Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing
Abstract
1. Introduction
2. The Skin between the Theory and the Physiology of Aging
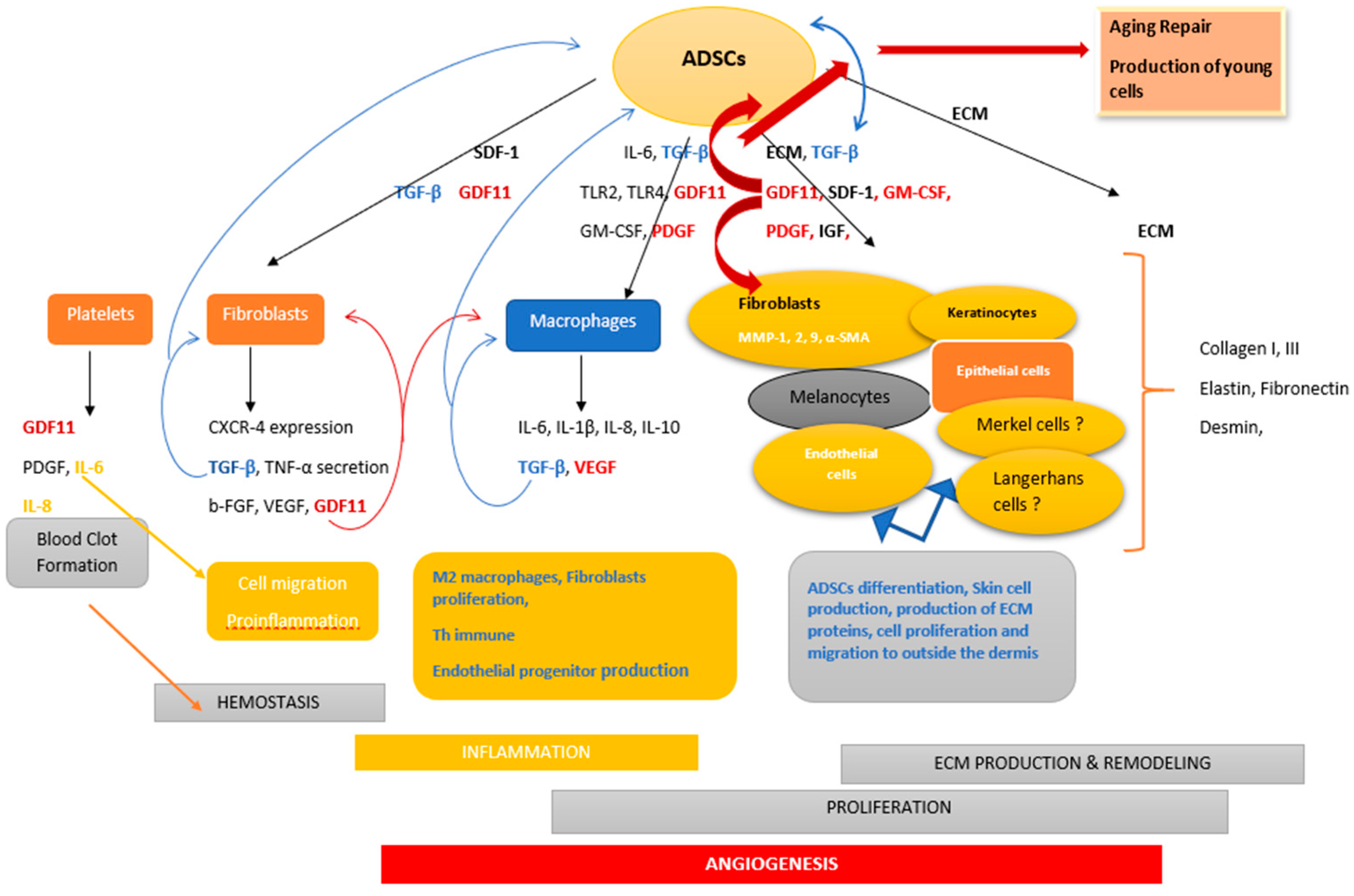
3. Role of the Microenvironment in ADSCs Induction
4. ADSCs Advancements in Skin Therapy
4.1. Inflammation Phase
4.2. Migration
4.3. Angiogenesis
4.4. Proliferation/Reepitelialization
5. ADSCs Issues in Skin Aging
6. ADSCs Issues between Hopes and Limits
7. Summary and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | adipose derived stem cells |
| GDF11 | growth differentiation factor |
| TGF-β | transforming growth factor |
| ECM | extracellular matrix |
| DF | dermal fibroblasts |
| ROS | reactive oxygen species |
| MSCs | mesenchymal stem cells |
| MMP1, 2, 9 | matrix metalloproteinase1, 2, 9 |
| IL-1, -6, -8, -10 | interleukin-1, -6, -8, -10 |
| TNF-α | tumor necrosis factor-α |
| VEGF | vascular endothelial growth factor |
| PDGF | platelets derived growth factor |
| α-SMA | α-smooth muscle actin |
| MCP-1 | monocyte chemoattractant protein-1 |
| b-FGF | basic-fibroblast growth factor |
| CXCR-4 | C motif chemokine receptor 4 |
| SDF-1 | stromal derived factor-1 |
| TLR2, 4 | toll-like receptor2,4 |
| GM-CSF | granulocyte monocyte-colony stimulating factor |
| IGF | insulin growth factor |
References
- Warren, R.; Chestnut, M.H.; Wong, T.K.; Otte, T.E.; Lammers, K.M.; Meili, M.L. An improved method for the isolation and cultivation of human scalp dermal papilla cells: Maintenance of extracellular matrix. Ann. N. Y. Acad. Sci. 1991, 642, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.A.; Pinxteren, J.; Roobrouck, V.D.; Luyckx, A.; van’t Hof, W.; Deans, R.; Verfaillie, C.M.; Waer, M.; Billiau, A.D.; Van Gool, S.W. Human multipotent adult progenitor cells are nonimmunogenic and exert potent immunomodulatory effects on alloreactive T-cell responses. Cell Transplant. 2013, 22, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Pachón-Peña, G.; Yu, G.; Tucker, A.; Wu, X.; Vendrell, J.; Bunnell, B.A.; Gimble, J.M. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J. Cell. Physiol. 2011, 226, 843–851. [Google Scholar] [CrossRef]
- Vishnubalaji, R.; Al-Nbaheen, M.; Kadalmani, B.; Aldahmash, A.; Ramesh, T. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 2012, 347, 419–427. [Google Scholar] [CrossRef]
- Dhar, S.; Yoon, E.S.; Kachgal, S.; Evans, G.R.D. Long-term maintenance of neuronally differentiated human adipose tissue-derived stem cells. Tissue Eng. 2007, 13, 2625–2632. [Google Scholar] [CrossRef]
- Huang, S.-H.; Lin, Y.-N.; Lee, S.-S.; Chai, C.-Y.; Chang, H.-W.; Lin, T.-M.; Lai, C.-S.; Lin, S.-D. New adipose tissue formation by human adipose-derived stem cells with hyaluronic acid gel in immunodeficient mice. Int. J. Med. Sci. 2015, 12, 154–162. [Google Scholar] [CrossRef]
- Choi, E.W.; Seo, M.K.; Woo, E.Y.; Kim, S.H.; Park, E.J.; Kim, S. Exosomes from human adipose-derived stem cells promote proliferation and migration of skin fibroblasts. Exp. Dermatol. 2018, 27, 1170–1172. [Google Scholar] [CrossRef]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef]
- Otsuki, Y.; Nakamura, Y.; Harada, S.; Yamamoto, Y.; Ogino, K.; Morikawa, K.; Ninomiya, H.; Miyagawa, S.; Sawa, Y.; Hisatome, I.; et al. Adipose stem cell sheets improved cardiac function in the rat myocardial infarction, but did not alter cardiac contractile responses to β-adrenergic stimulation. Biomed. Res. 2015, 36, 11–19. [Google Scholar] [CrossRef]
- Ferreira, A.D.F.; Gomes, D.A. Stem Cell Extracellular Vesicles in Skin Repair. Bioengineering (Basel) 2018, 6, 4. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Duscher, D.; Liu, Y.; Guo, G.; Kang, Y.; Xiong, H.; Zhan, P.; Wang, Y.; Wang, C.; et al. Microvesicles from human adipose stem cells promote wound healing by optimizing cellular functions via AKT and ERK signaling pathways. Stem Cell Res. Ther. 2019, 10, 47. [Google Scholar] [CrossRef]
- Lombardi, F.; Palumbo, P.; Augello, F.R.; Cifone, M.G.; Cinque, B.; Giuliani, M. Secretome of Adipose Tissue-Derived Stem Cells (ASCs) as a Novel Trend in Chronic Non-Healing Wounds: An Overview of Experimental In Vitro and In Vivo Studies and Methodological Variables. Int. J. Mol. Sci. 2019, 20, 3721. [Google Scholar] [CrossRef]
- Kucharzewski, M.; Rojczyk, E.; Wilemska-Kucharzewska, K.; Wilk, R.; Hudecki, J.; Los, M.J. Novel trends in application of stem cells in skin wound healing. Eur. J. Pharmacol. 2019, 843, 307–315. [Google Scholar] [CrossRef]
