An Overview of Orchid Protocorm-Like Bodies: Mass Propagation, Biotechnology, Molecular Aspects, and Breeding
Abstract
1. Introduction
2. Genus Phalaenopsis and Related
3. Oncidium Hybrids Group
4. Some News with Cymbidium, Dendrobium, and Others
5. Applications of IPR–PLB Technique on Orchid Propagation and Breeding and Main Limitations of the Technique
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chase, M.W.; Cameron, K.M.; Freudestein, J.V.; Pridgeon, A.M.; Salazar, G.; Van den Berg, C.; Schuiteman, A. An update classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- The Plant List. 2019. Orchidaceae Family. Available online: http://www.theplantlist.org/1.1/browse/A/Orchidaceae/#statistics (accessed on 31 December 2019).
- RHS 2019. The International Orchid Register. Available online: https://apps.rhs.org.uk/horticulturaldatabase/orchidregister/orchidregister.asp (accessed on 31 December 2019).
- Bulpitt, C.J.; Li, Y.; Bulpitt, P.F.; Wang, J. The use of orchids in Chinese medicine. J. R. Soc. Med. 2007, 100, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Zuraida, A.R.; Izzati, K.H.F.L.; Nazreena, O.A.; Zaliha, W.S.W.; Radziah, C.M.Z.C.; Zamri, Z.; Sreeramanan, S. A simple and efficient protocol for the mass propagation of Vanilla planifolia. Am. J. Plant Sci. 2013, 4, 1685–1692. [Google Scholar] [CrossRef]
- Chen, C. The Fundamental Issue in the Phalaenopsis Industry. 2018. Available online: http://amebse.nchu.edu.tw/orchids_cultivation21.htm (accessed on 30 December 2019).
- Cardoso, J.C. Dendrobium ‘Brazilian Fire 101’-New option of color of flowers for the orchid market. Hortic. Bras. 2012, 30, 561–564. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Martinelli, A.P.; Teixeira da Silva, J.A. A novel approach for the selection of Cattleya hybrids for precocious and season-independent flowering. Euphytica 2016, 210, 143–150. [Google Scholar] [CrossRef]
- Cardoso, J.C. Ionocidium ‘Cerrado101’: Intergeneric orchid hybrid with high quality of blooming. Ornam. Hortic. 2017, 23, 351–356. [Google Scholar] [CrossRef][Green Version]
- Ho, T.-T.; Kwon, A.-R.; Yoon, Y.-J.; Paek, K.-Y.; Park, S.-Y. Endoreduplication level affects flower size and development by increasing cell size in Phalaenopsis and Doritaenopsis. Acta Physiol. Plant 2016, 38, 190. [Google Scholar] [CrossRef]
- Lakshman, C.; Pathak, P.; Rao, A.N.; Rajeevan, P.K. Commercial Orchids; De Gruyter Open: Beijing, China, 2014; p. 322. [Google Scholar]
- Van den Berg, C. Reaching a compromise between conflicting nuclear and plastid phylogenetic trees: A new classification for the genus Cattleya (Epidendreae; Epidendroideae; Orchidaceae). Phytotaxa 2014, 186, 75–86. [Google Scholar] [CrossRef]
- Peraza-Flores, L.N.; Carnevali, G.; Van den Berg, C. A molecular phylogeny of the Laelia Alliance (Orchidaceae) and reassessment of Laelia and Schomburgkia. Taxon 2017, 65, 6. [Google Scholar] [CrossRef]
- Dalström, S.; Higgins, W.E. New combinations and transfers to Odontoglossum Oncidiinae (Orchidaceae): Avoid creating new names. Harv. Pap. Bot. 2016, 21, 97–104. [Google Scholar] [CrossRef]
- Yeung, E.C. A perspective on orchid seed and protocorm development. Bot. Stud. 2017, 58, 33. [Google Scholar] [CrossRef] [PubMed]
- Oneal, E.; Willis, J.H.; Franks, R. Disruption of endosperm development is a major cause of hybrid seed inviability between Mimulus guttatus and M. nudatus. New Phytol. 2010, 210, 029223. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Cardoso, J.C.; Dobránszki, J.; Zeng, S. Dendrobium micropropagation: A review. Plant Cell Rep. 2015, 34, 671–704. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Tsavkelova, E.A.; Ng, T.B.; Parthibhan, S.; Dobránszki, J.; Cardoso, J.C.; Rao, M.V.; Zeng, S. Asymbiotic in vitro seed propagation of Dendrobium. Plant Cell Rep. 2015, 34, 1685–1706. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Chen, X.-M.; Zhang, Y.; Cho, Y.-H.; Wang, A.-R.; Yeung, E.C.; Zeng, X.; Guo, S.-X.; Lee, Y.-I. Immunolocalization and changes of hydroxyproline-rich glycoproteins during symbiotic germination of Dendrobium officinale. Front. Plant Sci. 2018, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Mala, B.; Kuegkong, K.; Sa-ngiaemsri, N.; Nontachaiyapoom, S. Effect of germination media on in vitro symbiotic seed germination of three Dendrobium orchids. S. Afr. J. Bot. 2017, 112, 521–526. [Google Scholar] [CrossRef]
- Arditti, J. Factors affecting the germination of orchid seeds. Bot. Rev. 1967, 33, 1–97. [Google Scholar] [CrossRef]
- Santos, S.A.; Smidt, E.C.; Padial, A.A.; Ribas, L.L.F. Asymbiotic seed germination and in vitro propagation of Brasiliorchis picta. Afr. J. Biotech. 2016, 15, 134–144. [Google Scholar]
- Kunakhonnuruk, B.; Inthima, P.; Kongbangkerd, A. In vitro propagation of Epipactis flava Seidenf, an endangered rheophytic orchid: A first study on factors affecting asymbiotic seed germination, seedling development and greenhouse acclimatization. Plant Cell Tissue Organ Cult. 2018, 135, 419–432. [Google Scholar] [CrossRef]
- Rao, A.N. Tissue culture in orchid industry. In Applied and Fundamental Aspects of Plant Cell Tissue and Organ Culture; Reinert, J., Bajaj, Y.P.S., Eds.; Springer: Berlin, Germany, 1977; pp. 44–49. [Google Scholar]
- Parmar, G.; Pant, B. In vitro seed germination and seedling development of the orchid Coelogyne stricta (D. Don) Schltr. Afr. J. Biotechnol. 2016, 15, 105–109. [Google Scholar]
- Huang, H.; Zi, X.-M.; Lin, H.; Gao, J.-Y. Host-specificity of symbiotic mycorrhizal fungi for enhancing seed germination, protocorm formation and seedling development of over-collected medicinal orchid, Dendrobium devonianum. J. Microb. 2018, 56, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Fochi, V.; Chitarra, W.; Kohler, A.; Voyron, S.; Singan, V.R.; Lindquist, E.A.; Barry, K.W.; Girlanda, M.; Grigoriev, I.V.; Martin, F.; et al. Fungal and plant gene expression in the Tulasnella calospora-Serapias vomeracea symbiosis provides clues about nitrogen pathways in orchid mycorrizas. New Phytol. 2016, 213, 10–12. [Google Scholar]
- Lee, Y.; Hsu, S.; Yeung, E.C. Orchid protocorm-like bodies are somatic embryos. Am. J. Bot. 2013, 100, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Chen, J.C.; WEI, M.J. Protocorms and protocorm-like bodies are molecularly distinct from zygotic embryonic tissues. Plant Physiol. 2016, 171, 2682–2700. [Google Scholar] [CrossRef] [PubMed]
- Zanello, C.A.; Cardoso, J.C. PLBs induction and clonal plantlet regeneration from leaf segment of commercial hybrids of Phalaenopsis. J. Hortic. Sci. Biotech. 2019, 94, 627–631. [Google Scholar] [CrossRef]
- Chookoh, N.; Chiu, Y.; Chang, C.; Hu, W.; Dai, T. Micropropagation of Tolumnia orchids through induction of protocorm-like bodies from leaf segments. Hortscience 2019, 54, 1230–1236. [Google Scholar] [CrossRef]
- Li, S.H.; Kuoh, C.S.; Chen, Y.H.; Chen, H.H.; Chen, W.H. Osmotic sucrose enhancement of single-cell embryogenesis and transformation efficiency in Oncidium. Plant Cell Tissue Organ Cult. 2005, 81, 183–192. [Google Scholar] [CrossRef]
- Naing, A.H.; Chung, J.D.; Park, I.S.; Lim, K.B. Efficient plant regeneration of the endangered medicinal orchid, Coelogyne cristata using protocorm-like bodies. Acta Physiol. Plant 2011, 33, 659–666. [Google Scholar] [CrossRef]
- Kalyan, K.; Sil, S. Protocorm-like bodies and plant regeneration from foliar explants of Coelogyne flaccida, a horticulturally and medicinally important endangered orchid of eastern himalaya. Lanke 2015, 15, 151–158. [Google Scholar]
- Picolotto, D.R.N.; Paiva Neto, V.B.; Barros, F.; Padilha, D.R.C.; Cruz, A.C.F.; Otoni, W.C. Micropropagation of Cyrtopodium paludicolum (Orchidaceae) from root tip explants. Crop Breed. App. Biotech. 2017, 17, 191–197. [Google Scholar] [CrossRef][Green Version]
- Samala, S.; Te-chato, S.; Yenchon, S.; Thammasiri, K. Protocorm-like body of Grammatophyllum speciosum through asymbiotic seed germination. ScienceAsia 2014, 40, 379–383. [Google Scholar] [CrossRef][Green Version]
- Chen, C. Cost analysis of plant micropropagation of Phalaenopsis. Plant Cell Tissue Organ Cult. 2016, 126, 167–175. [Google Scholar] [CrossRef]
- Tanaka, M.; Sakanishi, Y. Clonal propagation of Phalaenopsis by leaf culture. Am. Orc. Soc. Bull. 1977, 46, 733–737. [Google Scholar]
- Tanaka, M.; Sakanishi, Y. Clonal propagation of Phalaenopsis through tissue culture. In Proc. 9th World Orchid Conference; Kashemsanta, M.R.S., Ed.; Amarin Press: Bangkok, Thailand, 1980; pp. 215–221. [Google Scholar]
- Tanaka, M.; Sakanishi, Y. Regenerative capacity of in vitro cultured leaf segments excised from mature Phalaenopsis plants. Bull. Univ. Osaka Ser. B 1985, 37, 1–4. [Google Scholar]
