β3-Adrenoreceptor Blockade Induces Stem Cells Differentiation in Melanoma Microenvironment
Abstract
1. Introduction
2. Results
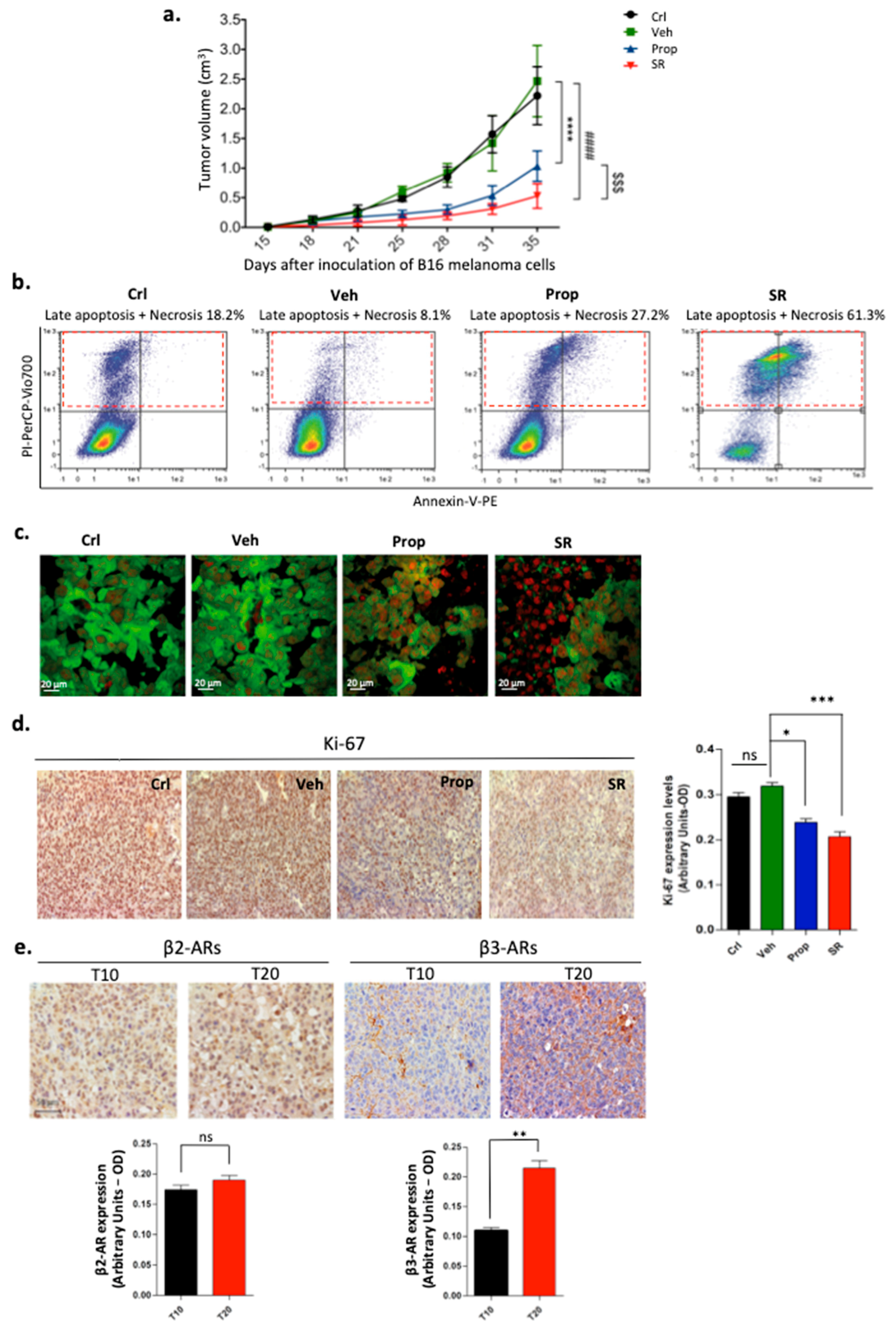
2.1. β-ARs Blockade Leads to Tumor Growth Reduction by Affecting Proliferation and Viability of Melanoma Cells
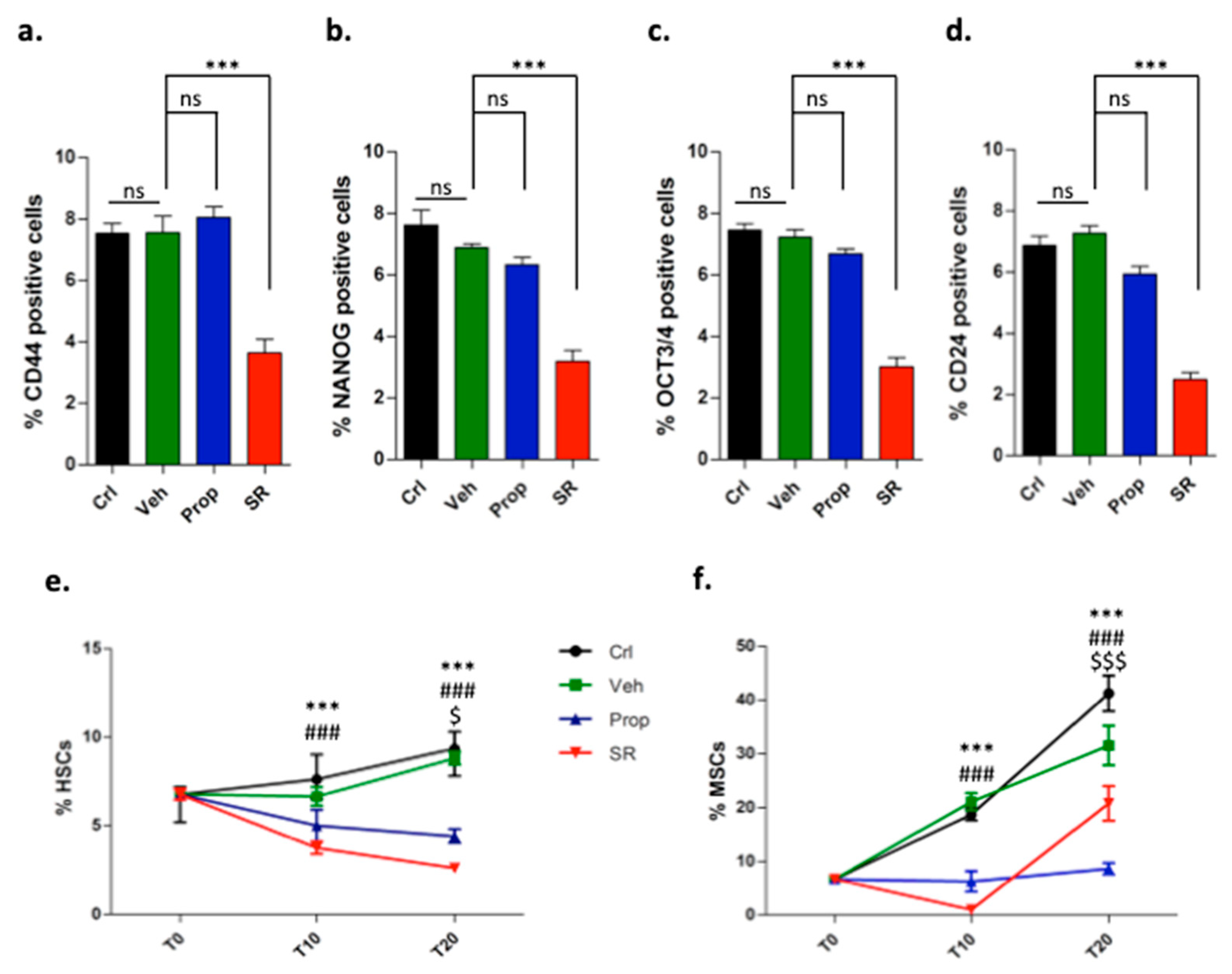
2.2. β-ARs Blockade Attenuates the Expression of Stemness Markers
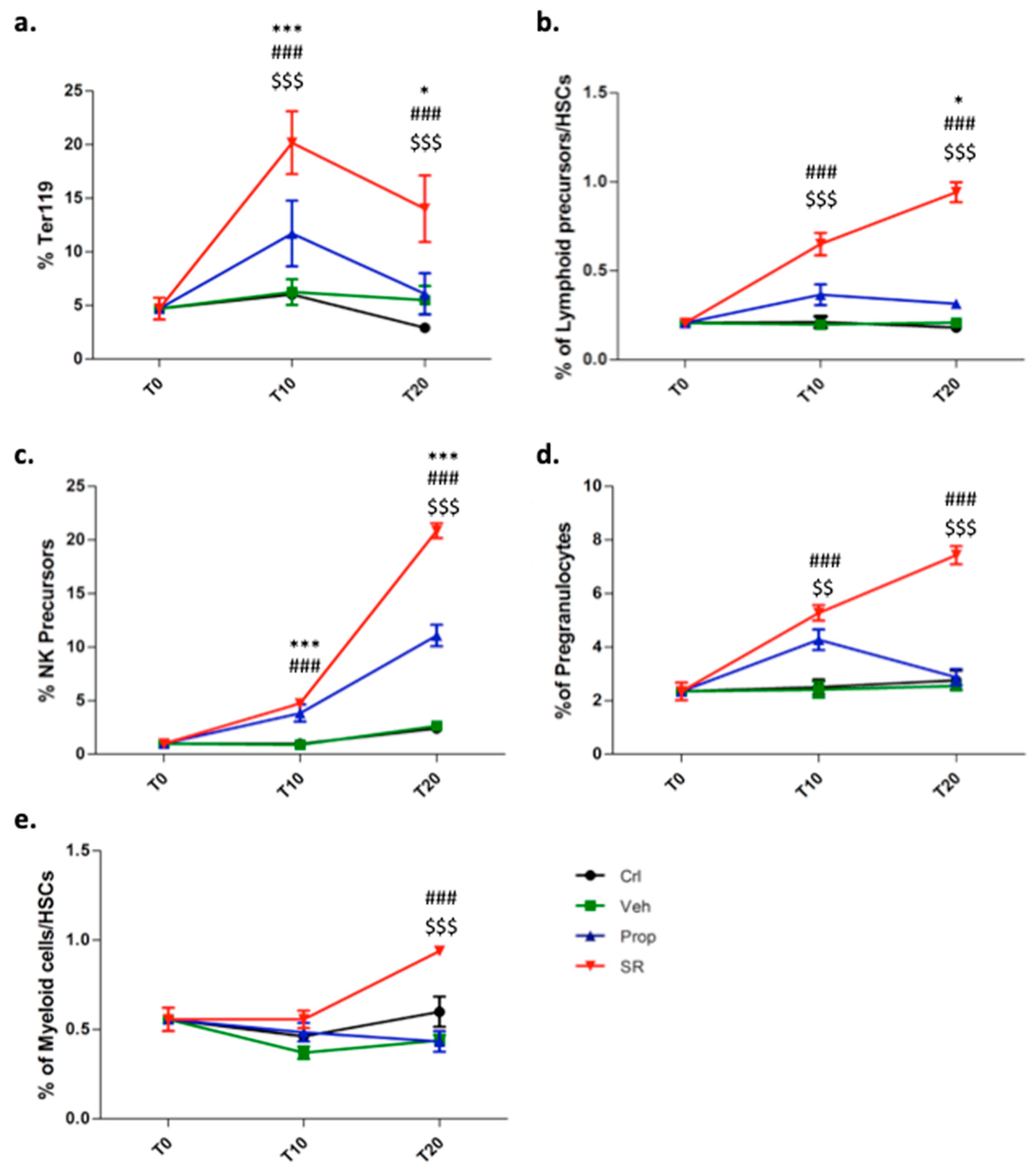
2.3. β3-AR Blockade Induces Tumor Stromal Cells Differentiation
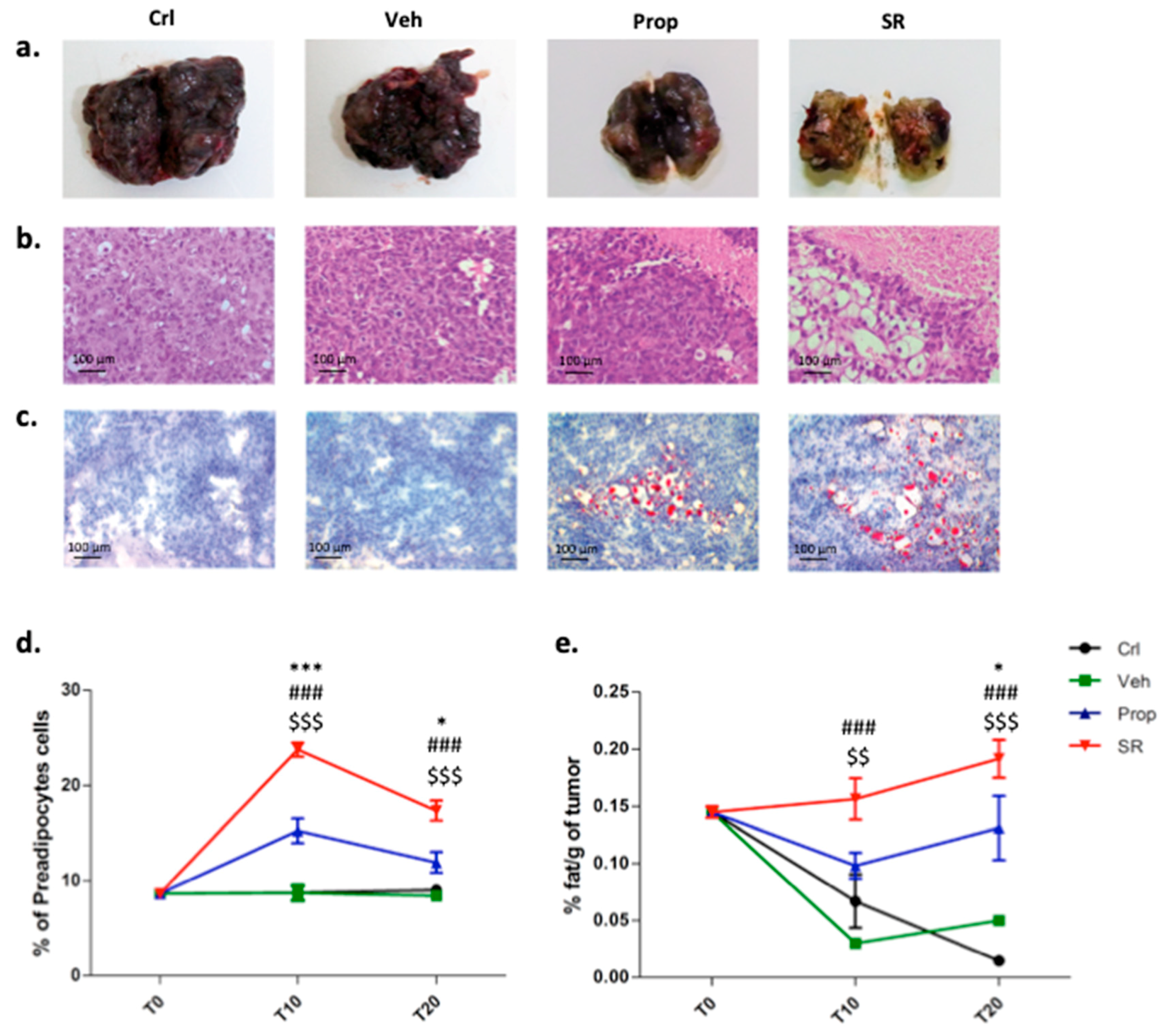
2.4. β-AR Antagonism Increases Pre-Adipocytes Formation in the Tumor Microenvironment
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Mice
4.3. Murine B16-F10 Syngeneic Model and Treatments
4.4. Isolation of Tumor Cells
4.5. Viability Assay
4.6. Extraction of Lipids from Tumors
4.7. Flow Cytometry and Morphology Analysis
4.8. Immunohistochemistry
4.9. Oil Red O Staining
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bocci, F.; Gearhart-Serna, L.; Boareto, M.; Ribeiro, M.; Ben-Jacob, E.; Devi, G.R.; Levine, H.; Onuchic, J.N.; Jolly, M.K. Toward understanding cancer stem cell heterogeneity in the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2019, 116, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Korkaya, H.; Liu, S.; Wicha, M.S. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J. Clin. Investig. 2011, 121, 3804–3809. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.; Zheng, J.; Umikawa, M.; Silvany, R.; Xie, X.J.; Wu, C.J.; Holzenberger, M.; Wang, Q.; Zhang, C.C. Components of the Hematopoietic Compartments in Tumor Stroma and Tumor-Bearing Mice. PLoS ONE 2011, 6, e18054. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; De Bari, C. The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 2010, 21, 1226–1238. [Google Scholar] [CrossRef]
- Rusciano, D. Differentiation and metastasis in melanoma. Crit. Rev. Oncog. 2000, 11, 147–163. [Google Scholar]
- Bao, B.; Ahmad, A.; Azmi, A.S.; Ali, S.; Sarkar, F.H. Overview of cancer stem cells (CSCs) and mechanisms of their regulation: Implications for cancer therapy. Curr. Protoc. Pharmacol. 2013, 61, 14–25. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Gandini, S.; Benemei, S.; Lotti, T.; Marchionni, N.; Geppetti, T. Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch. Intern. Med. 2011, 171, 779–781. [Google Scholar] [CrossRef]
- Yazawa, T.; Kaira, K.; Shimizu, K.; Shimizu, A.; Mori, K.; Nagashima, T.; Ohtaki, Y.; Oyama, T.; Mogi, A.; Kuwano, H. Prognostic significance of β2-adrenergic receptor expression in non-small cell lung cancer. Am. J. Transl. Res. 2016, 8, 5059–5070. [Google Scholar] [CrossRef]
- Childers, W.K.; Hollenbeak, C.S.; Cheriyath, P. β-Blockers Reduce Breast Cancer Recurrence and Breast Cancer Death: A Meta-Analysis. Clin. Breast Cancer 2015, 15, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Pelon, F.; Comito, G.; Taddei, M.L.; Moretti, S.; Innocenti, S.; Nassini, R.; Gerlini, G.; Borgognoni, L.; Bambi, F.; et al. Norepinephrine promotes tumor microenvironment reactivity through β3-adrenoreceptors during melanoma progression. Oncotarget 2015, 6, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Casini, G.; Filippi, L.; Nicchia, G.P.; Svelto, M.; Bagnoli, P. Functional involvement of β3-adrenergic receptors in melanoma growth and vascularization. J. Mol. Med. 2013, 91, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Sereni, F.; Dal Monte, M.; Filippi, L.; Bagnoli, P. Role of host β1- and β2-adrenergic receptors in a murine model of B16 melanoma: Functional involvement of β3-adrenergic receptors. Naunyn. Schmiedebergs Arch. Pharmacol. 2015, 388, 1317–1331. [Google Scholar] [CrossRef]