- Marfia, G.; Navone, S.E.; Di Vito, C.; Ughi, N.; Tabano, S.; Miozzo, M.; Tremolada, C.; Bolla, G.; Crotti, C.; Ingegnoli, F.; et al. Mesenchymal stem cells: Potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis 2015, 11, 183–206. [Google Scholar] [CrossRef]
- Balaji, S.; Keswani, S.G.; Crombleholme, T.M. The Role of Mesenchymal Stem Cells in the Regenerative Wound Healing Phenotype. Adv. Wound Care (New Rochelle) 2012, 1, 159–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Liu, D.; Liu, F.; Li, X.; Pan, L.; Pang, Y.; Chen, D. Role of growth differentiation factor 11 in development, physiology and disease. Oncotarget 2017, 8, 81604–81616. [Google Scholar] [CrossRef]
- Nurkovic, J.; Dolicanin, Z.; Mustafic, F.; Mujanovic, R.; Memic, M.; Grbovic, V.; Skevin, A.J.; Nurkovic, S. Mesenchymal stem cells in regenerative rehabilitation. J. Phys. Ther. Sci. 2016, 28, 1943–1948. [Google Scholar] [CrossRef]
- Senoo, M. Epidermal Stem Cells in Homeostasis and Wound Repair of the Skin. Adv. Wound Care (New Rochelle) 2013, 2, 273–282. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jung, M.; Kim, H.-S.; Kim, Y.-M.; Choi, E.-H. Adipose-derived stem cells as a new therapeutic modality for ageing skin. Exp. Dermatol. 2011, 20, 383–387. [Google Scholar] [CrossRef]
- Cappuzzello, C.; Doni, A.; Dander, E.; Pasqualini, F.; Nebuloni, M.; Bottazzi, B.; Mantovani, A.; Biondi, A.; Garlanda, C.; D’Amico, G. Mesenchymal Stromal Cell-Derived PTX3 Promotes Wound Healing via Fibrin Remodeling. J. Invest. Dermatol. 2016, 136, 293–300. [Google Scholar] [CrossRef]
- Ozpur, M.A.; Guneren, E.; Canter, H.I.; Karaaltin, M.V.; Ovali, E.; Yogun, F.N.; Baygol, E.G.; Kaplan, S. Generation of Skin Tissue Using Adipose Tissue-Derived Stem Cells. Plast. Reconstr. Surg. 2016, 137, 134–143. [Google Scholar] [CrossRef]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef]
- Cuevas-Diaz Duran, R.; González-Garza, M.T.; Cardenas-Lopez, A.; Chavez-Castilla, L.; Cruz-Vega, D.E.; Moreno-Cuevas, J.E. Age-related yield of adipose-derived stem cells bearing the low-affinity nerve growth factor receptor. Stem Cells Int. 2013, 2013, 372164. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Zarei, F.; Abbaszadeh, A. Stem cell and skin rejuvenation. J. Cosmet. Laser Ther. 2018, 20, 193–197. [Google Scholar] [CrossRef]
- Lunyak, V.V.; Amaro-Ortiz, A.; Gaur, M. Mesenchymal Stem Cells Secretory Responses: Senescence Messaging Secretome and Immunomodulation Perspective. Front. Genet. 2017, 8, 220. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef]
- Quan, C.; Cho, M.K.; Perry, D.; Quan, T. Age-associated reduction of cell spreading induces mitochondrial DNA common deletion by oxidative stress in human skin dermal fibroblasts: Implication for human skin connective tissue aging. J. Biomed. Sci. 2015, 22, 62. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Qin, Z.; Balimunkwe, R.M.; Quan, T. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br. J. Dermatol. 2017, 177, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- DiLoreto, R.; Murphy, C.T. The cell biology of aging. Mol. Biol. Cell 2015, 26, 4524–4531. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xu, Y.; He, T.; Kang, S.; Voorhees, J.J.; Fisher, G.J. Ultraviolet irradiation induces CYR61/CCN1, a mediator of collagen homeostasis, through activation of transcription factor AP-1 in human skin fibroblasts. J. Invest. Dermatol. 2010, 130, 1697–1706. [Google Scholar] [CrossRef]
- Naylor, E.C.; Watson, R.E.B.; Sherratt, M.J. Molecular aspects of skin ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef]
- Chakraborti, S.; Mandal, M.; Das, S.; Mandal, A.; Chakraborti, T. Regulation of matrix metalloproteinases: An overview. Mol. Cell. Biochem. 2003, 253, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Maheshwari, A.; Chandra, A. Biomarkers for wound healing and their evaluation. J. Wound Care 2016, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Maity, N.; Nema, N.K.; Abedy, M.K.; Sarkar, B.K.; Mukherjee, P.K. Exploring Tagetes erecta Linn flower for the elastase, hyaluronidase and MMP-1 inhibitory activity. J. Ethnopharmacol. 2011, 137, 1300–1305. [Google Scholar] [CrossRef]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal Stem Cells from Adipose Tissue in Clinical Applications for Dermatological Indications and Skin Aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef]
- Bell, J.T.; Spector, T.D. DNA methylation studies using twins: What are they telling us? Genome Biol. 2012, 13, 172. [Google Scholar] [CrossRef]