- Tokuhara, K.; Mii, M. Micropropagation of Phalaenopsis and Doritaenopsis by culturing shoot tips of flower stalk buds. Plant Cell Rep. 1993, 13, 7–11. [Google Scholar] [CrossRef]
- Ishii, Y.; Takamura, T.; Goi, M.; Tanaka, M. Callus induction and somatic embryogenesis of Phalaenopsis. Plant Cell Rep. 1998, 17, 446–450. [Google Scholar] [CrossRef]
- Vacin, E.F.; Went, F.W. Some pH in nutrient solutions. Bot. Gaz. 1949, 110, 605–617. [Google Scholar] [CrossRef]
- Huan, L.V.T.; Takamura, T.; Tanaka, M. Callus formation and plant regeneration from callus through somatic embryo structures in Cymbidium orchid. Plant Sci. 2004, 166, 1443–1449. [Google Scholar] [CrossRef]
- Ulisses, C.; Pereira, J.A.F.; Silva, S.S.; Arruda, E.; Morais, M. Indução e histologia de embriões somáticos primários e secundários do híbrido Phalaenopsis Classic Spotted Pink (Orchidaceae). Acta Biol. Col. 2016, 21, 571–580. [Google Scholar]
- Goussard, P.G.; Wiid, J.; Kasdor, G.G.F. The effectiveness of in vitro somatic embryogenesis in eliminating fanleaf virus and leafroll associated viruses from grapevines. S. Afr. J. Enol. Vitic. 1991, 12, 77–81. [Google Scholar] [CrossRef]
- Quainoo, A.K.; Wetten, A.C.; Allainguillaume, J. The effectiveness of somatic embryogenesis in eliminating the cocoa swollen shoot virus from infected cocoa trees. J. Virol. Met. 2008, 149, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Di Matteo, D.; Gribaudo, I. Elimination of Grapevine fanleaf virus from three Vitis vinifera cultivars by somatic embryogenesis. Eur. J. Plant Pathol. 2009, 123, 57–60. [Google Scholar] [CrossRef]
- Nkaa, F.A.; Ene-Obong, E.E.; Taylor, N.; Fauquet, C.; Mbanaso, E.N.A. Elimination of African Cassava Mosaic Virus (ACMV) and East African Cassava Mosaic Virus (EACMV) from cassava (Manihot esculenta Crantz) cv. ‘Nwugo’ via somatic embryogenesis. Am. J. Biotech. Molec. Sci. 2013, 3, 33–40. [Google Scholar]
- Chai, M.L.; Xu, C.-J.; Senthil, K.; Kim, J.Y. Stable transformation of protocorm-like bodies in Phalaenopsis orchid mediated by Agrobacterium tumefasciens. Sci. Hort. 2002, 96, 213–224. [Google Scholar] [CrossRef]
- Mishiba, K.; Chin, D.P.; Mii, M. Agrobacterium-mediated transformation of Phalaenopsis by targeting protocorms at an early stage after germination. Plant Cell Rep. 2005, 24, 297–303. [Google Scholar] [CrossRef]
- Huang, Y.W.; Tsai, Y.J.; Chen, F.C. Characterization and expression analysis of somatic embryogenesis receptor-like kinase genes from Phalaenopsis. Genet. Mol. Res. 2014, 13, 10690–10703. [Google Scholar] [CrossRef]
- Hecht, V.; Vielle-Calzada, J.P.; Hartog, M.V.; Schmidt, E.D.; Boutilier, K.; Grossniklaus, U.; De Vries, S.C. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol. 2001, 127, 803–816. [Google Scholar] [CrossRef]
- Chardin, C.; Girin, T.; Roudier, F.; Meyer, C.; Krapp, A. The plant RWP-RK transcription factors: Key regulators of nitrogen responses and of gametophyte development. J. Exp. Bot. 2014, 65, 5577–5587. [Google Scholar] [CrossRef]
- Mursyanti, E.; Purwantoro, A.; Moeljopawiro, S.; Semiarti, E. Induction of somatic embryogenesis through overexpression of ATRKD4 genes in Phalaenopsis “Sogo Vivien”. Ind. J. Biotech. 2015, 20, 42–53. [Google Scholar] [CrossRef]
- Setiari, N.; Purwantoro, A.; Moeljopawiro, S.; Semiarti, E. Micropropagation of Dendrobium phalaenopsis orchid through overexpression of embryo gene AtRKD4. Agriv. J. Agric. Sci. 2018, 40, 284–294. [Google Scholar] [CrossRef]
- Cai, J.; Liu, X.; Vanneste, K.; Proost, S.; Tsai, W.-C.; Liu, K.-W.; Chen, L.-J.; He, Y.; Xu, Q.; Bian, C.; et al. The genome sequence of the orchid Phalaenopsis equestris. Nat. Genet. 2015, 47, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Z.; Lin, C.-P.; Cheng, T.-C.; Huang, Y.-W.; Tsai, Y.-J.; Cheng, S.-Y.; Chen, Y.-W.; Lee, C.-P.; Chung, W.-C.; Chang, B.C.-H.; et al. The genome and transcriptome of Phalaenopsis yield insights into floral organ development and flowering regulation. PeerJ 2016, 4, e2017. [Google Scholar] [CrossRef] [PubMed]
- Gow, W.; Chen, J.; Chang, W. Effects of genotype, light regime, explant position, and orientation on direct somatic embryogenesis from leaf explants of Phalaenopsis orchid. Acta Physiol. Plant 2009, 31, 363–369. [Google Scholar] [CrossRef]
- Mehraj, H.; Alam, M.M.; Habiba, S.U.; Mehbub, H. LEDs combined with CHO sources and CCC priming PLB regeneration of Phalaenopsis. Horticulture 2019, 5, 34. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Winarto, B. Somatic embryogenesis in two orchid genera (Cymbidium, Dendrobium). Meth. Mol. Biol. 2016, 1359, 371–386. [Google Scholar]
- Reuter, E. The importance of propagating Phalaenopsis by tissue culture. Orchid Rev. 1983, 91, 199–201. [Google Scholar]
- Meilasari, D.; Prayogo, I. Regeneration of plantlets through PLB (protocorm-like body) formation in Phalaenopsis ‘Join Angle × Sogo Musadian’. J. Math. Fund. Sci. 2016, 48, 204–212. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chang, C.; Chang, W.C. A reliable protocol for plant regeneration from callus culture of Phalaenopsis. Vitr. Cell. Dev. Biol. Plant 2000, 36, 420–423. [Google Scholar] [CrossRef]
- Park, S.Y.; Murthy, H.N.; Paek, K.Y. Mass multiplication of protocorm-like bodies using bioreactor system and subsequent plant regeneration in Phalaenopsis. Plant Cell Tissue Organ Cult. 2000, 63, 67–72. [Google Scholar]
- Tokuhara, K.; Mii, M. Induction of embryogenic callus and cell suspension culture from shoot tips excised from flower stalk buds in Phalaenopsis (Orchidaceae). Vitr. Cell. Dev. Biol. Plant 2001, 37, 457–461. [Google Scholar] [CrossRef]
- Park, S.-Y.; Murthy, H.N.; Paek, K.Y. Rapid propagation of Phalaenopsis from flower stalk-derived leaves. Vitr. Cell. Dev. Biol. Plant 2002, 38, 168–172. [Google Scholar] [CrossRef]
- Park, S.Y.; Yeung, E.C.; Chakrabarty, D.; Paek, K.Y. An efficient direct induction of protocorm-like bodies from leaf subepidermal cells of Doritaenopsis hybrid using thin-section culture. Plant Cell Rep. 2002, 21, 46–51. [Google Scholar]
- Park, S.-Y.; Hosakatte, N.M.; Paek, K.Y. Protocorm-like body induction and subsequent plant regeneration from root tip cultures of Doritaenopsis. Plant Sci. 2003, 164, 919–923. [Google Scholar] [CrossRef]
- Tokuhara, K.; Mii, M. Highly-efficient somatic embryogenesis from cell suspension cultures of Phalaenopsis orchids by adjusting carbohydrate sources. Vitr. Cell. Dev. Biol. Plant 2003, 39, 635. [Google Scholar] [CrossRef]
- Kuo, H.; Chen, J.; Chang, W. Efficient plant regeneration through direct somatic embryogenesis from leaf explants of Phalaenopsis ‘Little Steve’. Vitr. Cell. Dev. Biol. Plant 2005, 41, 453–456. [Google Scholar] [CrossRef]
- Chen, J.T.; Chang, W.C. Direct somatic embryogenesis and plant regeneration from leaf explants of Phalaenopsis amabilis. Biol. Plant 2006, 50, 169–173. [Google Scholar] [CrossRef]
- Murdad, R.; Hwa, K.S.; Seng, C.K.; Latip, M.A.; Aziz, Z.A.; Ripin, R. High frequency multiplication of Phalaenopsis gigantea using trimmed bases protocorms technique. Sci. Hortic. 2006, 111, 73–79. [Google Scholar] [CrossRef]
- Minamiguchi, J.; Machado Neto, N.B. Embriogênese somática direta em folhas de Phalaenopsis: Orchidaceae. Colloq. Agrar. 2007, 3, 7–13. [Google Scholar] [CrossRef]
- Sinha, P.; Hakim, M.; Alam, M. Efficient micropropagation of Phalaenopsis amabilis (L.) BL. cv. ’Cool Breeze’ using inflorescence axis thin sections as explants. Propag. Ornam. Plants 2007, 7, 9–15. [Google Scholar]
- Ling, A.C.K.; Yap, C.P.; Shaib, J.M.; Vilasini, P. Induction and morphogenesis of Phalaenopsis callus. J. Trop. Agric. Food Sci. 2007, 35, 147–152. [Google Scholar]
- Gow, W.; Chen, J.; Chang, W. Influence of growth regulators on direct embryo formation from leaf explants of Phalaenopsis orchid. Acta Physiol. Plant. 2008, 30, 507–512. [Google Scholar] [CrossRef]
- Gow, W.; Chen, J.; Chang, W. Enhancement of direct somatic embryogenesis and plantlet growth from leaf explants of Phalaenopsis by adjusting culture period and explant length. Acta Physiol. Plant. 2010, 32, 621–627. [Google Scholar] [CrossRef]
- Chen, W.H.; Tang, C.Y.; Kao, Y.L. Ploidy doubling by in vitro culture of excised protocorms or protocorm-like bodies in Phalaenopsis species. Plant Cell Tissue Organ Cult. 2009, 98, 229–238. [Google Scholar] [CrossRef]
- Subramaniam, S.; Balasubramaniam, V.R.M.T.; Poobathy, R.; Sasidharan, S. Chemotaxis Movement and Attachment of Agrobacterium tumefaciens to Phalaenopsis violacea Orchid Tissues an Assessment of Early Factors Influencing the Efficiency of Gene Transfer. Trop. Life Sci. Res. 2009, 20, 39–49. [Google Scholar]
- Sinha, P.; Jahan, M.A.A.; Munshi, J.L.; Khatun, R. High frequency regeneration of Phalaenopsis amabilis (L.) Bl. cv. Lovely through in vitro culture. Plant Tissue Cult. Biotech. 2010, 20, 185–193. [Google Scholar] [CrossRef]
