Depletion of CD4 and CD8 Positive T Cells Impairs Venous Thrombus Resolution in Mice
Abstract
1. Introduction
2. Results
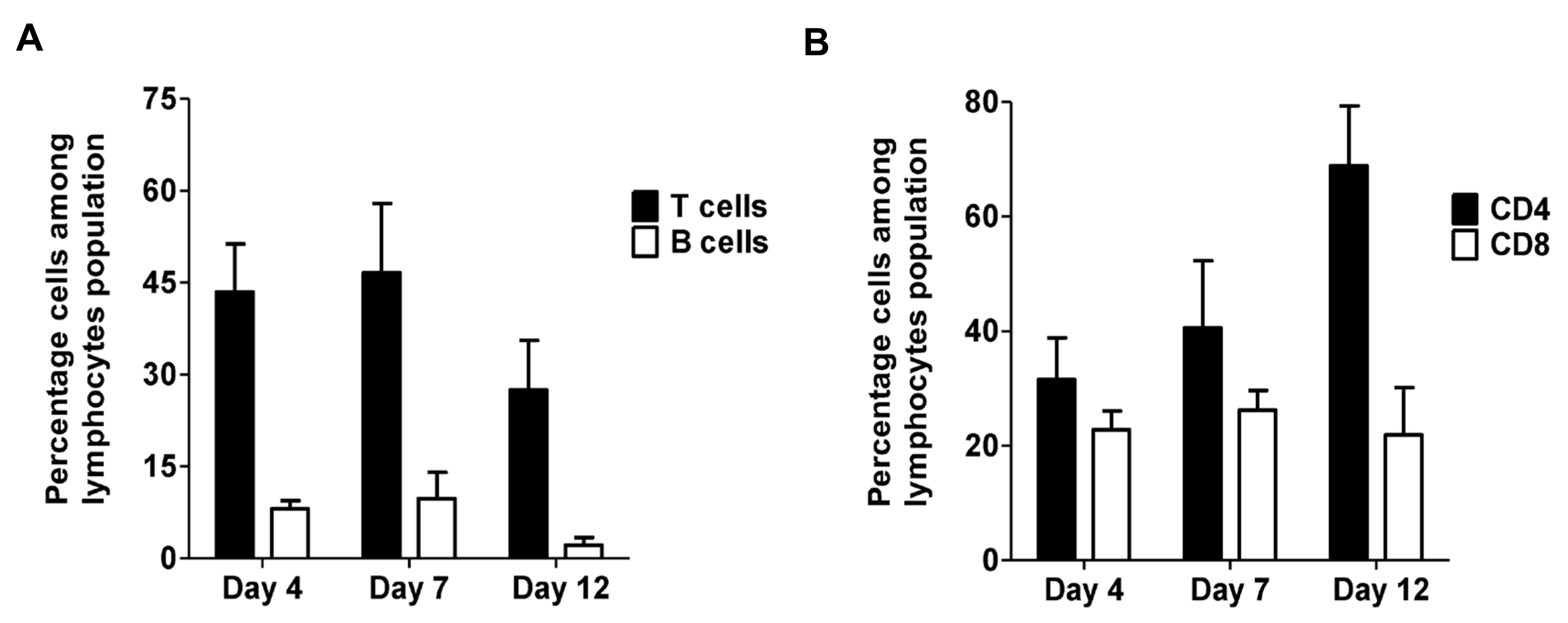
2.1. T Cells Are Present in Both Early and Late Stages of Venous Thrombosis
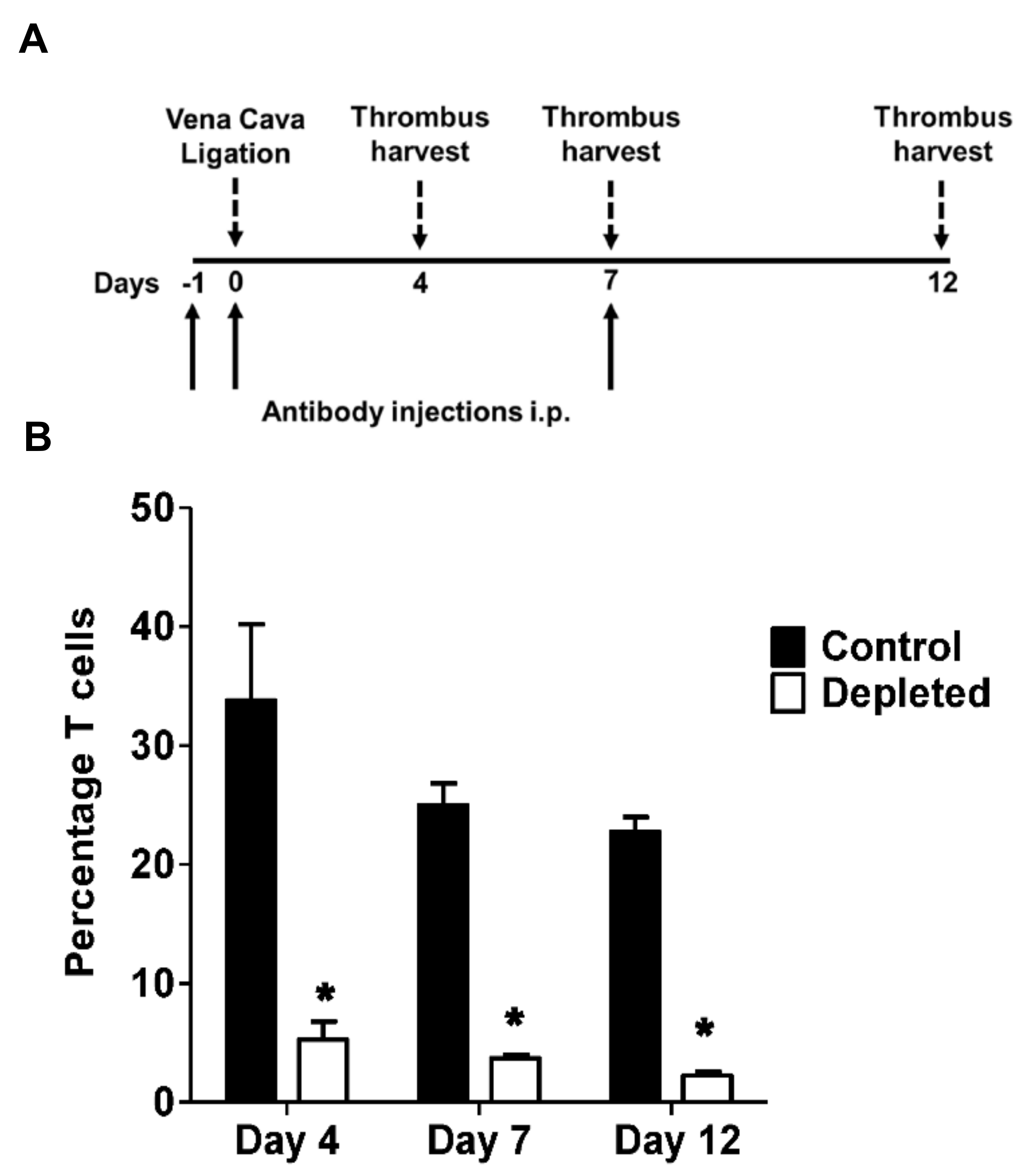
2.2. T Cell Depletion Impairs Venous Thrombus Resolution
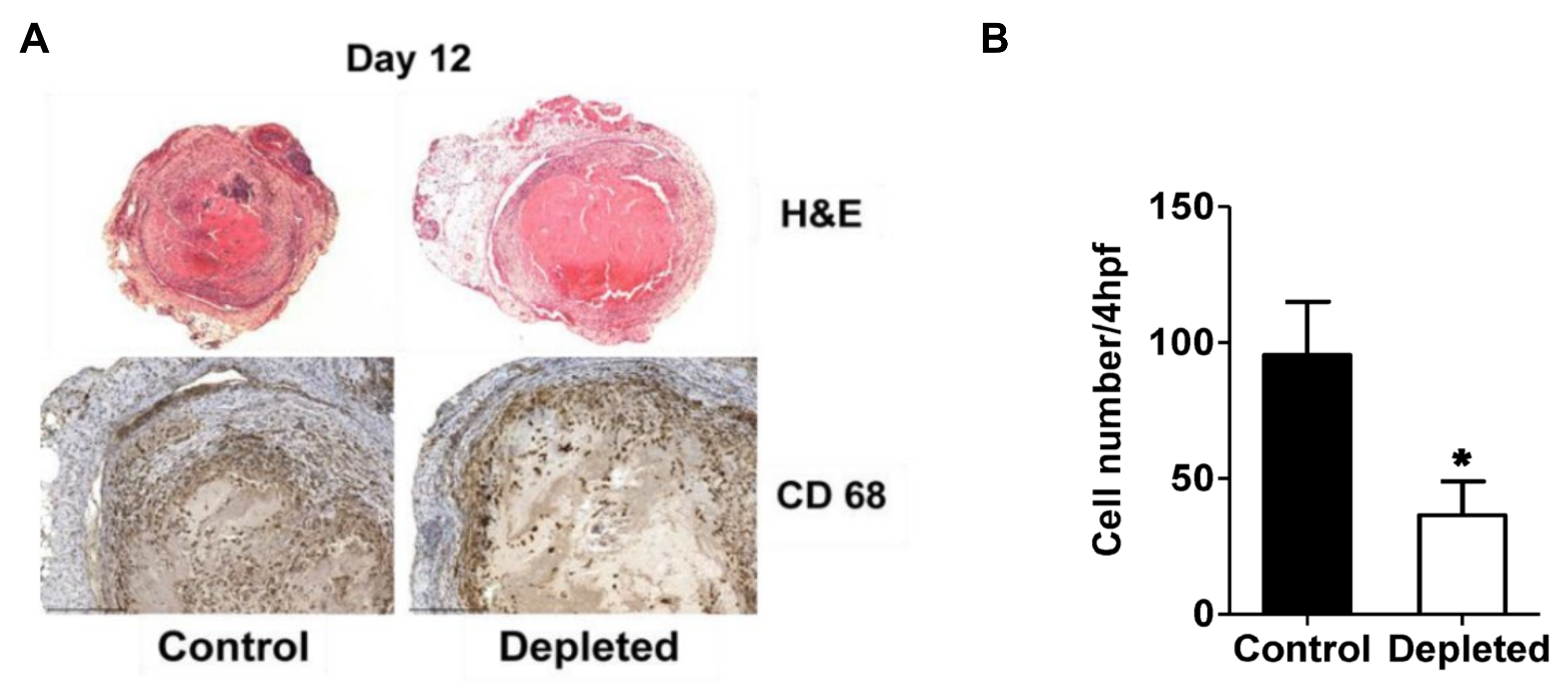
2.3. T Cell Depleted Thrombi Have Decreased Macrophage Infiltration
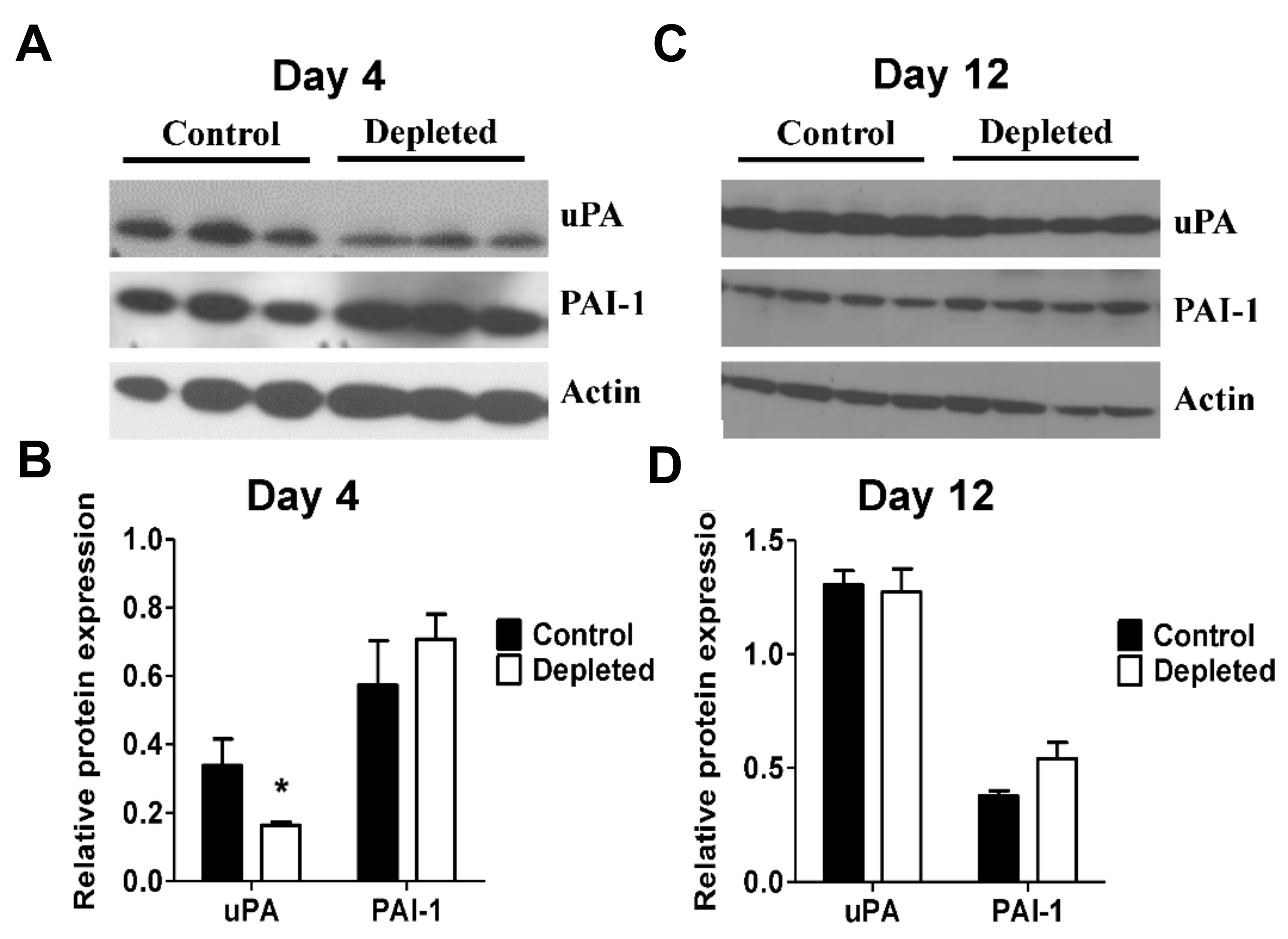
2.4. T Cell Depletion Decreases Fibrinolytic Activity in the Resolving Thrombus
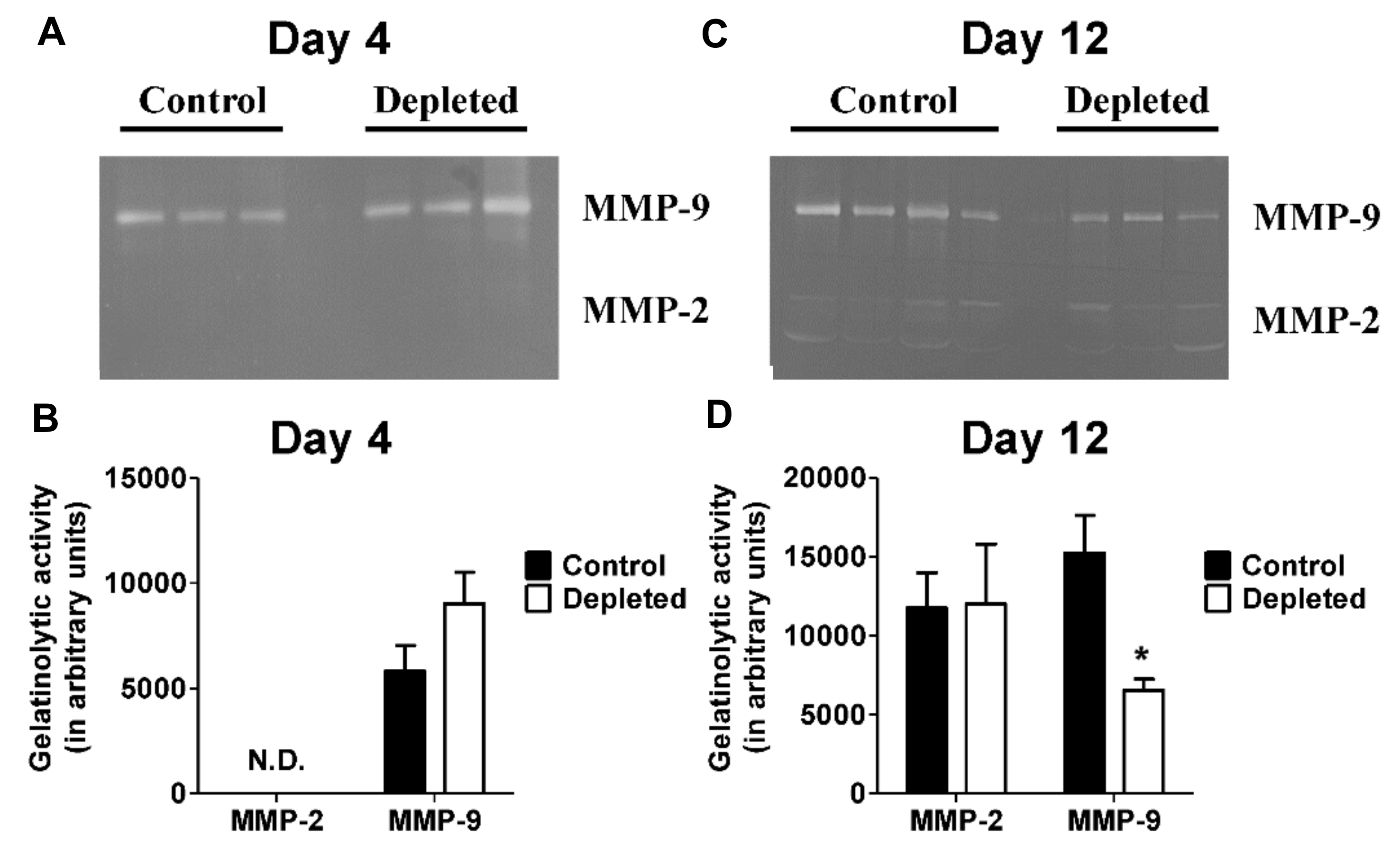
2.5. T Cell Depleted Thrombi Have Decreased MMP-9 Activity
3. Discussion
4. Materials and Methods
4.1. Murine Stasis Induced Venous Thrombosis
4.2. T Cell Depletion
4.3. Venous Thrombus Digestion
4.4. Flow Cytometric Analyses of Thrombus and Spleen
4.5. Immunohistochemistry
4.6. Immunoblotting
4.7. Gelatin Gel Zymography
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CTEPH | Chronic thromboembolic pulmonary hypertension |
| DVT | Deep vein thrombosis |
| IVC | Inferior vena cava |
| MMP | Matrix metalloproteinase |
| PAI-1 | Plasminogen activator inhibitor type I |
| PE | Pulmonary embolism |
| PTS | Post-thrombotic syndrome |
| TEM | Effector memory T cells |
| tPA | Tissue-type plasminogen activator |
| uPA | Urokinase-type plasminogen activator |
References
- Cushman, M. Epidemiology and Risk Factors for Venous Thrombosis. Semin. Hematol. 2007, 44, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Hudgens, S.A.; Cella, D.; Caprini, C.A.; Caprini, J.A. Deep Vein Thrombosis: Validation of a Patient-Reported Leg Symptom Index. Health Qual. Life Outcomes 2003, 1, 76. [Google Scholar] [CrossRef] [PubMed]
- Deitcher, S.R.; Carman, T.L. Deep Venous Thrombosis and Pulmonary Embolism. Curr. Treat. Options Cardiovasc. Med. 2002, 4, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Mahan, C.E.; Borrego, M.E.; Woersching, A.L.; Federici, R.; Downey, R.; Tiongson, J.; Bieniarz, M.C.; Cavanaugh, B.J.; Spyropoulos, A.C. Venous Thromboembolism: Annualised United States Models for Total, Hospital-Acquired and Preventable Costs Utilising Long-Term Attack Rates. Thromb. Haemost. 2012, 108, 291–302. [Google Scholar] [PubMed]