- Shibata, K.R.; Aoyama, T.; Shima, Y.; Fukiage, K.; Otsuka, S.; Furu, M.; Kohno, Y.; Ito, K.; Fujibayashi, S.; Neo, M.; et al. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells 2007, 25, 2371–2382. [Google Scholar] [CrossRef]
- Ludke, A.; Li, R.-K.; Weisel, R.D. The rejuvenation of aged stem cells for cardiac repair. Can. J. Cardiol. 2014, 30, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.; Munoz-Najar, U.; De Wit, J.; Renard, A.J.S.; Hoeijmakers, J.H.J.; Sedivy, J.M.; Van Blitterswijk, C.; De Boer, J. A link between the accumulation of DNA damage and loss of multi-potency of human mesenchymal stromal cells. J. Cell. Mol. Med. 2010, 14, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.-L.; Schumacher, B. DNA damage responses and p53 in the aging process. Blood 2018, 131, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Sperka, T.; Wang, J.; Rudolph, K.L. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590. [Google Scholar] [CrossRef]
- Voog, J.; Jones, D.L. Stem cells and the niche: A dynamic duo. Cell Stem Cell 2010, 6, 103–115. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol. 2006, 22, 339–373. [Google Scholar] [CrossRef]
- Blanpain, C.; Horsley, V.; Fuchs, E. Epithelial stem cells: Turning over new leaves. Cell 2007, 128, 445–458. [Google Scholar] [CrossRef]
- Monfort, A.; Soriano-Navarro, M.; García-Verdugo, J.M.; Izeta, A. Production of human tissue-engineered skin trilayer on a plasma-based hypodermis. J. Tissue Eng. Regen. Med. 2013, 7, 479–490. [Google Scholar] [CrossRef]
- Chan, R.K.; Zamora, D.O.; Wrice, N.L.; Baer, D.G.; Renz, E.M.; Christy, R.J.; Natesan, S. Development of a vascularized skin construct using adipose-derived stem cells from debrided burned skin. Stem Cells Int. 2012, 2012, 841203. [Google Scholar] [CrossRef]
- Baharlou, R.; Ahmadi-Vasmehjani, A.; Faraji, F.; Atashzar, M.R.; Khoubyari, M.; Ahi, S.; Erfanian, S.; Navabi, S.-S. Human adipose tissue-derived mesenchymal stem cells in rheumatoid arthritis: Regulatory effects on peripheral blood mononuclear cells activation. Int. Immunopharmacol. 2017, 47, 59–69. [Google Scholar] [CrossRef]
- Hyldig, K.; Riis, S.; Pennisi, C.P.; Zachar, V.; Fink, T. Implications of Extracellular Matrix Production by Adipose Tissue-Derived Stem Cells for Development of Wound Healing Therapies. Int. J. Mol. Sci. 2017, 18, 1167. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Malka, G. Growth Differentiation Factor 11 (GDF11)/Transforming Growth Factor-β (TGF- β)/Mesenchymal Stem Cells (MSCs) Balance: A Complicated Partnership in Skin Rejuvenation. J. Embryol. Stem Cell Res. 2019, 3, 000122. [Google Scholar]
- Waters, R.; Subham, S.; Pacelli, S.; Modaresi, S.; Chakravarti, A.R.; Paul, A. Development of MicroRNA-146a-Enriched Stem Cell Secretome for Wound-Healing Applications. Mol. Pharm. 2019, 16, 4302–4312. [Google Scholar] [CrossRef]
- Kronsteiner, B.; Wolbank, S.; Peterbauer, A.; Hackl, C.; Redl, H.; van Griensven, M.; Gabriel, C. Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 2011, 20, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Domenis, R.; Cifù, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 2018, 8, 13325. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, W.; Liu, L.; Qu, R.; Chen, X.; Qiu, C.; Li, J.; Hayball, J.; Liu, L.; Chen, J.; et al. GDF11 antagonizes TNF-α-induced inflammation and protects against the development of inflammatory arthritis in mice. FASEB J. 2019, 33, 3317–3329. [Google Scholar] [CrossRef]
- Li, M.; Zeng, L.; Liu, S.; Dangelmajer, S.; Kahlert, U.D.; Huang, H.; Han, Y.; Chi, X.; Zhu, M.; Lei, T. Transforming Growth Factor-β Promotes Homing and Therapeutic Efficacy of Human Mesenchymal Stem Cells to Glioblastoma. J. Neuropathol. Exp. Neurol. 2019, 78, 315–325. [Google Scholar] [CrossRef]
- Koivisto, L.; Bi, J.; Häkkinen, L.; Larjava, H. Integrin αvβ6: Structure, function and role in health and disease. Int. J. Biochem. Cell Biol. 2018, 99, 186–196. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Bachmann, S.; Jennewein, M.; Bubel, M.; Guthörl, S.; Pohlemann, T.; Oberringer, M. Interacting adipose-derived stem cells and microvascular endothelial cells provide a beneficial milieu for soft tissue healing. Mol. Biol. Rep. 2019, 47, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, S.; Liu, X.; Xv, N.; Zhang, S. Protective effects of adipose-derived stem cells secretome on human dermal fibroblasts from ageing damages. Int. J. Clin. Exp. Pathol. 2015, 8, 15739–15748. [Google Scholar] [PubMed]
