Cadmium Uptake, MT Gene Activation and Structure of Large-Sized Multi-Domain Metallothioneins in the Terrestrial Door Snail Alinda biplicata (Gastropoda, Clausiliidae)
Abstract
1. Introduction
2. Results and Discussion
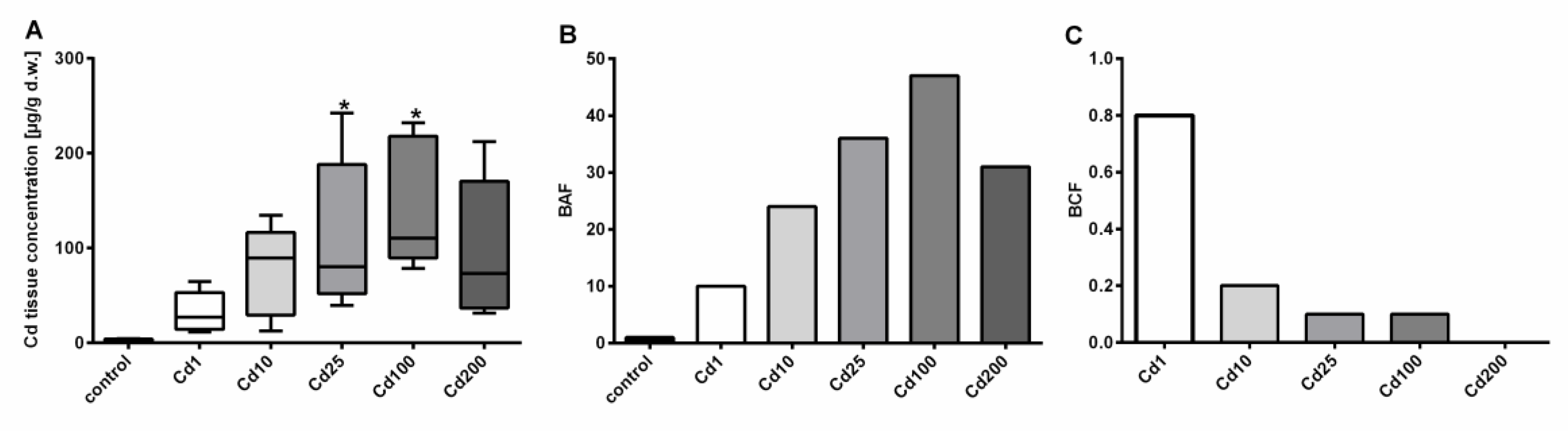
2.1. Cd Accumulation in Alinda biplicata Is Restrained beyond a High Threshold Level
2.2. Avoidance Behavior
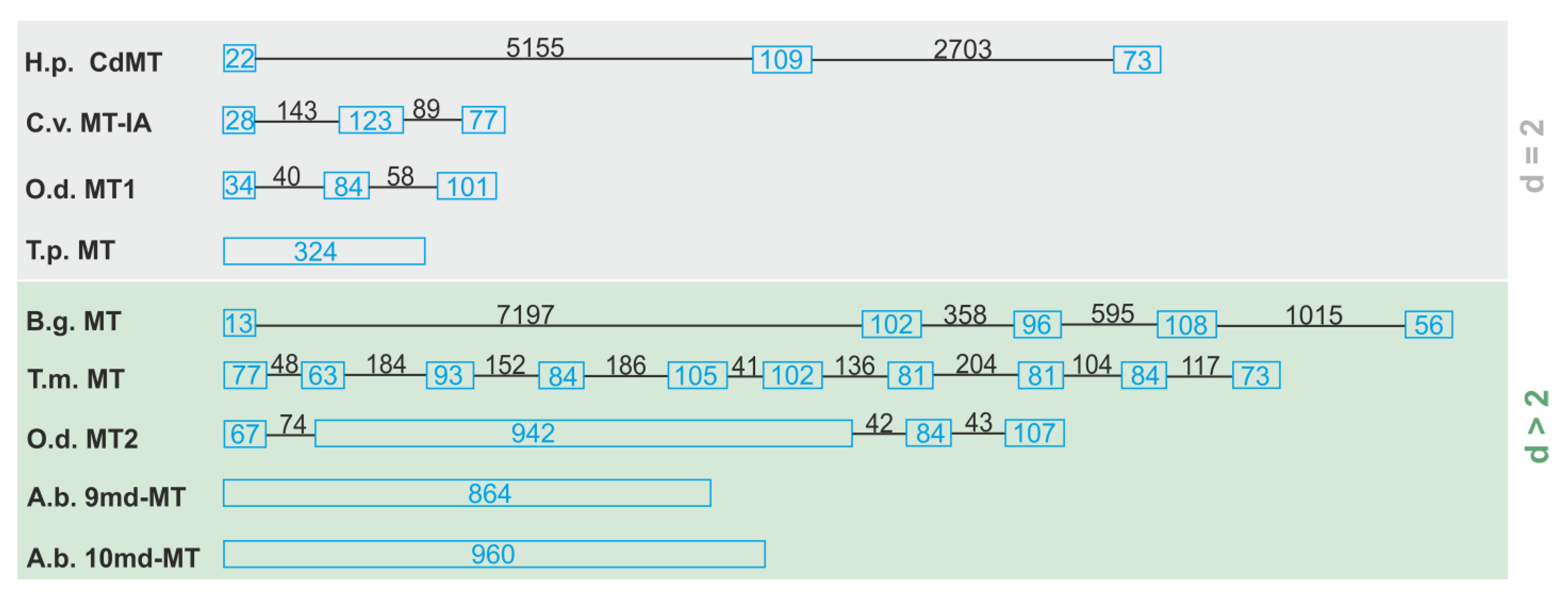
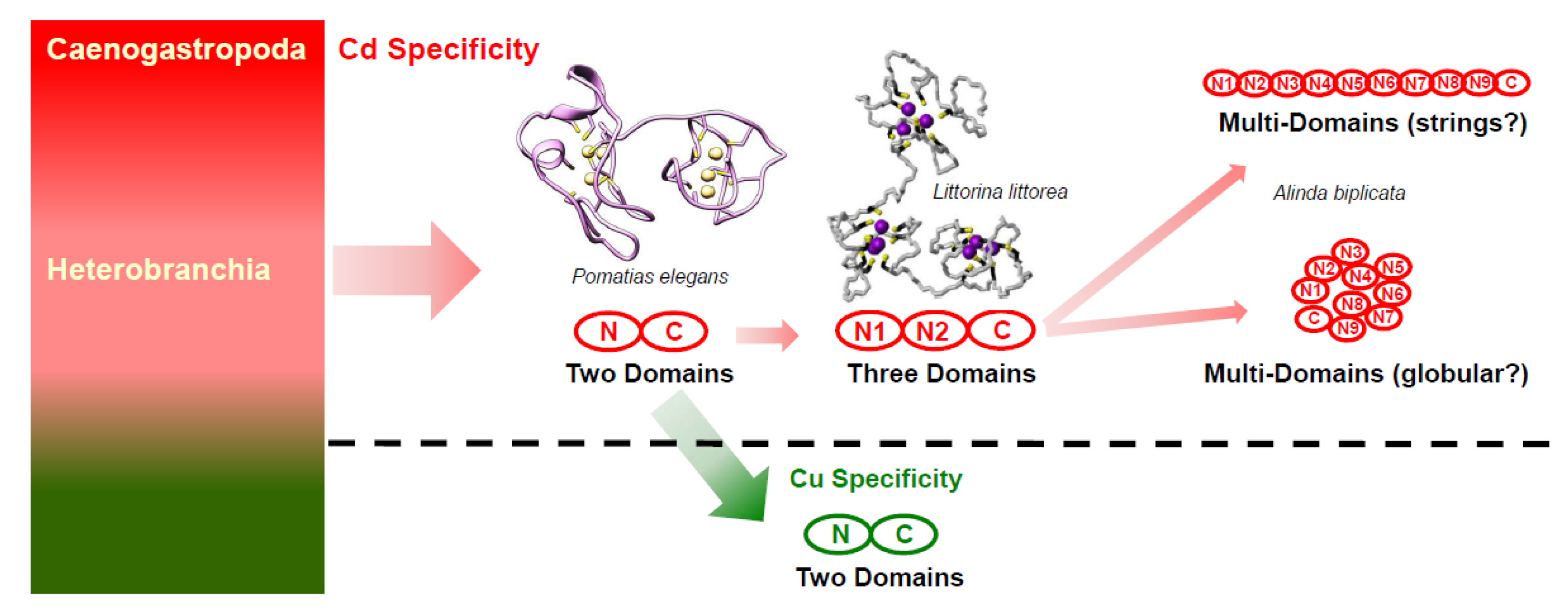
2.3. Characterization of Multi-Domain MT Genes in Alinda Biplicata
- 9md-MT: N2, N4, N5, N8 > N3, N9 > N7 > N1.
- 10md-MT: N3, N4, N6 > N5, N8, N2 > N9 > N7 > N1;
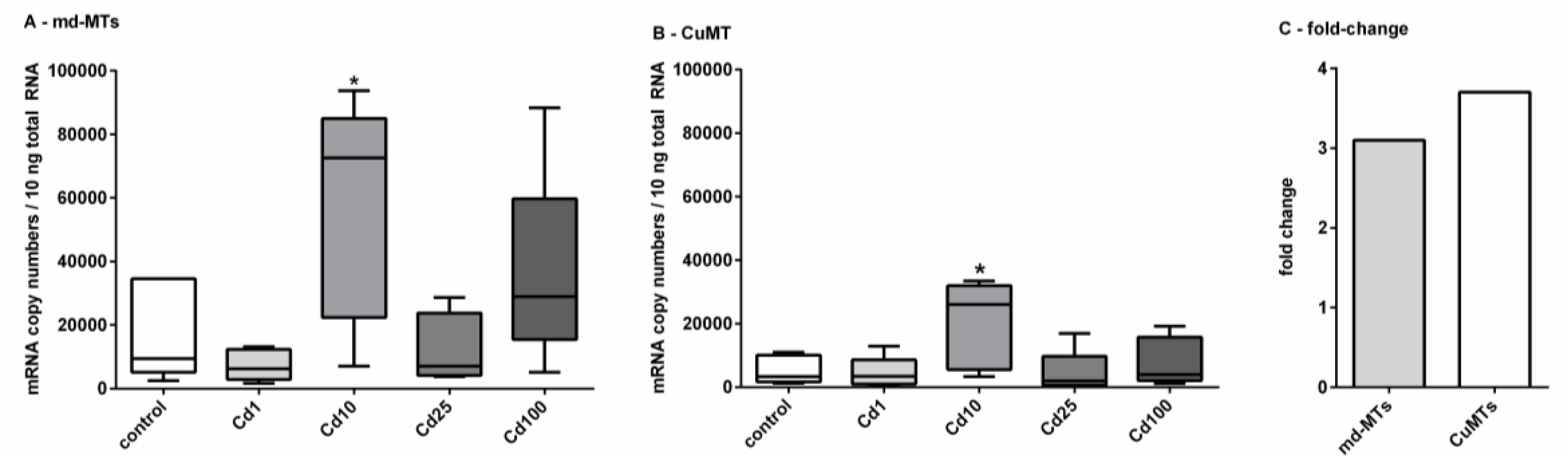
2.4. Impact of Cd Exposure on MT Gene Transcription in Alinda biplicata
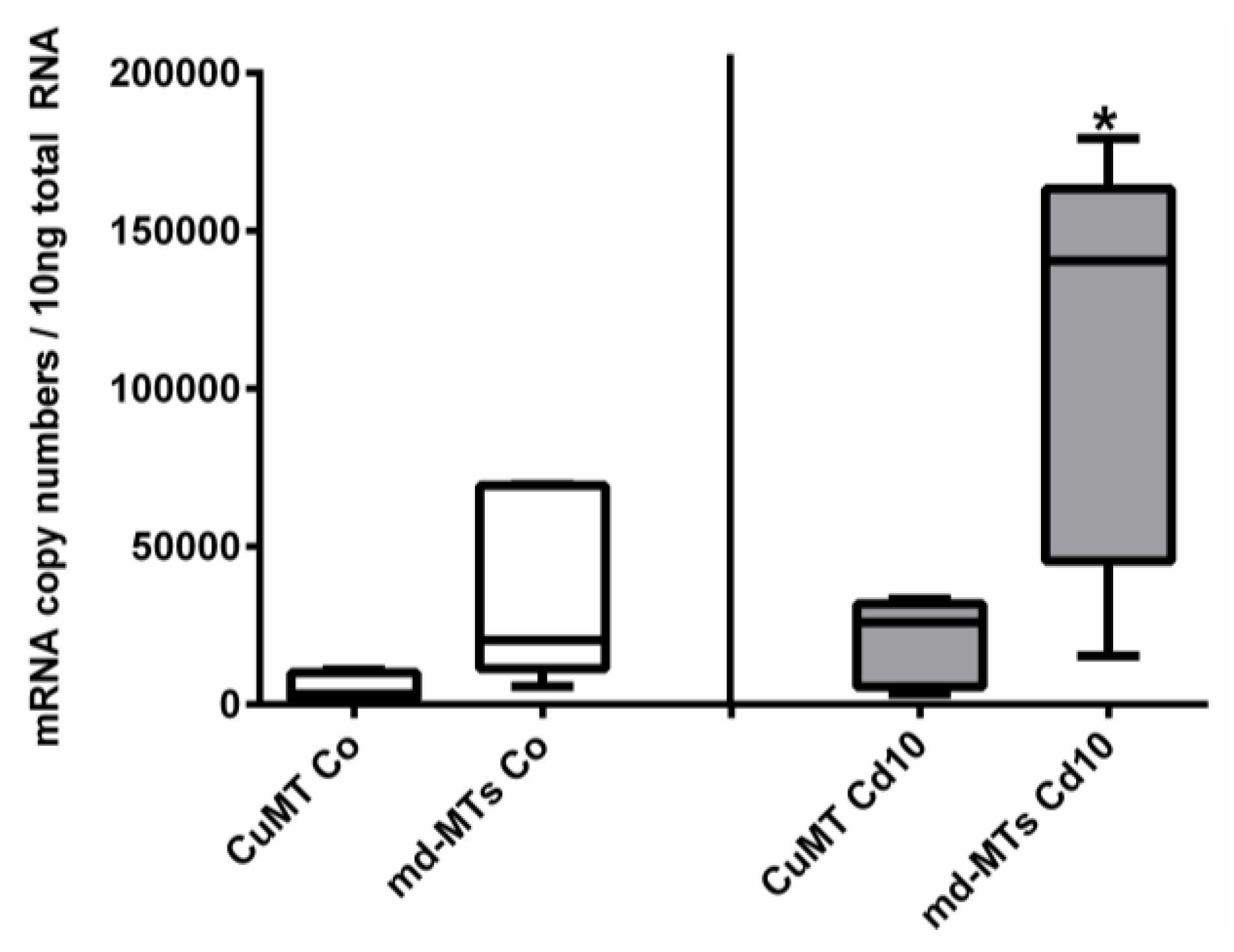
2.5. Are Alinda biplicata md-MTs Involved in Cd Detoxification?
2.6. Structural and Evolutionary Considerations of the Emergence of md-MTs in Alinda biplicata
3. Material and Methods
3.1. Animal Rearing and Acclimatization
3.2. Cd Exposure and Tissue Dissection
3.3. Cd Analysis
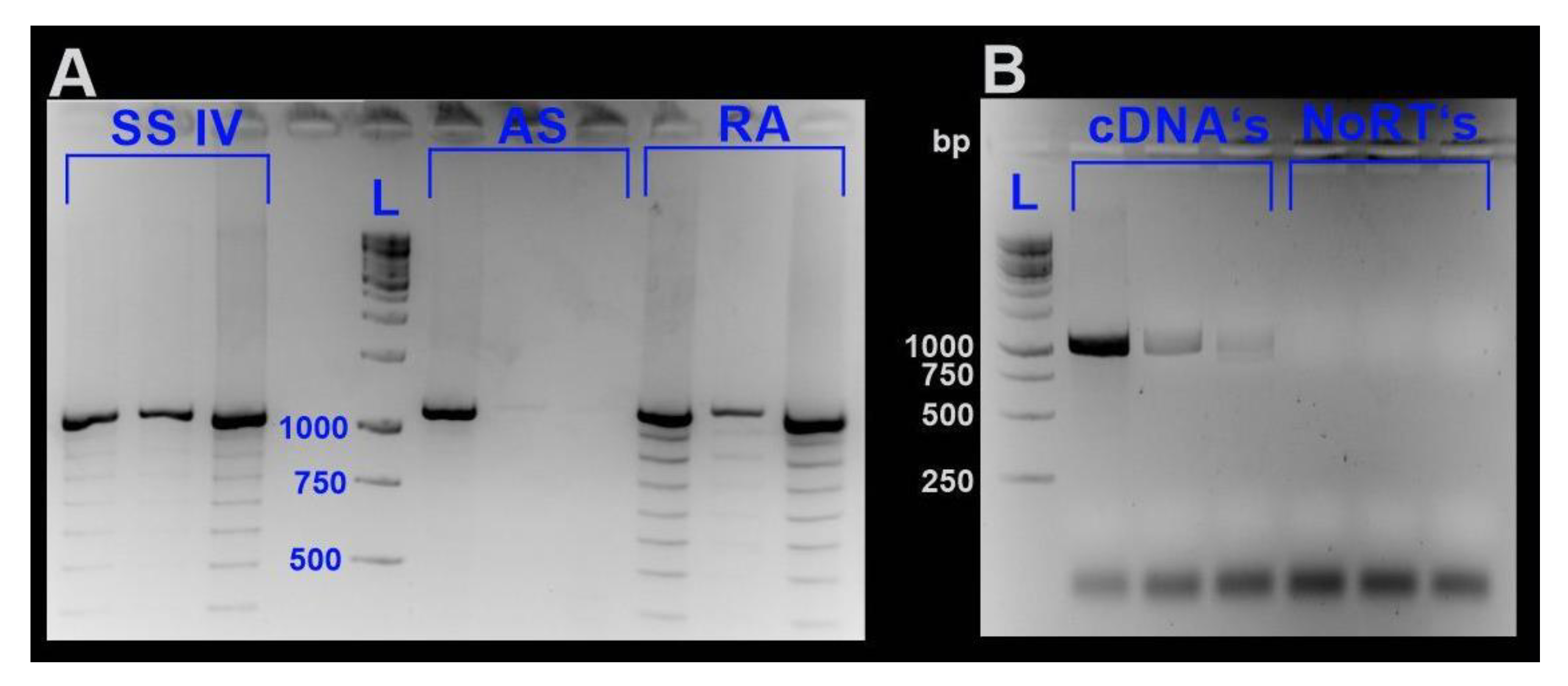
3.4. DNA Isolation and Long Distance (LP)-PCR of md-MT Genes
3.5. RNA Isolation, Quantification and cDNA Synthesis
3.6. Quantification of MT Gene Transcription by Quantitative RealTime PCR (qRT-PCR)
3.7. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kerney, J.M.P.; Cameron, R.A.D.; Jungbluth, J.H. Die Landschnecken Nord-und Mitteleuropas; Verlag Paul Parey: Hamburg/Berlin, Gernamy, 1983; p. 384. [Google Scholar]
- Maltz, T.K.; Sulikowska-Drozd, A. Life History of Alinda biplicata (Montagu, 1803) (Gastropoda: Pulmonata: Clausiliidae) Based on Five-Year Laboratory Observations. Ann. Zool. 2012, 62, 789–807. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Metabolic rate depression in animals: Transcriptional and translational controls. Biol. Rev. Camb. Philos. Soc. 2004, 79, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Aestivation: Signaling and hypometabolism. J. Exp. Biol. 2012, 215, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, R.; Lagg, B.; Egg, M.; Schipflinger, R.; Chabicovsky, M.-K. Cd Accumulation and Cd-Metallothionein as a Biomarker in Cepaea hortensis (Helicidae, Pulmonata) from Laboratory Exposure and Metal-polluted Habitats. Ecotoxicology 2004, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Hispard, F.; Schuler, D.; de Vaufleury, A.; Scheifler, R.; Badot, P.M.; Dallinger, R. Metal Distribution and Metallothionein Induction After Cadmium Exposure in the Terrestrial Snail Helix Aspersa. Environ. Toxicol. 2008, 27, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Chabicovsky, M.; Klepal, W.; Dallinger, R. Mechanisms of cadmium toxicity in terrestrial pulmonates: Programmed cell death and metallothionein overload. Environ. Toxicol. Chem. 2004, 23, 648–655. [Google Scholar] [CrossRef]
- Dallinger, R.; Berger, B.; Gruber, C.; Hunziker, P.; Stürzenbaum, S. Metallothioneins in terrestrial invertebrates: Structural aspects, biological significance and implications for their use as biomarkers. Cell. Mol. Biol. 2000, 46, 331–346. [Google Scholar]