- Di Nisio, M.; van Es, N.; Buller, H.R. Deep Vein Thrombosis and Pulmonary Embolism. Lancet 2016, 388, 3060–3073. [Google Scholar] [CrossRef]
- Lapner, S.T.; Kearon, C. Diagnosis and Management of Pulmonary Embolism. BMJ 2013, 346, f757. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Lang, I.M. Current Understanding of the Pathophysiology of Chronic Thromboembolic Pulmonary Hypertension. Thromb. Res. 2017, 164, 136–144. [Google Scholar] [CrossRef]
- Simonneau, G.; Torbicki, A.; Dorfmuller, P.; Kim, N. The Pathophysiology of Chronic Thromboembolic Pulmonary Hypertension. Eur. Respir. Rev. 2017, 26, 160112. [Google Scholar] [CrossRef]
- Tapson, V.F.; Humbert, M. Incidence and Prevalence of Chronic Thromboembolic Pulmonary Hypertension: From Acute to Chronic Pulmonary Embolism. Proc. Am. Thorac. Soc. 2006, 3, 564–567. [Google Scholar] [CrossRef]
- Kearon, C. Long-Term Management of Patients after Venous Thromboembolism. Circulation 2004, 110, I10–I18. [Google Scholar] [CrossRef]
- Hirsh, J.; Lee, A.Y. How We Diagnose and Treat Deep Vein Thrombosis. Blood 2002, 99, 3102–3110. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Hirsh, J. Diagnosis and Treatment of Venous Thromboembolism. Annu. Rev. Med. 2002, 53, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Noventa, F.; Lensing, A.W.; Prins, M.H.; Villalta, S. Post-Thrombotic Syndrome and the Risk of Subsequent Recurrent Thromboembolism. Thromb. Res. 2016, 141, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.J.; Moore, H.M.; Rudarakanchana, N.; Gohel, M.; Davies, A.H. Post-Thrombotic Syndrome: A Clinical Review. J. Thromb. Haemost. 