- Na, Y.K.; Ban, J.-J.; Lee, M.; Im, W.; Kim, M. Wound healing potential of adipose tissue stem cell extract. Biochem. Biophys. Res. Commun. 2017, 485, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Zografou, A.; Tsigris, C.; Papadopoulos, O.; Kavantzas, N.; Patsouris, E.; Donta, I.; Perrea, D. Improvement of skin-graft survival after autologous transplantation of adipose-derived stem cells in rats. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.N.M.; Wright, K.T.; Fuller, H.R.; MacNeil, S.; Johnson, W.E.B. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: An in vitro study of fibroblast and keratinocyte scratch assays. Exp. Cell Res. 2010, 316, 1271–1281. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Seo, D.H.; Lee, S.H.; Lee, S.-H.; An, G.-H.; Ahn, H.-J.; Kwon, D.; Seo, K.-W.; Kang, K.-S. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem. Biophys. Rep. 2018, 16, 96–102. [Google Scholar] [CrossRef]
- Jun, E.K.; Zhang, Q.; Yoon, B.S.; Moon, J.-H.; Lee, G.; Park, G.; Kang, P.J.; Lee, J.H.; Kim, A.; You, S. Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int. J. Mol. Sci. 2014, 15, 605–628. [Google Scholar] [CrossRef]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef]
- Michalek, J.; Moster, R.; Lukac, L.; Proefrock, K.; Petrasovic, M.; Rybar, J.; Capkova, M.; Chaloupka, A.; Darinskas, A.; Michalek, J.; et al. WITHDRAWN: Autologous adipose tissue-derived stromal vascular fraction cells application in patients with osteoarthritis. Cell Transplant 2015, 20, 1–36. [Google Scholar]
- Klar, A.S.; Zimoch, J.; Biedermann, T. Skin Tissue Engineering: Application of Adipose-Derived Stem Cells. BioMed Res. Int. 2017, 2017, 9747010. [Google Scholar] [CrossRef]
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.R. Evaluation of cellular and humoral immune responses to allogeneic adipose-derived stem/stromal cells. Methods Mol. Biol. 2011, 702, 133–150. [Google Scholar] [PubMed]
- Koh, K.S.; Oh, T.S.; Kim, H.; Chung, I.W.; Lee, K.W.; Lee, H.B.; Park, E.J.; Jung, J.S.; Shin, I.S.; Ra, J.C.; et al. Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann. Plast. Surg. 2012, 69, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, Y.; Luo, X.; Fu, M.-G.; Hu, X.; Dong, H.; Fan, Z.-H. [Cell-assisted lipotransfer for breast augmentation: A report of 18 patients]. Zhonghua Zheng Xing Wai Ke Za Zhi 2012, 28, 1–6. [Google Scholar] [PubMed]
- Yoshimura, K.; Asano, Y.; Aoi, N.; Kurita, M.; Oshima, Y.; Sato, K.; Inoue, K.; Suga, H.; Eto, H.; Kato, H.; et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J 2010, 16, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthet. Plast. Surg. 2008, 32, 48–55. [Google Scholar] [CrossRef]
- Bae, Y.C.; Song, J.S.; Bae, S.H.; Kim, J.H. Effects of human adipose-derived stem cells and stromal vascular fraction on cryopreserved fat transfer. Dermatol. Surg. 2015, 41, 605–614. [Google Scholar] [CrossRef]
- Zhou, X.; Ning, K.; Ling, B.; Chen, X.; Cheng, H.; Lu, B.; Gao, Z.; Xu, J. Multiple Injections of Autologous Adipose-Derived Stem Cells Accelerate the Burn Wound Healing Process and Promote Blood Vessel Regeneration in a Rat Model. Stem Cells Dev. 2019, 28, 1463–1472. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Kong, W.; Deng, W.; Wang, D.; Feng, X.; Zhao, C.; Hua, B.; Wang, H.; Sun, L. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: A long-term retrospective study. Stem Cell Res. Ther. 2018, 9, 312. [Google Scholar] [CrossRef]
- Moon, K.-C.; Chung, H.-Y.; Han, S.-K.; Jeong, S.-H.; Dhong, E.-S. Possibility of Injecting Adipose-Derived Stromal Vascular Fraction Cells to Accelerate Microcirculation in Ischemic Diabetic Feet: A Pilot Study. Int. J. Stem Cells 2019, 12, 107–113. [Google Scholar] [CrossRef]
- Raposio, E.; Bertozzi, N.; Bonomini, S.; Bernuzzi, G.; Formentini, A.; Grignaffini, E.; Pio Grieco, M. Adipose-derived Stem Cells Added to Platelet-rich Plasma for Chronic Skin Ulcer Therapy. Wounds 2016, 28, 126–131. [Google Scholar] [PubMed]