- Khoddamzadeh, A.A.; Sinniah, U.R.; Kadir, M.A.; Kadzimin, S.B.; Mahmood, M.; Sreeramanan, S. Detection of somaclonal variation by random amplified polymorphic DNA analysis during micropropagation of Phalaenopsis bellina (Rchb.f.) Christenson. Afr. J. Biotech. 2010, 9, 6632–6639. [Google Scholar]
- Khoddamzadeh, A.A.; Sinniah, U.R.; Kadir, M.A.; Kadzimin, S.B.; Mahmood, M.; Sreeramanan, S. In vitro induction and proliferation of protocorm-like bodies (PLBs) from leaf segments of Phalaenopsis bellina (Rchb.f.) Christenson. Plant Grow. Reg. 2011, 65, 381–387. [Google Scholar] [CrossRef]
- Niknejad, A.; Kadir, M.A.; Kadzimin, S.B. In vitro plant regeneration from protocorms-like bodies (PLBs) and callus of Phalaenopsis gigantea (Epidendroideae: Orchidaceae). Afr. J. Biotech. 2001, 10, 11808–11816. [Google Scholar]
- Sinha, P.; Jahan, M.A.A. Clonal propagation of Phalaenopsis amabilis (L.) BL. Cv. ‘Golden Horizon’ through in vitro culture of leaf segments. BangladeshJ. Sci. Ind. Res. 2011, 46, 163–168. [Google Scholar] [CrossRef]
- Van Thanh, P.; Teixeira Da Silva, J.A.; Huy, H.E.; Tanaka, M. The effects of permanent magnetic fields on in vitro growth of Phalaenopsis plantlets. J. Hortic. Sci. Biotech. 2011, 86, 473–478. [Google Scholar] [CrossRef]
- Rittirat, S.; Kongruk, S.; Te-Chato, S. Induction of protocorm-like bodies (PLBs) and plantlet regeneration from wounded protocorms of Phalaenopsis cornu-cervi (Breda) Blume & Rchb. f. J. Agric. Tech. 2012, 8, 2397–2407. [Google Scholar]
- Samarfard, S.; Kadir, M.A.; Kadzimin, S.B.; Ravanfar, S.; Saud, H.M. Genetic stability of in vitro multiplied Phalaenopsis gigantea protocorm-like bodies as affected by chitosan. Not. Bot. Horti Agrobot. 2013, 41, 177–183. [Google Scholar] [CrossRef]
- Antensari, F.; Mariani, T.S.; Wicaksono, A. Micropropagation of Phalaenopsis ‘R11 × R10’ Through Somatic Embryogenesis Method. Asian J. Appl. Sci. 2014, 2, 145–150. [Google Scholar]
- Huang, Y.-W.; Tsai, Y.-J.; Cheng, T.-C.; Chen, J.-J.; Chen, F.C. Physical wounding and ethylene stimulated embryogenic stem cell proliferation and plantlet regeneration in protocorm-like bodies of Phalaenopsis orchids. Genet. Mol. Res. 2014, 13, 9543–9557. [Google Scholar] [CrossRef] [PubMed]
- Rittirat, S.; Klaocheed, S.; Thammasiri, K. Enhanced efficiency for propagation of Phalaenopsis cornu-cervi (Breda) Blume & Rachb. F. using trimmed leaf technique. Int. J. Agric. Biosyst. Eng. 2014, 8, 336–339. [Google Scholar]
- Samarfard, S.; Kadir, M.A.; Kadzimin, S.B.; Saud, H.M.; Ravanfar, S.A.; Danaee, M. In vitro propagation and detection of somaclonal variation in Phalaenopsis gigantea as affected by chitosan and thidiazuron combinations. Hortscience 2014, 49, 82–88. [Google Scholar] [CrossRef]
- Soe, K.W.; Myint, K.T.; Naing, A.H.; Kim, C.K. Optimization of efficient protocorm-like body (PLB) formation of Phalaenopsis and Dendrobium hybrids. Curr. Res. Agric. Life Sci. 2014, 32, 179–183. [Google Scholar] [CrossRef]
- Feng, J.; Chen, J. A novel in vitro protocol for inducing direct somatic embryogenesis in Phalaenopsis aphrodite without taking explants. Sci. World J. 2014, 2014, 263642. [Google Scholar] [CrossRef]
- Balilashaki, K.; Vahedi, M.; Karimi, R. In vitro direct regeneration from node and leaf explants of Phalaenopsis cv. ‘Surabaya’. Plant Tissue Cult. Biotech. 2015, 25, 193–205. [Google Scholar] [CrossRef]
- Sultana, K.S.; Hasan, K.M.; Hasan, K.M.; Sultana, S.; Mehraj, H.; Ahasan, M.; Shimasaki, K.; Habiba, S.U. Effect of two elicitors on organogenesis in protocorm-like bodies (PLBs) of Phalaenopsis ‘Fmk02010’ cultured in vitro. World Appl. Sci. J. 2015, 33, 1528–1532. [Google Scholar]
- Balilashaki, K.; Ghehsareh, M.G. Micropropagation of Phalaenopsis amabilis var. ’Manila’ by leaves obtained from in vitro culturing the nodes of flower stalks. Not. Sci. Biol. 2016, 8, 164–169. [Google Scholar] [CrossRef][Green Version]
- Mose, W.; Indrianto, A.; Purwantoro, A.; Semiarti, E. The influence of Thidiazuron on direct somatic embryo formation from various types of explant in Phalaenopsis amabilis Blume Orchid. Hayati J. Biosci. 2017, 24, 201–205. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kano, K. Studies on the media for orchid seed germination. Mem. Fac. Agri. Kagawa Univ. 1965, 20, 1–68. [Google Scholar]
- Ernst, R. Effects of thidiazuron on in vitro propagation of Phalaenopsis and Doritaenopsis (Orchidaceae). Plant Cell Tissue Organ Cult. 1994, 39, 273–275. [Google Scholar] [CrossRef]
- Semiarti, E.; Indrianto, A.; Purwantoro, Y.H.; Martiwi, I.N.A.; Feroniasanti, Y.M.A.; Nadifah, F.; Mercuriana, I.S.; Dwiyani, R.; Iwakawa, H.; Yoshioka, Y.; et al. High-frequency genetic transformation of Phalaenopsis amabilis orchid using tomato extract-enriched medium for the pre-culture of protocorms. J. Hortic. Sci. Biotech. 2010, 85, 205–210. [Google Scholar] [CrossRef]
- Chuanjun, X.; Zhiwei, R.; Ling, L.; Biyu, Z.; Junmei, H.; Wen, H.; Ou, H. The effects of polyphenol oxidase and cycloheximide on the early stage of Browning in Phalaenopsis explants. Hortic. Plant J. 2015, 1, 172–180. [Google Scholar]
- Novak, S.D.; Luna, L.J.; Gamage, R.N. Role of auxin in orchid development. Plant Sign. Behav. 2014, 9, e972277. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Grow. Reg. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Raynalta, E.; Elina, J.; Sudarsono, S.; Sukma, D. Clonal fidelity of micropropagated Phalaenopsis plantlets based on assessment using eighteen Ph-Pto SNAP marker loci. J. Agric. Sci. 2018, 40, 390–402. [Google Scholar]
- Park, S.I.; Yeung, E.C.; Paek, K.Y. Endoreduplication in Phalaenopsis is affected by light quality from light-emitting diodes during somatic embryogenesis. Plant Biotec. Rep. 2010, 4, 303–309. [Google Scholar] [CrossRef]
- Young, P.S.; Murthy, H.N. Clonal fidelity of micropropagated Phalaenopsis plantlets based on assessment using eighteen Ph-Pto SNAP marker loci. Yoeup, P.K. Mass multiplication of protocorm-like bodies using bioreactor system Clonal fidelity of micropropagated Phalaenopsis plantlets based on assessment using eighteen Ph-Pto SNAP marker loci. and subsequent plant regeneration in Phalaenopsis. Plant Cell Tissue Organ Cult. 2000, 63, 67–72. [Google Scholar]
- Liu, M.; Xu, Z.; Yang, Y.; Feng, Y. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tissue Organ Cult. 2011, 106, 1–10. [Google Scholar]
- Wei, C.H. Optimization of PLB induction conditions for Oncidium. Fuj. J. Agr. Sci. 2007, 22, 332–335. [Google Scholar]
- Li, W.-L.; Zhai, L.-S.; Ziu, Y.-P. Study on induction and culture of Oncidium protocorm-like body (PLB). Hen. Sci. 2004, 3. [Google Scholar]
- Tran, M.V.; Nguyen, K.V.; Hoa, B.T. Rapid micropropagation of Vu Nu Orchid (Oncidium sp.) by using tissue culture technique. In Proceedings of the CBU International Conference, Prague, Czech Republic, 22–24 March 2017. [Google Scholar]
- Yang, J.F.; Piao, X.C.; Sun, D.; Lian, M.L. Production of protocorm-like bodies with bioreactor and regeneration in vitro of Oncidium ‘Sugar Sweet’. Sci. Hortic. 2010, 125, 712–717. [Google Scholar] [CrossRef]
- Chen, J.T.; Chang, W.C. Effects of auxins and cytokinins on direct somatic embryogenesis from leaf explants of Oncidium ‘Gower Ramsey’. Plant Growth Regul. 2001, 34, 229–232. [Google Scholar] [CrossRef]
- Chen, J.-T.; Chang, C.; Chang, W.C. Direct somatic embryogenesis from leaf explants of Oncidium ‘Gower Ramsey’ and subsequent plant regeneration. Plant Cell Rep. 1999, 19, 143–149. [Google Scholar] [CrossRef]
- Chen, J.-T.; Chang, W.C. Plant regeneration via embryo and shoot bud formation from flower-stalk explants of Oncidium Sweet Sugar. Plant Cell. Tiss. Organ Cult. 2000, 62, 95–100. [Google Scholar] [CrossRef]
- Chen, J.; Chang, W. Efficient plant regeneration through somatic embryogenesis from callus cultures of Oncidium (Orchidaceae). Plant Sci. 2000, 160, 87–93. [Google Scholar] [CrossRef]
- Chen, J.-T.; Chang, W.-C. Effects of GA3, ancymidol, cycocel and paclobutrazol on direct somatic embryogenesis of Oncidium in vitro. Plant Cell Tissue Organ Cult. 2003, 72, 105–108. [Google Scholar] [CrossRef]
- Kerbauy, G.B. Plant regeneration of Oncidium varicosum (Orchidaceae) by means of root tip culture. Plant Cell Rep. 1984, 3, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Flachsland, E.A.; Graciela-Mroginski, L.A. Regeneración de Protocormos y Yemas de Oncidium bifolium Sims. Por cultivo in vitro de láminas foliares. 2001. Available online: researchgate.net/publication/267300737_Regeneracion_de_protocormos_y_yemas_de_Oncidium_bifolium_Sims_por_cultivo_in_vitro_de_laminas_foliares (accessed on 30 January 2020).