2013, 11, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Pengo, V.; Lensing, A.W.; Prins, M.H.; Marchiori, A.; Davidson, B.L.; Tiozzo, F.; Albanese, P.; Biasiolo, A.; Pegoraro, C.; Iliceto, S.; et al. Incidence of Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Embolism. N. Engl. J. Med. 2004, 350, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Dorffler-Melly, J.; Schwarte, L.A.; Ince, C.; Levi, M. Mouse Models of Focal Arterial and Venous Thrombosis. Basic Res. Cardiol. 2000, 95, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N. Mouse Models of Venous Thrombosis Are Not Equal. Blood 2016, 127, 2510–2511. [Google Scholar] [CrossRef][Green Version]
- Mackman, N. Mouse Models, Risk Factors, and Treatments of Venous Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 554–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cleuren, A.C.; van Vlijmen, B.J.; Reitsma, P.H. Transgenic Mouse Models of Venous Thrombosis: Fulfilling the Expectations? Semin. Thromb. Hemost. 2007, 33, 610–616. [Google Scholar] [CrossRef]
- Diaz, J.A.; Obi, A.T.; Myers, D.D., Jr.; Wrobleski, S.K.; Henke, P.K.; Mackman, N.; Wakefield, T.W. Critical Review of Mouse Models of Venous Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 556–562. [Google Scholar] [CrossRef]
- von Bruhl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Kollnberger, M.; et al. Monocytes, Neutrophils, and Platelets Cooperate to Initiate and Propagate Venous Thrombosis in Mice in Vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.J. Neutrophils and Deep Venous Thrombosis. Haemostasis 1993, 23 (Suppl. 1), 127–140. [Google Scholar] [CrossRef] [PubMed]
- Heestermans, M.; Salloum-Asfar, S.; Salvatori, D.; Laghmaniel, H.; Luken, B.M.; Zeerleder, S.S.; Spronk, H.M.; Korporaal, S.J.; Wagenaar, G.T.; Reitsma, P.H.; et al. Role of Platelets, Neutrophils, and Factor Xii in Spontaneous Venous Thrombosis in Mice. Blood 2016, 127, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Downing, L.J.; Strieter, R.M.; Kadell, A.M.; Wilke, C.A.; Brown, S.L.; Wrobleski, S.K.; Burdick, M.D.; Hulin, M.S.; Fowlkes, J.B.; Greenfield, L.J.; et al. Neutrophils Are the Initial Cell Type Identified in Deep Venous Thrombosis Induced Vein Wall Inflammation. ASAIO J. 1996, 42, M677–M682. [Google Scholar] [CrossRef] [PubMed]
