Generation of a Quantitative Luciferase Reporter for Sox9 SUMOylation
Abstract
1. Introduction
2. Results
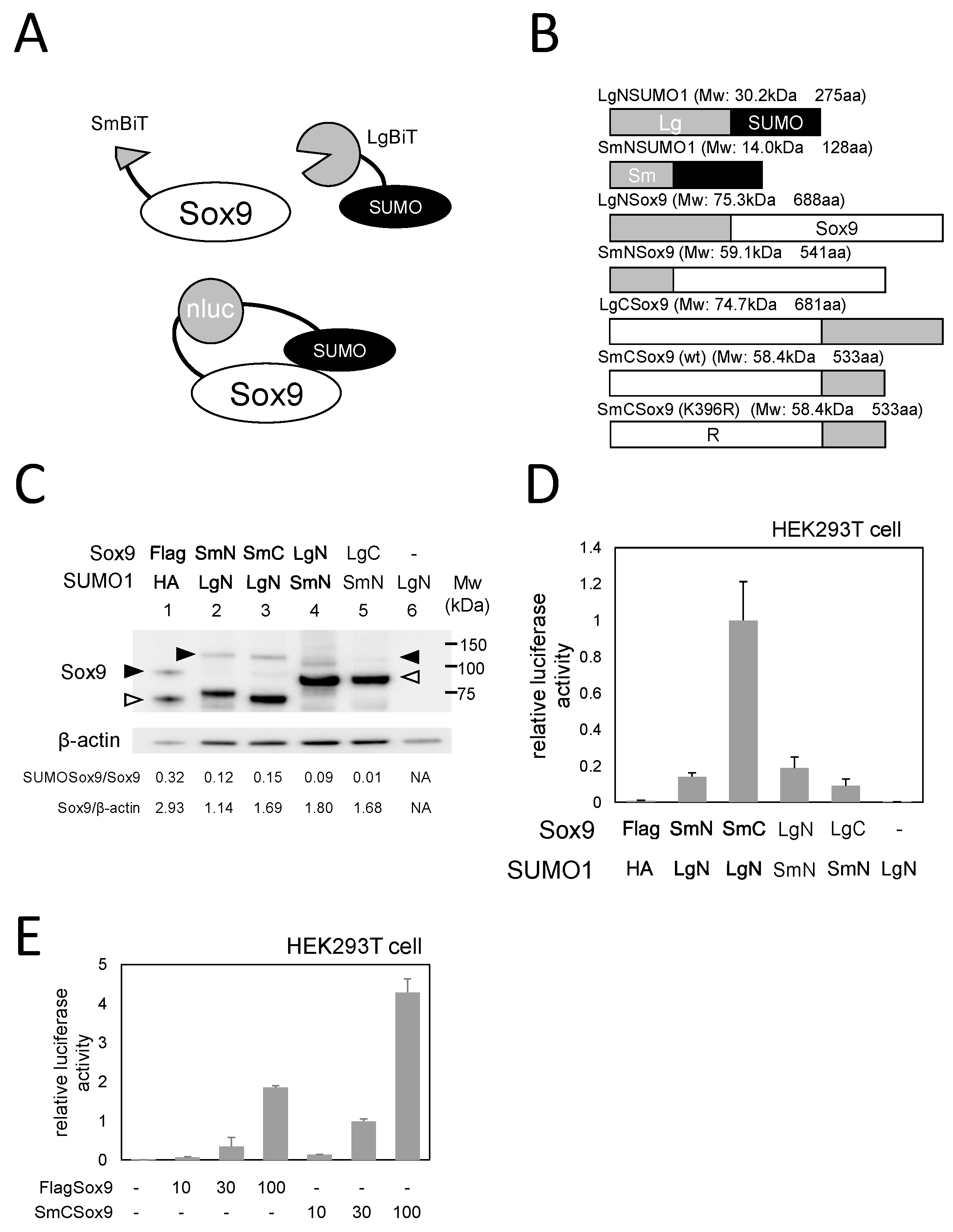
2.1. Generation of a NanoBiT reporter for Sox9 SUMOylation
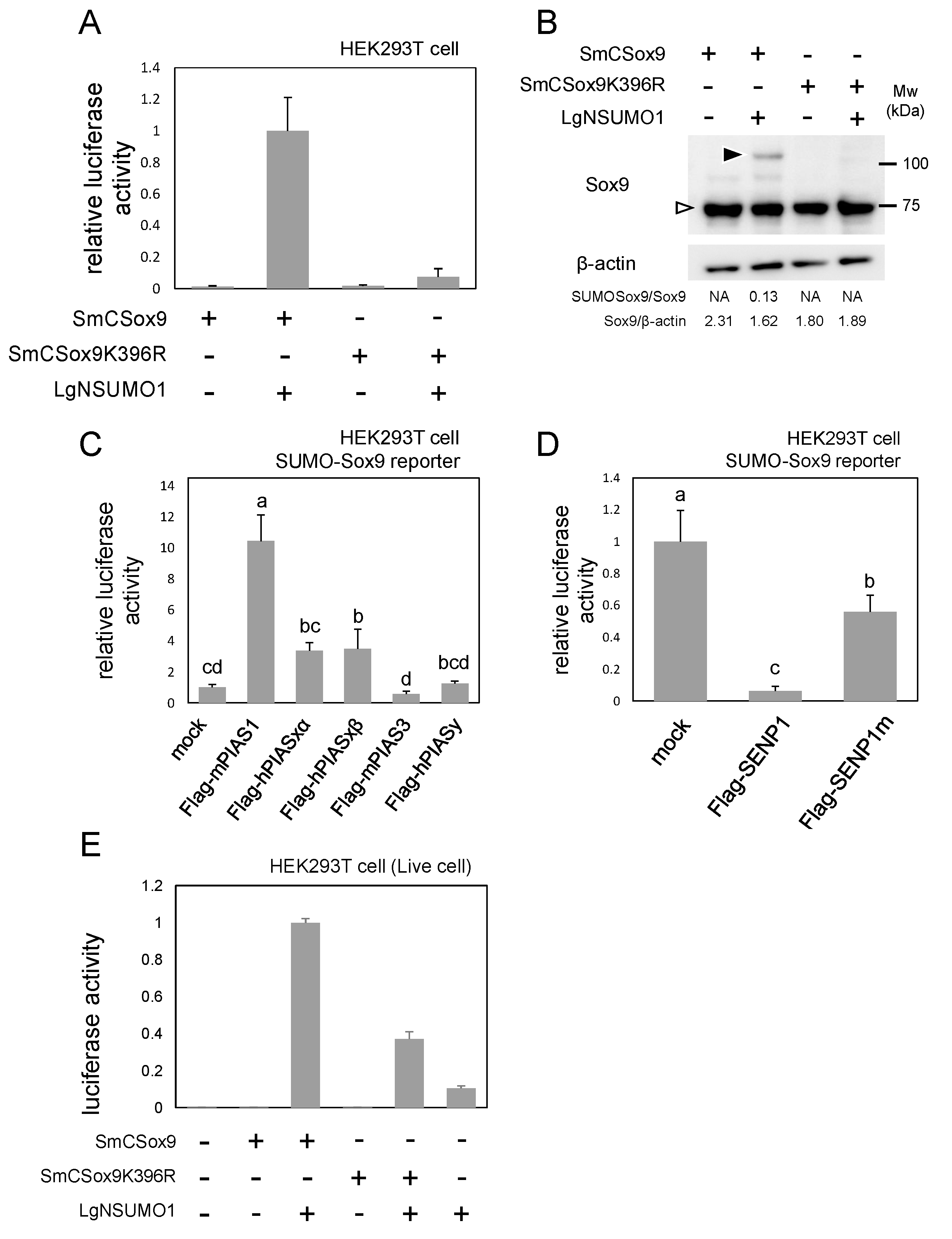
2.2. SUMO-Sox9 Reporter Activity Depends on Sox9 SUMOylation and Is Quantitatively Dynamic
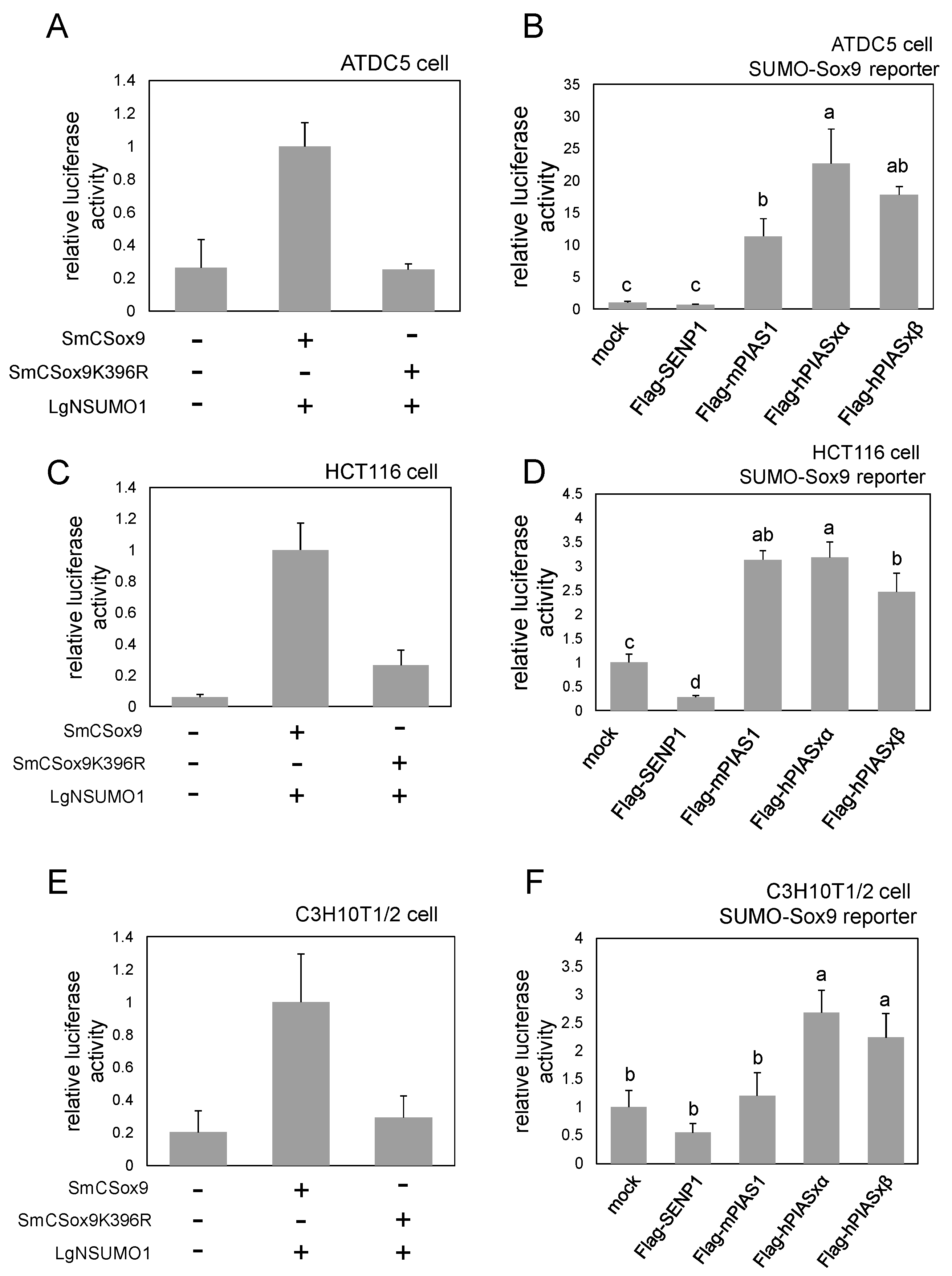
2.3. SUMO-Sox9 Reporter Is Active also in Other Cell Lines
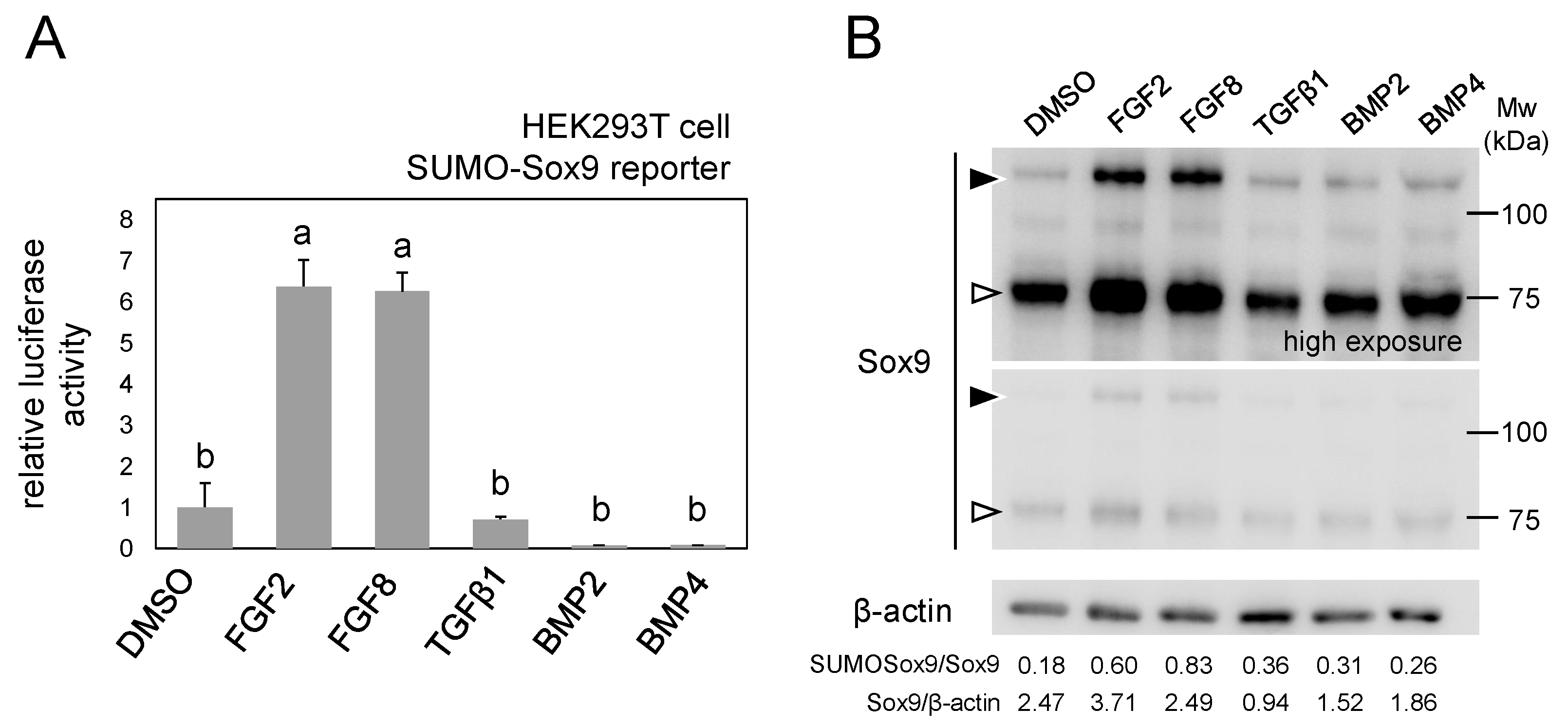
2.4. SUMO-Sox9 Reporter Revealed that FGF Signal Increases the Amount of SUMOylated Sox9
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Plasmid Construction
4.3. Western Blotting
4.4. Luciferase Assay
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the Many Facets of Its Regulation in the Chondrocyte Lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Sox9 Is Required for Cartilage Formation. Nat. Genet. 1999, 22, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Huang, W.; Whitworth, D.J.; Deng, J.M.; Zhang, Z.; Behringer, R.R.; de Crombrugghe, B. Haploinsufficiency of Sox9 Results in Defective Cartilage Primordia and Premature Skeletal Mineralization. Proc. Natl. Acad. Sci. USA 2001, 98, 6698–6703. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Wirth, J.; Meyer, J.; Zabel, B.; Held, M.; Zimmer, J.; Pasantes, J.; Bricarelli, F.D.; Keutel, J.; Hustert, E.; et al. Autosomal Sex Reversal and Campomelic Dysplasia Are Caused by Mutations in and Around the SRY-Related Gene SOX9. Cell 1994, 79, 1111–1120. [Google Scholar] [CrossRef]