- Capdevila, M.; Bofil, R.; Palacios, Ò.; Atrian, S. State-of-the-art of Metallothioneins at the beginning of the 21st century. Coord. Chem. Rev. 2012, 256, 46–62. [Google Scholar] [CrossRef]
- Egli, D.; Yepiskoposyan, H.; Selvaraj, A.; Balamurugan, K.; Rajaram, R.; Simons, A.; Multhaup, G.; Mettler, S.; Vardanyan, A.; Georgiev, O.; et al. A family knockout of all four Drosophila metallothioneins reveals a central role in copper homeostasis and detoxification. Mol. Cell. Biol. 2006, 26, 2286. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The functions of metallothionein and ZIP and ZnT transporters: An overview and perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef]
- Baird, S.K.; Kurz, T.; Brunk, U.T. Metallothionein protects against oxidative stress-induced lysosomal destabilization. Biochem. J. 2006, 394, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Egg, M.; Höckner, M.; Brandstätter, A.; Schuler, D.; Dallinger, R. Structural and bioinformatic analysis of the Roman snail Cd-Metallothionein gene uncovers molecular adaptation towards plasticity in coping with multifarious environmental stress. Mol. Ecol. 2009, 18, 2426–2443. [Google Scholar] [CrossRef] [PubMed]
- Pedrini-Martha, V.; Niederwanger, M.; Kopp, R.; Schnegg, R.; Dallinger, R. Physiological, Diurnal and Stress-Related Variability of Cadmium-Metallothionein Gene Expression in Land Snails. PLoS ONE 2016, 11, e0150442. [Google Scholar] [CrossRef]
- Tamait, K.T.; Liu, X.; Silar, P.; Sosinowski, T.; Thiele, D.J. Heat Shock Transcription Factor Activates Yeast Metallothionein Gene Expression in Response to Heat and Glucose Starvation via Distinct Signalling Pathways. Mol. Cell. Biol. 1994, 14, 8155–8165. [Google Scholar] [CrossRef] [PubMed]
- Moschovaki-Filippidou, F.; Itziou, A.; Dimitriadis, V.K. Effect of starvation and hibernation on the values of five biomarkers of general and specific stress using the land snail Eobania Vermiculata. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 165, 236–242. [Google Scholar] [CrossRef]
- Kojima, Y.; Binz, P.A.; Kägi, J.H.R. Nomenclature of metallothionein: Proposal for a revision. In Metallothionein IV; Klaassen, C.D., Ed.; Birkhäuser Basel: Basel, Switzerland, 1999; pp. 3–6. ISBN 978-3-0348-8847-9. [Google Scholar]
- Pérez-Rafael, S.; Mezger, A.; Lieb, B.; Dallinger, R.; Capdevila, M.; Palacios, Ò.; Atrian, S. The metal binding abilities of Megathura crenulata metallothionein (McMT) in the frame of Gastropoda MTs. J. Inorg. Biochem. 2012, 108, 84–90. [Google Scholar] [CrossRef]
- Dallinger, R.; Zerbe, O.; Baumann, C.; Egger, B.; Capdevila, M.; Palacios, O.; Albalat, R.; Calatayud, S.; Ladurner, P.; Schlick-Steiner, B.; et al. Metallomics reveal a persisting impact of cadmium on the evolution of metal-selective metallothioneins. Metallomics 2020. accepted. [Google Scholar]
- Dallinger, R.; Berger, B. Function of metallothioneins in terrestrial gastropods. Sci. Total Environ. 1993, 134, 607–615. [Google Scholar] [CrossRef]