- Henke, P.K.; Varga, A.; De, S.; Deatrick, C.B.; Eliason, J.; Arenberg, D.A.; Sukheepod, P.; Thanaporn, P.; Kunkel, S.L.; Upchurch, G.R., Jr.; et al. Deep Vein Thrombosis Resolution Is Modulated by Monocyte Cxcr2-Mediated Activity in a Mouse Model. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Laurance, S.; Bertin, F.R.; Ebrahimian, T.; Kassim, Y.; Rys, R.N.; Lehoux, S.; Lemarie, C.A.; Blostein, M.D. Gas6 Promotes Inflammatory (Ccr2(Hi)Cx3cr1(Lo)) Monocyte Recruitment in Venous Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1315–1322. [Google Scholar] [CrossRef]
- Humphries, J.; McGuinness, C.L.; Smith, A.; Waltham, M.; Poston, R.; Burnand, K.G. Monocyte Chemotactic Protein-1 (Mcp-1) Accelerates the Organization and Resolution of Venous Thrombi. J. Vasc. Surg. 1999, 30, 894–899. [Google Scholar] [CrossRef]
- Ali, T.; Humphries, J.; Burnand, K.; Sawyer, B.; Bursill, C.; Channon, K.; Greaves, D.; Rollins, B.; Charo, I.F.; Smith, A. Monocyte Recruitment in Venous Thrombus Resolution. J. Vasc. Surg. 2006, 43, 601–608. [Google Scholar] [CrossRef]
- Henke, P.K.; Pearce, C.G.; Moaveni, D.M.; Moore, A.J.; Lynch, E.M.; Longo, C.; Varma, M.; Dewyer, N.A.; Deatrick, K.B.; Upchurch, G.R.; et al. Targeted Deletion of Ccr2 Impairs Deep Vein Thombosis Resolution in a Mouse Model. J. Immunol. 2006, 177, 3388–3397. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Johnson, T.A.; Sarkar, R.; Antalis, T.M. Serpins in Venous Thrombosis and Venous Thrombus Resolution. Methods Mol. Biol. 2018, 1826, 197–211. [Google Scholar]
- Deatrick, K.B.; Luke, C.E.; Elfline, M.A.; Sood, V.; Baldwin, J.; Upchurch, G.R., Jr.; Jaffer, F.A.; Wakefield, T.W.; Henke, P.K. The Effect of Matrix Metalloproteinase 2 and Matrix Metalloproteinase 2/9 Deletion in Experimental Post-Thrombotic Vein Wall Remodeling. J. Vasc. Surg. 2013, 58, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Deatrick, K.B.; Obi, A.; Luke, C.E.; Elfline, M.A.; Sood, V.; Upchurch, G.R., Jr.; Jaffer, F.; Wakefield, T.W.; Henke, P.K. Matrix Metalloproteinase-9 Deletion Is Associated with Decreased Mid-Term Vein Wall Fibrosis in Experimental Stasis Dvt. Thromb. Res. 2013, 132, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.P.; McGilvray, K.C.; Puttlitz, C.M.; Mukhopadhyay, S.; Chabasse, C.; Sarkar, R. Matrix Metalloproteinase 9 (Mmp-9) Regulates Vein Wall Biomechanics in Murine Thrombus Resolution. PLoS ONE 2015, 10, e0139145. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Inui, M.; Mukaida, N.; Kondo, T. Absence of Ifn-Gamma Accelerates Thrombus Resolution through Enhanced Mmp-9 and Vegf Expression in Mice. J. Clin. Investig. 2011, 121, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Gabre, J.; Chabasse, C.; Cao, C.; Mukhopadhyay, S.; Siefert, S.; Bi, Y.; Netzel-Arnett, S.; Sarkar, R.; Zhang, L. Activated Protein C Accelerates Venous Thrombus Resolution through Heme Oxygenase-1 Induction. J. Thromb. Haemost. 2014, 12, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Antalis, T.M.; Nguyen, K.P.; Hoofnagle, M.H.; Sarkar, R. Myeloid P53 Regulates Macrophage Polarization and Venous Thrombus Resolution by Inflammatory Vascular Remodeling in Mice. Blood 2017, 129, 3245–3255. [Google Scholar] [CrossRef]
- Siefert, S.A.; Chabasse, C.; Mukhopadhyay, S.; Hoofnagle, M.H.; Strickland, D.K.; Sarkar, R.; Antalis, T.M. Enhanced Venous Thrombus Resolution in Plasminogen Activator Inhibitor Type-2 Deficient Mice. J. Thromb. Haemost. 2014, 12, 1706–1716. [Google Scholar] [CrossRef]
- Wakefield, T.W.; Strieter, R.M.; Wilke, C.A.; Kadell, A.M.; Wrobleski, S.K.; Burdick, M.D.; Schmidt, R.; Kunkel, S.L.; Greenfield, L.J. Venous Thrombosis-Associated Inflammation and Attenuation with Neutralizing Antibodies to Cytokines and Adhesion Molecules. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 258–268. [Google Scholar] [CrossRef]