- Dal Monte, M.; Calvani, M.; Cammalleri, M.; Favre, C.; Filippi, L.; Bagnoli, P. β-Adrenoceptors as drug targets in melanoma: Novel preclinical evidence for a role of β(3)-adrenoceptors. Br. J. Pharmacol. 2019, 176, 2496–2508. [Google Scholar] [CrossRef]
- Calvani, M.; Bruno, G.; Dal Monte, M.; Nassini, R.; Fontani, F.; Casini, A.; Cavallini, L.; Becatti, M.; Bianchini, F.; De Logu, F.; et al. β(3)-Adrenoceptor as a potential immuno-suppressor agent in melanoma. Br. J. Pharmacol. 2019, 176, 2509–2524. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Giles, A.J.; Chien, C.D.; Reid, C.M.; Fry, T.J.; Park, D.M.; Kaplan, R.N.; Gilbert, M.R. The functional interplay between systemic cancer and the hematopoietic stem cell niche. Pharmacol. Ther. 2016, 168, 53–60. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L.; MacDougald, O.A. Adipose tissue stem cells meet preadipocyte commitment: Going back to the future. J. Lipid Res. 2012, 53, 227–246. [Google Scholar] [CrossRef]
- Rafii, S.; Avecilla, S.; Shmelkov, S.; Shido, K.; Tejada, R.; Moore, M.A.; Heissig, B.; Hattori, K. Angiogenic factors reconstitute hematopoiesis by recruiting stem cells from bone marrow microenvironment. Ann. N. Y. Acad. Sci. 2003, 996, 49–60. [Google Scholar] [CrossRef]
