Salicylic Acid Protects Photosystem II by Alleviating Photoinhibition in Arabidopsis thaliana under High Light
Abstract
1. Introduction
2. Results
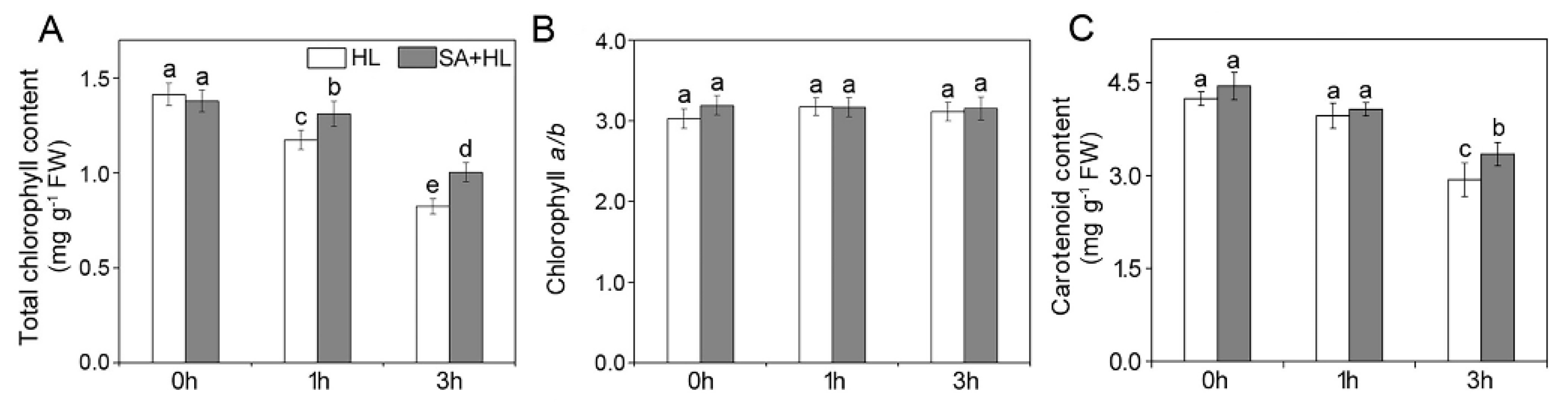
2.1. Chl Content and Carotenoid Content
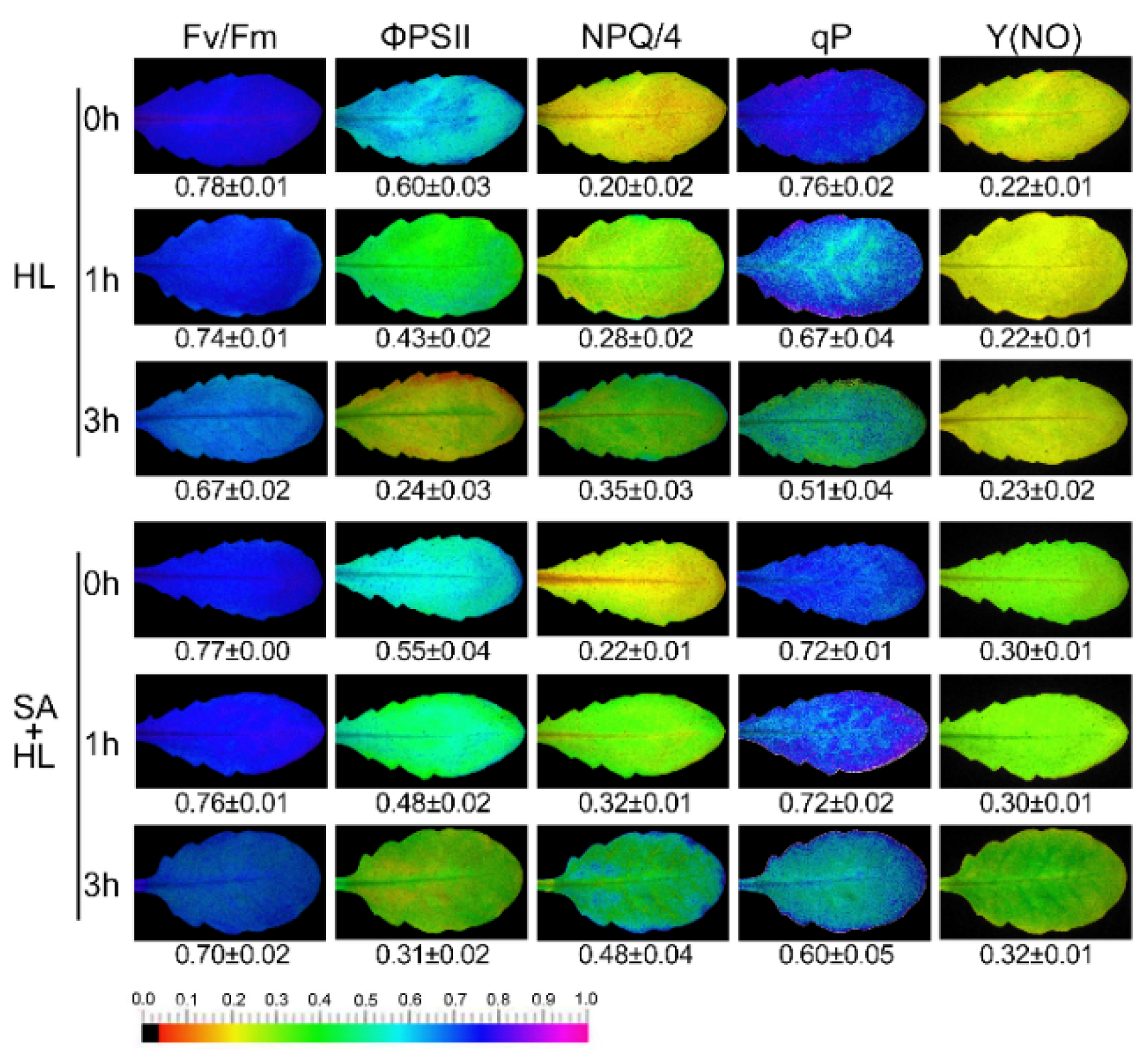
2.2. SA Improved Photosynthetic Efficiency under High Light
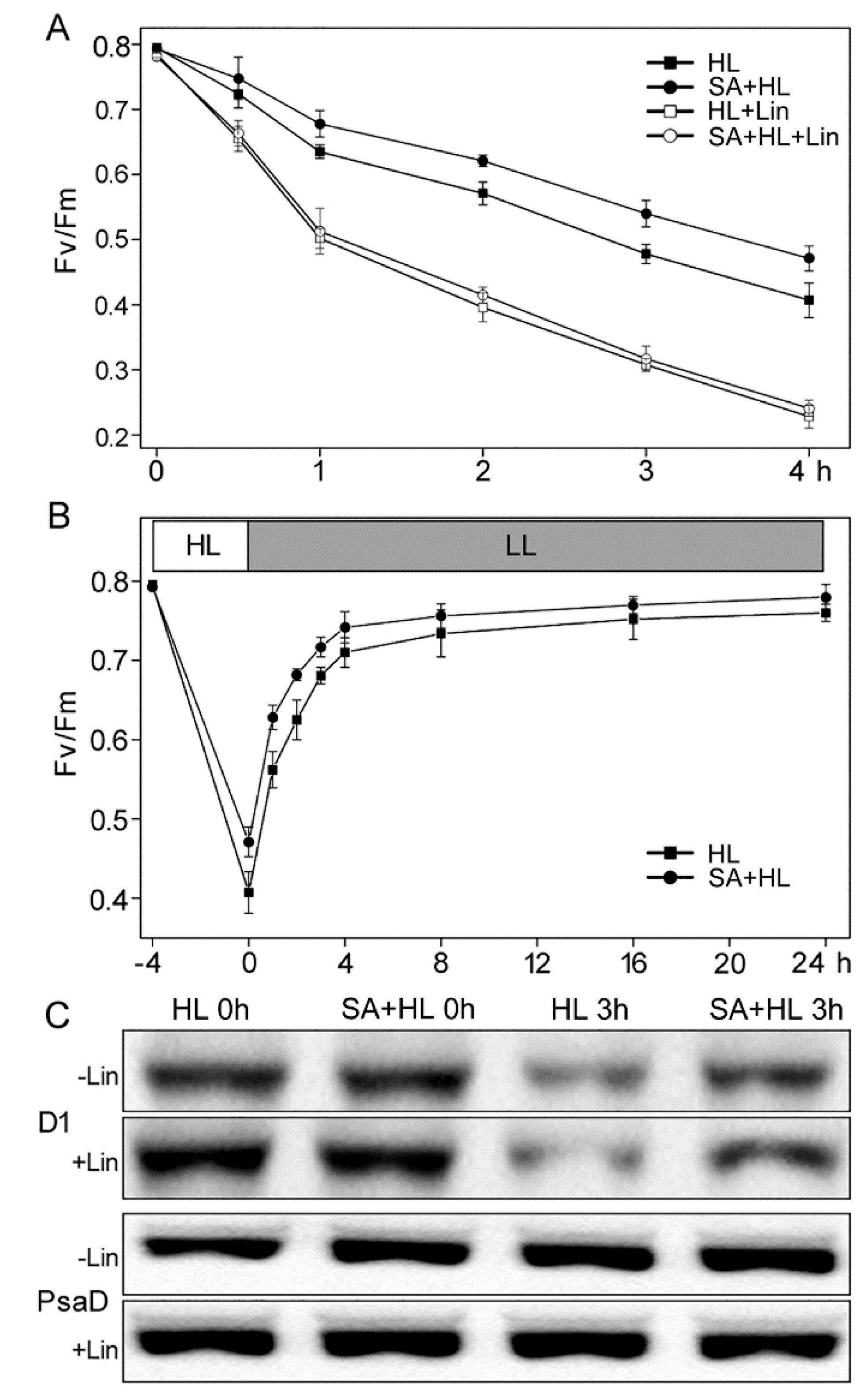
2.3. PSII Photoinhibition Analysis
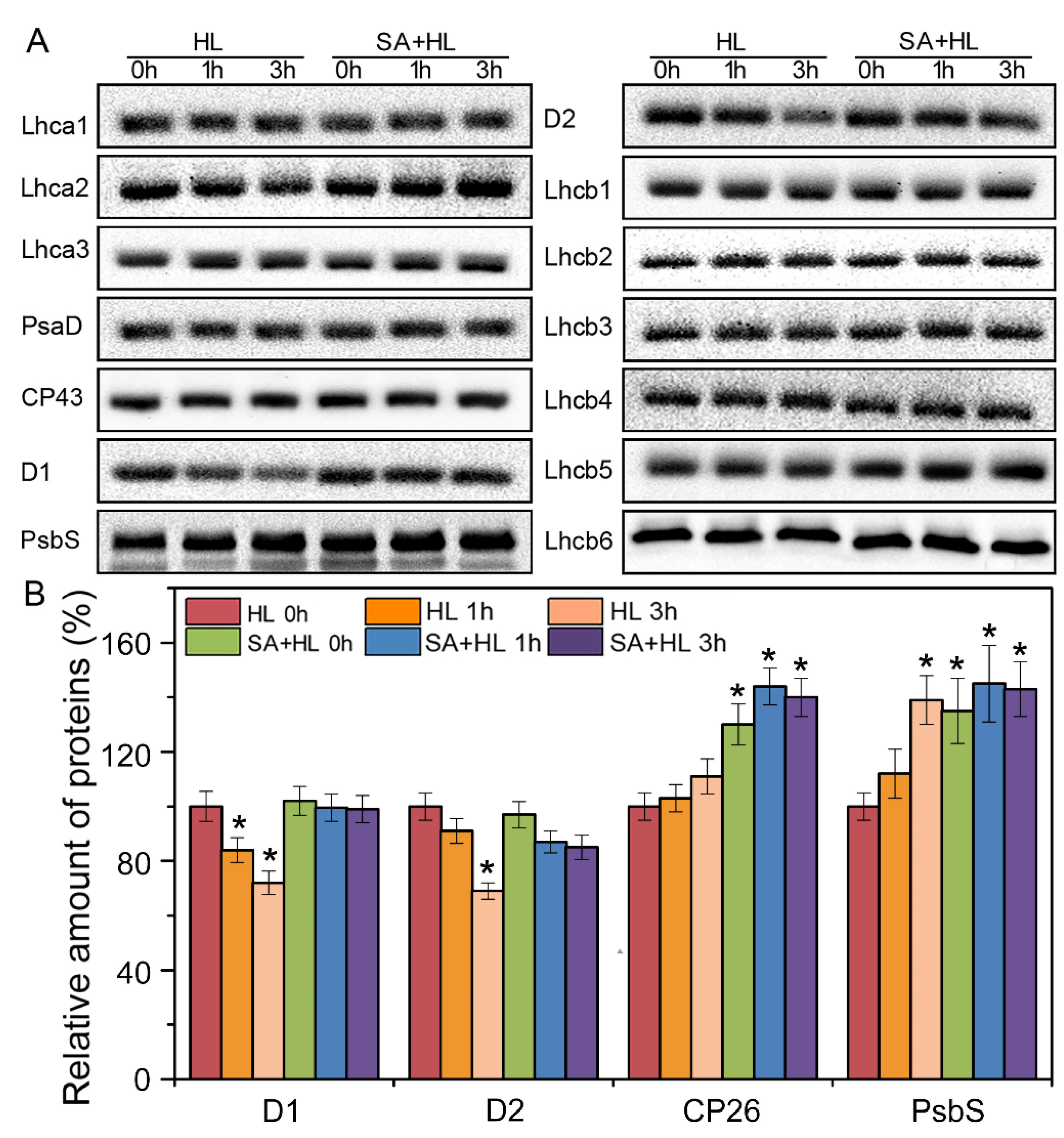
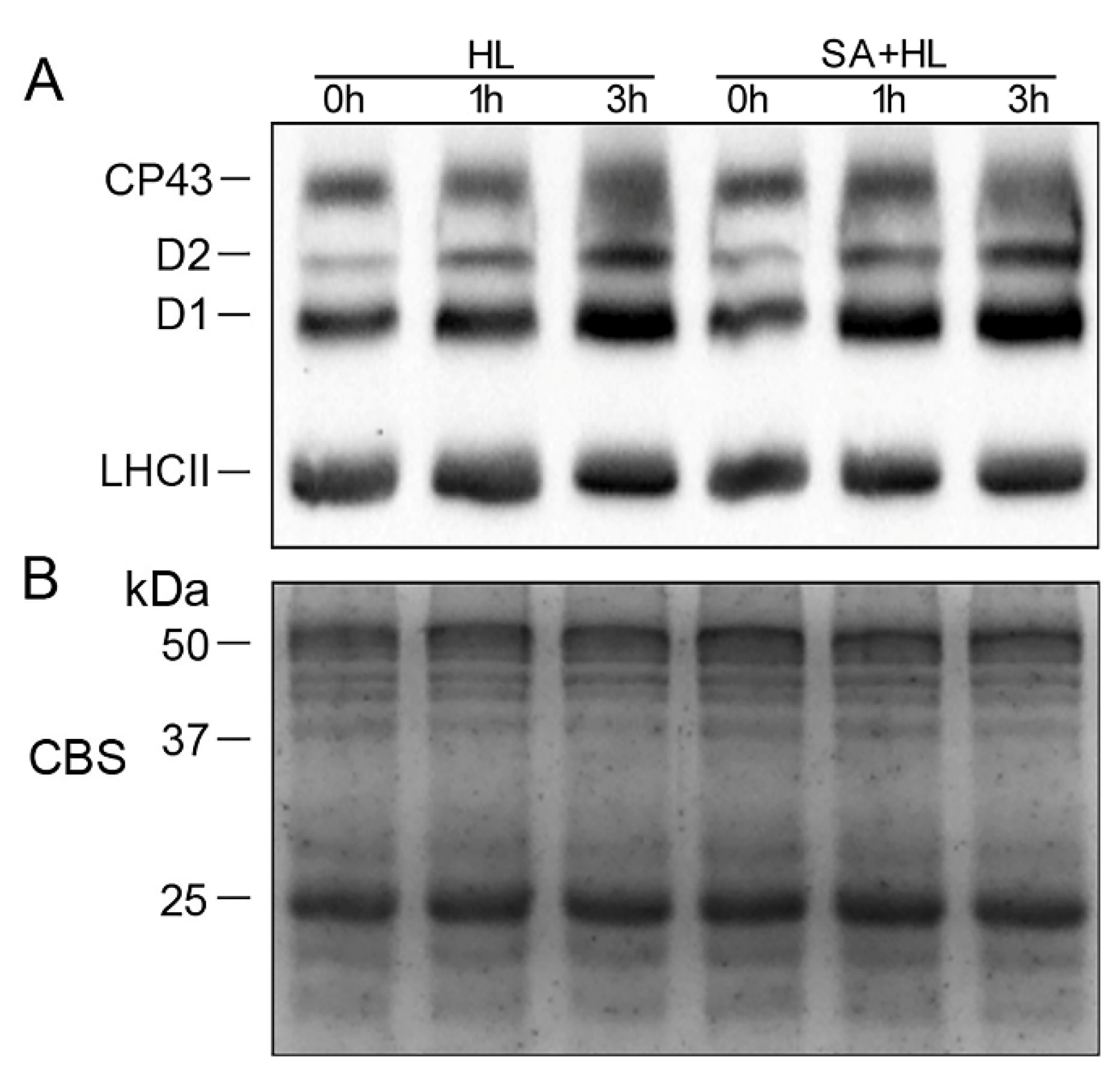
2.4. Changes in Thylakoid Proteins and Phosphorylation under High Light
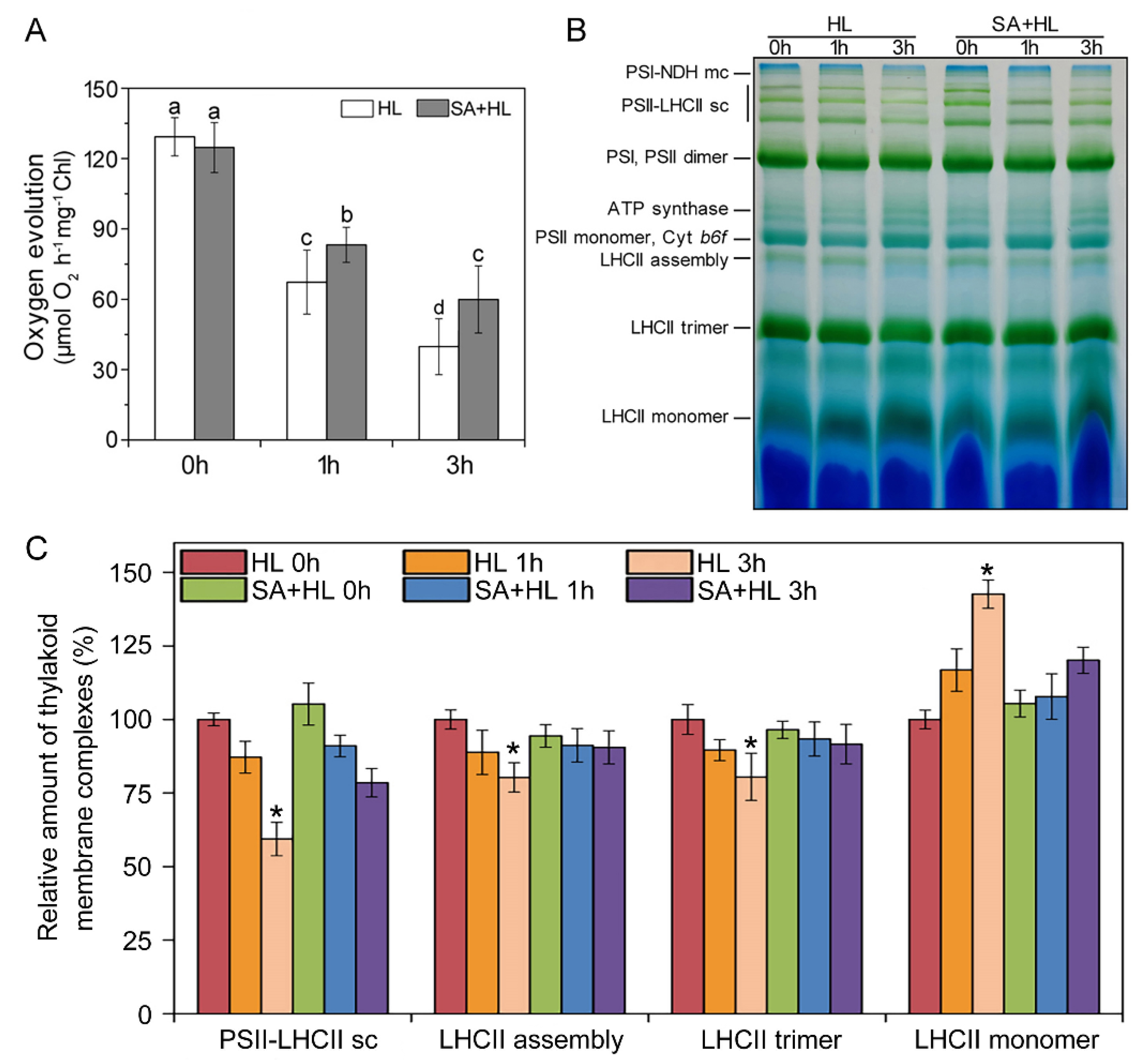
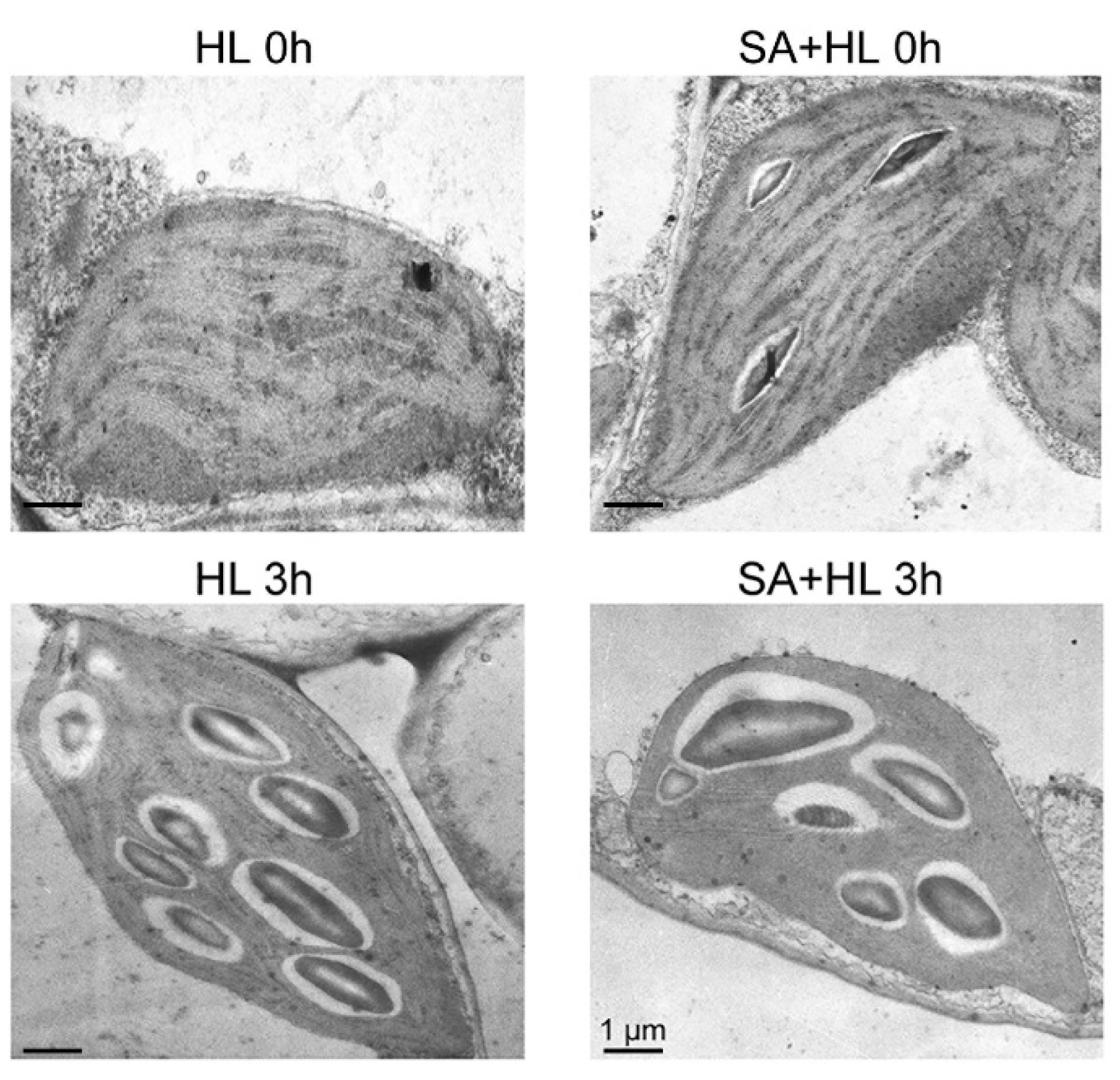
2.5. Alterations in Oxygen Evolution, Thylakoid Membrane Complexes, and Chloroplast Organization
3. Discussion
4. Material and Methods
4.1. Plant Materials and Stress Treatments
4.2. Measurements of Chlorophyll and Carotenoid Contents
4.3. Measurements of P700 Parameters and Chl Fluorescence
4.4. Measurements of Gas Exchange Parameters and Oxygen Evolution
4.5. Analysis of Photoinhibition and Recovery
4.6. Immunoblotting Analysis
4.7. Blue Native PAGE
4.8. Electron Microscopy
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pagliano, C.; Saracco, G.; Barber, J. Structural, functional and auxiliary proteins of photosystem II. Photosynth. Res. 2013, 116, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Adir, N.; Zer, H.; Shochat, S.; Ohad, I. Photoinhibition—A historical perspective. Photosynth. Res. 2003, 76, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K.; Truong, T.B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 2013, 16, 307–314. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Cazzaniga, S.; Bressan, M.; Paleček, D.; Židek, K.; Niyogi, K.K.; Fleming, G.R.; Zigmantas, D.; Bassi, R. Two mechanisms for dissipation of excess light in monomeric and trimeric light-harvesting complexes. Nat. Plants 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef]