- Frey, M.K.; Winter, M.-P.; Alimohammadi, A.; Panzenboeck, A.; Puthenkalam, S.; Bonderman, D.; Lang, I. Resolution of Venous Thrombus Is Depending on B-Lymphocytes. Eur. Respir. J. 2012, 40, 3909. [Google Scholar]
- Luther, N.; Shahneh, F.; Brahler, M.; Krebs, F.; Jackel, S.; Subramaniam, S.; Stanger, C.; Schonfelder, T.; Kleis-Fischer, B.; Reinhardt, C.; et al. Innate Effector-Memory T-Cell Activation Regulates Post-Thrombotic Vein Wall Inflammation and Thrombus Resolution. Circ. Res. 2016, 119, 1286–1295. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Paulnock, D.M. Macrophage Activation by T Cells. Curr. Opin. Immunol. 1992, 4, 344–349. [Google Scholar] [CrossRef]
- Underhill, D.M.; Bassetti, M.; Rudensky, A.; Aderem, A. Dynamic Interactions of Macrophages with T Cells During Antigen Presentation. J. Exp. Med. 1999, 190, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T Cell Responses: Naive to Memory and Everything in Between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef]
- Emeson, E.E.; Shen, M.L.; Bell, C.G.; Qureshi, A. Inhibition of Atherosclerosis in Cd4 T-Cell-Ablated and Nude (Nu/Nu) C57bl/6 Hyperlipidemic Mice. Am. J. Pathol. 1996, 149, 675–685. [Google Scholar]
- Taraseviciene-Stewart, L.; Nicolls, M.R.; Kraskauskas, D.; Scerbavicius, R.; Burns, N.; Cool, C.; Wood, K.; Parr, J.E.; Boackle, S.A.; Voelkel, N.F. Absence of T Cells Confers Increased Pulmonary Arterial Hypertension and Vascular Remodeling. Am. J. Respir. Crit. Care Med. 2007, 175, 1280–1289. [Google Scholar] [CrossRef]
- Hansson, G.K.; Holm, J.; Holm, S.; Fotev, Z.; Hedrich, H.J.; Fingerle, J. T Lymphocytes Inhibit the Vascular Response to Injury. Proc. Natl. Acad. Sci. USA 1991, 88, 10530–10534. [Google Scholar] [CrossRef]
- Dimayuga, P.C.; Chyu, K.Y.; Kirzner, J.; Yano, J.; Zhao, X.; Zhou, J.; Shah, P.K.; Cercek, B. Enhanced Neointima Formation Following Arterial Injury in Immune Deficient Rag-1-/- Mice Is Attenuated by Adoptive Transfer of Cd8 T Cells. PLoS ONE 2011, 6, e20214. [Google Scholar] [CrossRef]