- Foster, J.W.; Dominguez-Steglich, M.A.; Guioli, S.; Kwok, C.; Weller, P.A.; Stevanović, M.; Weissenbach, J.; Mansour, S.; Young, I.D.; Goodfellow, P.N. Campomelic Dysplasia and Autosomal Sex Reversal Caused by Mutations in an SRY-Related Gene. Nature 1994, 372, 525–530. [Google Scholar] [CrossRef]
- Akiyama, H.; Lyons, J.P.; Mori-Akiyama, Y.; Yang, X.; Zhang, R.; Zhang, Z.; Deng, J.M.; Taketo, M.M.; Nakamura, T.; Behringer, R.R.; et al. Interactions Between Sox9 and Beta-Catenin Control Chondrocyte Differentiation. Genes Dev. 2004, 18, 1072–1087. [Google Scholar] [CrossRef]
- Wilhelm, D.; Koopman, P. The Makings of Maleness: Towards an Integrated View of Male Sexual Development. Nat. Rev. Genet. 2006, 7, 620–631. [Google Scholar] [CrossRef]
- Seymour, P.A.; Freude, K.K.; Dubois, C.L.; Shih, H.-P.; Patel, N.A.; Sander, M. A Dosage-Dependent Requirement for Sox9 in Pancreatic Endocrine Cell Formation. Dev. Biol. 2008, 323, 19–30. [Google Scholar] [CrossRef]
- Jo, T.; Denduluri, S.; Zhang, B.; Wang, Z.; Yin, L.; Yan, Z.; Kang, R.; Shi, L.; Mok, J.; Lee, M.; et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2004, 1, 149–161. [Google Scholar] [CrossRef]