- Minagawa, J. State transitions—The molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 897–905. [Google Scholar] [CrossRef]
- Tikkanen, M.; Nurmi, M.; Kangasjärvi, S.; Aro, E.M. Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 1432–1437. [Google Scholar] [CrossRef]
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physiol. Plant 2016, 158, 225–235. [Google Scholar] [CrossRef]
- Nixon, P.J.; Michoux, F.; Yu, J.; Boehm, M.; Komenda, J. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 2010, 106, 1–16. [Google Scholar] [CrossRef]
- Su, X.; Wu, S.; Yang, L.; Xue, R.; Li, H.; Wang, Y.; Zhao, H. Exogenous progesterone alleviates heat and high light stress- induced inactivation of photosystem II in wheat by enhancing antioxidant defense and D1 protein stability. Plant Growth Regul. 2014, 74, 311–318. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Guo, F.; Meng, J.J.; Li, X.G.; Dong, S.T.; Wan, S.B. Exogenous calcium alleviates photoinhibition of PSII by improving the xanthophyll cycle in peanut (Arachis hypogaea) leaves during heat stress under high irradiance. PLoS ONE 2013, 8, e71214. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- De Bianchi, S.; Ballottari, M.; Dall’Osto, L.; Bassi, R. Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem. Soc. Trans. 2010, 38, 651–660. [Google Scholar] [CrossRef]