- Socie, G.; Schmoor, C.; Bethge, W.A.; Ottinger, H.D.; Stelljes, M.; Zander, A.R.; Volin, L.; Ruutu, T.; Heim, D.A.; Schwerdtfeger, R.; et al. TG-Fresenius Trial Group. Chronic Graft-Versus-Host Disease: Long-Term Results from a Randomized Trial on Graft-Versus-Host Disease Prophylaxis with or without Anti-T-Cell Globulin Atg-Fresenius. Blood 2011, 117, 6375–6382. [Google Scholar] [CrossRef]
- Mohty, M. Mechanisms of Action of Antithymocyte Globulin: T-Cell Depletion and Beyond. Leukemia 2007, 21, 1387–1394. [Google Scholar] [CrossRef]
- Reichert, T.; DeBruyere, M.; Deneys, V.; Totterman, T.; Lydyard, P.; Yuksel, F.; Chapel, H.; Jewell, D.; Van Hove, L.; Linden, J.; et al. Lymphocyte Subset Reference Ranges in Adult Caucasians. Clin. Immunol. Immunopathol. 1991, 60, 190–208. [Google Scholar] [CrossRef]
- Singh, I.; Burnand, K.G.; Collins, M.; Luttun, A.; Collen, D.; Boelhouwer, B.; Smith, A. Failure of Thrombus to Resolve in Urokinase-Type Plasminogen Activator Gene-Knockout Mice: Rescue by Normal Bone Marrow-Derived Cells. Circulation 2003, 107, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Gossage, J.A.; Humphries, J.; Modarai, B.; Burnand, K.G.; Smith, A. Adenoviral Urokinase-Type Plasminogen Activator (Upa) Gene Transfer Enhances Venous Thrombus Resolution. J. Vasc. Surg. 2006, 44, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.; Gossage, J.A.; Modarai, B.; Burnand, K.G.; Sisson, T.H.; Murdoch, C.; Smith, A. Monocyte Urokinase-Type Plasminogen Activator up-Regulation Reduces Thrombus Size in a Model of Venous Thrombosis. J. Vasc. Surg. 2009, 50, 1127–1134. [Google Scholar] [CrossRef]
- Harrison, C. Cardiovascular Disorders: Resolving Blood Clots. Nat. Rev. Drug Discov. 2011, 10, 578. [Google Scholar] [CrossRef]
- Josefowicz, S.Z.; Lu, L.F.; Rudensky, A.Y. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef]
- Lu, B.; Ferrandino, A.F.; Flavell, R.A. Gadd45beta Is Important for Perpetuating Cognate and Inflammatory Signals in T Cells. Nat. Immunol. 2004, 5, 38–44. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. Cd4 T Cells: Fates, Functions, and Faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef]