- Heissig, B.; Hattori, K.; Dias, S.; Friedrich, M.; Ferris, B.; Hackett, N.R.; Crystal, R.G.; Besmer, P.; Lyden, D.; Moore, M.A.; et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 2002, 109, 625–637. [Google Scholar] [CrossRef]
- Hattori, K.; Heissig, B.; Rafii, S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk. Lymphoma 2003, 44, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Fu, S. The correlation of hematopoietic stem cells with cancer stem cells through the regulation of stromal cells in tumor microenvironment. Med. Hypotheses 2013, 80, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gorain, M.; Kundu, G.; Kundu, G.C. Therapeutic implications of cellular and molecular biology of cancer stem cells in melanoma. Mol.Cancer. 2017, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, T.; Ouyang, T.; Xin, S.; Ma, Y.; Chang, M. Propranolol enhanced adipogenesis instead of induction of apoptosis of hemangiomas stem cells. Int. J. Clin. Exp. Pathol. 2014, 7, 3809–3817. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvani, M.; Bruno, G.; Dabraio, A.; Subbiani, A.; Bianchini, F.; Fontani, F.; Casazza, G.; Vignoli, M.; De Logu, F.; Frenos, S.; et al. β3-Adrenoreceptor Blockade Induces Stem Cells Differentiation in Melanoma Microenvironment. Int. J. Mol. Sci. 2020, 21, 1420. https://doi.org/10.3390/ijms21041420