- Nath, K.; Jajoo, A.; Poudyal, R.S.; Timilsina, R.; Park, Y.S.; Aro, E.M.; Nam, H.G.; Lee, C.H. Towards a critical understanding of the photosystem II repair mechanism and its regulation during stress conditions. FEBS Lett. 2013, 587, 3372–3381. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 742–749. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Pancheva, T.V.; Popova, L.P.; Uzunova, A.N. Effects of salicylic acid on growth and photosynthesis in barley plants. J. Plant Physiol. 1996, 149, 57–63. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef]
- Boatwright, J.L.; Pajerowska-Mukhtar, K. Salicylic acid: An old hormone up to new tricks. Mol. Plant Pathol. 2013, 14, 623–634. [Google Scholar] [CrossRef]
- Janda, T.; Gondor, O.K.; Yordanova, R.; Szalai, G.; Pál, M. Salicylic acid and photosynthesis: Signalling and effects. Acta Physiol. Plant 2014, 36, 2537–2546. [Google Scholar] [CrossRef]
- Yuan, S.; Lin, H.H. Role of salicylic acid in plant abiotic stress. Zeitschrift für Naturforschung C 2008, 63, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Fan, L.; Loescher, W.; Duan, W.; Liu, G.J.; Cheng, J.S.; Luo, H.B.; Li, S.H. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Cui, J.M.; Li, G.X.; Yuan, M.; Zhang, Z.W.; Yuan, S.; Zhang, H.Y. Effect of salicylic acid on the antioxidant system and photosystem II in wheat seedlings. Biol. Plant 2016, 60, 139–147. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hou, P.; Su, X.; Zhao, P.; Zhao, H.; Liu, S. Foliar-applied salicylic acid alleviates heat and high light stress induced photoinhibition in wheat (Triticum aestivum) during the grain filling stage by modulating the psbA gene transcription and antioxidant defense. Plant Growth Regul. 2014, 73, 289–297. [Google Scholar] [CrossRef]
- Gururani, M.A.; Mohanta, T.K.; Bae, H. Current understanding of the interplay between phytohormones and photosynthesis under environmental stress. Int. J. Mol. Sci. 2015, 16, 19055–19085. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L.F. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef]
