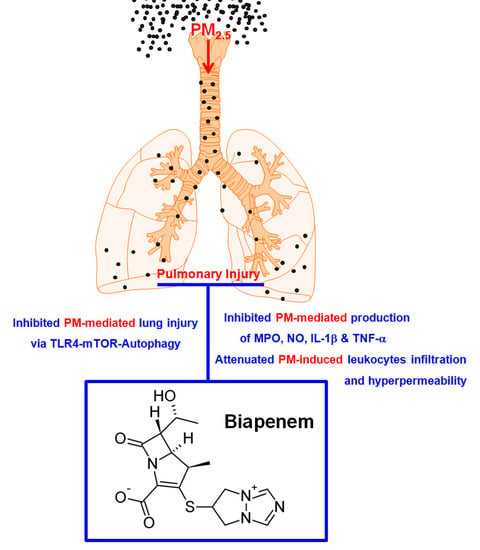
Biapenem as a Novel Insight into Drug Repositioning against Particulate Matter-Induced Lung Injury
Abstract
1. Introduction
2. Results
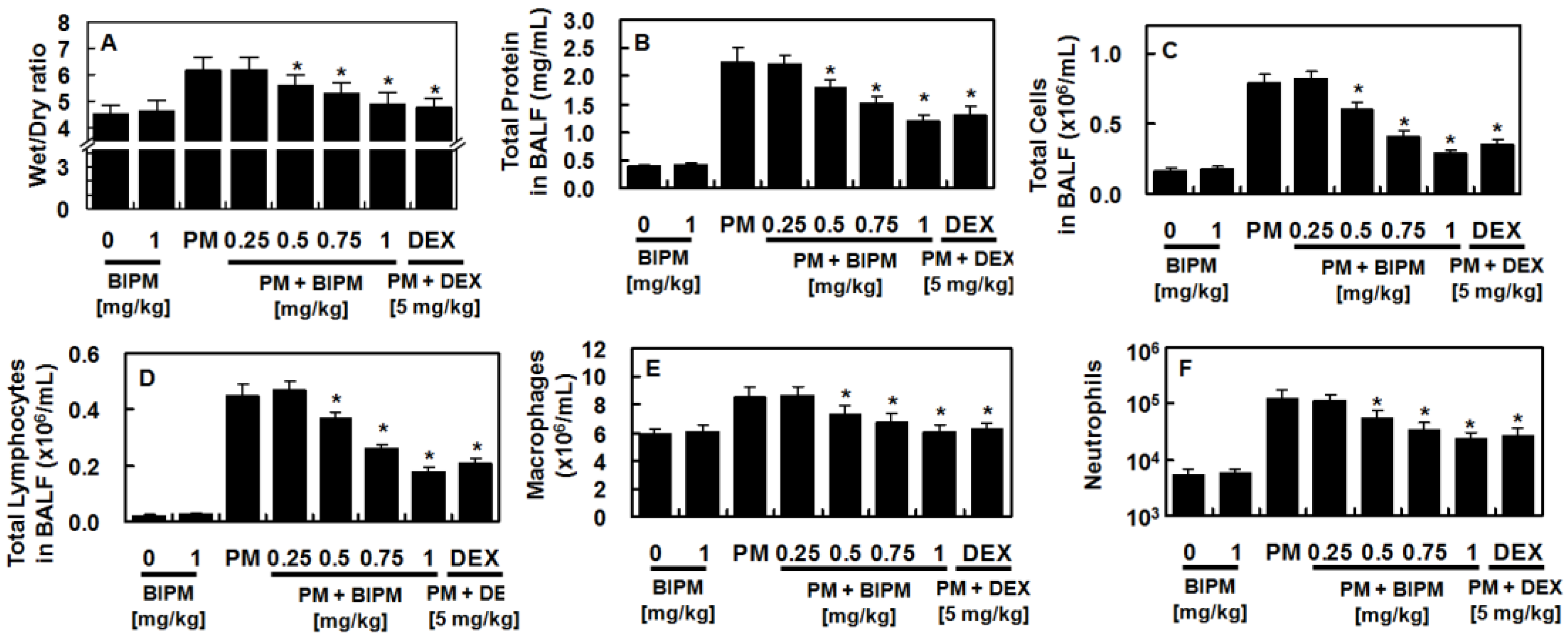
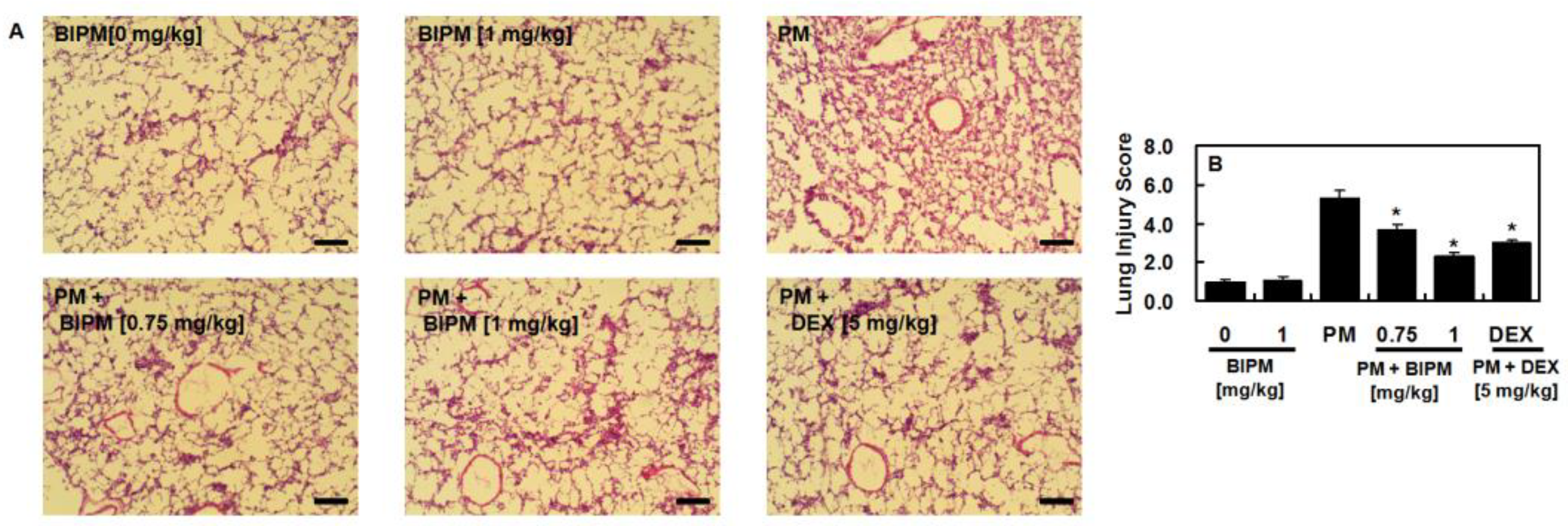
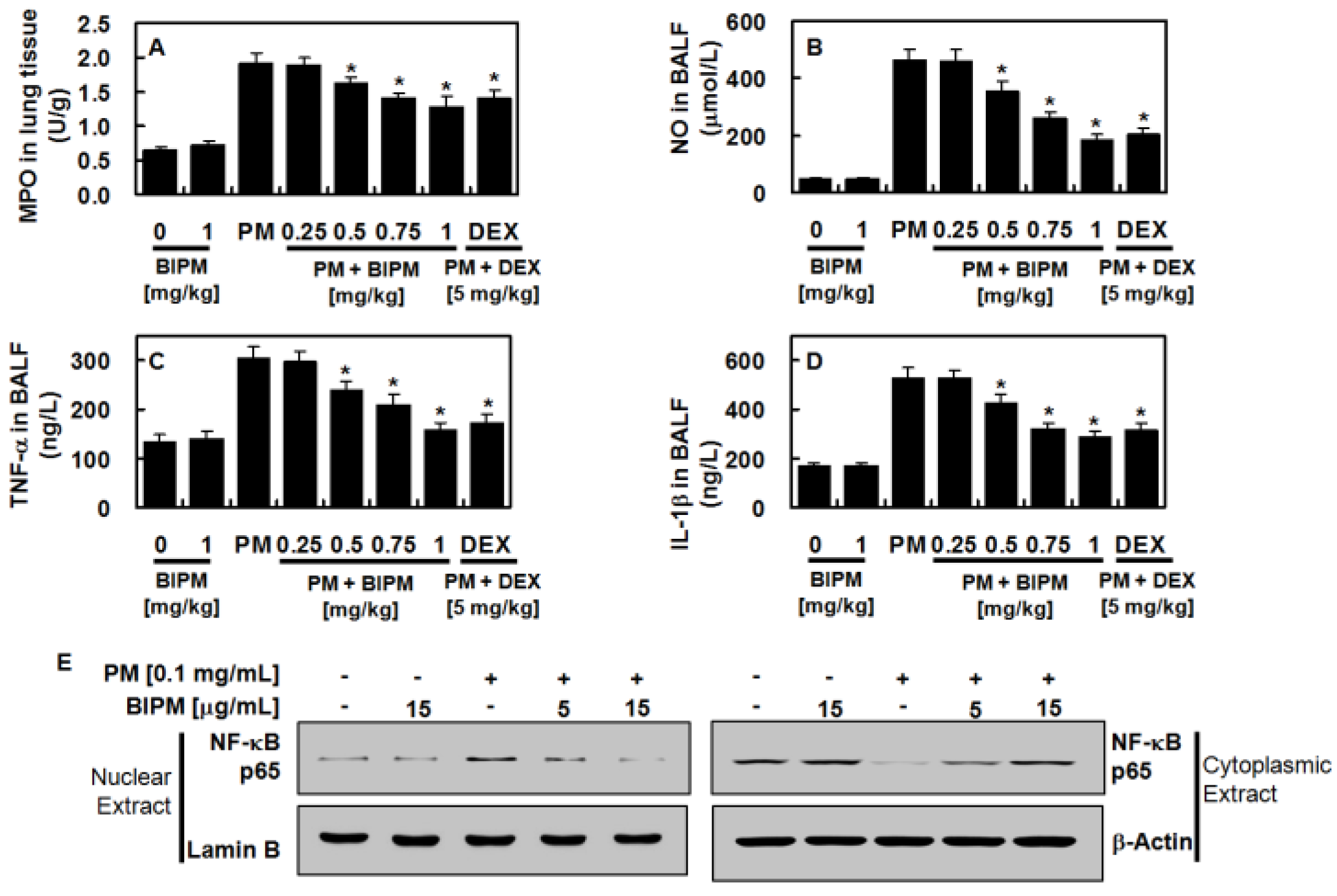
2.1. Effects of BIPM on PM2.5-Induced Lung Damage
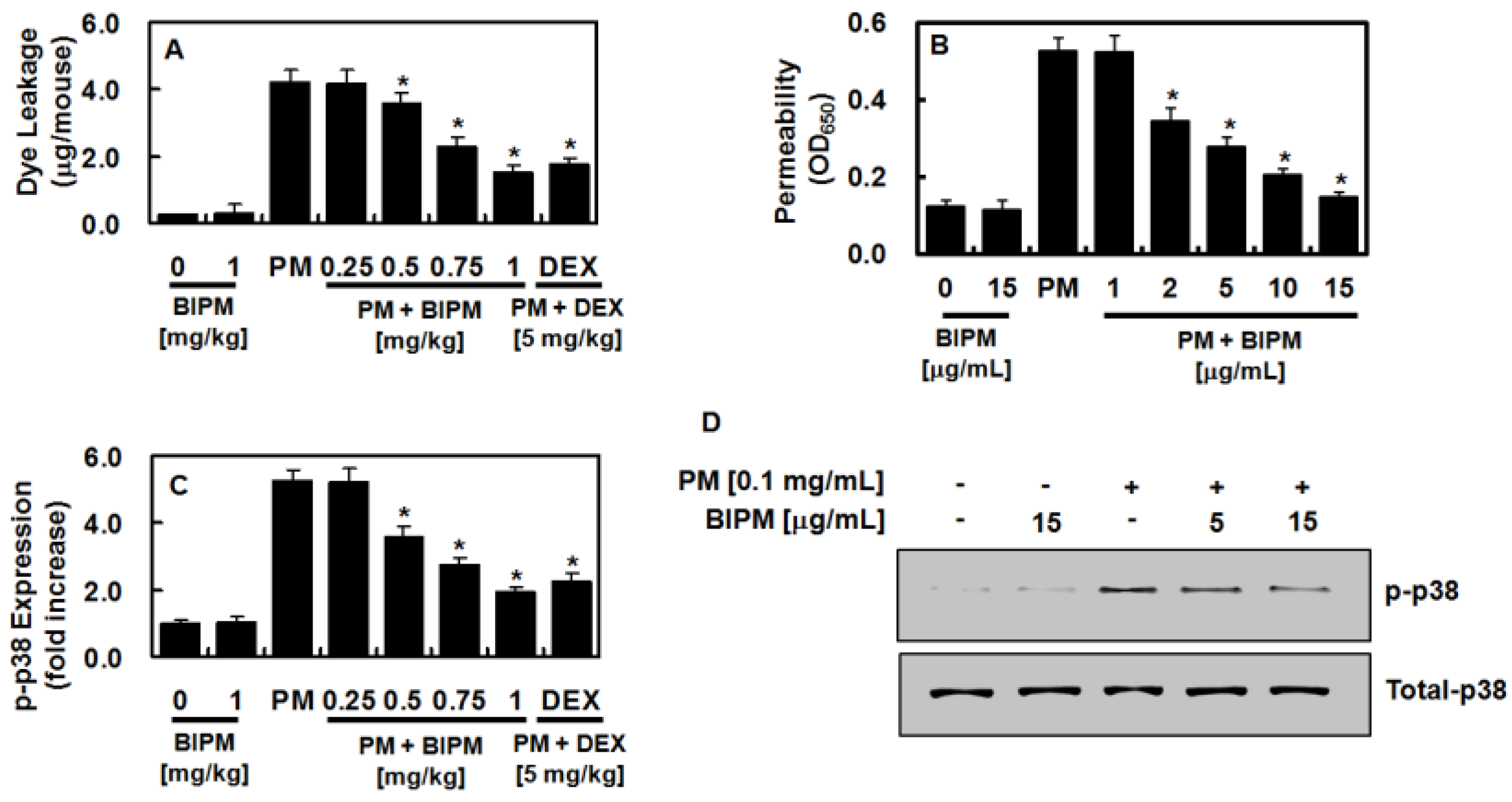
2.2. Effects of BIPM on PM2.5-Mediated Vascular Barrier Disruption
2.3. Effects of BIPM on PM2.5-Induced Pulmonary Inflammation
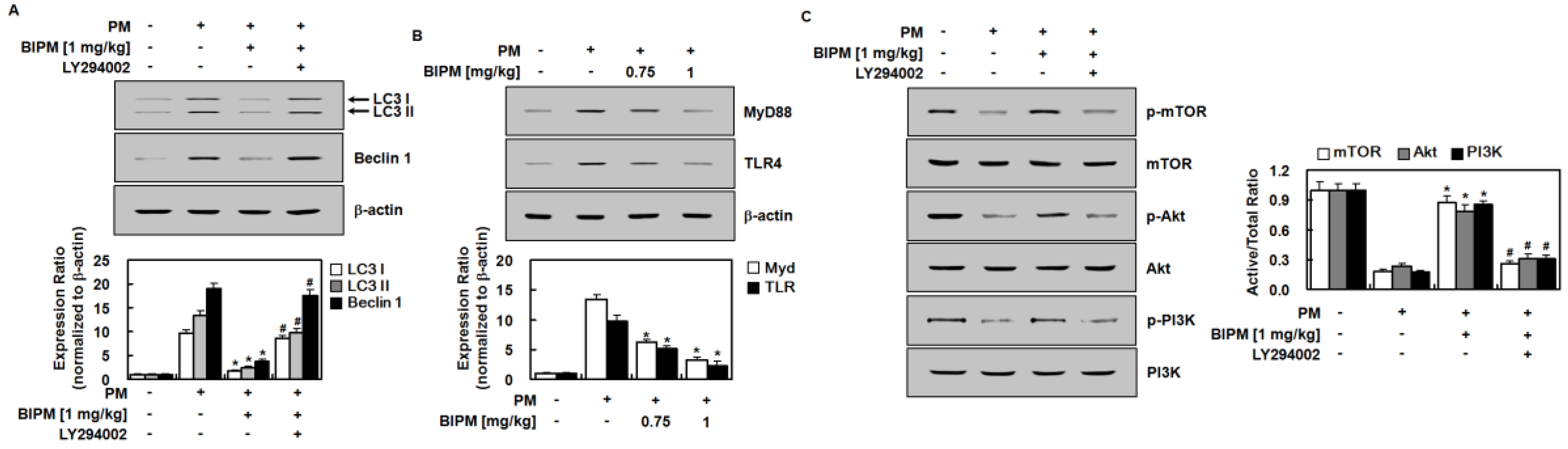
2.4. Effects of BIPM on PM2.5-Induced Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals and Husbandry
4.3. Primary Culture of Mouse Lung Microvascular Endothelial Cells (MLMVECs)
4.4. Lung Wet/Dry Weight Ratio
4.5. Hematoxylin and Eosin (H&E) Staining
4.6. ELISA of Phosphorylated p38 MAPK, MPO, NO, IL-1 β, and TNF-α
4.7. Protein Concentration and Cell Count in the BALF
4.8. Permeability Assays
4.9. Western Blot Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Baek, M.C.; Jung, B.; Kang, H.; Lee, H.S.; Bae, J.S. Novel insight into drug repositioning: Methylthiouracil as a case in point. Pharm. Res. 2015, 99, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Padhy, B.M.; Gupta, Y.K. Drug repositioning: Re-investigating existing drugs for new therapeutic indications. J. Postgrad. Med. 2011, 57, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Giulianotti, M.A.; Welmaker, G.S.; Houghten, R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov. Today 2013, 18, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Langedijk, J.; Mantel-Teeuwisse, A.K.; Slijkerman, D.S.; Schutjens, M.H. Drug repositioning and repurposing: Terminology and definitions in literature. Drug Discov. Today 2015. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Yu, Z.; Ding, H.; Ma, Z. The influence of PM2.5 on lung injury and cytokines in mice. Exp. Med. 2019, 18, 2503–2511. [Google Scholar] [CrossRef]
- Losacco, C.; Perillo, A. Particulate matter air pollution and respiratory impact on humans and animals. Environ. Sci. Pollut Res. Int. 2018, 25, 33901–33910. [Google Scholar] [CrossRef]
- Ning, X.; Ji, X.; Li, G.; Sang, N. Ambient PM2.5 causes lung injuries and coupled energy metabolic disorder. Ecotoxicol. Environ. Saf. 2019, 170, 620–626. [Google Scholar] [CrossRef]
- Li, Y.; Lin, T.; Wang, F.; Ji, T.; Guo, Z. Seasonal variation of polybrominated diphenyl ethers in PM2.5 aerosols over the east china sea. Chemosphere 2015, 119, 675–681. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, X.; Ku, T.; Li, G.; Sang, N. Heavy metals bound to fine particulate matter from northern china induce season-dependent health risks: A study based on myocardial toxicity. Environ. Pollut. 2016, 216, 380–390. [Google Scholar] [CrossRef]
- Gent, J.F.; Triche, E.W.; Holford, T.R.; Belanger, K.; Bracken, M.B.; Beckett, W.S.; Leaderer, B.P. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA 2003, 290, 1859–1867. [Google Scholar] [CrossRef]