- Chen, J.T.; Chang, W.C. Effects of tissue culture conditions and explant characteristics on direct somatic embryogenesis in Oncidium ‘Gower Ramsey’. Plant Cell Tissue Organ Cult. 2002, 69, 41–44. [Google Scholar] [CrossRef]
- Rahman, S.M.M.; Islam, M.S.; Sen, P.K.; Begum, F. In vitro propagation of Oncidium taka. Biotechnology 2005, 4, 225–229. [Google Scholar]
- Jheng, F.Y.; Do, Y.Y.; Liauh, Y.W.; Chung, J.P.; Huang, P.L. Enhancement of growth and regeneration efficiency from embryogenic callus cutures of Oncidium “Gower Ramsey” by adjusting carbohydrate sources. Plant Sci. 2006, 170, 1133–1140. [Google Scholar] [CrossRef]
- Hong, P.I.; Chen, J.T.; Chang, W.C. Promotion of direct somatic embryogenesis of Oncidium by adjusting carbon sources. Biol. Plant. 2008, 52, 597–600. [Google Scholar] [CrossRef]
- Wang, A.-S.; Lin, M.-G.; Liu, F.-X. Rapid propagation of cut flower varieties of Oncidium by tissue culture. Guangxi Agric. Sci. 2009, 40, 801–806, (In Chinese with abstract in English). [Google Scholar]
- Chung, J.P.; Huang, C.Y.; Dai, T.E. Spectral effects on embryogenesis and plantlet growth of Oncidium Gower Ramsey. Sci. Hortic. 2010, 124, 511–516. [Google Scholar] [CrossRef]
- Mayer, J.L.S.; Stancato, G.C.; Appezzato-da-Glória, B. Direct regeneration of Protocorm-like bodies (PLBs) from leaf apices of Oncidium flexuosum Sims (Orchidaceae). Plant Cell Tissue Organ Cult. 2010, 103, 411–416. [Google Scholar] [CrossRef]
- Chen, J.-T. Induction of petal-bearing embryos from root-derived callus of Oncidium ‘Gower Ramsey’. Acta Physiol. Plant 2012, 34, 1337–1343. [Google Scholar] [CrossRef]
- Gomes, L.R.P.; Franceschi, C.R.B.; Ribas, L.L.F. Micropropagation of Brasilidium forbesii (Orchidaceae) through transverse and longitudinal thin cell layer culture. Acta Sci. Biol. Sci. 2015, 37, 143–149. [Google Scholar] [CrossRef]
- Mahesh, R.; Kumar, H.G.A.; Satyanarayana, S. Synthesis and characterization of 2-mercapto-N methyl imidazole substituted benzimidazole derivatives and investigation of their effect on production of plantlets in Oncidium Gower Ramsey. Mater. Today Proc. 2018, 5, 21505–21511. [Google Scholar]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Int. Plant Propag. Soc. Proc. 1980, 30, 421–427. [Google Scholar]
- Chen, Y.H.; Chang, Y.S.; Chen, W.H. Tissue culture advances for mass propagation of Oncidium mericlones. Rep. Taiwan Sugar Res. Inst. 2001, 173, 67–76. [Google Scholar]
- Wang, T.C.; Zhang, M.; Tong, Y.O. Molecular specstrum of somaclonal variation in PLB-regenerated Oncidium revealed by SLAF-seq. Plant Cell Tissue Organ Cult. 2019, 137, 541–552. [Google Scholar] [CrossRef]
- Mohanty, P.; Paul, S.; Das, M.C.; Kumaria, S.; Tandon, P. A simple and efficient protocol for the mass propagation of Cymbidium mastersii: An ornamental orchid from Northeast India. Aob Plants. 2012, 2012, pls023. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Tanaka, M. Multiple regeneration pathways via Thin Cell Layers in hybrid Cymbidium (Orchidaceae). J. Plant Growth Regul. 2006, 25, 203. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Mulgund, G.S.; Kallappa, N. Micropropagation of Dendrobium nobile from shoot tip sections. J. Plant Physiol. 2005, 162, 473–478. [Google Scholar] [CrossRef]
- Parthibhan, S.; Venkateswara Rao, M.; Teixeira da Silva, J.A.; Senthil Kumar, T. Somatic embryogenesis from stem thin cell layers of Dendrobium aqueum. Biol. Plant. 2018, 62, 439–450. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Jin, X.; Dobránszki, J.; Lu, J.; Wang, H.; Zotz, G.; Cardoso, J.C.; Zeng, S. Advances in Dendrobium molecular research: Applications in genetic variations, identification and breeding. Mol. Phylogen. Evol. 2016, 95, 196–216. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumaria, S.; Tandon, P. High frequency regeneration protocol for Dendrobium nobile: A model tissue culture approach for propagation of medicinally important orchid species. S. Afr. J. Bot. 2016, 104, 232–243. [Google Scholar] [CrossRef]
- Chien, K.W.; Agrawal, D.C.; Tsay, H.S.; Chang, C.A. Elimination of mixed ‘Odontoglossum ringspot’ and ‘Cymbidium mosaic’ viruses from Phalaenopsis hybrid ‘V3’ through shoot-tip culture and protocorm-like body selection. Crop Protect. 2015, 67, 1–6. [Google Scholar] [CrossRef]
- Chen, F.C. Phalaenopsis in Vitro Cloning: Strategy for PLB or Shoots? Taiwan International Orchid Symposium: Taipei, Taiwan, 2009. [Google Scholar]
- Miguel, T.P.; Leonhardt, K.W. In vitro polyploid induction of orchids using oryzalin. Sci. Hortic. 2011, 130, 314–319. [Google Scholar] [CrossRef]
- Sarathum, S.; Hegele, M.; Tantiviwat, S.; Nanakorn, M. Effect of concentration and duration of colchicine treatment on polyploid induction in Dendrobium scabrilingue L. Eur. J. Hort. Sci. 2010, 75, 123–127. [Google Scholar]
- Wannajindaporn, A.; Kativat, C.; Tantasawat, P.A. Mutation induction of Dendrobium ‘Earsakul’ using sodium azide. Hortscience 2016, 51, 1363–1370. [Google Scholar] [CrossRef]
- Billore, V.; Mirajkar, S.J.; Suprasanna, P.; Jain, M. Gamma irradiation induced effects on in vitro shoot cultures and influence of monochromatic light regimes on irradiated shoot cultures of Dendrobium sonia orchid. Biotech. Rep. 2019, 22, e00343. [Google Scholar] [CrossRef]
- Chew, Y.-C.; Abdullah, W.; Kok, D.A.; Ong-Abdullah, J.; Mahmood, M.; Lai, K.-S. Development of an efficient particle bombardment transformation system for de endemic orchid Phalaenopsis bellina. Sains Malays. 2019, 48, 1867–1877. [Google Scholar] [CrossRef]
- Mii, M.; Chin, D.P. Genetic transformation of orchid species: An overview of approaches and methodologies. In Orchid Propagation: From Laboratories to Greenhouses – Methods and Protocol; Lee, Y.I., Yeung, E.T., Eds.; Humana Press: New York, NY, USA, 2018; pp. 347–365. [Google Scholar]
- Liau, C.-H.; You, S.-J.; Prasad, V.; Hsiao, H.-H.; Lu, J.-C.; Yang, N.-S.; Chan, M.-T. Agrobacterium tumefasciens-mediated transformation of Oncidium orchid. Plant Cell Rep. 2003, 21, 993–998. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Hsu, W.-H.; Yang, C.-H. Phosphomannose-isomerase as a selectable marker to recover transgenic orchid plants (Oncidium Gower Ramsey). Plant Cell Tissue Organ Cult. 2011, 104, 239–246. [Google Scholar] [CrossRef]
- Morel, G.M. Producing virus free Cymbidiums. Am. Orchid Soc. Bull. 1960, 29, 495–497. [Google Scholar]
- Pradhan, S.; Regmi, T.; Ranjit, M.; Pant, B. Production of virus-free orchid Cymbidium aloifolium (L.) Sw. by various tissue culture techniques. Heliyon 2016, 2, e00176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khentry, Y.; Paradornuwat, A.; Tantiwiwat, S.; Phansiri, S.; Thaveechail, N. Incidence of Cymbidium mosaic virus and Odontoglossum Ringspot Virus on in vitro Thai native orchid seedlings and cultivated orchid Mericlones. Kasertsat J. Nat. Sci. 2006, 40, 49–57. [Google Scholar]
- Shen, R.-S.; Hsu, S.-T. Virus elimination through meristem culture and rapid clonal propagation using a temporary immersion system. In Orchid Propagation: From Laboratories to Greenhouses–Methods and Protocols; Lee, Y.I., Yeung, E.T., Eds.; Humana Press: New York, NY, USA, 2018; pp. 267–282. [Google Scholar]
- Saiprasad, G.V.S.; Polisetty, R. Propagation of three orchid genera using encapsulated protocorm-like bodies. Vitr. Cell. Dev. Biol. Plant 2003, 39, 42–48. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Jin, M.-Y.; Paek, K.-Y.; Piao, X.-C.; Lian, M.-L. An efficient strategy for enhancement of bioactive compounds by protocorm-like body culture of Dendrobium candidum. Ind. Crop. Prod. 2016, 84, 121–130. [Google Scholar] [CrossRef]
- Paek, K.Y.; Hahn, E.J.; Park, S.Y. Micropropagation of Phalaenopsis orchids via protocorms and protocorm-like bodies. Methods Mol. Biol. 2011, 710, 293–306. [Google Scholar] [PubMed]
- Hsu, C.-C.; Lai, P.-H.; Chen, T.-C.; Tsai, W.-C.; Hsu, J.-L.; Hsiao, Y.-Y.; Wu, W.-L.; Tsai, C.-H.; Chen, W.-H.; Chen, H.-H. PePIF1, a P-lineage of PIF-like transposable element identified in protocorm-like bodies of Phalaenopsis orchids. Bmc Genom. 2019, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.K.; Lee, Z.H.; Mubbarakh, S.A.; Antony, J.J.J.; Chew, B.L.; Subramanian, S. Effects of plant growth regulators and activated charcoal on somaclonal variations of protocorm-like bodies (PLBs) of Dendrobium Sabin Blue orchid. Biocatal. Agric. Biotech. 2019, 22, 101426. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, T.M.; Fu, Y.M.; Hsieh, R.M. Studies on somaclonal variation in Phalaenopsis. Plant Cell Rep. 1998, 18, 7–13. [Google Scholar] [CrossRef]