- De Bianchi, S.; Betterle, N.; Kouril, R.; Cazzaniga, S.; Boekema, E.; Bassi, R.; Dall’Osto, L. Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted photosystem II macrostructure and are defective in photoprotection. Plant Cell 2011, 23, 2659–2679. [Google Scholar] [CrossRef]
- Nath, K.; Poudyal, R.S.; Eom, J.S.; Park, Y.S.; Zulfugarov, I.S.; Mishra, S.R.; Tovuu, A.; Ryoo, N.; Yoon, H.S.; Nam, H.G.; et al. Loss-of-function of OsSTN8 suppresses the photosystem II core protein phosphorylation and interferes with the photosystem II repair mechanism in rice (Oryza sativa). Plant J. 2013, 76, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Zhang, C.M.; Su, Y.Q.; Ma, J.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Responses of photosystem II and antioxidative systems to high light and high temperature co-stress in wheat. Environ. Exp. Bot. 2017, 135, 45–55. [Google Scholar] [CrossRef]
- Mateo, A.; Funck, D.; Muhlenbock, P.; Kular, B.; Mullineaux, P.M. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. Exp. Bot. 2006, 57, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Sytar, O.; Allakhverdiev, S.I. Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth. Res. 2015, 126, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhou, B.; Ma, C.; Xu, X.; Xu, J.; Jiang, Y.; Liu, C.; Li, G.; Herbert, S.J.; Hao, L. Salicylic acid-altering Arabidopsis mutants’ response to NO2 exposure. Bull. Environ. Contam. Toxicol. 2010, 84, 106–111. [Google Scholar] [CrossRef]