- Gong, H., Jr.; Linn, W.S.; Clark, K.W.; Anderson, K.R.; Geller, M.D.; Sioutas, C. Respiratory responses to exposures with fine particulates and nitrogen dioxide in the elderly with and without copd. Inhal. Toxicol. 2005, 17, 123–132. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, G.; Li, Y.; Tan, M.; Wang, W.; Chen, J.; Hwu, Y.; Hsu, P.C.; Je, J.H.; Margaritondo, G.; et al. Synchrotron microradiography study on acute lung injury of mouse caused by pm(2.5) aerosols. Eur. J. Radiol. 2006, 58, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Mengersen, K.; Kimlin, M.; Zhou, M.; Tong, S.; Fang, L.; Wang, B.; Hu, W. Lung cancer and particulate pollution: A critical review of spatial and temporal analysis evidence. Environ. Res. 2018, 164, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Zong, Z.; Yu, R.; Lv, X.; Xin, J.; Tong, C.; Hao, Q.; Qin, Z.; Xiong, Y.; et al. Biapenem versus meropenem in the treatment of bacterial infections: A multicenter, randomized, controlled clinical trial. Indian J. Med. Res. 2013, 138, 995–1002. [Google Scholar]
- Pei, G.; Yin, W.; Zhang, Y.; Wang, T.; Mao, Y.; Sun, Y. Efficacy and safety of biapenem in treatment of infectious disease: A meta-analysis of randomized controlled trials. J. Chemother. 2016, 28, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yamamoto, Y.; Yanagihara, K.; Araki, N.; Harada, Y.; Morinaga, Y.; Izumikawa, K.; Kakeya, H.; Hasegawa, H.; Kohno, S.; et al. In vivo efficacy and pharmacokinetics of biapenem in a murine model of ventilator-associated pneumonia with pseudomonas aeruginosa. J. Infect. Chemother. 2012, 18, 472–478. [Google Scholar] [CrossRef]
- Wang, T.; Shimizu, Y.; Wu, X.; Kelly, G.T.; Xu, X.; Wang, L.; Qian, Z.; Chen, Y.; Garcia, J.G.N. Particulate matter disrupts human lung endothelial cell barrier integrity via rho-dependent pathways. Pulm. Circ. 2017, 7, 617–623. [Google Scholar] [CrossRef]