- Tarallo, M.; Fino, P.; Ribuffo, D.; Casella, D.; Toscani, M.; Spalvieri, C.; Lattanzi, W.; Di Taranto, G. Liposuction Aspirate Fluid Adipose-Derived Stem Cell Injection and Secondary Healing in Fingertip Injury: A Pilot Study. Plast. Reconstr. Surg. 2018, 142, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Charles-de-Sá, L.; Gontijo-de-Amorim, N.F.; Maeda Takiya, C.; Borojevic, R.; Benati, D.; Bernardi, P.; Sbarbati, A.; Rigotti, G. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast. Reconstr. Surg. 2015, 135, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, G.; Charles-de-Sá, L.; Gontijo-de-Amorim, N.F.; Takiya, C.M.; Amable, P.R.; Borojevic, R.; Benati, D.; Bernardi, P.; Sbarbati, A. Expanded Stem Cells, Stromal-Vascular Fraction, and Platelet-Rich Plasma Enriched Fat: Comparing Results of Different Facial Rejuvenation Approaches in a Clinical Trial. Aesthet. Surg. J. 2016, 36, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Hersant, B.; Sid-Ahmed, M.; Braud, L.; Jourdan, M.; Baba-Amer, Y.; Meningaud, J.-P.; Rodriguez, A.-M. Platelet-Rich Plasma Improves the Wound Healing Potential of Mesenchymal Stem Cells through Paracrine and Metabolism Alterations. Stem Cells Int. 2019, 2019, 1234263. [Google Scholar] [CrossRef] [PubMed]
- Cowper, M.; Frazier, T.; Wu, X.; Curley, L.; Ma, M.H.; Mohuiddin, O.A.; Dietrich, M.; McCarthy, M.; Bukowska, J.; Gimble, J.M. Human Platelet Lysate as a Functional Substitute for Fetal Bovine Serum in the Culture of Human Adipose Derived Stromal/Stem Cells. Cells 2019, 8, 724. [Google Scholar] [CrossRef] [PubMed]
- Böttcher-Haberzeth, S.; Biedermann, T.; Klar, A.S.; Widmer, D.S.; Neuhaus, K.; Schiestl, C.; Meuli, M.; Reichmann, E. Characterization of pigmented dermo-epidermal skin substitutes in a long-term in vivo assay. Exp. Dermatol. 2015, 24, 16–21. [Google Scholar] [CrossRef]
- Klar, A.S.; Güven, S.; Zimoch, J.; Zapiórkowska, N.A.; Biedermann, T.; Böttcher-Haberzeth, S.; Meuli-Simmen, C.; Martin, I.; Scherberich, A.; Reichmann, E.; et al. Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatr. Surg. Int. 2016, 32, 17–27. [Google Scholar] [CrossRef]
- Klar, A.S.; Güven, S.; Biedermann, T.; Luginbühl, J.; Böttcher-Haberzeth, S.; Meuli-Simmen, C.; Meuli, M.; Martin, I.; Scherberich, A.; Reichmann, E. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 2014, 35, 5065–5078. [Google Scholar] [CrossRef]
- Debels, H. Advances in tissue engineering; a novel technology making use of an in vivo vascularized chamber. Acta Chir. Belg. 2015, 115, 104–110. [Google Scholar] [CrossRef]
- Trottier, V.; Marceau-Fortier, G.; Germain, L.; Vincent, C.; Fradette, J. IFATS collection: Using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells 2008, 26, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, S.; Capri, M.; Valensin, S.; Tieri, P.; Monti, D.; Ottaviani, E.; Franceschi, C. Inflamm-aging, cytokines and aging: State of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr. Pharm. Des. 2006, 12, 3161–3171. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Jia, S.-X.; Xie, P.; Xu, W.; Leung, K.P.; Mustoe, T.A.; Galiano, R.D. Topically delivered adipose derived stem cells show an activated-fibroblast phenotype and enhance granulation tissue formation in skin wounds. PLoS ONE 2013, 8, e55640. [Google Scholar] [CrossRef] [PubMed]
- Klar, A.S.; Michalak-Mićka, K.; Biedermann, T.; Simmen-Meuli, C.; Reichmann, E.; Meuli, M. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatr. Surg. Int. 2018, 34, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.; Najman, S. The Effect of Conditioned Media of Stem Cells Derived from Lipoma and Adipose Tissue on Macrophages’ Response and Wound Healing in Indirect Co-culture System In Vitro. Int. J. Mol. Sci. 2019, 20, 1671. [Google Scholar] [CrossRef]
- Klar, A.S.; Biedermann, T.; Simmen-Meuli, C.; Reichmann, E.; Meuli, M. Comparison of in vivo immune responses following transplantation of vascularized and non-vascularized human dermo-epidermal skin substitutes. Pediatr. Surg. Int. 2017, 33, 377–382. [Google Scholar] [CrossRef]
- Ji, F.; Wang, Y.; Yuan, J.; Wu, Q.; Wang, J.; Liu, D. The potential role of stromal cell-derived factor-1α/CXCR4/CXCR7 axis in adipose-derived mesenchymal stem cells. J. Cell. Physiol. 2019, 235, 3548–3557. [Google Scholar] [CrossRef]
- Rennert, R.C.; Sorkin, M.; Garg, R.K.; Gurtner, G.C. Stem cell recruitment after injury: Lessons for regenerative medicine. Regen. Med. 2012, 7, 833–850. [Google Scholar] [CrossRef]
- Wu, Q.; Ji, F.-K.; Wang, J.-H.; Nan, H.; Liu, D.-L. Stromal cell-derived factor 1 promoted migration of adipose-derived stem cells to the wounded area in traumatic rats. Biochem. Biophys. Res. Commun. 2015, 467, 140–145. [Google Scholar] [CrossRef]