- Habibi, G. Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol. Szeged. 2012, 56, 57–63. [Google Scholar]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Su, Y.Q.; Zhang, C.M.; Ma, J.; Zhang, Z.W.; Yuan, M.; Liu, W.J.; Zhang, H.Y.; Yuan, S. Comparison of phosphorylation and assembly of photosystem complexes and redox homeostasis in two wheat cultivars with different drought resistance. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Javaheri, M.; Mashayekhi, K.; Dadkhah, A.; Tavallaee, F.Z. Effects of salicylic acid on yield and quality characters of tomato fruit (Lycopersicum esculentum Mill.). Int. J. Agric. Crop Sci. 2012; 4, 1184–1187. [Google Scholar]
- Tikkanen, M.; Mekala, N.R.; Aro, E.M. Photosystem II photoinhibition-repair cycle protects photosystem I from irreversible damage. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 210–215. [Google Scholar] [CrossRef]
- Li, L.; Aro, E.M.; Millar, A.H. Mechanisms of photodamage and protein turnover in photoinhibition. Trends Plant Sci. 2018, 23, 667–676. [Google Scholar] [CrossRef]
- Ruban, A.V.; Berera, R.; Ilioaia, C.; van Stokum, I.H.; Kennis, J.T.; Pascal, A.A.; van Amerongen, H.; Robert, B.; Horton, P.; van Grondelle, R. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 2007, 450, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Norman, C.; Howell, K.A.; Millar, A.H.; Whelan, J.M.; Day, D.A. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol. 2004, 134, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, A.; Jensen, P.E.; Lunde, C.; Scheller, H.V. Balance of power: A view of the mechanism of photosynthetic state transitions. Trends Plant Sci. 2001, 6, 301–305. [Google Scholar] [CrossRef]
- Faried, H.N.; Ayyub, C.M.; Amjad, M.; Ahmed, R.; Wattoo, F.M.; Butt, M.; Bashir, M.; Shaheen, M.R.; Waqas, M.A. Salicylic acid confers salt tolerance in potato plants by improving water relations, gaseous exchange, antioxidant activities and osmoregulation. J. Sci. Food Agric. 2017, 97, 1868–1875. [Google Scholar] [CrossRef]