- Berger, B.; Dallinger, R.; Gehrig, P.; Hunziker, P.E. Primary structure of a copper-binding metallothionein from mantle tissue of the terrestrial gastropod Helix pomatia L. J. Biochem. 1997, 328, 219–224. [Google Scholar] [CrossRef]
- Pedrini-Martha, V.; Schnegg, R.; Baurand, P.E.; de Vaufleury, A.; Dallinger, R. The physiological role and toxicological significance of the non-metal-selective cadmium/copper-metallothionein isoform differ between embryonic and adult helicid snails. Comp. Biochem. Physiol. Part C Toxicol. Pharm. 2017, 199, 38–47. [Google Scholar] [CrossRef]
- Dallinger, R.; Berger, B.; Hunziker, P.; Kägi, H.R.J. Metallothionein in snail Cd and Cu metabolism. Nature 1997, 143, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rafael, S.; Monteiro, F.; Dallinger, R.; Atrian, S.; Palacios, Ò.; Capdevila, M. Cantareus aspersus metallothionein metal binding abilities: The unspecific CaCd/CuMT isoform provides hints about the metal preference determinants in metallothioneins. Biochim. Biophys. Acta Proteins Proteom. 2014, 1844, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Beil, A.; Jurt, S.; Walser, R.; Schönhut, T.; Güntert, P.; Palacios, Ò.; Atrian, S.; Capdevila, M.; Dallinger, R.; Zerbe, O. The Solution Structure and Dynamics of Cd-Metallothionein from Helix pomatia Reveal Optimization for Binding Cd over Zn. Biochemistry 2019, 58, 4570–4581. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Beil, A.; Jurt, S.; Niederwanger, M.; Palacios, O.; Capdevila, M.; Atrian, S.; Dallinger, R.; Zerbe, O. Structural Adaptation of a Protein to Increased Metal Stress: NMR Structure of a Marine Snail Metallothionein with an Additional Domain. Angew. Chem. Int. Ed. 2017, 56, 4617–4622. [Google Scholar] [CrossRef]
- Schmielau, L.; Dvorak, M.; Niederwanger, M.; Dobieszewski, N.; Pedrini-Martha, V.; Ladurner, P.; Pedregal, J.R.G.; Maréchal, J.D.; Dallinger, R. Differential response to Cadmium exposure by expression of a two and a three-domain metallothionein isoform in the land winkle Pomatias elegans: Valuating the marine heritage of a land snail. Sci. Total Environ. 2019, 648, 561–571. [Google Scholar] [CrossRef]
- Palacios, Ò.; Jiménez-Martí, E.; Niederwanger, M.; Gil-Moreno, S.; Zerbe, O.; Atrian, S.; Dallinger, R.; Capdevila, M. Analysis of metal-binding features of the wild type and two domain-truncated mutant variants of littorina littorea metallothionein reveals its cd-specific character. Int. J. Mol. Sci. 2017, 18, 1452. [Google Scholar] [CrossRef]
- Maroni, G.; Wise, J.; Young, J.E.; Otto, E. Metallothionein Gene Duplications and Metal Tolerance in Natural Populations of Drosophila Melanogaster. Genetics 1987, 117, 739–744. [Google Scholar]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Trzcińska, M.; Pawlik-Skowrońska, B. Soil algal communities inhabiting zinc and lead mine spoils. J. Appl. Phycol. 2008, 20, 341–348. [Google Scholar] [CrossRef]
- Coughtrey, P.; Jones, C.; Martin, M.; Shales, S. Litter Accumulation in Woodlands Contaminated. Oecologia 1979, 39, 51–60. [Google Scholar] [CrossRef]
- Lepp, N.W.; Madejón, P. Cadmium and zinc in vegetation and litter of a voluntary woodland that has developed on contaminated sediment-derived soil. Proc. J. Environ. Qual. 2007, 36, 1123–1131. [Google Scholar] [CrossRef]