- Kozhemyakina, E.; Lassar, A.B.; Zelzer, E. A Pathway to Bone: Signaling Molecules and Transcription Factors Involved in Chondrocyte Development and Maturation. Development 2015, 142, 817–831. [Google Scholar] [CrossRef]
- Kawakami, Y.; Rodriguez-León, J.; Belmonte, J.C.I. The Role of TGFβs and Sox9 during Limb Chondrogenesis. Curr. Opin. Cell Biol. 2006, 18, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Angelozzi, M.; Haseeb, A. SOX9 in Cartilage Development and Disease. Curr. Opin. Cell Biol. 2019, 61, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Geiss-Friedlander, R.; Melchior, F. Concepts in Sumoylation: A Decade on. Nat. Rev. Mol. Cell Biol. 2007, 8, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Kido, T.; Lau, Y.-F.C. PIAS1 Interacts with and Represses SOX9 Transactivation Activity. Mol. Reprod. Dev. 2007, 74, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Mizusaki, H.; Mukai, T.; Ogawa, H.; Baba, D.; Shirakawa, M.; Hatakeyama, S.; Nakayama, K.I.; Yamamoto, H.; Kikuchi, A.; et al. Small Ubiquitin-Like Modifier 1(SUMO-1) Modification of the Synergy Control Motif of Ad4 Binding Protein/Steroidogenic Factor 1(Ad4BP/SF-1) Regulates Synergistic Transcription Between Ad4BP/SF-1 and Sox9. Mol. Endocrinol. 2004, 18, 2451–2462. [Google Scholar] [CrossRef]
- Hattori, T.; Eberspaecher, H.; Lu, J.; Zhang, R.; Nishida, T.; Kahyo, T.; Yasuda, H.; de Crombrugghe, B. Interactions Between PIAS Proteins and SOX9 Result in an Increase in the Cellular Concentrations of SOX9. J. Biol. Chem. 2006, 281, 14417–14428. [Google Scholar] [CrossRef]