- Qiu, C.; Ji, W.; Guo, Y. Effects of high temperature and strong light on chlorophyll fluorescence, the D1 protein, and Deg1 protease in Satsuma mandarin, and the protective role of salicylic acid. Acta Ecol. Sin. 2011, 31, 3802–3810. [Google Scholar]
- Kapri-Pardes, E.; Naveh, L.; Adam, Z. The thylakoid lumen protease Deg1 is involved in the repair of photosystem II from photoinhibition in Arabidopsis. Plant Cell 2007, 19, 1039–1047. [Google Scholar] [CrossRef]
- Aro, E.M.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II inactivation, protein damage and turnover. Biochim. Biophys. Acta Bioenerg. 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Kato, Y.; Sakamoto, W. Protein quality control in chloroplasts: A current model of D1 protein degradation in the photosystem II repair cycle. J. Biochem. 2009, 146, 463–469. [Google Scholar] [CrossRef]
- Zhao, H.J.; Zhao, X.J.; Ma, P.F.; Wang, Y.X.; Hu, W.W.; Li, L.H.; Zhao, Y.D. Effect of salicylic acid on protein kinase activity and chloroplast D1 protein degradation in wheat leaves subjected to heat and high light stress. Acta Ecol. Sin. 2011, 31, 259–263. [Google Scholar] [CrossRef]
- De Bianchi, S.; Dall’Osto, L.; Tognon, G.; Morosinotto, T.; Bassi, R. Minor antenna proteins CP24 and CP26 affect the interactions between photosystem II subunits and the electron transport rate in grana membranes of Arabidopsis. Plant Cell 2008, 20, 1012–1028. [Google Scholar] [CrossRef]