- Bairu, M.W.; Stirk, W.A.; Dolezal, K.; van Staden, J. The role of topolins in micropropagation and somaclonal variation of banana cultivars ‘Williams’ and ‘Grand Naine’ (Musa spp. AAA). Plant Cell Tissue Organ Cult. 2008, 95, 373–379. [Google Scholar] [CrossRef]
- Tokuhara, K.; Mii, M. Somaclonal variation in flower and inflorescence axis in micropropagated plants through flower stalk bud culture of Phalaenopsis and Doritaenopsis. Plant Biotech. 1998, 15, 23–28. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tsai, Y.J.; Huang, J.Z.; Chen, F.C. Transcription analysis of peloric mutants of Phalaenopsis orchids derived from tissue culture. Cell Res. 2005, 15, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.J.J.; Shanshir, R.A.; Poobathy, R.; Chew, B.L.; Subramanian, S. Somaclonal variations were not induced by cryopreservation: Levels of somaclonal variation of in vitro and thawed protocorms of Dendrobium Bobby Messina analysed by SCoT and TRAP DNA markers. South Afr. J. Bot. 2015, 100, 148–157. [Google Scholar] [CrossRef]
- Chen, F.-C.; Yu, J.-Y.; Chen, P.-Y.; Huang, Y.-W. Somaclonal variation in orchids and the application of biotechnology. Acta Hortic. 2008, 766, 315–322. [Google Scholar] [CrossRef]
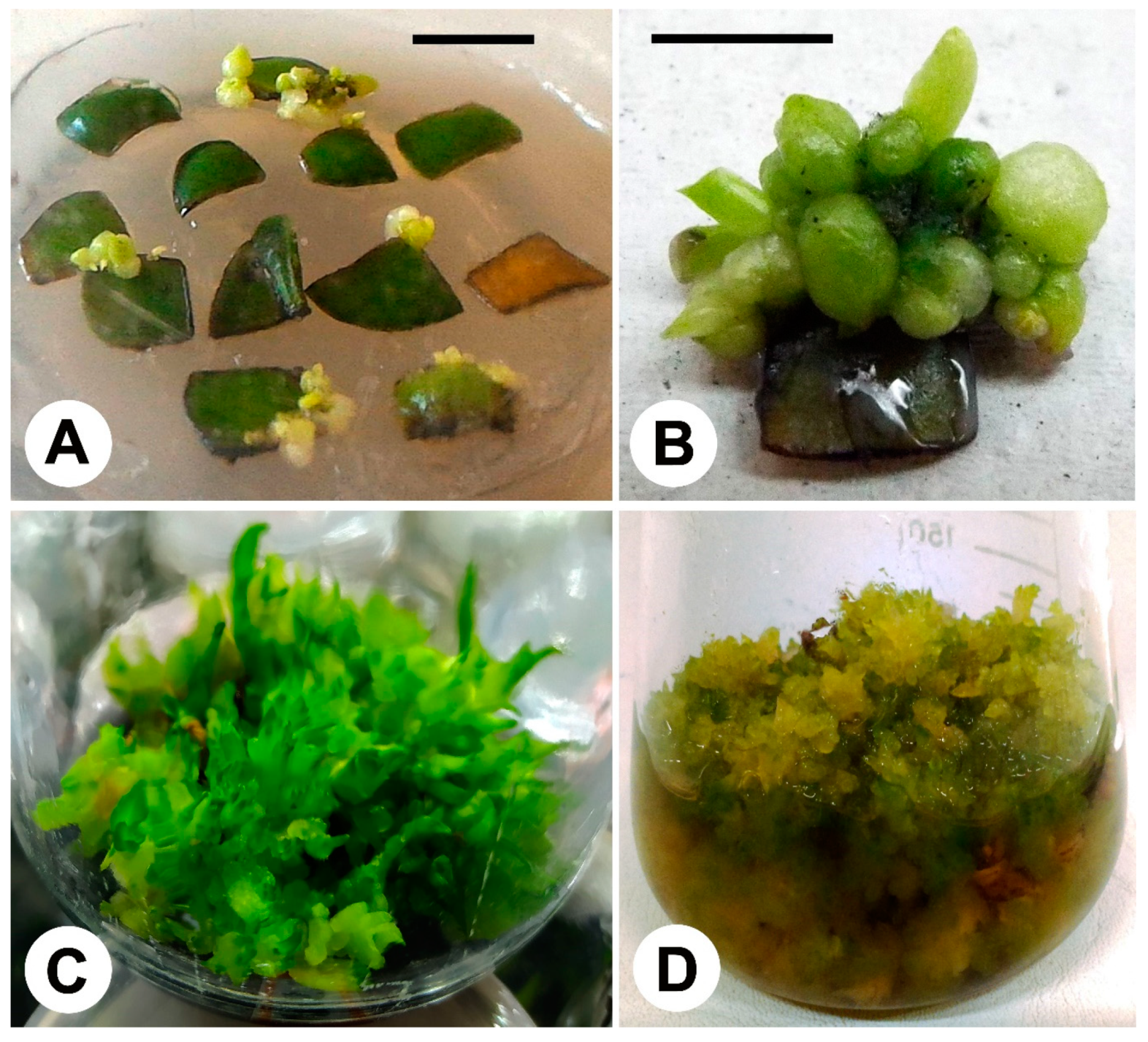
| Species or Hybrids | Origin and Age of Explants | Culture Media | Growth Conditions | Main Results | Evaluation and Detection of SV | Reference |
|---|---|---|---|---|---|---|
| 12 cultivars of Phalaenopsis | Shoot tips derived from flower stalk buds | NDM added 10 g L−1 sucrose, 2 g L−1 Gelrite, 0.1 mg L−1 NAA and 1–5 mg L−1 BA | 23 ± 1 °C, 14-h photoperiod, 33 µmol m−2 s−1 | 93–100% survival rate of explants, 33–40% PLB formation, green color of PLBs showed multiplication, 27–28% PLBs formed shoots | Non-evaluated | [41] |
| Phalaenopsis Nebula | Calluses derived from 1–2 months protocorms | MS½ + 100 mg L−1 myo-inositol + 0.5 mg L−1 niacin and pyridoxine + 0.1 mg L−1 thiamine + 2.0 mg L−1 glycine + 170 mg L−1 NaH2PO4 + 20 g L−1 sucrose + 2.2 g L−1 Gelrite, pH 5.2 | 26 ± 2 °C, 16-h photoperiod, PPFD 28–36 µmol m−2 s−1 | Both TDZ and BA were able to induce PLBs in calluses, but interestingly equal number of PLBs per callus (74) was obtained when callus was transferred to free-PGR medium | Not observed any phenotypic abnormality and no chromosome number alterations were observed in 2–3 months plantlets | [64] |
| Phalaenopsis Hybrid with pink striped flowers | Section transversely cutted from apical meristems (2-mm in size) of PLBs obtained from leaf segments | Liquid Hyponex modified medium (Kano, 1965—1 g L−1 of 6.5N − 4.5P − 19K + 1 g L−1 20N − 20P − 20K + 1% potato homogenate) | 25 ± 2 °C, 16-h photoperiod, PPFD 60 µmol m−2 s−1, white fluorescent light, under shaker at 100 rpm or temporary our continuous immersion bioreactor system | 100 ml medium per 0.5 g inoculum under agitation (9.2 PLBs/PLB section) or air-lift balloon with 10.0 g inoculum (12.6 PLBs/PLB section); charcoal filter attached to bioreactor increased to 17 PLBs/PLB section; Hyponex medium increased percentage of PLB regeneration, rooting and fresh weight of plantlets | Non-evaluated | [65] |
| 9 genotypes of Phalaenopsis | Shoot tips from flower stalk buds and callus from cell suspension cultures | Shoot tips to PLBS, NDM + 2 g L−1 Gellan gum, pH 5.4; Cell suspension, liquid NDM + 58.4 mM sucrose; Induction of PLBs from calluses, NDM + 29.2 μM sucrose + 2 g L−1 Gellan gum | 23 ± 1 °C, 14-h photoperiod, 33 µmol m−2 s−1, cell suspension culture were obtained in liquid medium under agitation 0f 80 rpm | 44.4% PLB formation from shoot tips were obtained with 0.5 µM NAA and 4.44 µM BA and 29.2 mM sucrose; increases in sucrose concentration (58.4 mM increased callus formation); calluses could induced to PLBs with 29.2 mM sucrose | The type and frequency of morphological variants were large dependent on genotype: in P. Snow Parade and P. Little Steve any variants was reported, while 47.9% variants were observed in P. Reichentea | [66] |
| Phalaenopsis Tinny Sunshine ‘Annie’; ‘Taisuco Hatarot’; Teipei Gold ‘Golden Star’; Tinny Galaxy ‘Annie’ | Young leaf segments (10 × 5 mm) derived in vitro shoots from flower stalk nodes | induction of PLBs: MS½ + 10% coconut water/Proliferation of PLBs: different saline formulation + 2 g L−1 peptone + 3% potato homogenate + 0.05% activated charcoal + 30 g L−1 sucrose | Temp 25 ± 1 °C, 16-h photoperiod by cool white fluorescent lamps, PPFD 30 µmol m−2 s−1; liquid media in shaker at 50 rpm | 70–90% of explants with PLBs depending on cultivar; 85% explants with PLBs and 12 PLBs/explant with 88.8 µM BA + 5.4 µM NAA; 45 g L−1 sucrose showed highest number PLBs per explant (6) and low light intensity (10 µmol m−2 s−1) resulted in best PLBs induction (90%) and number of PLBs/explant (12); liquid with cotton raft support Hyponex medium increased PLBs proliferation (20.5 PLBs) | Non-evaluated | [67] |
| Doritaenopsis ’New Candy’ × (D. ’Mary Anes’ × D. ’Ever Spring’ | Leaf segments 1 mm thick from three months old leaves from in vitro plantlets | MS½ + 20% coconut water + 10 mg L−1 adenine sulphate + 2.3 g L−1 Gelrite, pH 5.5 | 1 week in dark at 27 °C followed by 25 ± 1 °C, 16-h photoperiod by cool white fluorescent lamps, PPFD 10 µmol m−2 s−1 | 9.0 µM TDZ resulted in best PLB formation (72.3%); Thin leaf segments—1 mm— resulted in best PLB formation (>50%) than thick leaf sections—5 mm (10%) and are correlated with ethylene content (ppm) | Irregular shaped bodies (CLBs) increased with increases in concentrations of TDZ (0.57% at free-PGR to 11.56% at 22.5 µM) and BA (32.14% at 4.4 µM); However, no phenotypic variations were observed in vegetative growth in greenhouse | [68] |
| Doritaenopsis ’New Candy’ × (D. ’Mary Anes’ × D. ’Ever Spring’ | Root tips (<0.5 cm) from 3-months old in vitro plantlets | MS + 20% coconut water + 10 mg L−1 adenine sulphate + 2.3 g L−1 Gelrite, pH 5.5 | Temp 25 °C, cool white fluorescent lamps, PPFD 30 µmol m−2 s−1, 16-h photoperiod | TDZ at 2.3 µM showed best PLB formation (47.2% of root tips with 2–6 PLBs each) compared to BA and Zea; most of PLBs originated from cortex tissues of root | Non-evaluated | [69] |
| Phalaenopsis Snow Parade and Wedding Promenade, Doritaenopsis New Toyohashi | Cell suspension from calluses | NDM + 2 g L−1 gellan gum, pH 5.4 | 23 ± 1 °C, 14-h photoperiod, 33 µmol m−2 s−1, cell suspension culture were obtained in liquid medium under agitation 0f 80 rpm | The response were genotype-dependent: Glucose at 58.4 mM and sucrose at 29.2 mM showed several increases in number (>2000) and fresh weight of PLBs for P. Snow Parade, while glucose at 14.6–29.2 mM showed highest number of PLBs in P. Wedding Promenade | Non-evaluated | [70] |
| Phalaenopsis ’Little Steve’ | Leaf explants (1cm2) derived from flower stalk buds eighteen-month-old in vitro plants | MS½ added 4.54 μM TDZ, 100 mg L−1 myo-inositol + 0.5 mg L−1 niacin + 0.5 mg L−1 pyridoxine + 0.1 mg L−1 thiamine + 2.0 mg L−1 glycine + 1000 mg L−1 peptone + 2.2 g L−1 Gelrite + 20 g L−1 sucrose, pH 5.2 | Dark for 2 months followed by 16-h photoperiod | 40% explants with PLBs; not reported the number of PLBs per explant | Non-evaluated | [71] |
| Phalaenopsis amabilis var. formosa | Leaf tip segments obtained from in vitro germinated seedlings and leaf-derived nodular masses | M½ S added 3 mg L −1 TDZ, 100 mg L−1 myo-inositol + 0.5 mg L−1 niacin + 0.5 mg L−1 pyridoxine + 0.1 mg L−1 thiamine + 2.0 mg L−1 glycine + 1000 mg L−1 peptone + 2.2 g L−1 Gelrite + 20 g L−1 sucrose, pH 5.2 | Temp 26 ± 1 °C; 16-h photoperiod | 93.8% explants with PLBs and 19.4 PLBs per explant for leaf tip segments; 5.4 proliferation rate and 13.8 PLBs per explant for leaf-derived embryogenic masses | Non-evaluated | [72] |
| Phalaenopsis gigantea | Trimmer base protocorms 1 mm from 150-d in vitro germinated protocorms | XER medium (Ernst, 1994) + 20 g L−1 fructose + 1% agar, pH 5.7 | Temp 25 ± 2 °C, under continuous illumination from cool fluorescent lamps, PPFD 20–50 µmol m−2 s−1 | Trimmed protocorms increased PLBs proliferation (56.8%) and number of PLBs/protocorm (4.24) using 15% coconut water and 2.5 g L−1 activated charcoal, compared to untrimmed (4.56% and 0.56 PLB/protocorm) and shoot regeneration from PLBs were increased using only coconut water at 10% (33.56% shoot formation) | [73] | |
