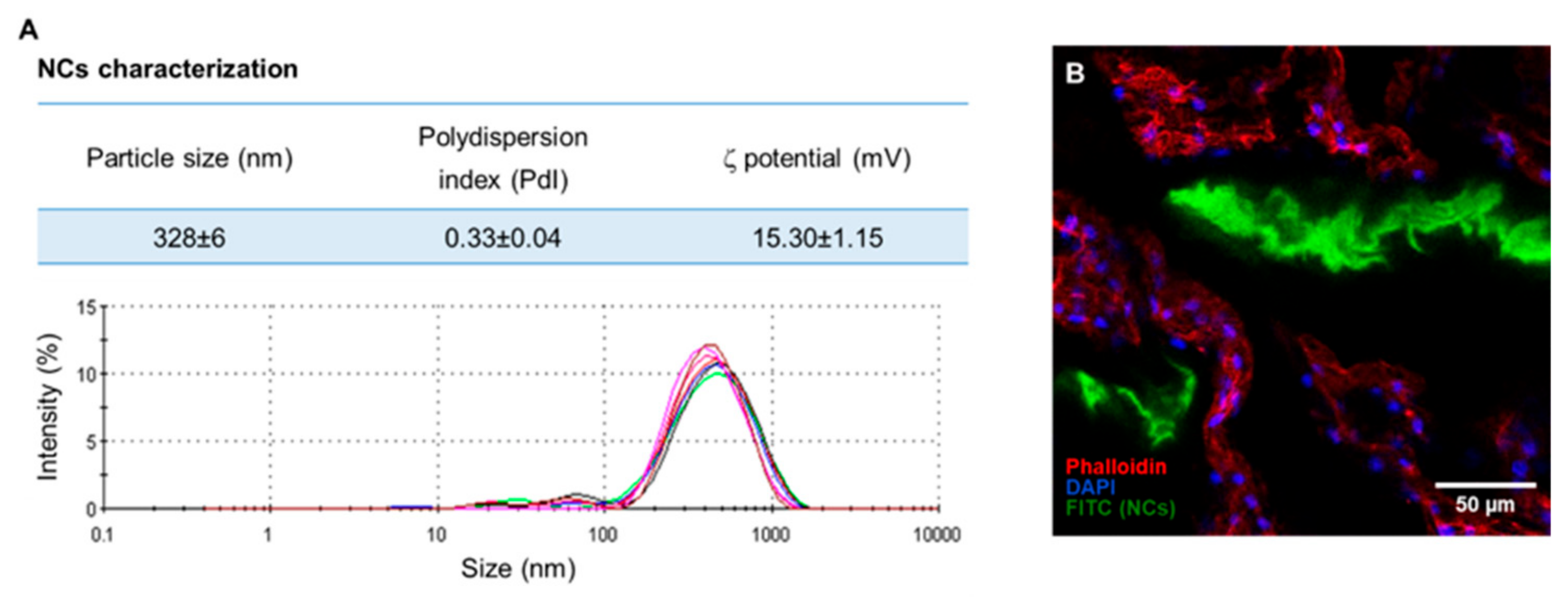
Appendix B
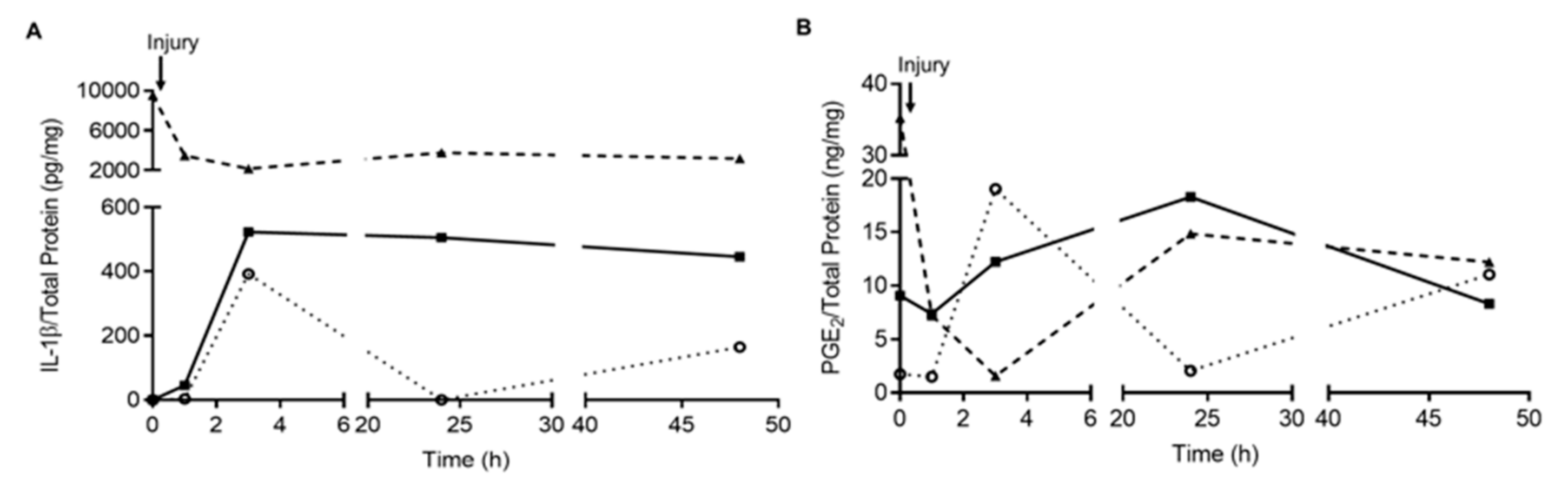
References
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Goncalves, R.M.; Barbosa, M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 2015, 12, 20141191. [Google Scholar] [CrossRef]
- Raj, P.P. Intervertebral disc: Anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef]
- Shen, F.H.; Samartzis, D.; Andersson, G.B. Nonsurgical management of acute and chronic low back pain. J. Am. Acad. Orthop. Surg. 2006, 14, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.M.; Yerramalli, C.S.; Beckstein, J.C.; Boxberger, J.I.; Johannessen, W.; Vresilovic, E.J. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine 2008, 33, 588–596. [Google Scholar] [CrossRef]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, K.; Aota, Y.; Muehleman, C.; Imai, Y.; Okuma, M.; Thonar, E.J.; Andersson, G.B.; An, H.S. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: Correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 2005, 30, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Iatridis, J.C.; Michalek, A.J.; Purmessur, D.; Korecki, C.L. Localized intervertebral disc injury leads to organ level changes in structure, cellularity, and biosynthesis. Cell Mol. Bioeng. 2009, 2, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Illien-Jünger, S.; Pattappa, G.; Peroglio, M.; Benneker, L.M.; Stoddart, M.J.; Sakai, D.; Mochida, J.; Grad, S.; Alini, M. Homing of mesenchymal stem cells in induced degenerative intervertebral discs in a whole organ culture system. Spine 2012, 37, 1865–1873. [Google Scholar] [CrossRef]
- Yang, H.; Cao, C.; Wu, C.; Yuan, C.; Gu, Q.; Shi, Q.; Zou, J. TGF-βl suppresses inflammation in cell therapy for intervertebral disc degeneration. Sci. Rep. 2015, 5, 13254. [Google Scholar] [CrossRef] [Green Version]
- Cunha, C.; Lamas, S.; Gonçalves, R.M.; Barbosa, M.A. Joint analysis of IVD herniation and degeneration by rat caudal needle puncture model. J. Orthop. Res. 2017, 35, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Cunha, C.; Almeida, C.R.; Almeida, M.I.; Silva, A.M.; Molinos, M.; Lamas, S.; Pereira, C.L.; Teixeira, G.Q.; Monteiro, A.T.; Santos, S.G.; et al. Systemic delivery of bone marrow mesenchymal stem cells for in situ intervertebral disc regeneration. Stem Cells Transl. Med. 2017, 6, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.; Ghosh, P.; Jenkin, G.; Oehme, D.; Goldschlager, T. A review of animal models of intervertebral disc degeneration: Pathophysiology, regeneration, and translation to the clinic. Biomed. Res. Int. 2016, 2016, 5952165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.M.; Gonçalves, R.M.; Almeida, C.R.; Pereira, I.O.; Oliveira, M.I.; Neves, N.; Silva, A.M.; Ribeiro, A.C.; Cunha, C.; Almeida, A.R.; et al. Fibrinogen scaffolds with immunomodulatory properties promote in vivo bone regeneration. Biomaterials 2016, 111, 163–178. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, D.P.; Costa, M.; Neves, N.; Teixeira, J.H.; Vasconcelos, D.M.; Santos, S.G.; Águas, A.P.; Barbosa, M.A.; Barbosa, J.N. Chitosan porous 3D scaffolds embedded with resolvin D1 to improve in vivo bone healing. J. Biomed. Mater. Res. A 2018, 106, 1626–1633. [Google Scholar] [CrossRef]