- Dallinger, R. Strategies of metal detoxification in terrestrial invertebrates. In Ecotoxicology of Metals in Invertebrates; Dallinger, R., Rainbow, P.S., Eds.; Lewis Publisher: Boca Rota, FL, USA, 1993; pp. 245–289. ISBN 9780873717342. [Google Scholar]
- Regoli, F.; Gorbi, S.; Fattorini, D.; Tedesco, S.; Notti, A.; Machella, N.; Bocchetti, R.; Benedetti, M.; Piva, F. Use of the land snail Helix aspersa sentinel organism for monitoring ecotoxicologic effects of urban pollution: An integrated approach. Environ. Health Perspect. 2006, 114, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, R.; Berger, B.; Triebskorn-Köhler, R.; Köhler, A.H. 4 Soil Biology and Ecotoxicology. In The Biology of Terrestrial Mollsucs; Barker, G.M., Ed.; CABI Publishing: Oxon, UK, 2001; pp. 489–525. [Google Scholar]
- De Vaufleury, A.G.; Bispo, A. Methods for toxicity assessment of contaminated soil by oral or dermal uptake in land snails. 1. Sublethal effects on growth. Environ. Sci. Technol. 2000, 34, 1865–1870. [Google Scholar] [CrossRef]
- Coeurdassier, M.; Gomot-De Vaufleury, A.; Lovy, C.; Badot, P.-M. Is the cadmium uptake from soil important in bioaccumulation and toxic effects for snails? Ecotoxicol. Environ. Saf. 2002, 53, 425–431. [Google Scholar] [CrossRef]
- Laskowski, R.; Hopkin, S.P. Effect of Zn, Cu, Pb, and Cd on Fitness in Snails (Helix aspersa). Ecotoxicol. Environ. Saf. 1996, 34, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Notten, M.J.M.; Oosthoek, A.J.P.; Rozema, J.; Aerts, R. Heavy metal pollution affects consumption and reproduction of the landsnail Cepaea nemoralis fed on naturally polluted Urtica dioica leaves. Ecotoxicology 2006, 15, 295–304. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, K.S.; Radwan, M.A.; Gad, A.F. Feeding and growth responses of the snail theba pisana to dietary metal exposure. Arch. Environ. Contam. Toxicol. 2011, 60, 272–280. [Google Scholar] [CrossRef]
- Schüder, I.; Port, G.; Bennison, J. The behavioural response of slugs and snails to novel molluscicides, irritants and repellents. Pest Manag. Sci. 2004, 60, 1171–1177. [Google Scholar] [CrossRef]
- Lefcort, H.; Abbott, D.P.; Cleary, D.A.; Howell, E.; Keller, N.C.; Smith, M.M. Aquatic Snails from Mining Sites Have Evolved to Detect and Avoid Heavy Metals. Arch. Environ. Contam. Toxicol. 2004, 46, 478–484. [Google Scholar] [CrossRef]
- Cain, D.J.; Croteau, M.N.; Fuller, C.C.; Ringwood, A.H. Dietary Uptake of Cu Sorbed to Hydrous Iron Oxide is Linked to Cellular Toxicity and Feeding Inhibition in a Benthic Grazer. Environ. Sci. Technol. 2016, 50, 1552–1560. [Google Scholar] [CrossRef]