- Heirani-Tabasi, A.; Toosi, S.; Mirahmadi, M.; Mishan, M.A.; Bidkhori, H.R.; Bahrami, A.R.; Behravan, J.; Naderi-Meshkin, H. Chemokine Receptors Expression in MSCs: Comparative Analysis in Different Sources and Passages. Tissue Eng. Regen. Med. 2017, 14, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Barzelay, A.; Weisthal Algor, S.; Niztan, A.; Katz, S.; Benhamou, M.; Nakdimon, I.; Azmon, N.; Gozlan, S.; Mezad-Koursh, D.; Neudorfer, M.; et al. Adipose-Derived Mesenchymal Stem Cells Migrate and Rescue RPE in the Setting of Oxidative Stress. Stem Cells Int. 2018, 2018, 9682856. [Google Scholar] [CrossRef] [PubMed]
- Stuermer, E.K.; Lipenksy, A.; Thamm, O.; Neugebauer, E.; Schaefer, N.; Fuchs, P.; Bouillon, B.; Koenen, P. The role of SDF-1 in homing of human adipose-derived stem cells. Wound Repair Regen. 2015, 23, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, A.; Tao, C.; Li, X.; Jin, P. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem. Biophys. Res. Commun. 2013, 441, 675–680. [Google Scholar] [CrossRef]
- Bellei, B.; Migliano, E.; Tedesco, M.; Caputo, S.; Papaccio, F.; Lopez, G.; Picardo, M. Adipose tissue-derived extracellular fraction characterization: Biological and clinical considerations in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 207. [Google Scholar] [CrossRef]
- Planat-Benard, V.; Silvestre, J.-S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Kilroy, G.E.; Foster, S.J.; Wu, X.; Ruiz, J.; Sherwood, S.; Heifetz, A.; Ludlow, J.W.; Stricker, D.M.; Potiny, S.; Green, P.; et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J. Cell. Physiol. 2007, 212, 702–709. [Google Scholar] [CrossRef]
- Xiao, W.; Tang, H.; Wu, M.; Liao, Y.; Li, K.; Li, L.; Xu, X. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 2017, 37, BSR20170658. [Google Scholar] [CrossRef]
- He, Y.; Xia, J.; Chen, H.; Wang, L.; Deng, C.; Lu, F. Human adipose liquid extract induces angiogenesis and adipogenesis: A novel cell-free therapeutic agent. Stem Cell Res. Ther. 2019, 10, 252. [Google Scholar] [CrossRef]
- Komaki, M.; Numata, Y.; Morioka, C.; Honda, I.; Tooi, M.; Yokoyama, N.; Ayame, H.; Iwasaki, K.; Taki, A.; Oshima, N.; et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res. Ther. 2017, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Sumi, M.; Sata, M.; Toya, N.; Yanaga, K.; Ohki, T.; Nagai, R. Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci. 2007, 80, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Romaldini, A.; Mastrogiacomo, M.; Cancedda, R.; Descalzi, F. Platelet Lysate Activates Human Subcutaneous Adipose Tissue Cells by Promoting Cell Proliferation and Their Paracrine Activity Toward Epidermal Keratinocytes. Front. Bioeng. Biotechnol. 2018, 6, 203. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care (New Rochelle) 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhao, J.; Nie, F.; Qin, Z.; Xue, H.; Wang, G.; Li, D. Exosomes from Adipose-Derived Stem Cells (ADSCs) Overexpressing miR-21 Promote Vascularization of Endothelial Cells. Sci. Rep. 2019, 9, 12861. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Daganzo, R.; Regidor, C.; Martín-Donaire, T.; Rico, M.A.; Bautista, G.; Krsnik, I.; Forés, R.; Ojeda, E.; Sanjuán, I.; García-Marco, J.A.; et al. Results of a pilot study on the use of third-party donor mesenchymal stromal cells in cord blood transplantation in adults. Cytotherapy 2009, 11, 278–288. [Google Scholar] [CrossRef]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Kim, H.; Hyun, M.R.; Kim, S.W. The Effect of Adipose-Derived Stem Cells on Wound Healing: Comparison of Methods of Application. Stem Cells Int. 2019, 2019, 2745640. [Google Scholar] [CrossRef]
- Yoon, B.S.; Moon, J.-H.; Jun, E.K.; Kim, J.; Maeng, I.; Kim, J.S.; Lee, J.H.; Baik, C.S.; Kim, A.; Cho, K.S.; et al. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 2010, 19, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Sundaresan, N.R.; Gupta, M.P. Regulation of Akt signaling by sirtuins: Its implication in cardiac hypertrophy and aging. Circ. Res. 2014, 114, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Khorraminejad-Shirazi, M.; Farahmandnia, M.; Kardeh, B.; Estedlal, A.; Kardeh, S.; Monabati, A. Aging and stem cell therapy: AMPK as an applicable pharmacological target for rejuvenation of aged stem cells and achieving higher efficacy in stem cell therapy. Hematol. Oncol. Stem Cell Ther. 2018, 11, 189–194. [Google Scholar] [CrossRef]
- Chavez-Munoz, C.; Nguyen, K.T.; Xu, W.; Hong, S.-J.; Mustoe, T.A.; Galiano, R.D. Transdifferentiation of adipose-derived stem cells into keratinocyte-like cells: Engineering a stratified epidermis. PLoS ONE 2013, 8, e80587. [Google Scholar] [CrossRef] [PubMed]