- Wang, T.; Chiang, E.T.; Moreno-Vinasco, L.; Lang, G.D.; Pendyala, S.; Samet, J.M.; Geyh, A.S.; Breysse, P.N.; Chillrud, S.N.; Natarajan, V.; et al. Particulate matter disrupts human lung endothelial barrier integrity via ros- and p38 mapk-dependent pathways. Am. J. Respir. Cell Mol. Biol. 2010, 42, 442–449. [Google Scholar] [CrossRef]
- Qin, Y.H.; Dai, S.M.; Tang, G.S.; Zhang, J.; Ren, D.; Wang, Z.W.; Shen, Q. Hmgb1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of mapk p38 through receptor for advanced glycation end products. J. Immunol. 2009, 183, 6244–6250. [Google Scholar] [CrossRef]
- Sun, C.; Liang, C.; Ren, Y.; Zhen, Y.; He, Z.; Wang, H.; Tan, H.; Pan, X.; Wu, Z. Advanced glycation end products depress function of endothelial progenitor cells via p38 and erk 1/2 mitogen-activated protein kinase pathways. Basic Res. Cardiol. 2009, 104, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ros-mapk-nf-kappab signaling pathway. J. Thorac. Dis. 2017, 9, 4398–4412. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Lee, T.L.; Chen, Y.C.; Liang, C.J.; Wang, S.H.; Lue, J.H.; Tsai, J.S.; Lee, S.W.; Chen, S.H.; Yang, Y.F.; et al. PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the il-6/akt/stat3/nf-kappab-dependent pathway. Part. Fibre Toxicol. 2018, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Qiu, X.; Hu, X.; Shang, Y.; Pardo, M.; Fang, Y.; Wang, J.; Rudich, Y.; Zhu, T. Effects on il-1beta signaling activation induced by water and organic extracts of fine particulate matter (PM2.5) in vitro. Environ. Pollut. 