- Gonçalves, R.M.; Pereira, A.C.; Pereira, I.O.; Oliveira, M.J.; Barbosa, M.A. Macrophage response to chitosan/poly-(γ-glutamic acid) nanoparticles carrying an anti-inflammatory drug. J. Mat. Sci. Mat. Med. 2015, 26, 167. [Google Scholar] [CrossRef]
- Castro, F.; Pinto, M.L.; Silva, A.M.; Pereira, C.L.; Teixeira, G.Q.; Gomez-Lazaro, M.; Santos, S.G.; Barbosa, M.A.; Gonçalves, R.M.; Oliveira, M.J. Pro-inflammatory chitosan/poly(gamma-glutamic acid) nanoparticles modulate human antigen-presenting cells phenotype and revert their pro-invasive capacity. Acta Biomater. 2017, 63, 96–109. [Google Scholar] [CrossRef]
- Teixeira, G.Q.; Boldt, A.; Nagl, I.; Pereira, C.L.; Benz, K.; Wilke, H.J.; Ignatius, A.; Barbosa, M.A.; Gonçalves, R.M.; Neidlinger-Wilke, C. A degenerative/proinflammatory intervertebral disc organ culture: An ex vivo model for anti-inflammatory drug and cell therapy. Tissue Eng. Part C Methods 2016, 22, 8–19. [Google Scholar] [CrossRef]
- Kobayashi, S.; Meir, A.; Kokubo, Y.; Uchida, K.; Takeno, K.; Miyazaki, T.; Yayama, T.; Kubota, M.; Nomura, E.; Mwaka, E.; et al. Ultrastructural analysis on lumbar disc herniation using surgical specimens: Role of neovascularization and macrophages in hernias. Spine 2009, 34, 655–662. [Google Scholar] [CrossRef]
- Castro, F.; Pinto, M.L.; Almeida, R.; Pereira, F.; Silva, A.M.; Pereira, C.L.; Santos, S.G.; Barbosa, M.A.; Gonçalves, R.M.; Oliveira, M.J. Chitosan/poly(γ-glutamic acid) nanoparticles incorporating IFN-γ for immune response modulation in the context of colorectal cancer. Biomater. Sci. 2019, 7, 3386–3403. [Google Scholar] [CrossRef]
- Stout, A. Discography. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Evashwick-Rogler, T.W.; Lai, A.; Watanabe, H.; Salandra, J.M.; Winkelstein, B.A.; Cho, S.K.; Hecht, A.C.; Iatridis, J.C. Inhibiting tumor necrosis factor-alpha at time of induced intervertebral disc injury limits long-term pain and degeneration in a rat model. JOR Spine 2018, 1, e1014. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.Q.; Pereira, C.L.; Castro, F.; Ferreira, J.R.; Gomez-Lazaro, M.; Aguiar, P.; Barbosa, M.A.; Neidlinger-Wilke, C.; Goncalves, R.M. Anti-inflammatory Chitosan/Poly-γ-glutamic acid nanoparticles control inflammation while remodeling extracellular matrix in degenerated intervertebral disc. Acta Biomater. 2016, 42, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, P.; Guo, H.F.; Liu, L.; Liu, X.D. Pharmacokinetic-pharmacodynamic modeling of diclofenac in normal and Freund’s complete adjuvant-induced arthritic rats. Acta Pharmacol. Sin. 2012, 33, 1372–1378. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, N.; Sekiguchi, M.; Kikuchi, S.; Konno, S. Anti-nociceptive effect of bovine milk-derived lactoferrin in a rat lumbar disc herniation model. Spine 2010, 35, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Jiang, L.; Zhuang, C.; Yang, Y.; Zhang, Z.; Chen, W.; Zheng, T. Intradiscal injection therapy for degenerative chronic discogenic low back pain with end plate Modic changes. Spine, J. 2011, 11, 100–106. [Google Scholar] [CrossRef]
- Kang, S.S.; Hwang, B.M.; Son, H.J.; Cheong, I.Y.; Lee, S.J.; Lee, S.H.; Chung, T.Y. The dosages of corticosteroid in transforaminal epidural steroid injections for lumbar radicular pain due to a herniated disc. Pain Physician 2011, 14, 361–370. [Google Scholar]
- Kato, M.; Nishida, S.; Kitasato, H.; Sakata, N.; Kawai, S. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs: Investigation using human peripheral monocytes. J. Pharm. Pharmacol. 2001, 53, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Cornefjord, M.; Olmarker, K.; Otani, K.; Rydevik, B. Nucleus pulposus-induced nerve root injury: Effects of diclofenac and ketoprofen. Eur. Spine J. 2002, 11, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Richy, F.; Bruyere, O.; Ethgen, O.; Rabenda, V.; Bouvenot, G.; Audran, M.; Herrero-Beaumont, G.; Moore, A.; Eliakim, R.; Haim, M.; et al. Time dependent risk of gastrointestinal complications induced by non-steroidal anti-inflammatory drug use: A consensus statement using a meta-analytic approach. Ann. Rheum. Dis. 2004, 63, 759–766. [Google Scholar] [CrossRef] [Green Version]
- Matta, A.; Karim, M.Z.; Isenman, D.E.; Erwin, W.M. Molecular therapy for degenerative disc disease: Clues from secretome analysis of the notochordal cell-rich nucleus pulposus. Sci. Rep. 2017, 7, 45623. [Google Scholar] [CrossRef] [Green Version]
- Antunes, J.C.; Pereira, C.L.; Teixeira, G.Q.; Silva, R.V.; Caldeira, J.; Grad, S.; Gonçalves, R.M.; Barbosa, M.A. Poly(gamma-glutamic acid) and poly(gamma-glutamic acid)-based nanocomplexes enhance type II collagen production in intervertebral disc. J. Mater. Sci. Mater. Med. 2017, 28, 6. [Google Scholar] [CrossRef]
- Antunes, J.C.; Tsaryk, R.; Gonçalves, R.M.; Pereira, C.L.; Landes, C.; Brochhausen, C.; Ghanaati, S.; Barbosa, M.A.; Kirkpatrick, C.J. Poly(γ-glutamic acid) as an exogenous promoter of chondrogenic differentiation of human mesenchymal stem/stromal cells. Tissue Eng. Part A 2015, 21, 1869–1885. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, G.D.; Vresilovic, E.J.; Elliott, D.M. Comparison of animals used in disc research to human lumbar disc geometry. Spine 2007, 32, 328–333. [Google Scholar] [CrossRef]