| Alba flower hybrid’ of Phalaenopsis | Nodular masses | NDM culture medium added 1.0 mg L−1 BA and 0.1 mg L−1 NAA, 100 mg L−1 myo-inositol + 1.0 mg L−1 (niacin, pyridoxine, thiamine, cysteine, calcium pantothenate) + 0.1 0 mg L−1 biotin + 20 g L−1 sucrose + 2.0 g L−1 Phytagel, pH 5.8 | Not reported growth conditions | 8.5 PLBs per explant; not reported percentage of explants with PLBs | Non-evaluated | [74] |
| Phalaenopsis amabilis cv. ’Cool Breeze’ | Inflorescence axis thin sections | MS½ added 2,0 mg L−1 BA, 0,5 mg L−1 NAA, 2% sucrose, 10% coconut water, 2 g L−1 peptone and 1 g L−1 activated charcoal | 20 PLBs/explant after 12 weeks | Non-evaluated | [75] | |
| Phalaenopsis var. Hawaiian Clouds × Phalaenopsis Carmela’s Dream | Clumps of callus (8 mm diameter) | NDM culture medium added 1 mg L−1 TDZ, 10 g L−1 maltose, 2.8 g L−1 Gelrite | Temp 25 ± 2 °C, in the dark | 52.5% callus with PLBs | Non-evaluated | [76] |
| Phal. amabilis; Phal. ’Nebula’ | Cut end of leaf explants (1.0 cm length); clonal plantlets of P. amabilis and in vitro germinated seedlings for P. ’Nebula’ | MS½ added 3 mg L−1 TDZ, 100 mg L−1 myo-inositol + 0.5 mg L−1 niacin + 0.5 mg L−1 pyridoxine + 0.1 mg L−1 thiamine + 2.0 mg L−1 glycine + 1000 mg L−1 peptone + 2.2 g L−1 Gelrite + 20 g L−1 sucrose, pH 5.2 | Temp 26 ± 1 °C; dark for 60-d (induction) 45-d for subculture period; | 50% explants with PLBS and 8.2 PLBs/explant for P. amabilis; 80% explants with PLBs and 3.5 PLBs for P. ’Nebula’ | Non-evaluated | [59,77,78] |
| 10 genotypes of Phalaenopsis | Basal portion of sectioned horizontally protocorms (3–5 mm) were placed upward in contact with the culture medium | 3.5 g L−1 HyponexTM #1 + 1 g L−1 tryptone + 0.1 g L−1 citric acid + 1 g L−1 activated charcoal + 20 g L−1 sucrose + 20 g L−1 homogenized potato + 25 g L−1 homogenized banana + 7.5 g L−1 agar, pH 5.5 | Temp 25 ± 2 °C, 16-h photoperiod with PPFD 10 µmol m−2 s−1 | 22% of sectioned protocorms induced PLBs and 17.5 PLBs per responsive protocorms were obtained | High endopolyploidy were observed in Phalaenopsis protocorms; from 22 diploid protocorms used as explant, 34.1% of derived-PLBs were polyploidy at first cycle and 51.7% at second cycle of proliferation | [79] |
| Phalaenopsis violacea | Leaf segments (1 × 1 cm) from in vitro shoots derived from flower stalks | MS½ + 5% banana extract | Temp 25 °C, 16-h photoperiod, PPFD 40 µmol m−2 s−1 by white fluorescent tubes | 70% of leaf segments formed PLBs with 0.8 µM BAP, while TDZ were able to induce PLBs only in 40% of explants and BAP (0.6 µM) was more effective to PLBs proliferation than TDZ and Zea | Non-evaluated | [80] |
| Phal. amabilis cv. Lovely (purple flowers) | Young emerging leaves from in vivo plants | MS1/2 + 2% sucrose + 10% coconut water + 2 g L-1 peptone + 1 g L-1 activated charcoal + 2.2 g L-1 Gelrite, pH 5.6; | Temp 24 ± 1 °C, cool white fluorescent light, PPFD 30 µmol m−2 s−1, 16-h photoperiod | 2.0 mg L−1 BA and 0.5 mg L−1 NAA resulted in 75.5% explants formed PLBs and 10 PLBs/explant; MS½ + 10% coconut water + 150 mg L−1 glutamine showed best proliferation rate of PLBs (200.5 PLBs/explant) | Non-evaluated | [81] |
| Phalaenopsis bellina | In vivo leaf | MS½ + 100 mg L−1 myo-inositol + 0.5 mg L−1 niacin and pyridoxine + 0.1 mg L−1 thiamine + 2.0 mg L−1 glycine + 3.0 mg L−1 TDZ + 2% sucrose + 3.0 g L−1 Gelrite + 10% fresh banana extract, pH 5.6 | PLBs from leaf (S0), PLBs proliferation after 3 months (S1) and after six months (S2) | Efficiency of induction and regeneration of PLBs not presented by authors | Minimal dissimilarity in P. bellina by RAPD markers; S0 presented 96% similarity, S1 87% and S2 80% similarity to the mother plant | [82] |
| Phalaenopsis bellina | Young leaves (1.5 cm2) of a nursery plant | MS½ + 2% sucrose + 100 mg L−1 myo-inositol + 0.5 mg L−1 niacin and pyridoxine + 0.1 mg L−1 thiamine + 2.0 mg L−1 glycine + 10% fresh ripen banana extract + 3.0 mg L−1 TDZ + 3.0 g L−1 Gelrite, pH 5.6 | Temp 25 ± 2 °C, 14-h photoperiod for 12–16 weeks | 71.9–78.1% explants with PLBs; 14.3–14.8 PLBs per flask; MS1/2 was the best for PLB proliferation compared to VW | Non-evaluated | [83] |
| Phalaenopsis gigantea | Leaf tip segments (1.0 cm length) from in vitro germinated seedlings | NDM culture medium, sucrose 20 g L−1 + 1.0 mg L−1 NAA and 0.1 mg L−1 TDZ | Temp 25 ± 2 °C, 12-h photoperiod for 6 weeks | The authors only report that NAA and TDZ treatment was the best for callus induction and PLBs after 6 weeks of culture. | Non-evaluated | [84] |
| Phalaenopsis amabilis cv. ’Golden Horizon’ | Young emerging leaves from in vivo plants | MS½ + 2% sucrose + 10% coconut water + 2 g L−1 peptone + 1 g L−1 activated charcoal + 2.2 g L−1 Gelrite, pH 5.6 | Temp 24 ± 1 °C, cool white fluorescent light, PPFD 30 µmol m−2 s−1, 16-h photoperiod | BA at 2.0 mg L−1 combined with NAA 0.5 mg L−1 resulted in 80.5% explants with PLBs and 15 PLBs/explant; MS½ + 10% coconut water + 150 mg L−1 glutamine showed best proliferation rate of PLBs (250.5 PLBs/explant) | Non-evaluated | [85] |
| Phalaenopsis Gallant Beau ’George Vasquez’ | Longitudinally bisected PLBs (2–3 mm in diameter) and 2-months old | Miracle Pack® culture system with liquid VW + 20% coconut water without sucrose, pH 5.3 | Temp 25 °C, 16-h photoperiod, PPFD 45 µmol m−2 s−1, plant growth fluorescent lamps, under magnetic fields | Although higher Fresh weight of PLBs was obtained with 0.1 Tesla–South (237.4 g), best number of PLBs was obtained in control without magnetic fields; 0.15 Tesla for 7 weeks (South) also increased PLB fresh weight, control treatment not differed from the best results using magnetic fields | Non-evaluated | [86] |
| Phalaenopsis cornu-cervi | Leaf segments from in vitro germinated seedlings with 2-months | MS½ added 0.1 mg L−1 NAA, 0.1 mg L−1 TDZ and 15% coconut water | Temp 25 ± 1 °C; 16-h photoperiod for 45 days | 100% explants with PLBs; 35 PLBs per explant | Non-evaluated | [87] |
| Phalaenopsis gigantea | PLBs obtained from leaf tip segments (1.5 cm length) from young leaves | Liquid medium with 20% coconut water, pH 5.4. | Temp 25 ± 2 °C, under 16-h photoperiod using fluorescent lighting 30 µmol m−2 s−1, 60 rpm rotary shaker | VW medium with 10 mg L−1 chitosan resulted in higher number of PLBs (177) and fresh weight of PLBs (8.4 g) | ISSR, non-detected somaclonal variations in P. gigantea related to mother plants | [88] |
| Phalaenopsis ’R11 × R10’ | Leaves, root tips and stem explants from eight months (plantlets or seedlings?) | MS½ + 15% coconut water + 0.01% activated charcoal + 0.03% polyvinylpyrrolidone (PVP) + 88.8 µM BA + 5.37 µM NAA + 0.025% Phytagel, pH 5.6–5.8 | Temp. 25 °C, 16-h photoperiod | Stem segments were interesting explant for PLB induction; sucrose at 3% (71.2 PLBs) was more effective than maltose (39 PLBs) in PLBs proliferation | Non-evaluated | [89] |
| Phalaenopsis Tropican Lady | Young etiolated shoots leaves segments (5 × 10 mm) from flower stalk nodes for induction and PLBs for proliferation | PLB induction: ¼ macroelements and full-strength microelements, glycine and vitamins of MS + 30 g L−1 sucrose + 0.5 mg L−1 TDZ + 7 g L−1 agar / PLB Proliferation: 3 g L−1 Hyponex (7-6-19) + 1 g L−1 tryptone + 50 g L−1 potato homogenate + 50 g L−1 banana homogenate + 30 g L−1 sucrose + 2 g L−1 activated charcoal + 7.5 g L−1 agar, pH 5.6 | Temp 25 ± 2 °C, under 12-h photoperiod by cool white fluorescent lamps, PPFD 23.2 µmol m−2 s−1, | Basal part of sectioned of bi or trisectioned PLBs resulted in highest explants with PLB formation (46.8–96.3%) and number of PLBs/explant (15.4–22.9); wounding stimulate ethylene production and gene expression for stimulation of cell division | Non-evaluated | [90] |
| Phalaenopsis cornu-cervi | Whole leaves and leaf-segments (proximal, middle and distal regions) from 120-d old seedlings | MS½ + 3% sucrose + 15% coconut water + 0.23% Gelrite, pH 5.6 | Temp 25 ± 1 °C, under 16-h photoperiod, cool white fluorescent lamps, PPFD 20 µmol m−2 s−1 or pre-treated with 1 week in the dark before photoperiod | Highest percentage of explants with PLBs (30%) and number of PLBs per leaf segment (5.3) were obtained with 9 µM of TDZ under without dark period. Dark period reduced number of PLBs/explant | Non-evaluated | [91] |