2018, 237, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.H.; van Eeden, S.F. Particulate matter air pollution exposure: Role in the development and exacerbation of chronic obstructive pulmonary disease. Int. J. Chron Obs. Pulmon. Dis. 2009, 4, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, W.; Kim, E.; Ku, S.K.; Bae, J.S. Inhibitory effects of collismycin c and pyrisulfoxin a on particulate matter-induced pulmonary injury. Phytomedicine 2019, 62, 152939. [Google Scholar] [CrossRef]
- Lee, W.; Jeong, S.Y.; Gu, M.J.; Lim, J.S.; Park, E.K.; Baek, M.C.; Kim, J.S.; Hahn, D.; Bae, J.S. Inhibitory effects of compounds isolated from dioscorea batatas decne peel on particulate matter-induced pulmonary injury in mice. J. Toxicol. Environ. Health A 2019, 82, 727–740. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, G.E.; Song, G.Y.; Bae, J.S. Pulmonary protective functions of rare ginsenoside rg4 on particulate matter-induced inflammatory responses. Biotechnol. Bioprocess. Eng. 2019, 24, 445–453. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Fontanari, C.; Borducchi, E.; Keller, A.C.; Russo, M.; Soares, E.G.; Albuquerque, D.A.; Faccioli, L.H. Anti-inflammatory effects of lafoensia pacari and ellagic acid in a murine model of asthma. Eur. J. Pharm. 2008, 580, 262–270. [Google Scholar] [CrossRef]
- Yoder, M.C., Jr.; Chua, R.; Tepper, R. Effect of dexamethasone on pulmonary inflammation and pulmonary function of ventilator-dependent infants with bronchopulmonary dysplasia. Am. Rev. Respir. Dis. 1991, 143, 1044–1048. [Google Scholar] [CrossRef]