- Li, X.P.; Gilmore, A.M.; Caffarri, S.; Bassi, R.; Golari, T.; Kramer, D.; Niyogi, K.K. Regulation of photosynthetic light harvesting involves in thylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 2004, 279, 22866–22874. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Aro, E.M. Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.M.; Ohad, I. Redox regulation of thylakoid protein phosphorylation. Antioxid. Redox Signal. 2003, 5, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Yuan, S.; Lezhneva, L.; Meurer, J.; Schwenkert, S.; Mamedov, F.; Schröder, W.P. The low molecular mass photosystem II protein PsbTn is important for light acclimation. Plant Physiol. 2019, 179, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Koivuniemi, A.; Aro, E.M.; Andersson, B. Degradation of the D1- and D2-proteins of photosystem II in higher plants is regulated by reversible phosphorylation. Biochemistry 1995, 34, 16022–16029. [Google Scholar] [CrossRef]
- Chen, Y.E.; Yuan, S.; Du, J.B.; Xu, M.Y.; Zhang, Z.W.; Lin, H.H. Phosphorylation of photosynthetic antenna protein CP29 and photosystem II structure changes in monocotyledonous plants under environmental stresses. Biochemistry 2009, 48, 9757–9763. [Google Scholar] [CrossRef]
- Chang, L.; Wang, L.; Peng, C.; Tong, Z.; Wang, D.; Ding, G.; Xiao, J.; Guo, A.; Wang, X. The chloroplast proteome response to drought stress in cassava leaves. Plant Physiol. Biochem. 2019, 142, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Muramatsu, S.; Makino, A.; Mae, T. Relationship between the suppression of photosynthesis and starch accumulation in the pod-removed bean. Aust. J. Plant Physiol. 2000, 27, 167–173. [Google Scholar] [CrossRef]