- Zhang, N.; Bevan, M.J. Cd8(+) T Cells: Foot Soldiers of the Immune System. Immunity 2011, 35, 161–168. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Johnson, T.A.; Duru, N.; Buzza, M.S.; Pawar, N.R.; Sarkar, R.; Antalis, T.M. Fibrinolysis and Inflammation in Venous Thrombus Resolution. Front. Immunol. 2019, 10, 1348. [Google Scholar] [CrossRef]
- Tormey, V.J.; Faul, J.; Leonard, C.; Burke, C.M.; Dilmec, A.; Poulter, L.W. T-Cell Cytokines May Control the Balance of Functionally Distinct Macrophage Populations. Immunology 1997, 90, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Azoury, S.C.; Straughan, D.M.; Shukla, V. Immune Checkpoint Inhibitors for Cancer Therapy: Clinical Efficacy and Safety. Curr. Cancer Drug Targets 2015, 15, 452–462. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhopadhyay, S.; Gabre, J.; Chabasse, C.; Bromberg, J.S.; Antalis, T.M.; Sarkar, R. Depletion of CD4 and CD8 Positive T Cells Impairs Venous Thrombus Resolution in Mice. Int. J. Mol. Sci. 2020, 21, 1650. https://doi.org/10.3390/ijms21051650
Mukhopadhyay S, Gabre J, Chabasse C, Bromberg JS, Antalis TM, Sarkar R. Depletion of CD4 and CD8 Positive T Cells Impairs Venous Thrombus Resolution in Mice. International Journal of Molecular Sciences. 2020; 21(5):1650. https://doi.org/10.3390/ijms21051650
Chicago/Turabian StyleMukhopadhyay, Subhradip, Joel Gabre, Christine Chabasse, Jonathan S. Bromberg, Toni M. Antalis, and Rajabrata Sarkar. 2020. "Depletion of CD4 and CD8 Positive T Cells Impairs Venous Thrombus Resolution in Mice" International Journal of Molecular Sciences 21, no. 5: 1650. https://doi.org/10.3390/ijms21051650
APA StyleMukhopadhyay, S., Gabre, J., Chabasse, C., Bromberg, J. S., Antalis, T. M., & Sarkar, R. (2020). Depletion of CD4 and CD8 Positive T Cells Impairs Venous Thrombus Resolution in Mice. International Journal of Molecular Sciences, 21(5), 1650. https://doi.org/10.3390/ijms21051650