- Meng, L.; Li, L.; Lu, S.; Li, K.; Su, Z.; Wang, Y.; Fan, X.; Li, X.; Zhao, G. The protective effect of dexmedetomidine on lps-induced acute lung injury through the hmgb1-mediated tlr4/nf-kappab and pi3k/akt/mtor pathways. Mol. Immunol. 2018, 94, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Mizumura, K.; Cloonan, S.M.; Haspel, J.A.; Choi, A.M.K. The emerging importance of autophagy in pulmonary diseases. Chest 2012, 142, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, J.; Wu, Y.F.; Lou, J.; Mao, Y.Y.; Shen, H.H.; Chen, Z.H. Mtor and autophagy in regulation of acute lung injury: A review and perspective. Microbes Infect. 2014, 16, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lou, J.; Mao, Y.Y.; Lai, T.W.; Liu, L.Y.; Zhu, C.; Zhang, C.; Liu, J.; Li, Y.Y.; Zhang, F.; et al. Activation of mtor in pulmonary epithelium promotes lps-induced acute lung injury. Autophagy 2016, 12, 2286–2299. [Google Scholar] [CrossRef] [PubMed]
- Woodward, N.C.; Levine, M.C.; Haghani, A.; Shirmohammadi, F.; Saffari, A.; Sioutas, C.; Morgan, T.E.; Finch, C.E. Toll-like receptor 4 in glial inflammatory responses to air pollution in vitro and in vivo. J. Neuroinflamm. 2017, 14, 84. [Google Scholar] [CrossRef]
- Cadwell, K. Crosstalk between autophagy and inflammatory signalling pathways: Balancing defence and homeostasis. Nat. Rev. Immunol. 2016, 16, 661–675. [Google Scholar] [CrossRef]
- Zeng, M.C.; Sang, W.H.; Chen, S.; Chen, R.; Zhang, H.L.; Xue, F.; Li, Z.M.; Liu, Y.; Gong, Y.S.; Zhang, H.Y.; et al. 4-pba inhibits lps-induced inflammation through regulating er stress and autophagy in acute lung injury models. Toxicol. Lett. 2017, 271, 26–37. [Google Scholar] [CrossRef]