- Cuéllar, J.M.; Borges, P.M.; Cuéllar, V.G.; Yoo, A.; Scuderi, G.J.; Yeomans, D.C. Cytokine expression in the epidural space: A model of noncompressive disc herniation-induced inflammation. Spine 2013, 38, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbir, A.; Godburn, K.E.; Michalek, A.J.; Lai, A.; Monsey, R.D.; Iatridis, J.C. Effects of torsion on intervertebral disc gene expression and biomechanics, using a rat tail model. Spine 2011, 36, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLean, J.J.; Roughley, P.J.; Monsey, R.D.; Alini, M.; Iatridis, J.C. In vivo intervertebral disc remodeling: Kinetics of mRNA expression in response to a single loading event. J. Orthop. Res. 2008, 26, 579–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, H.S.; Takegami, K.; Kamada, H.; Nguyen, C.M.; Thonar, E.J.; Singh, K.; Andersson, G.B.; Masuda, K. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine 2005, 30, 25–32. [Google Scholar] [CrossRef]
- Walsh, A.J.; Bradford, D.S.; Lotz, J.C. In vivo growth factor treatment of degenerated intervertebral discs. Spine 2004, 29, 156–163. [Google Scholar] [CrossRef]
- Teixeira, J.H.; Silva, A.M.; Almeida, M.I.; Bessa-Gonçalves, M.; Cunha, C.; Barbosa, M.A.; Santos, S.G. The systemic immune response to collagen-induced arthritis and the impact of bone injury in inflammatory conditions. Int. J. Mol. Sci. 2019, 20, 5436. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Cao, P.; Gao, Y.; Wu, M.; Lin, Y.; Tian, Y.; Yuan, W. Differential expression of p38 MAPK alpha, beta, gamma, delta isoforms in nucleus pulposus modulates macrophage polarization in intervertebral disc degeneration. Sci. Rep. 2016, 6, 22182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamji, M.F.; Setton, L.A.; Jarvis, W.; So, S.; Chen, J.; Jing, L.; Bullock, R.; Isaacs, R.E.; Brown, C.; Richardson, W.J. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010, 62, 1974–1982. [Google Scholar] [PubMed] [Green Version]
- Akyol, S.; Tanriverdi, T.; Hanci, M. Do immunologic events in degenerative disc tissue alter the peripheral immune tolerance? WScJ 2012, 3, 45–49. [Google Scholar]
- Weber, K.T.; Satoh, S.; Alipui, D.O.; Virojanapa, J.; Levine, M.; Sison, C.; Quraishi, S.; Bloom, O.; Chahine, N.O. Exploratory study for identifying systemic biomarkers that correlate with pain response in patients with intervertebral disc disorders. Immunol. Res. 2015, 63, 170–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klyne, D.M.; Barbe, M.F.; van den Hoorn, W.; Hodges, P.W. ISSLS PRIZE IN CLINICAL SCIENCE 2018: Longitudinal analysis of inflammatory, psychological, and sleep-related factors following an acute low back pain episode-the good, the bad, and the ugly. Eur. Spine J. 2018, 27, 763–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallab, N.J.; Bao, Q.B.; Brown, T. Assessment of epidural versus intradiscal biocompatibility of PEEK implant debris: An in vivo rabbit model. Eur. Spine J. 2013, 22, 2740–2751. [Google Scholar] [CrossRef] [Green Version]
- Ogunleye, A.; Bhat, A.; Irorere, V.U.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.I.; Santos, S.G.; Oliveira, M.J.; Torres, A.L.; Barbosa, M.A. Chitosan drives anti-inflammatory macrophage polarisation and pro-inflammatory dendritic cell stimulation. Eur. Cells Mat. 2012, 24, 152–153. [Google Scholar] [CrossRef]