| Phalaenopsis gigantea | Leaf tip segments from young leaves of in vitro seedlings | NDM medium added 0.1 mg L−1 TDZ, 10 mg L−1 chitosan, 0.2% Gelrite and pH 5.7 | Temp 25 ± 2 °C, 16-h photoperiod, 33 µmol m−2 s−1 | 353 PLBs per explant and 4.8 g PLBs fresh weight | ISSR, SV detected after the subculture four (5 to 20%) | [92] |
| Phalaenopsis hybrids | Intact and transversally divided protocorms (two or four divisions) 1.0–1.5 mm width | MS + 15% coconut water + 7.0 g L−1 agar | Temp 25 ± 2 °C, 16-h photoperiod, 25 µmol m−2 s−1 | No PLBs formed in intact protocorms; Middle and Basal part of sectioned protocorms showed 40 and 44% PLB formation and 11.7 and 13.3 PLBs per explant in Free-PGR culture medium, respectively; Four division of protocorms increased PLBs formation and number of PLBs | Non-evaluated | [93] |
| P. aphrodite subsp. formosana | in vitro germinated seedlings with 2-months | Using 2-step method: Liquid MS½ for 2 months and then transferred to solid MS (half strength) with 1 cm of medium Liquid MS (half strength) for a further 2 months. All media with 1 mg L−1 TDZ. | Temp 25 ± 2 °C; followed by 16-h photoperiod | 44 PLBs per seedling | Non-evaluated | [94] |
| Phalaenopsis amabilis cv. ’Surabaya’ | Leaf segments from in vitro shoots obtained from inflorescence stalk segments | - | Temp 25 ± 1 °C; 16-h photoperiod, subcultured each 14-d | 5 mg L−1 BA + 2 mg L−1 NAA produced 8.7 number of PLBs and TDZ at 3.0 mg L−1 showed 22.45 PLBs | non reported by authors that acclimatized and cultivated regenerated plantlets until flowering stage | [95] |
| Phalaenopsis ’Fmk02010’ | Single PLBs | MS with 412.5 mg L−1 NH4NO3 and 950 mg L−1 of KNO3 + 20;0 g L−1 sucrose + 2.0 g L−1 Phytagel, pH 5.5–5.8 | - | Hyaluronic acid 9 and 12, at 0.1 mg L−1, increased percentage of explants with PLBs (100%), PLB number (18.2 to 23.3) and fresh weight of PLBs (0.291 to 0.596 g) compared to control (86.7%, 12.9 and 0.198 g) | no malformation was observed in regenerated plantlets | [96] |
| P. ‘Join Angle × Sogo Musadian’ | In vitro roots | MS½ added NAA (0.5 ppm), BA (5 ppm) and IAA (0.5 ppm) | Temp 26 ± 1 °C; dark for 1 month (induction) followed by 16-h photoperiod (4 weeks) | 49.33 PLBs per explant; not reported percentage of explants with PLBs | Non-evaluated | [63] |
| Phalaenopsis Classic Spoted Pink | leaf segments (1.0 cm2) with 90-d obtained from in vitro shoots | MS½ added NAA (0,537μM) and TDZ (13,621μM) | Temp 25 ± 2 °C, dark for 90-d (induction) followed by 16-h photoperiod | The percentage of explants in regeneration and the number of PLBs/explant were not described | Non-evaluated | [45] |
| Phalaenopsis amabilis var. ’Manila’ | Leaf segments (1 cm × 0.5 cm) obtained from in vitro flower stalk nodes | MS added 15 mg L−1 BA and 3 mg L−1 NAA | Temp 25 ± 1 °C; 16-h photoperiod | 50.65 PLBs per explant after 6 weeks | Non-evaluated | [97] |
| Phalaenopsis amabilis | Protocorms (4 weeks-old), roots, leaves and stems (6-month-old) cut transversely | NP (New Phalaenopsis) medium added 3 mg L−1 TDZ | 25 ± 1 ºC with 1000 lux intensity of continuous light; 8 weeks | Protocorm: 100% explants with PLBs and 23.3 PLBs/explant; Leaf: 100% explants with PLBs and 7.75 PLBs/explant; Root: 80% explants with PLBs and 8.25 PLBs/explant; Stem: 100% explants with PLBs and 28.25 PLBs/explant | Non-evaluated | [98] |
| Phalaenopsis ’Fmk02010’ | Single PLBs | MS with 412.5 mg L−1 NH4NO3 and 950 mg L−1 of KNO3 + 2.2 g L−1 Phytagel, pH 5.5–5.8 | Temp 25 ± 2 °C, 16-h photoperiod, PPFD 54 µmol m−2 s−1 | Highest number of PLBs (54.13) were obtained with Red-White LEDs and with sucrose at 20 g L−1 and highest fresh weight of PLBs (0.167 g) was obtained with Red-Blue-White LEDs and trehalose (20 g L−1) | Non-evaluated | [60] |
| Phalaenopsis ’RP3’ and ’908’ | Leaf segments (0.4–0.5 cm2) obtained from in vitro shoots | NDM culture medium added 0.25 mg L−1 TDZ (908) or 1.0 mg L−1 NAA, 20.0 mg L−1 BA and 0.125 mg L−1 TDZ (RP3) | Temp 25 ± 2 °C, dark for 60-d (induction) followed by 14-h photoperiod | 45% (908) and 10% (RP3) explants with PLBs; 25 and 2 PLBs/explant respectively | Non-evaluated | [30] |
| Species or Hybrids | Origin and Age of Explants | Culture Media | Growth Conditions | Main Results | Evaluation and Detection of SV | Reference |
|---|---|---|---|---|---|---|
| Oncidium varicosum | Root tips 1.5 mm long from seedlings | Modified VW (replace Fe2(C4H4O6)3 by 27.8 mg L−1 Fe-EDTA + 15% coconut water (PLBs proliferation from PLB) + 1.25 mg L−1 NAA (callus and PLB induction), pH 5.5 | 25 ± 1 °C, Gro-lux bulbs with 16-h photoperiod and 500 lux | Only one callus formed PLB and proliferation of PLBs occurred only in liquid medium with 15% coconut water | Non-evaluated | [119] |
| Oncidium ‘Gower Ramsey’ | Leaf segments 5 mm in length from in vitro plantlets leaves of 2–4 cm and 5–7 cm | MS½ + 100 mg L−1 inositol + niacin and pyridoxine (0.5 mg L-1) + thiamine (0.1 mg L-1) + glycine (2.0 mg L-1), peptone (1000 mg L-1), NaH2PO4 (170 mg L-1), sucrose (20,000 mg L-1) + Gelrite (2,500 mg L-1), pH 5.2 | Temp 26 ± 2 °C, PPFD 28–36 µmol m−2 s−1, daylight fluorescent tubes, 16-h photoperiod | Donor leaves with 5–7 cm long showed higher percentage formed PLBs (25–35%) and number of PLBs/leaf segment (17–24.4) than 2–4 cm donor leaves (15–25% and 5.3–13.0) using 1–3 mg L−1 TDZ; proliferation of PLBs was highest with 0.3 mg L−1 TDZ, and regeneration of PLBs showed best response in absence of PGRs | Non-evaluated | [115] |
| Oncidium ‘Gower Ramsey’ | Leaves 2–4 and 5–7 cm, stem internodes 5mm and root tips 1 cm | MS½ + 100 mg L−1 inositol + niacin and pyridoxine (0,5 mg L-1) + thiamine (0.1 mg L-1)+ glycine (2.0 mg L-1), peptone (1000 mg L-1), NaH2PO4 (170 mg L-1), sucrose (20,000 mg L-1) + Gelrite (2200 mg L-1), pH 5.2: callus phase, 3.0 mg L−1 2,4-D + 3.0 mg L−1 TDZ; PLBs, 0.1 NAA + 3.0 mg L−1 TDZ | Temp 26 ± 1 °C, PPFD 28–36 µmol m−2 s−1 white cool fluorescente, 16-h photoperiod | 10% and 25% callusing from stem and root tips, 3.38 and 3.86 callus proliferation rate from stem and root tips, until 93.8 callus forming embryos and 29.1 embryos/callus from roots | Different callus lines showed large differential response to PLBs induction (0% to 93.8%) and number of PLBs/explant (0 to 29.1) | [117] |
| Oncidium ‘Gower Ramsey’ and O. ‘Sweet Sugar’ | Internodes 5 mm length from 15–20 cm inflorescence length | MS½ + 100 mg L−1 inositol + niacin and pyridoxine (0,5 mg L-1) + thiamine (0.1 mg L-1)+ glycine (2.0 mg L-1), peptone (1000 mg L-1), NaH2PO4 (170 mg L-1), sucrose (20,000 mg L-1) + Gelrite (2200 mg L-1), pH 5.2 | Temp 26 ± 1 °C, PPFD 28–36 µmol m−2 s−1, daylight fluorescent tubes, 16-h photoperiod | TDZ 1–3 mg L−1 increased explants produced PLBs directly in O. Sweet Sugar, but not in O. Gower Ramsey. Callus from explants on NAA + TDZ both at 1.0 mg L−1 showed 19 PLBs/callus. PLBs regeneration into shoots occurred in free-PGR MS½ | Non-evaluated | [116] |
| Oncidium ‘Gower Ramsey’ | Leaf explants 1 cm in length from two-month old donor in vitro plantlets | MS½ + 100 mg L−1 inositol + niacin and pyridoxine (0,5 mg L-1) + thiamine (0.1 mg L-1)+ glycine (2.0 mg L-1), peptone (1000 mg L-1), NaH2PO4 (170 mg L-1), sucrose (20,000 mg L-1) + Gelrite (2200 mg L-1), pH 5.2 | Temp 26 ± 1 °C, PPFD 28–36 µmol m−2 s−1, daylight fluorescent tubes, 16-h photoperiod | Auxins IAA, NAA, IBA and 2,4-D inhibited direct PLB induction, while cytokinins promoted; TDZ 0.3–3.0 mg L−1 increased percentage of explants formed PLBs (60–75% in leaf tips and 25–40% in adaxial surfaces, with 9.5–10.7 PLBs/explant | Non-evaluated | [114] |
| Oncidium bifolium | Leaf segments 4 × 4 mm from germinated seedlings | MS½ + 2% sucrose + 2 g L−1 Phytagel + 1.0 mg L−1 TDZ, pH 5.5 | 27 ± 2 °C, 14-h photoperiod | 25.5% of leaf segments formed PLBs and 12 PLBs/explant | Non-evaluated | [120] |
| Oncidium ‘Gower Ramsey’ | Leaf explants 1-cm length from two month old in vitro donor plantlets | MS½ + 1.0 mg L−1 TDZ, pH 5.2 | Temp 26 ± 1 °C, PPFD 28–36 µmol m−2 s−1, daylight fluorescent tubes, 16-h photoperiod | Leaf tips and leaves with adaxial surface in contact with culture medium was the best region for PLB induction, sucrose at 10–20 g L−1, NaH2PO4 170 mg L−1, peptone 1.0 g L−1 (65–80% explants with PLBs and 10.7 to 11.2 PLBs/explant); | Non-evaluated | [121] |