- Taylor, K.M.; LaBonne, C. SoxE Factors Function Equivalently During Neural Crest and Inner Ear Development and Their Activity Is Regulated by SUMOylation. Dev. Cell 2005, 9, 593–603. [Google Scholar] [CrossRef]
- Lee, P.C.; Taylor-Jaffe, K.M.; Nordin, K.M.; Prasad, M.S.; Lander, R.M.; LaBonne, C. SUMOylated SoxE Factors Recruit Grg4 and Function as Transcriptional Repressors in the Neural Crest. J. Cell Biol. 2012, 198, 799–813. [Google Scholar] [CrossRef]
- Dixon, A.S.; Schwinn, M.K.; Hall, M.P.; Zimmerman, K.; Otto, P.; Lubben, T.H.; Butler, B.L.; Binkowski, B.F.; Machleidt, T.; Kirkland, T.A.; et al. NanoLuc Complementation Reporter Optimized for Accurate Measurement of Protein Interactions in Cells. ACS Chem. Biol. 2016, 11, 400–408. [Google Scholar] [CrossRef]
- Schwinn, M.K.; Hoang, T.; Yang, X.; Zhao, X.; Ma, J.; Li, P.; Wood, K.V.; Mallender, W.D.; Bembenek, M.E.; Yan, Z.-H. Antibody-Free Detection of Cellular Neddylation Dynamics of Cullin1. Anal. Biochem. 2018, 555, 67–72. [Google Scholar] [CrossRef]
- Martin, S.F.; Tatham, M.H.; Hay, R.T.; Samuel, I.D.W. Quantitative Analysis of Multi-Protein Interactions Using FRET: Application to the SUMO Pathway. Protein. Sci. 2008, 17, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Madahar, V.; Liao, J. Development of FRET Assay Into Quantitative and High-Throughput Screening