- Idkowiak-Baldys, J.; Santhanam, U.; Buchanan, S.M.; Pfaff, K.L.; Rubin, L.L.; Lyga, J. Growth differentiation factor 11 (GDF11) has pronounced effects on skin biology. PLoS ONE 2019, 14, e0218035. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Chintala, S.K.; Jung, J.C.; Villar, W.V.L.; McCabe, F.; Russo, L.A.; Lee, Y.; McCarthy, B.E.; Wollenberg, K.R.; Jester, J.V.; et al. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J. Biol. Chem. 2002, 277, 2065–2072. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kim, H.; Liu, X.; Sugiura, H.; Kohyama, T.; Fang, Q.; Wen, F.-Q.; Abe, S.; Wang, X.; Atkinson, J.J.; et al. Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L1006–L1015. [Google Scholar] [CrossRef]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H. Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch. Dermatol. Res. 2009, 301, 329–336. [Google Scholar] [CrossRef]
- Park, B.-S.; Jang, K.A.; Sung, J.-H.; Park, J.-S.; Kwon, Y.H.; Kim, K.J.; Kim, W.-S. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol. Surg. 2008, 34, 1323–1326. [Google Scholar]
- Shi, Y.-Y.; Nacamuli, R.P.; Salim, A.; Longaker, M.T. The osteogenic potential of adipose-derived mesenchymal cells is maintained with aging. Plast. Reconstr. Surg. 2005, 116, 1686–1696. [Google Scholar] [CrossRef]
- Ehrlich, M.; Rao, J.; Pabby, A.; Goldman, M.P. Improvement in the appearance of wrinkles with topical transforming growth factor beta(1) and l-ascorbic acid. Dermatol. Surg. 2006, 32, 618–625. [Google Scholar] [PubMed]
- Decean, H.P.; Brie, I.C.; Tatomir, C.B.; Perde-Schrepler, M.; Fischer-Fodor, E.; Virag, P. Targeting MAPK (p38, ERK, JNK) and inflammatory CK (GDF-15, GM-CSF) in UVB-Activated Human Skin Cells with Vitis vinifera Seed Extract. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 261–272. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, R.; Lôbo, M.; Trindade, K.; Silva, D.F.; Pereira, N. Fibroblast Growth Factors: A Controlling Mechanism of Skin Aging. Skin Pharmacol. Physiol. 2019, 32, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Maddaluno, L.; Urwyler, C.; Werner, S. Fibroblast growth factors: Key players in regeneration and tissue repair. Development 2017, 144, 4047–4060. [Google Scholar] [CrossRef] [PubMed]
- Żerańska, J.; Pasikowska, M.; Szczepanik, B.; Mlosek, K.; Malinowska, S.; Dębowska, R.M.; Eris, I. A study of the activity and effectiveness of recombinant fibroblast growth factor (Q40P/S47I/H93G rFGF-1) in anti-aging treatment. Postepy Dermatol. Alergol. 2016, 33, 28–36. [Google Scholar] [CrossRef]
- Zakrzewska, M.; Krowarsch, D.; Wiedlocha, A.; Otlewski, J. Design of fully active FGF-1 variants with increased stability. Protein Eng. Des. Sel. 2004, 17, 603–611. [Google Scholar] [CrossRef]
- Weiss, R.A.; Weiss, M.A. Evaluation of a novel anti-aging topical formulation containing cycloastragenol, growth factors, peptides, and antioxidants. J. Drugs Dermatol. 2014, 13, 1135–1139. [Google Scholar]
- Yang, L.; Zhang, D.; Wu, H.; Xie, S.; Zhang, M.; Zhang, B.; Tang, S. Basic Fibroblast Growth Factor Influences Epidermal Homeostasis of Living Skin Equivalents through Affecting Fibroblast Phenotypes and Functions. Skin Pharmacol. Physiol. 2018, 31, 229–237. [Google Scholar] [CrossRef]
- Wang, Y.; Viennet, C.; Robin, S.; Berthon, J.-Y.; He, L.; Humbert, P. Precise role of dermal fibroblasts on melanocyte pigmentation. J. Dermatol. Sci. 2017, 88, 159–166. [Google Scholar] [CrossRef]
- Lee, H.C.; An, S.G.; Lee, H.W.; Park, J.-S.; Cha, K.S.; Hong, T.J.; Park, J.H.; Lee, S.Y.; Kim, S.-P.; Kim, Y.D.; et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ. J. 2012, 76, 1750–1760. [Google Scholar] [CrossRef]
- Argentati, C.; Morena, F.; Bazzucchi, M.; Armentano, I.; Emiliani, C.; Martino, S. Adipose Stem Cell Translational Applications: From Bench-to-Bedside. Int. J. Mol. Sci. 2018, 19, 3475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-A.; Zhou, B.-R.; Xu, Y.; Chen, X.; Liu, J.; Gozali, M.; Wu, D.; Yin, Z.-Q.; Luo, D. MiR-23a-depressed autophagy is a participant in PUVA- and UVB-induced premature senescence. Oncotarget 2016, 7, 37420–37435. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Jinnin, M.; Wang, Z.; Hirano, A.; Tomizawa, Y.; Kira, T.; Igata, T.; Masuguchi, S.; Fukushima, S.; Ihn, H. The expression of miR-124 increases in aged skin to cause cell senescence and it decreases in squamous cell carcinoma. Biosci. Trends 2017, 10, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Keck, M.; Zeyda, M.; Gollinger, K.; Burjak, S.; Kamolz, L.-P.; Frey, M.; Stulnig, T.M. Local anesthetics have a major impact on viability of preadipocytes and their differentiation into adipocytes. Plast. Reconstr. Surg. 2010, 126, 1500–1505. [Google Scholar] [CrossRef]