- Shao, X.; Lai, D.; Zhang, L.; Xu, H. Induction of autophagy and apoptosis via pi3k/akt/tor pathways by azadirachtin a in spodoptera litura cells. Sci. Rep. 2016, 6, 35482. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, Y.; Huang, Y.; Lu, Q.; Zheng, L.; Hu, D.; Feng, W.K.; Liu, Y.L.; Ji, K.T.; Zhang, H.Y.; et al. Bfgf regulates autophagy and ubiquitinated protein accumulation induced by myocardial ischemia/reperfusion via the activation of the pi3k/akt/mtor pathway. Sci. Rep. 2015, 5, 9287. [Google Scholar] [CrossRef]
- Herrero, R.; Sanchez, G.; Lorente, J.A. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann. Transl. Med. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. Mtor signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Bergvall, C.; Westerholm, R. Determination of dibenzopyrenes in standard reference materials (srm) 1649a, 1650, and 2975 using ultrasonically assisted extraction and lc-gc-ms. Anal. Bioanal. Chem. 2006, 384, 438–447. [Google Scholar] [CrossRef]
- Yan, X.D.; Wang, Q.M.; Tie, C.; Jin, H.T.; Han, Y.X.; Zhang, J.L.; Yu, X.M.; Hou, Q.; Zhang, P.P.; Wang, A.P.; et al. Polydatin protects the respiratory system from PM2.5 exposure. Sci. Rep. 2017, 7, 40030. [Google Scholar] [CrossRef] [PubMed]
- Ozdulger, A.; Cinel, I.; Koksel, O.; Cinel, L.; Avlan, D.; Unlu, A.; Okcu, H.; Dikmengil, M.; Oral, U. The protective effect of n-acetylcysteine on apoptotic lung injury in cecal ligation and puncture-induced sepsis model. Shock 2003, 19, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, W.; Yang, S.; Cho, S.H.; Baek, M.C.; Song, G.Y.; Bae, J.S. Suppressive effects of rare ginsenosides, rk1 and rg5, on hmgb1-mediated septic responses. Food Chem. Toxicol. 