- Khan, J.; Noboru, N.; Imamura, Y.; Eliav, E. Effect of Pregabalin and Diclofenac on tactile allodynia, mechanical hyperalgesia and pro inflammatory cytokine levels (IL-6, IL-1β) induced by chronic constriction injury of the infraorbital nerve in rats. Cytokine 2018, 104, 124–129. [Google Scholar] [CrossRef]
- Muroi, C.; Hugelshofer, M.; Seule, M.; Keller, E. The impact of nonsteroidal anti-inflammatory drugs on inflammatory response after aneurysmal subarachnoid hemorrhage. Neurocrit. Care 2014, 20, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.L.; Antunes, J.C.; Gonçalves, R.M.; Ferreira-da-Silva, F.; Barbosa, M.A. Biosynthesis of highly pure poly-γ-glutamic acid for biomedical applications. J. Mater. Sci. Mater. Med. 2012, 23, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Pereira, C.L.; Molinos, M.; Ferreira-da-Silva, F.; Dessì, M.; Gloria, A.; Ambrosio, L.; Gonçalves, R.M.; Barbosa, M.A. Layer-by-layer self-assembly of chitosan and poly(γ-glutamic acid) into polyelectrolyte complexes. Biomacromolecules 2011, 12, 4183–4195. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ma, H.; Cen, N.; Zhou, A.; Tao, H. A pharmacokinetic study of diclofenac sodium in rats. Biomed. Rep. 2017, 7, 179–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Ruifrok, A.C.; Johnston, D.A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, C.; Q. Teixeira, G.; Ribeiro-Machado, C.; L. Pereira, C.; Ferreira, J.R.; Molinos, M.; G. Santos, S.; Barbosa, M.A.; M. Goncalves, R. Modulation of the In Vivo Inflammatory Response by Pro- Versus Anti-Inflammatory Intervertebral Disc Treatments. Int. J. Mol. Sci. 2020, 21, 1730. https://doi.org/10.3390/ijms21051730
Cunha C, Q. Teixeira G, Ribeiro-Machado C, L. Pereira C, Ferreira JR, Molinos M, G. Santos S, Barbosa MA, M. Goncalves R. Modulation of the In Vivo Inflammatory Response by Pro- Versus Anti-Inflammatory Intervertebral Disc Treatments. International Journal of Molecular Sciences. 2020; 21(5):1730. https://doi.org/10.3390/ijms21051730
Chicago/Turabian StyleCunha, Carla, Graciosa Q. Teixeira, Cláudia Ribeiro-Machado, Catarina L. Pereira, Joana R. Ferreira, Maria Molinos, Susana G. Santos, Mário A. Barbosa, and Raquel M. Goncalves. 2020. "Modulation of the In Vivo Inflammatory Response by Pro- Versus Anti-Inflammatory Intervertebral Disc Treatments" International Journal of Molecular Sciences 21, no. 5: 1730. https://doi.org/10.3390/ijms21051730
APA StyleCunha, C., Q. Teixeira, G., Ribeiro-Machado, C., L. Pereira, C., Ferreira, J. R., Molinos, M., G. Santos, S., Barbosa, M. A., & M. Goncalves, R. (2020). Modulation of the In Vivo Inflammatory Response by Pro- Versus Anti-Inflammatory Intervertebral Disc Treatments. International Journal of Molecular Sciences, 21(5), 1730. https://doi.org/10.3390/ijms21051730