- Moore, M.N.; Shaw, J.P.; Ferrar Adams, D.R.; Viarengo, A. Anti-oxidative cellular protection effect of fasting-induced autophagy as a mechanism for hormesis. Mar. Environ. Res. 2015, 107, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Moleirinho, A.; Carneiro, J.; Matthiesen, R.; Silva, R.M.; Amorim, A.; Azevedo, L. Gains, losses and changes of function after gene duplication: Study of the metallothionein family. PLoS ONE 2011, 6, e18487. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, S.; Garcia-Risco, M.; Rojas, N.S.; Espinosa-Sánchez, L.; Artime, S.; Palacios, O.; Cañestro, C.; Albalat, R. Metallothioneins of the urochordate Oikopleura dioica have Cys-rich tandem repeats, large size and cadmium-binding preference. Metallomics 2018, 10, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Niederwanger, M.; Dvorak, M.; Schnegg, R.; Pedrini-Martha, V.; Bacher, K.; Bidoli, M.; Dallinger, R. Challenging the Metallothionein (MT) gene of Biomphalaria glabrata: Unexpected response patterns due to cadmium exposure and temperature stress. Int. J. Mol. Sci. 2017, 18, 1747. [Google Scholar] [CrossRef] [PubMed]
- Iturbe-Espinoza, P.; Gil-Moreno, S.; Lin, W.; Calatayud, S.; Palacios, Ò.; Capdevila, M.; Atrian, S. The fungus Tremella mesenteric encodes the longest metallothionein currently known: Gene, protein and metal binding characterization. PLoS ONE 2016, 11, e0148651. [Google Scholar] [CrossRef] [PubMed]
- Jenny, M.J.; Ringwood, A.H.; Schey, K.; Warr, G.W.; Chapman, R.W. Diversity of metallothioneins in the American oyster, Crassostrea virginica, revealed by transcriptomic and proteomic approaches. Eur. J. Biochem. 2004, 271, 1702–1712. [Google Scholar] [CrossRef]
- Piccinni, E.; Bertaggia, D.; Santovito, G.; Miceli, C.; Kraev, A. Cadmium metallothionein gene of Tetrahymena pyriformis k. Gene 1999, 234, 51–59. [Google Scholar] [CrossRef]
- Piccinni, E.; Irato, P.; Coppellotti, O.; Guidolin, L. Biochemical and ultrastructural data on Tetrahymena pyriformis treated with copper and cadmium. J. Cell Sci. 1987, 88, 283–293. [Google Scholar]
- Hung, M.S.; Lin, Y.C.; Mao, J.H.; Kim, I.J.; Xu, Z.; Yang, C.T.; Jablons, D.M.; You, L. Functional polymorphism of the CK2α intronless gene plays oncogenic roles in lung cancer. PLoS ONE 2010, 5, e11418. [Google Scholar] [CrossRef]
- Hunt, C.; Calderwood, S. Characterization and sequence of a mouse hsp70 gene and its expression in mouse cell lines. Gene 1990, 87, 199–204. [Google Scholar] [CrossRef]
- Grzybowska, E.A. Human intronless genes: Functional groups, associated diseases, evolution, and mRNA processing in absence of splicing. Biochem. Biophys. Res. Commun. 2012, 424, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Louhichi, A.; Fourati, A.; Rebaï, A. IGD: A resource for intronless genes in the human genome. Gene 2011, 488, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, S.A.; Ogurtsov, A.Y.; Spiridonov, A.N.; Novichkov, P.S.; Spiridonov, N.A.; Koonin, E.V. Distinct patterns of expression and evolution of intronless and intron-containing mammalian genes. Mol. Biol. Evol. 2010, 27, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Otto, E.; Young, J.E.; Maroni, G. Structure and expression of a tandem duplication of the Drosophila metallothionein gene (gene amplification/cadmium resistance/Min gene). Genetics 1986, 83, 6025–6029. [Google Scholar]