| Oncidium ‘Gower Ramsey’ | Leaf tips 1-cm length from two month old in vitro donor plantlets | MS½ + 100 mg L−1 inositol + niacin and pyridoxine (0,5 mg L-1) + thiamine (0.1 mg L-1)+ glycine (2.0 mg L-1), peptone (1000 mg L-1), NaH2PO4 (170 mg L-1), sucrose (20,000 mg L-1) + Gelrite (2200 mg L-1), pH 5.2 | Temp 26 ± 1 °C, PPFD 28–36 µmol m−2 s−1, daylight fluorescent tubes, 16-h photoperiod | GA3 inhibited PLB formation, while anti-gibberellins Ancymidol (2.5 mg L−1) and paclobutrazol (10 mg L−1) increased explants formed PLBs (80–87.5% leaf tips formed PLBs and 154.8–193.2 PLBs/petri dish) | Non-evaluated | [118] |
| Oncidium taka | Axillary buds 0.5–1.0 cm lenght | MS + 3% sucrose + 0.7% agar, pH 5.7–5.8. PLBs induction at 1.0 mg L−1 BA + 0.5 mg L−1 NAA; PLBs regeneration at 2.0 mg L−1 BA + 1.0 mg L−1 BA | 26 ± 2 °C, 12-h photoperiod, 3000 lux cool white fluorescent light | 90% explants with PLBs and 9.4 shoots per culture | Non-evaluated | [122] |
| Oncidium ‘Gower Ramsey’ | Shoot tips 2–3 mm length for callus induction and 9-months age callus line for PLBs induction | MS½ + thiamine (1.0 mg L-1) + nicotinic acid and pyridoxine (0.5 mg L-1) + glycine (2.0 mg L-1) + inositol (100 mg L-1) + 2% sucrose + 7.5 g L−1 Agar, pH 5.7: callus proliferation, 1.0 mg L−1 2,4-D + 0.5–1.0 mg L−1 TDZ / PLBs induction, 0.1 mg L−1 NAA and 0.4 mg L−1 BA with sucrose, maltose or trehalose | callus induction and proliferation in dark for 60-d (induction), subcultured every 2-weeks; PLBs induction, Temp 26 ± 2 °C, PPFD 57 µmol m−2 s−1, 16-h photoperiod | 680–732 g callus FW (1.0 mg L−1 2,4-D and 0.5–1.0 mg L−1 TDZ); 1478 PLBs/0.25 g callus (Sucrose 10–20 g L−1); 24 to 52.9 efficiency of plantlet conversion from PLBs (trehalose at 20 g L−1) | Non-evaluated | [123] |
| Oncidium ‘Gower Ramsey’ and O. ‘Sweet Sugar’ | Leave tips 1-cm long from in vitro plantlets | MS½ + 1.0 mg L−1 TDZ | Temp 26 ± 1 °C, PPFD 28–36 µmol m−2 s−1 daylight fluorescent tubes, 16-h photoperiod | Leaf tips and Adaxial region of leaves showed most response to PLB formation; 95% explants with PLBs with 20 g L−1 fructose in two cultivars; 31.1 (O. Sweet Sugar) to 33.7 (O. Gower Ramsey) PLBs/explant with 20 and 30 g L−1 sucrose, respectively | non evaluated | [124] |
| Cut flower varieties of Oncidium | New lateral buds | MS + 25 g L−1 sucrose + 10% coconut water + 7.5 g L−1 agar + 3.0 mg L−1 BA + 0.3 mg L−1 NAA, pH 5.6 | 25 ± 2 °C, 10–12-h photoperiod, 2000–2500 lux cool white fluorescent light | proliferation of 2.96 | Non-evaluated | [125] |
| Oncidium ‘Gower Ramsey’ | PLBs from callus | Method described by ref. [123] using 10 g L−1 maltose | Callus at 26 ± 2 °C in the darkness; PLBs from callus in 50 µmol m−2 s−1 for 16-h photoperiod, under blue (455 nm), red (660 nm) and Far-red (730 nm) | 2986 PLBs under fluorescent lamps statistically equal to red + blue + far red LEDs (3114 PLBs) | Non-evaluated | [126] |
| Oncidium flexuosum | Leaf apices 0.5 cm in length from 4-m seedlings | MS½ + 30 g L−1 sucrose + myo-inositol 100 mg L−1 + 5 g L−1 agar + nicotinic acid and Pyridoxine (0.5 mg L-1) + thiamine (0.1 mg L-1) + glycine (2.0 mg L-1), pH 5.8 | Temp 25 ± 2 °C, PPFD 40 µmol m−2 s−1, 16-h photoperiod | Darkness for 90-d before photoperiod increased explants regenerating PLBs from 5 (Light) to 80% (Dark) and 10.8 PLBs per explant using 1.5 mg L−1 TDZ. Until 29.3 PLBs/explant 60-d after transfer PLBs to free-PGR MS | Non-evaluated | [127] |
| Oncidium ‘Sugar Sweet’ | Shoot tips 0.5 mm length for callus induction and PLBs obtained from callus | Callus: MS½ +2.0 mg L−1 BA + 0.3 mg L−1 NAA + 30 g L−1 sucrose + 7.0 g L−1 agar, pH 5.7; PLBs proliferation in MS + 30 g L−1 sucrose + 1.0 mg L−1 BA + 0.2 mg L−1 NAA, pH 5.8; PLBs regeneration, MS + 2.0 mg L−1 BA + 0.1 mg L−1 NAA + 30 g L−1 sucrose + 7.0 g L−1 agar | PLBs proliferation: 25 °C, 16-h photoperiod, white fluorescent light at 30 µmol m−2 s−1 at 5 l balloon type air lift bioreactor, 20 g fresh weight PLBs per bioreactor | 3335.5 g fresh weight PLBs per vessel and 16.8 growth ratio; until 4.3 shoots/PLB and 1.17 g fresh weight per explant | Non-evaluated | [113] |
| Oncidium ‘Gower Ramsey’ | Shoot tips 5 mm for PLB induction and PLBs sections 3–4 mm diameter | PLBs induction: MS½ + 30 g L−1 sucrose + 6.0 g L−1 agar + 1.0 mg L−1 BA / PLBs proliferation: MS + 30 g L−1 sucrose + 6.0 g L−1 agar + 1.0 mg L−1 BA + 0.5 mg L−1 NAA | Temp 25 ± 2 °C, 16-h photoperiod | Red LEDs (660 nm) resulted in best induction rate (83.3% explants), Fresh weight (≡ 20 g) and propagation rate (>6) of PLBs, while Blue LEDs showed 90% of differentiation rate of PLBs into shoots | Non-evaluated | [109] |
| Oncidium ‘Gower Ramsey’ | Root tips segments 1 cm in length from 6-months old in vitro plantlets | Callus induction: MS½, pH 5.2 / PLB induction: MS½ + 0.1 mg L−1 NAA + 3.0 mg L−1 TDZ | Temp 25 ± 1 °C, darkness | Age of callus from 0.5 to 2 years resulted in best percentage (80–100%) of callus produced PLBs and number of PLBs/callus (6.2–6.6); the increase in age of callus reduced it embryogenesis capacity | Different callus lines showed large differential response to PLBs induction. However, 3-years old plantlets greenhouse cultivated showed same color, size and morphology of O. Gower Ramsey | [128] |
| Oncidium forbesii (Brasilidium forbesii) | Transverse and lateral Thin cell layers 1mm thickness from in vitro germinated protocorms | WPM + 3% sucrose + 0.6% agar, pH 5.8 | Temp 25 ± 1 °C/19 ± 1 °C (day/night), 16-h photoperiod, white fluorescent tubes 40 µmol m−2 s−1 | Lateral thin cell layers in culture medium with BA at 2.0 µM increased PLB induction in 64 to 82% explants and both from lateral and transversal TCL at 1.0 µM promoted the number of PLBs obtained/explant (17.1–24.6) | Non-evaluated | [129] |
| Oncidium ‘Gower Ramsey’ | PLBs sections obtained from nodal explants from inflorescences | MS½ (full strength MS vitamins) + 1 g L−1 tryptone + 20 g L−1 sucrose + 1 g L−1 activated charcoal + 65 g L−1 potato tuber + 8 g L−1 agar + 5 μM TDZ (TDZ, vitamins and glycine were filter sterilized) | Temp 22 ± 2 °C, 16-h photoperiod | PLBs regeneration from PLBs section increased with addition of chloro or methyl or nitro derivatives (compounds 5a–5c) using 2–5 µM, from 41 (control) until 95 plantlets per culture bottle using 5 µM of 5c compound | Non-evaluated | [130] |
| Oncidium sp. (Vu Nu Orchids) | In vitro shoots | MS½ + 20 g L−1 sucrose + 10% coconut water + agar, pH 5.8 | Temp 26 ± 2 °C, PPFD 22.2 µmol m−2 s−1, 12-h photoperiod | NAA 0.75 mg L−1 produced highest number of PLBs/callus (98) and 1 mg L−1 BA promoted PLBs regeneration into shoots (12.42/PLB) | Non-evaluated | [112] |
| Tolumnia Snow Fairy | Leaf segments from different in vitro plantlets height and leaf positions | MS½ (with Fe-NaEDTA, vitamins and glycine at full-strength MS) + 100 mg L−1 myo-inositol + NaH2PO4 (170 mg L−1), 30 g L−1 sucrose + 8.0 g L−1 agar, pH 5.2 | Temp 25 ± 2 °C, 8-weeks in dark and transferred to dim light, PPFD 5 µmol m−2 s−1, cool white fluorescent tubes, 12-h photoperiod | Leaves from 1–2 cm plantlet height showed highest explants induced PLBs using 2.0 mg L−1 BA (16.7%), but highest number of embryos was obtained with 4.0 mg L−1 BA and from plantlets with 2–3 cm (41 PLBs/explant), upper wounding region of bigger PLBs improved PLBs proliferation and number of PLBs per explant | Plants were transferred to plastic pots and flowered after one-year without reports of somaclonal variations in vegetative and reproductive phase | [31] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, J.C.; Zanello, C.A.; Chen, J.-T. An Overview of Orchid Protocorm-Like Bodies: Mass Propagation, Biotechnology, Molecular Aspects, and Breeding. Int. J. Mol. Sci. 2020, 21, 985. https://doi.org/10.3390/ijms21030985
Cardoso JC, Zanello CA, Chen J-T. An Overview of Orchid Protocorm-Like Bodies: Mass Propagation, Biotechnology, Molecular Aspects, and Breeding. International Journal of Molecular Sciences. 2020; 21(3):985. https://doi.org/10.3390/ijms21030985
Chicago/Turabian StyleCardoso, Jean Carlos, Cesar Augusto Zanello, and Jen-Tsung Chen. 2020. "An Overview of Orchid Protocorm-Like Bodies: Mass Propagation, Biotechnology, Molecular Aspects, and Breeding" International Journal of Molecular Sciences 21, no. 3: 985. https://doi.org/10.3390/ijms21030985
APA StyleCardoso, J. C., Zanello, C. A., & Chen, J.-T. (2020). An Overview of Orchid Protocorm-Like Bodies: Mass Propagation, Biotechnology, Molecular Aspects, and Breeding. International Journal of Molecular Sciences, 21(3), 985. https://doi.org/10.3390/ijms21030985