2019, 124, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Bae, J.S. Pelargonidin protects against renal injury in a mouse model of sepsis. J. Med. Food 2019, 22, 57–61. [Google Scholar] [CrossRef]
- Lee, W.; Cho, S.H.; Kim, J.E.; Lee, C.; Lee, J.H.; Baek, M.C.; Song, G.Y.; Bae, J.S. Suppressive effects of ginsenoside rh1 on hmgb1-mediated septic responses. Am. J. Chin. Med. 2019, 47, 119–133. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.; Baek, M.-C.; Kim, K.-M.; Bae, J.-S. Biapenem as a Novel Insight into Drug Repositioning against Particulate Matter-Induced Lung Injury. Int. J. Mol. Sci. 2020, 21, 1462. https://doi.org/10.3390/ijms21041462
Lee W, Baek M-C, Kim K-M, Bae J-S. Biapenem as a Novel Insight into Drug Repositioning against Particulate Matter-Induced Lung Injury. International Journal of Molecular Sciences. 2020; 21(4):1462. https://doi.org/10.3390/ijms21041462
Chicago/Turabian StyleLee, Wonhwa, Moon-Chang Baek, Kyung-Min Kim, and Jong-Sup Bae. 2020. "Biapenem as a Novel Insight into Drug Repositioning against Particulate Matter-Induced Lung Injury" International Journal of Molecular Sciences 21, no. 4: 1462. https://doi.org/10.3390/ijms21041462
APA StyleLee, W., Baek, M.-C., Kim, K.-M., & Bae, J.-S. (2020). Biapenem as a Novel Insight into Drug Repositioning against Particulate Matter-Induced Lung Injury. International Journal of Molecular Sciences, 21(4), 1462. https://doi.org/10.3390/ijms21041462