- Richardson, S.R.; Salvador-Palomeque, C.; Faulkner, G.J. Diversity through duplication: Whole-genome sequencing reveals novel gene retrocopies in the human population. BioEssays 2014, 36, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Richards, R.I. Human metallothionein genes-primary structure of the metallothionein-II gene and a related processed gene. Nature 1982, 299, 797–802. [Google Scholar] [CrossRef]
- Poliseno, L.; Salmena, L.; Zhang, J.; Carver, B.; Haveman, W.J.; Pandolfi, P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010, 465, 1033–1038. [Google Scholar] [CrossRef]
- Casola, C.; Betrán, E. The genomic impact of gene retrocopies: What have we learned from comparative genomics, population genomics, and transcriptomic analyses? Genome Biol. Evol. 2017, 9, 1351–1373. [Google Scholar] [CrossRef]
- Roy, S.W.; Gilbert, W. Rates of intron loss and gain: Implications for early eukaryotic evolution. PNAS 2005, 102, 5773–5778. [Google Scholar] [CrossRef]
- Loh, Y.H.; Brenner, S.; Venkatesh, B. Investigation of loss and gain of introns in the compact genomes of pufferfishes (Fugu and Tetraodon). Mol. Biol. Evol. 2008, 25, 526–535. [Google Scholar] [CrossRef][Green Version]
- Robertson, G.; Schein, J.; Chiu, R.; Corbett, R.; Field, M.; Jackman, S.D.; Mungall, K.; Lee, S.; Okada, H.M.; Qian, J.Q.; et al. De novo assembly and analysis of RNA-seq data. Nat. Methods 2010, 7, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Cocquet, J.; Chong, A.; Zhang, G.; Veitia, R.A. Reverse transcriptase template switching and false alternative transcripts. Genomics 2006, 88, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Houseley, J.; Tollervey, D. Apparent non-canonical trans-splicing is generated by reverse transcriptase in vitro. PLoS ONE 2010, 5, e12271. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Lekstrom, K.; Leto, T.L. Analysis of mRNA transcripts from the NAD(P)H oxidase 1 (Nox1) gene: Evidence against production of the NADPH oxidase homolog-1 short (NOH-1s) transcript variant. J. Biol. Chem. 2004, 279, 51661–51668. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, Y.; Chen, Y.; Yin, C.; Chen, J.J.; Liu, S. MiR-214 protects erythroid cells against oxidative stress by targeting ATF4 and EZH2. Free Radic. Biol. Med. 2016, 92, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Quail, M.A.; Artymiuk, P.J.; Guest, J.R.; Green, J. Escherichia coli aconitases and oxidative stress: Post-transcriptional regulation of sodA expression. Microbiology 2002, 148, 1027–1037. [Google Scholar] [CrossRef]
- Koritzinsky, M.; Seigneuric, R.; Magagnin, M.G.; Van Den Beucken, T.; Lambin, P.; Wouters, B.G. The hypoxic proteome is influenced by gene-specific changes in mRNA translation. Proc. Radiother. Oncol. 2005, 76, 177–186. [Google Scholar] [CrossRef]
- Dallinger, R.; Chabicovsky, M.; Berger, B. Isoform-specific quantification of metallothionein in the terrestrial gastropod Helix pomatia. I. Molecular, biochemical, and methodical background. Environ. Toxicol. Chem. 2004, 23, 890–901. [Google Scholar] [CrossRef]
- Banni, M.; Messaoudi, I.; Said, L.; El Heni, J.; Kerkeni, A.; Said, K. Metallothionein gene expression in liver of rats exposed to cadmium and supplemented with zinc and selenium. Arch. Environ. Contam. Toxicol. 2010, 59, 513–519. [Google Scholar] [CrossRef]