- Roato, I.; Alotto, D.; Belisario, D.C.; Casarin, S.; Fumagalli, M.; Cambieri, I.; Piana, R.; Stella, M.; Ferracini, R.; Castagnoli, C. Adipose Derived-Mesenchymal Stem Cells Viability and Differentiating Features for Orthopaedic Reparative Applications: Banking of Adipose Tissue. Stem Cells Int. 2016, 2016, 4968724. [Google Scholar] [CrossRef]
- Girard, A.-C.; Atlan, M.; Bencharif, K.; Gunasekaran, M.K.; Delarue, P.; Hulard, O.; Lefebvre-d’Hellencourt, C.; Roche, R.; Hoareau, L.; Festy, F. New insights into lidocaine and adrenaline effects on human adipose stem cells. Aesthet. Plast. Surg. 2013, 37, 144–152. [Google Scholar] [CrossRef]
- Breu, A.; Eckl, S.; Zink, W.; Kujat, R.; Angele, P. Cytotoxicity of local anesthetics on human mesenchymal stem cells in vitro. Arthroscopy 2013, 29, 1676–1684. [Google Scholar] [CrossRef]
- Seo, Y.-S.; Ko, I.O.; Park, H.; Jeong, Y.J.; Park, J.-A.; Kim, K.S.; Park, M.-J.; Lee, H.-J. Radiation-Induced Changes in Tumor Vessels and Microenvironment Contribute to Therapeutic Resistance in Glioblastoma. Front. Oncol. 2019, 9, 1259. [Google Scholar] [CrossRef]
- Dabrowski, F.A.; Burdzinska, A.; Kulesza, A.; Sladowska, A.; Zolocinska, A.; Gala, K.; Paczek, L.; Wielgos, M. Comparison of the paracrine activity of mesenchymal stem cells derived from human umbilical cord, amniotic membrane and adipose tissue. J. Obstet. Gynaecol. Res. 2017, 43, 1758–1768. [Google Scholar] [CrossRef]
- Park, J.E.; Barbul, A. Understanding the role of immune regulation in wound healing. Am. J. Surg. 2004, 187, 11S–16S. [Google Scholar] [CrossRef]
- Anderson, P.H. Vitamin D Activity and Metabolism in Bone. Curr. Osteoporos. Rep. 2017, 15, 443–449. [Google Scholar] [CrossRef]
- Othmani, A.E.; Rouam, S.; Abbad, A.; Erraoui, C.; Harriba, S.; Boukind, H.; Nourlil, J.; Malka, G.; Mazini, L. Cryopreservation Impacts Cell Functionality of Long Term Expanded Adipose-Derived Stem Cells. J. Stem Cell Res. Ther. 2019, 09, 445. [Google Scholar] [CrossRef]
- Ikegame, Y.; Yamashita, K.; Hayashi, S.-I.; Mizuno, H.; Tawada, M.; You, F.; Yamada, K.; Tanaka, Y.; Egashira, Y.; Nakashima, S.; et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011, 13, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Ahn, K.-S.; Oh, S.-R.; Kim, K.H.; Joo, M. Neutrophilic Lung Inflammation Suppressed by Picroside II Is Associated with TGF-β Signaling. Evid. Based Complement. Alternat. Med. 2015, 2015, 897272. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Xiang, G.; Li, Y.; Li, H.; Xiang, L.; Lu, J.; Xiang, L.; Dong, J.; Liu, M. GDF11 Protects against Endothelial Injury and Reduces Atherosclerotic Lesion Formation in Apolipoprotein E-Null Mice. Mol. Ther. 2016, 24, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Dreesen, O. Biomarkers of Cellular Senescence and Skin Aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Das, T.; Chen, Z.; Hendriks, R.W.; Kool, M. A20/Tumor Necrosis Factor α-Induced Protein 3 in Immune Cells Controls Development of Autoinflammation and Autoimmunity: Lessons from Mouse Models. Front. Immunol. 2018, 9, 104. [Google Scholar] [CrossRef]
- Niada, S.; Giannasi, C.; Gualerzi, A.; Banfi, G.; Brini, A.T. Differential Proteomic Analysis Predicts Appropriate Applications for the Secretome of Adipose-Derived Mesenchymal Stem/Stromal Cells and Dermal Fibroblasts. Stem Cells Int. 2018, 2018, 7309031. [Google Scholar] [CrossRef]
- Phelps, J.; Sanati-Nezhad, A.; Ungrin, M.; Duncan, N.A.; Sen, A. Bioprocessing of Mesenchymal Stem Cells and Their Derivatives: Toward Cell-Free Therapeutics. Stem Cells Int. 2018, 2018, 9415367. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. https://doi.org/10.3390/ijms21041306
Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. International Journal of Molecular Sciences. 2020; 21(4):1306. https://doi.org/10.3390/ijms21041306
Chicago/Turabian StyleMazini, Loubna, Luc Rochette, Brahim Admou, Said Amal, and Gabriel Malka. 2020. "Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing" International Journal of Molecular Sciences 21, no. 4: 1306. https://doi.org/10.3390/ijms21041306
APA StyleMazini, L., Rochette, L., Admou, B., Amal, S., & Malka, G. (2020). Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. International Journal of Molecular Sciences, 21(4), 1306. https://doi.org/10.3390/ijms21041306