Technology Platforms for Protein-Protein Interactions. Ann. Biomed. Eng. 2011, 39, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Kan, M.; McKeehan, W.L.; de Crombrugghe, B. Up-Regulation of the Chondrogenic Sox9 Gene by Fibroblast Growth Factors Is Mediated by the Mitogen-Activated Protein Kinase Pathway. Proc. Natl. Acad. Sci. USA 2000, 97, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Inui, M.; Miyado, M.; Igarashi, M.; Tamano, M.; Kubo, A.; Yamashita, S.; Asahara, H.; Fukami, M.; Takada, S. Rapid Generation of Mouse Models with Defined Point Mutations by the CRISPR/Cas9 System. Sci. Rep. 2014, 4, 5396. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liao, J.; Rao, X.; Kushner, S.A.; Chung, C.D.; Chang, D.D.; Shuai, K. Inhibition of Stat1-Mediated Gene Activation by PIAS1. Proc. Natl. Acad. Sci. USA 1998, 95, 10626–10631. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.D.; Liao, J.; Liu, B.; Rao, X.; Jay, P.; Berta, P.; Shuai, K. Specific Inhibition of Stat3 Signal Transduction by PIAS3. Science 1997, 278, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gross, M.; ten Hoeve, J.; Shuai, K. A Transcriptional Corepressor of Stat1 with an Essential LXXLL Signature Motif. Proc. Natl. Acad. Sci. USA 2001, 98, 3203–3207. [Google Scholar] [CrossRef]