- Bourdineaud, J.P.; Baudrimont, M.; Gonzalez, P.; Moreau, J.L. Challenging the model for induction of metallothionein gene expression. Biochimie 2006, 88, 1787–1792. [Google Scholar] [CrossRef]
- Cheung, A.P.L.; Lam, T.H.J.; Chan, K.M. Regulation of Tilapia metallothionein gene expression by heavy metal ions. Proc. Mar. Environ. Res. 2004, 58, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Rhee, J.S.; Dahms, H.U.; Lee, Y.M.; Han, K.N.; Lee, J.S. The yellow catfish, Pelteobagrus fulvidraco (Siluriformes) metallothionein cDNA: Molecular cloning and transcript expression level in response to exposure to the heavy metals Cd, Cu, and Zn. Fish Physiol. Biochem. 2012, 38, 1331–1342. [Google Scholar] [CrossRef]
- Brulle, F.; Mitta, G.; Leroux, R.; Lemière, S.; Leprêtre, A.; Vandenbulcke, F. The strong induction of metallothionein gene following cadmium exposure transiently affects the expression of many genes in Eisenia fetida: A trade-off mechanism? Comp. Biochem. Physiol. C Toxicol. Pharm. 2007, 144, 334–341. [Google Scholar] [CrossRef]
- Palacios, Ò.; Espart, A.; Espín, J.; Ding, C.; Thiele, D.J.; Atrian, S.; Capdevila, M. Full characterization of the Cu-, Zn-, and Cd-binding properties of CnMT1 and CnMT2, two metallothioneins of the pathogenic fungus Cryptococcus neoformans acting as virulence factors. Proc. Met. 2014, 6, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Niederwanger, M.; Calatayud, S.; Zerbe, O.; Atrian, S.; Albalat, R.; Capdevila, M.; Palacios, Ò.; Dallinger, R. Biomphalaria glabrata metallothionein: Lacking metal specificity of the protein and missing gene upregulation suggest metal sequestration by exchange instead of through selective binding. Int. J. Mol. Sci. 2017, 18, 1457. [Google Scholar] [CrossRef]
- Zangger, K.; Öz, G.; Otvos, J.D.; Armitage, I.M. Three-Dimensional Solution Structure of Mouse [Cd7]-Metallothionein-1 by Homonuclear and Heteronuclear NMR Spectroscopy; Cambridge University Press: Cambridge, UK, 1999; p. 8. [Google Scholar]
- Palacios, Ò.; Pérez-Rafael, S.; Pagani, A.; Dallinger, R.; Atrian, S.; Capdevila, M. Cognate and noncognate metal ion coordination in metal-specific metallothioneins: The Helix pomatia system as a model. J. Biol. Inorg. Chem. 2014, 19, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rafael, S.; Pagani, A.; Palacios, Ò.; Dallinger, R.; Capdevila, M.; Atrian, S. The role of histidine in a copper-specific metallothionein. Z. Fur Anorg. Und Allg. Chem. 2013, 639, 1356–1360. [Google Scholar] [CrossRef]
- Palacios, O.; Pagani, A.; Pérez-Rafael, S.; Egg, M.; Höckner, M.; Brandstätter, A.; Capdevila, M.; Atrian, S.; Dallinger, R. Shaping mechanisms of metal specificity in a family of metazoan metallothioneins: Evolutionary differentiation of mollusc metallothioneins. BMC Biol. 2011, 9, 4. [Google Scholar] [CrossRef]
- Höckner, M.; Stefanon, K.; De Vaufleury, A.; Monteiro, F.; Pérez-Rafael, S.; Palacios, Ò.; Capdevila, M.; Atrian, S.; Dallinger, R. Physiological relevance and contribution to metal balance of specific and non-specific Metallothionein isoforms in the garden snail, Cantareus Aspersus. Biometals 2011, 24, 1079–1092. [Google Scholar] [CrossRef]