- Luo, M.H.; Yuan, S.; Chen, Y.E.; Liu, W.J.; Du, J.B.; Lei, T.; Wang, M.B.; Lin, H.H. Effects of salicylic acid on the photosystem 2 of barley seedlings under osmotic stress. Biol. Plant 2009, 53, 663–669. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedmann, P.E. Determination of accurate extinction coeffcients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700 absorbance changes at 830 nm. Planta 1994, 192, 261–268. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 345, 659–668. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Yuan, S.; Schröder, W.P. Comparison of methods for extracting thylakoid membranes of Arabidopsis plants. Physiol. Plant. 2016, 156, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-E.; Mao, H.-T.; Wu, N.; Mohi Ud Din, A.; Khan, A.; Zhang, H.-Y.; Yuan, S. Salicylic Acid Protects Photosystem II by Alleviating Photoinhibition in Arabidopsis thaliana under High Light. Int. J. Mol. Sci. 2020, 21, 1229. https://doi.org/10.3390/ijms21041229
Chen Y-E, Mao H-T, Wu N, Mohi Ud Din A, Khan A, Zhang H-Y, Yuan S. Salicylic Acid Protects Photosystem II by Alleviating Photoinhibition in Arabidopsis thaliana under High Light. International Journal of Molecular Sciences. 2020; 21(4):1229. https://doi.org/10.3390/ijms21041229
Chicago/Turabian StyleChen, Yang-Er, Hao-Tian Mao, Nan Wu, Atta Mohi Ud Din, Ahsin Khan, Huai-Yu Zhang, and Shu Yuan. 2020. "Salicylic Acid Protects Photosystem II by Alleviating Photoinhibition in Arabidopsis thaliana under High Light" International Journal of Molecular Sciences 21, no. 4: 1229. https://doi.org/10.3390/ijms21041229
APA StyleChen, Y.-E., Mao, H.-T., Wu, N., Mohi Ud Din, A., Khan, A., Zhang, H.-Y., & Yuan, S. (2020). Salicylic Acid Protects Photosystem II by Alleviating Photoinhibition in Arabidopsis thaliana under High Light. International Journal of Molecular Sciences, 21(4), 1229. https://doi.org/10.3390/ijms21041229