- Arora, T.; Liu, B.; He, H.; Kim, J.; Murphy, T.L.; Murphy, K.M.; Modlin, R.L.; Shuai, K. PIASx Is a Transcriptional Co-Repressor of Signal Transducer and Activator of Transcription 4. J. Biol. Chem. 2003, 278, 21327–21330. [Google Scholar] [CrossRef]
- Cheng, J.; Kang, X.; Zhang, S.; Yeh, E.T.H. SUMO-Specific Protease 1 Is Essential for Stabilization of HIF1alpha During Hypoxia. Cell 2007, 131, 584–595. [Google Scholar] [CrossRef]
- Zhou, G.; Lefebvre, V.; Zhang, Z.; Eberspaecher, H.; de Crombrugghe, B. Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J. Biol. Chem. 1998, 273, 14989–14997. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saotome, H.; Ito, A.; Kubo, A.; Inui, M. Generation of a Quantitative Luciferase Reporter for Sox9 SUMOylation. Int. J. Mol. Sci. 2020, 21, 1274. https://doi.org/10.3390/ijms21041274
Saotome H, Ito A, Kubo A, Inui M. Generation of a Quantitative Luciferase Reporter for Sox9 SUMOylation. International Journal of Molecular Sciences. 2020; 21(4):1274. https://doi.org/10.3390/ijms21041274
Chicago/Turabian StyleSaotome, Hideka, Atsumi Ito, Atsushi Kubo, and Masafumi Inui. 2020. "Generation of a Quantitative Luciferase Reporter for Sox9 SUMOylation" International Journal of Molecular Sciences 21, no. 4: 1274. https://doi.org/10.3390/ijms21041274
APA StyleSaotome, H., Ito, A., Kubo, A., & Inui, M. (2020). Generation of a Quantitative Luciferase Reporter for Sox9 SUMOylation. International Journal of Molecular Sciences, 21(4), 1274. https://doi.org/10.3390/ijms21041274