- Ding, C.; Festa, R.A.; Chen, Y.L.; Espart, A.; Palacios, Ò.; Espín, J.; Capdevila, M.; Atrian, S.; Heitman, J.; Thiele, D.J. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe 2013, 13, 265–276. [Google Scholar] [CrossRef]
- Dallinger, R. Gastropod Metallothioneins in Evolution: New Rules for an Old Protein Family; Hobmayer, B., Micura, R., Striessnig, J., Eds.; CMBI: Innsbruck, Austria, 2018; pp. 24–25. [Google Scholar]
- Pérez, L.M.; Fittipaldi, M.; Adrados, B.; Morató, J.; Codony, F. Error estimation in environmental DNA targets quantification due to PCR efficiencies differences between real samples and standards. Folia Microbiol. 2013, 58, 657–662. [Google Scholar] [CrossRef] [PubMed]



| MT | # of C | K/N Ratio | # of H | # caas | % caas | Length |
|---|---|---|---|---|---|---|
| Helix pomatia CdMT | 18 | 9/1 | 0 | 16 | 23.90 | 67 |
| Cornu aspersum CdMT | 18 | 8/2 | 0 | 14 | 20.90 | 67 |
| Littorina littorea CdMT | 27 | 10/2 | 0 | 21 | 21 | 100 |
| Alinda biplicata 9md-MT | 81 | 36/10 | 0 | 66 | 23 | 287 |
| Alinda biplicata 10md-MT | 90 | 40/13 | 0 | 74 | 23.20 | 319 |
| Alinda biplicata CuMT | 17 | 6/6 | 1 | 11 | 16.90 | 65 |
| Helix pomatia CuMT | 18 | 5/6 | 1 | 12 | 18.50 | 65 |
| Cornu aspersum CuMT | 17 | 3/9 | 1 | 11 | 16.90 | 65 |
| Primer | Sequence 5′3′ | Length [bp] | Conc. [nm] | Primer Efficiency |
|---|---|---|---|---|
| CuMT S | GCC TGC AAC AGC AAT CCA T | 19 | 900 | 94% |
| CuMT AS | AAC AGG CAG CCC CAC ATT T | 19 | 900 | |
| md-MT S | GTG GTG ATG GCT GCA CAT GT | 20 | 900 | 88% |
| md-MT AS | CGC TGG GCC TGT ACA CTC TT | 20 | 900 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrini-Martha, V.; Köll, S.; Dvorak, M.; Dallinger, R. Cadmium Uptake, MT Gene Activation and Structure of Large-Sized Multi-Domain Metallothioneins in the Terrestrial Door Snail Alinda biplicata (Gastropoda, Clausiliidae). Int. J. Mol. Sci. 2020, 21, 1631. https://doi.org/10.3390/ijms21051631
Pedrini-Martha V, Köll S, Dvorak M, Dallinger R. Cadmium Uptake, MT Gene Activation and Structure of Large-Sized Multi-Domain Metallothioneins in the Terrestrial Door Snail Alinda biplicata (Gastropoda, Clausiliidae). International Journal of Molecular Sciences. 2020; 21(5):1631. https://doi.org/10.3390/ijms21051631
Chicago/Turabian StylePedrini-Martha, Veronika, Simon Köll, Martin Dvorak, and Reinhard Dallinger. 2020. "Cadmium Uptake, MT Gene Activation and Structure of Large-Sized Multi-Domain Metallothioneins in the Terrestrial Door Snail Alinda biplicata (Gastropoda, Clausiliidae)" International Journal of Molecular Sciences 21, no. 5: 1631. https://doi.org/10.3390/ijms21051631
APA StylePedrini-Martha, V., Köll, S., Dvorak, M., & Dallinger, R. (2020). Cadmium Uptake, MT Gene Activation and Structure of Large-Sized Multi-Domain Metallothioneins in the Terrestrial Door Snail Alinda biplicata (Gastropoda, Clausiliidae). International Journal of Molecular Sciences, 21(5), 1631. https://doi.org/10.3390/ijms21051631