Calvani M, Bruno G, Dabraio A, Subbiani A, Bianchini F, Fontani F, Casazza G, Vignoli M, De Logu F, Frenos S, et al. β3-Adrenoreceptor Blockade Induces Stem Cells Differentiation in Melanoma Microenvironment. International Journal of Molecular Sciences. 2020; 21(4):1420. https://doi.org/10.3390/ijms21041420
Chicago/Turabian StyleCalvani, Maura, Gennaro Bruno, Annalisa Dabraio, Angela Subbiani, Francesca Bianchini, Filippo Fontani, Gabriella Casazza, Marina Vignoli, Francesco De Logu, Stefano Frenos, and et al. 2020. "β3-Adrenoreceptor Blockade Induces Stem Cells Differentiation in Melanoma Microenvironment" International Journal of Molecular Sciences 21, no. 4: 1420. https://doi.org/10.3390/ijms21041420
APA StyleCalvani, M., Bruno, G., Dabraio, A., Subbiani, A., Bianchini, F., Fontani, F., Casazza, G., Vignoli, M., De Logu, F., Frenos, S., Filippi, L., & Favre, C. (2020). β3-Adrenoreceptor Blockade Induces Stem Cells Differentiation in Melanoma Microenvironment. International Journal of Molecular Sciences, 21(4), 1420. https://doi.org/10.3390/ijms21041420






